Epitaxially Integrated Hierarchical ZnO/Au/SrTiO3 and ZnO/Ag/Al2O3 Heterostructures: Three-Dimensional Plasmo-Photonic Nanoarchitecturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Epitaxially Grown Vertical Au and Ag Plasmonic Nanoplatforms
2.2. Synthesis of 3D Hierarchical Au–ZnO and Ag–ZnO Plasmo-Photonic Nanoarchitectures
2.3. Characterization
3. Results
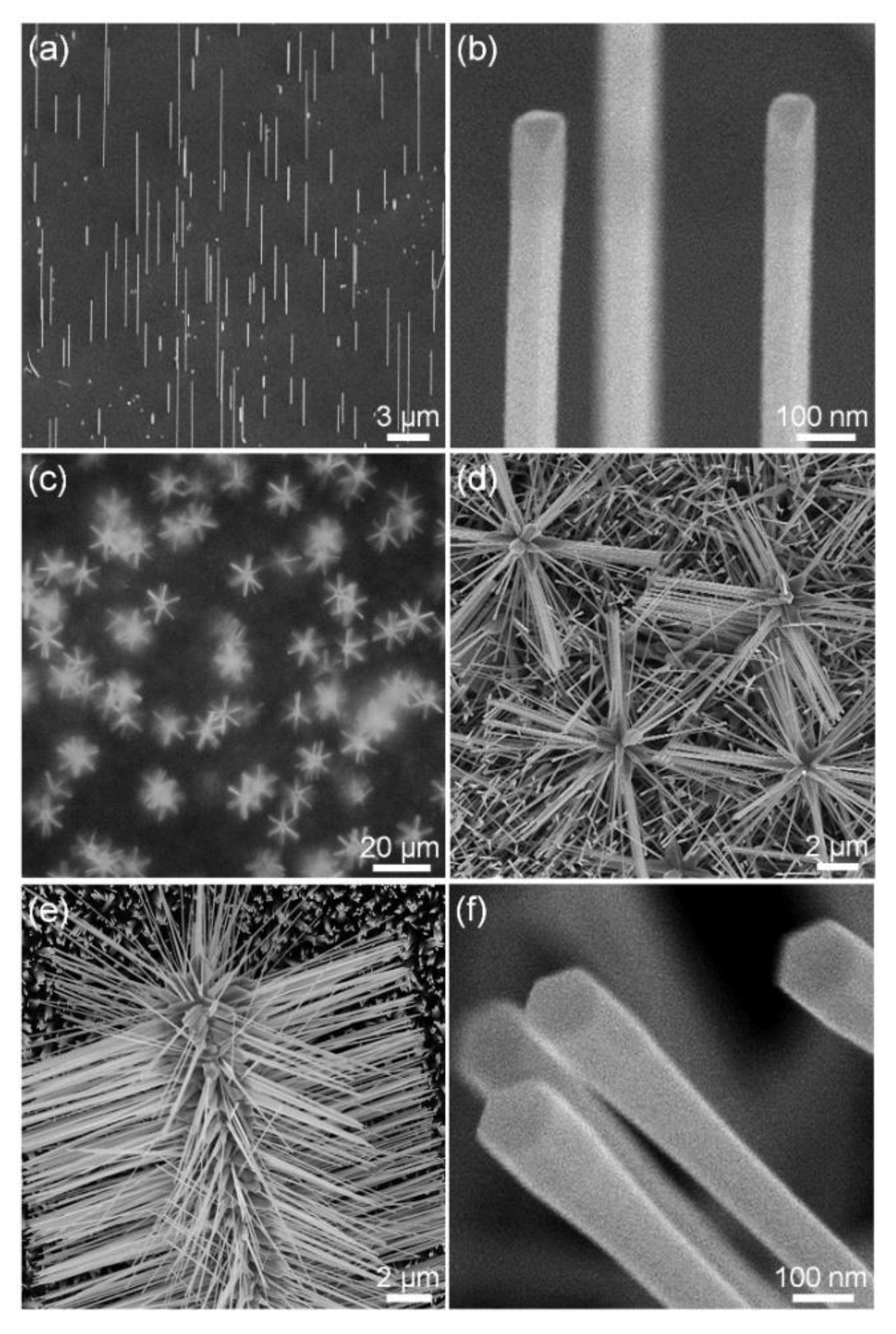
3.1. Vertical Au Nanowires and Three-Dimensional Hierarchical Au–ZnO Nanostructures
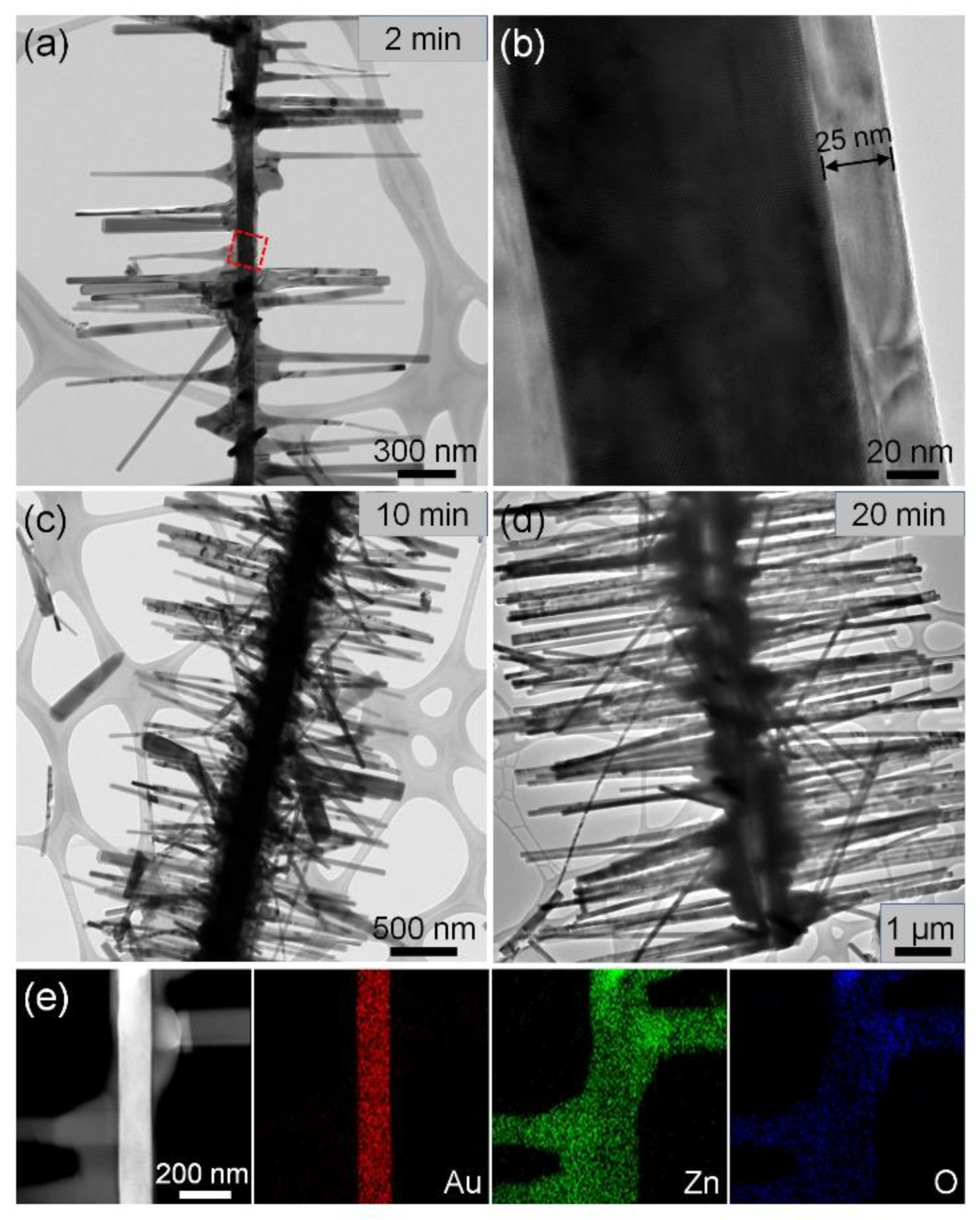
3.2. Growth Process of Three-Dimensional Hierarchical Au–ZnO Nanostructures
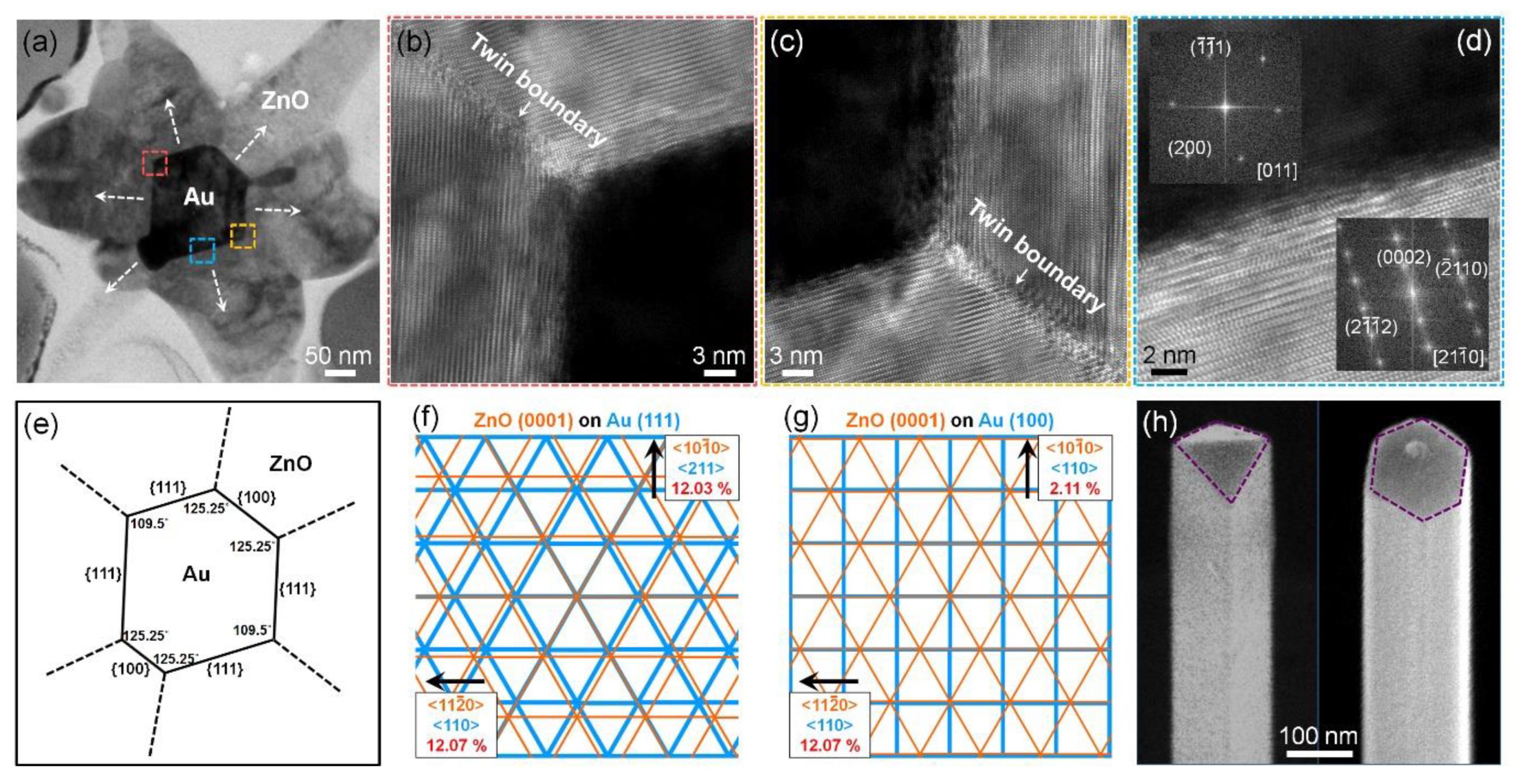
3.3. Internal Crystal Structure of Three-Dimensional Hierarchical Au–ZnO Nanostructures
3.4. Proposed Growth Mechanism of Three-Dimensional Hierarchical Au–ZnO Nanostructures
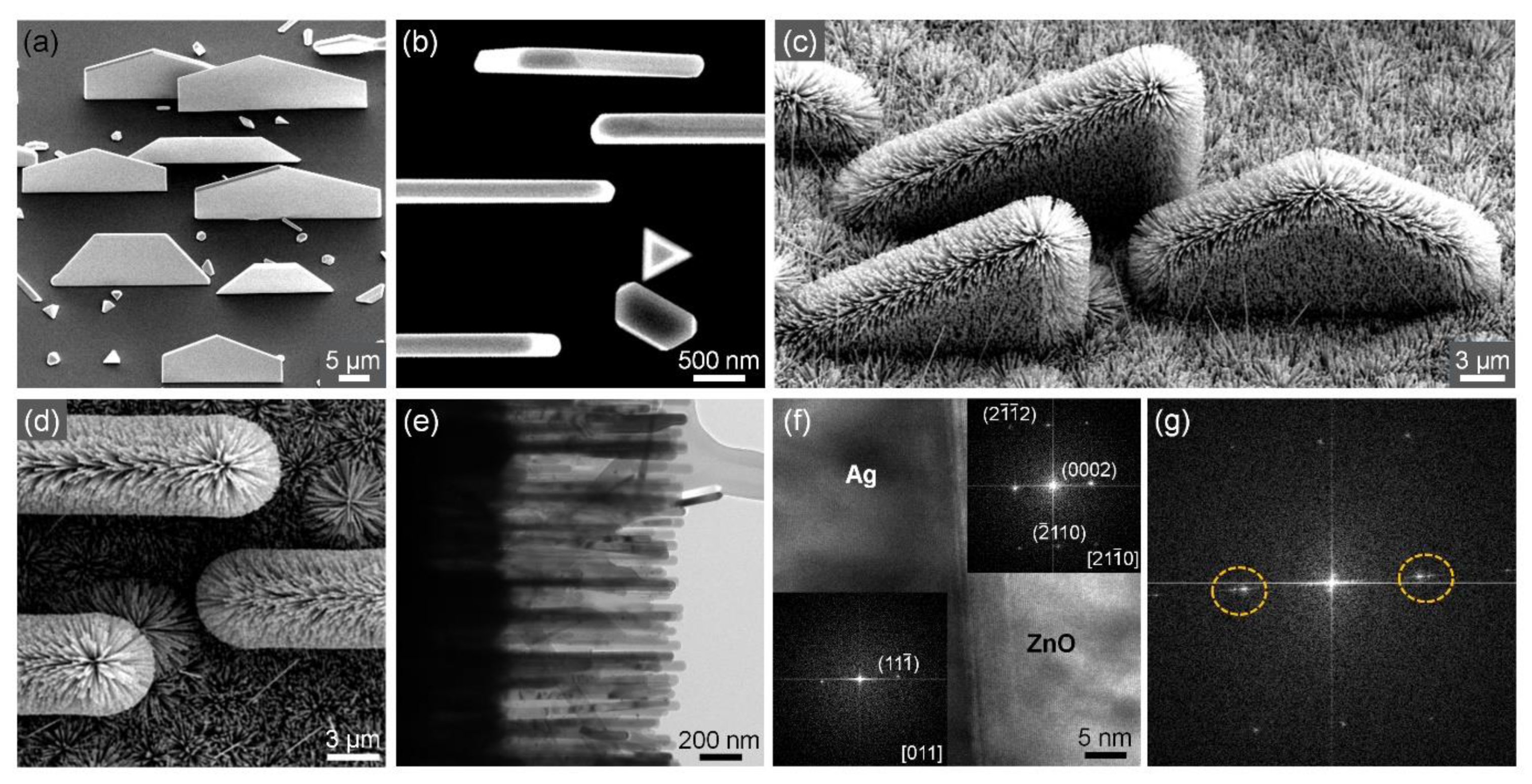
3.5. Vertical Ag Nanoplates and Three-Dimensional Hierarchical Ag-ZnO Nanostructures
3.6. Discussion on Potential Applications of Three-Dimensional Hierarchical Au–ZnO and Ag-ZnO Nanostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bierman, M.J.; Jin, S. Potential applications of hierarchical branching nanowires in solar energy conversion. Energy Environ. Sci. 2009, 2, 1050–1059. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, Y.; Wu, Y.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Sun, L.; Hou, J. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Fang, M.; Dong, G.; Wei, R.; Ho, J.C. Hierarchical Nanostructures: Design for Sustainable Water Splitting. Adv. Energy Mater. 2017, 7, 1700559. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, S. Hierarchical nanostructures of metal oxides for enhancing charge separation and transport in photoelec-trochemical solar energy conversion systems. Nanoscale Horiz. 2016, 1, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Fan, H.J. Branched nanowires: Synthesis and energy applications. Nano Today 2012, 7, 327–343. [Google Scholar] [CrossRef]
- Trepka, B.; Erler, P.; Selzer, S.; Kollek, T.; Boldt, K.; Fonin, M.; Nowak, U.; Wolf, D.; Lubk, A.; Polarz, S. Nanomorphology Effects in Semiconductors with Native Ferromagnetism: Hierarchical Europium (II) Oxide Tubes Prepared via a Topotactic Nanostructure Transition. Adv. Mater. 2017, 30, 1703612. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xiao, Y.; Chen, Y.; Peng, S.; Wang, D.; Hong, T.; Yang, Z.; Sun, Y.; Gao, X.; Zhao, L.-D. Hierarchical structures lead to high thermoelectric performance in Cum+nPb100SbmTe100Se2m (CLAST). Energy Environ. Sci. 2021, 14, 451–461. [Google Scholar] [CrossRef]
- Yan, R.; Pausauskie, P.; Huang, J.; Yang, P. Direct photonic–plasmonic coupling and routing in single nanowires. Proc. Natl. Acad. Sci. USA 2009, 106, 21045–21050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Qiu, M.; Bao, J.; Wiley, B.; Yang, Q.; Zhang, X.; Ma, Y.; Yu, H.; Tong, L. Direct Coupling of Plasmonic and Photonic Nanowires for Hybrid Nanophotonic Components and Circuits. Nano Lett. 2009, 9, 4515–4519. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Jiang, R.; Li, B.; Fang, C.; Wang, J. Metal/Semiconductor Hybrid Nanostructures for Plasmon-Enhanced Applications. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef]
- Thiyagarajan, P.; Ahn, H.-J.; Lee, J.-S.; Yoon, J.-C.; Jang, J.-H. Hierarchical Metal/Semiconductor Nanostructure for Efficient Water Splitting. Small 2013, 9, 2341–2347. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, H.; Marshall, A.F.; Barnett, D.M.; Nix, W.D.; Cui, Y. Formation of chiral branched nanowires by the Eshelby Twist. Nat. Nanotechnol. 2008, 3, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Morin, S.A.; Forticaux, A.; Jin, S. Screw Dislocation Driven Growth of Nanomaterials. Acc. Chem. Res. 2013, 46, 1616–1626. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, B.; Xiang, J.; Qian, F.; Zheng, G.; Wang, H.; Mai, L.; Lieber, C.M. Rational growth of branched nanowire het-erostructures with synthetically encoded properties and function. Proc. Natl. Acad. Sci. USA 2011, 108, 12212–12216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Qian, F.; Yang, C.; Zhong, A.Z.; Lieber, C.M. Rational Growth of Branched and Hyperbranched Nanowire Structures. Nano Lett. 2004, 4, 871–874. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Y.; Chun, J.; Hu, S.; Tang, M.; Li, D. Effects of catalyst droplets on wire growth and the resulting branched structures during VLS growth. Nanoscale 2020, 12, 7538–7543. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Y.; Zhang, T.; Chen, G.; Zhang, F.; Liu, D.; Cai, W.; Li, Y.; Yang, X.; Li, C. Complete Au@ZnO core–shell nano-particles with enhanced plasmonic absorption enabling significantly improved photocatalysis. Nanoscale 2016, 8, 10774–10782. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.; Yan, Y.; Yang, L.; Li, X.; Lu, Z.; Zhai, H.; Han, D.; Huo, P. Two Hybrid Au–ZnO Heterostructures with Different Hierarchical Structures: Towards Highly Efficient Photocatalysts. Sci. Rep. 2019, 9, 16863. [Google Scholar] [CrossRef]
- Kavitha, R.; Kumar, S.G. A review on plasmonic Au–ZnO heterojunction photocatalysts: Preparation, modifications and re-lated charge carrier dynamics. Mater. Sci. Semicond. Process. 2019, 93, 59–91. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Tsai, C.-Y.; Chen, H.-A.; Liang, Y.-T.; Chen, K.-P.; Gradečak, S.; Gwo, S.; Chen, L.-J. Plasmonic enhancement of Au nanoparticle—Embedded single-crystalline ZnO nanowire dye-sensitized solar cells. Nano Energy 2016, 20, 264–271. [Google Scholar] [CrossRef]
- Herman, I.; Yeo, J.; Hong, S.; Lee, D.; Nam, K.H.; Choi, J.-H.; Hong, W.-H.; Lee, D.; Grigoropoulos, C.; Ko, S.H. Hierarchical weeping willow nano-tree growth and effect of branching on dye-sensitized solar cell efficiency. Nanotechnology 2012, 23, 194005. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; López-Puente, V.; Wang, Q.; Polavarapu, L.; Pastoriza-Santos, I.; Xu, Q.-H. Plasmon-enhanced light harvesting: Applications in enhanced photocatalysis, photodynamic therapy and photovoltaics. RSC Adv. 2015, 5, 29076–29097. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ueno, K.; Oshikiri, T.; Sun, Q.; Sasaki, K.; Misawa, H. Enhanced water splitting under modal strong coupling conditions. Nat. Nanotechnol. 2018, 13, 953–958. [Google Scholar] [CrossRef]
- Mascaretti, L.; Dutta, A.; Kment, Š.; Shalaev, V.M.; Boltasseva, A.; Zbořil, R.; Naldoni, A. Plasmon-Enhanced Photoelectro-chemical Water Splitting for Efficient Renewable Energy Storage. Adv. Mater. 2019, 31, 1805513. [Google Scholar] [CrossRef]
- Yoo, Y.; Seo, K.; Han, S.; Varadwaj, K.S.K.; Kim, H.Y.; Ryu, J.H.; Lee, H.M.; Ahn, J.P.; Ihee, H.; Kim, B. Steering Epitaxial Alignment of Au, Pd, and AuPd Nanowire Arrays by Atom Flux Change. Nano Lett. 2010, 10, 432–438. [Google Scholar] [CrossRef]
- Yoo, Y.; Kim, S.-i.; Han, S.; Lee, H.; Kim, J.; Kim, H.S.; Ahn, J.-P.; Kang, T.; Choo, J.; Kim, B. Epitaxially aligned submillime-ter-scale silver nanoplates grown by simple vapor transport. Nanoscale 2019, 11, 17436–17443. [Google Scholar] [CrossRef]
- Güniat, L.; Caroff, P.; Fontcuberta i Morral, A. Vapor Phase Growth of Semiconductor Nanowires: Key Developments and Open Questions. Chem. Rev. 2019, 119, 8958–8971. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, S.J.; Yu, T.; Wu, T. Controlling the Growth Mechanism of ZnO Nanowires by Selecting Catalysts. J. Phys. Chem. C 2007, 111, 17500–17505. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Yan, B.; Yu, T. Growth mechanism and diameter control of well-aligned small-diameter ZnO nanowire arrays synthesized by a catalyst-free thermal evaporation method. Nanotechnology 2009, 20, 495604. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.; Kim, M.; Kim, B. Epitaxially Integrated Hierarchical ZnO/Au/SrTiO3 and ZnO/Ag/Al2O3 Heterostructures: Three-Dimensional Plasmo-Photonic Nanoarchitecturing. Nanomaterials 2021, 11, 3262. https://doi.org/10.3390/nano11123262
Yoo Y, Kim M, Kim B. Epitaxially Integrated Hierarchical ZnO/Au/SrTiO3 and ZnO/Ag/Al2O3 Heterostructures: Three-Dimensional Plasmo-Photonic Nanoarchitecturing. Nanomaterials. 2021; 11(12):3262. https://doi.org/10.3390/nano11123262
Chicago/Turabian StyleYoo, Youngdong, Minjung Kim, and Bongsoo Kim. 2021. "Epitaxially Integrated Hierarchical ZnO/Au/SrTiO3 and ZnO/Ag/Al2O3 Heterostructures: Three-Dimensional Plasmo-Photonic Nanoarchitecturing" Nanomaterials 11, no. 12: 3262. https://doi.org/10.3390/nano11123262
APA StyleYoo, Y., Kim, M., & Kim, B. (2021). Epitaxially Integrated Hierarchical ZnO/Au/SrTiO3 and ZnO/Ag/Al2O3 Heterostructures: Three-Dimensional Plasmo-Photonic Nanoarchitecturing. Nanomaterials, 11(12), 3262. https://doi.org/10.3390/nano11123262






