Light-Excited Ag-Doped TiO2−CoFe2O4 Heterojunction Applied to Toluene Gas Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Material Characterization
2.3. Sensing Performance Evaluation
3. Results and Discussion
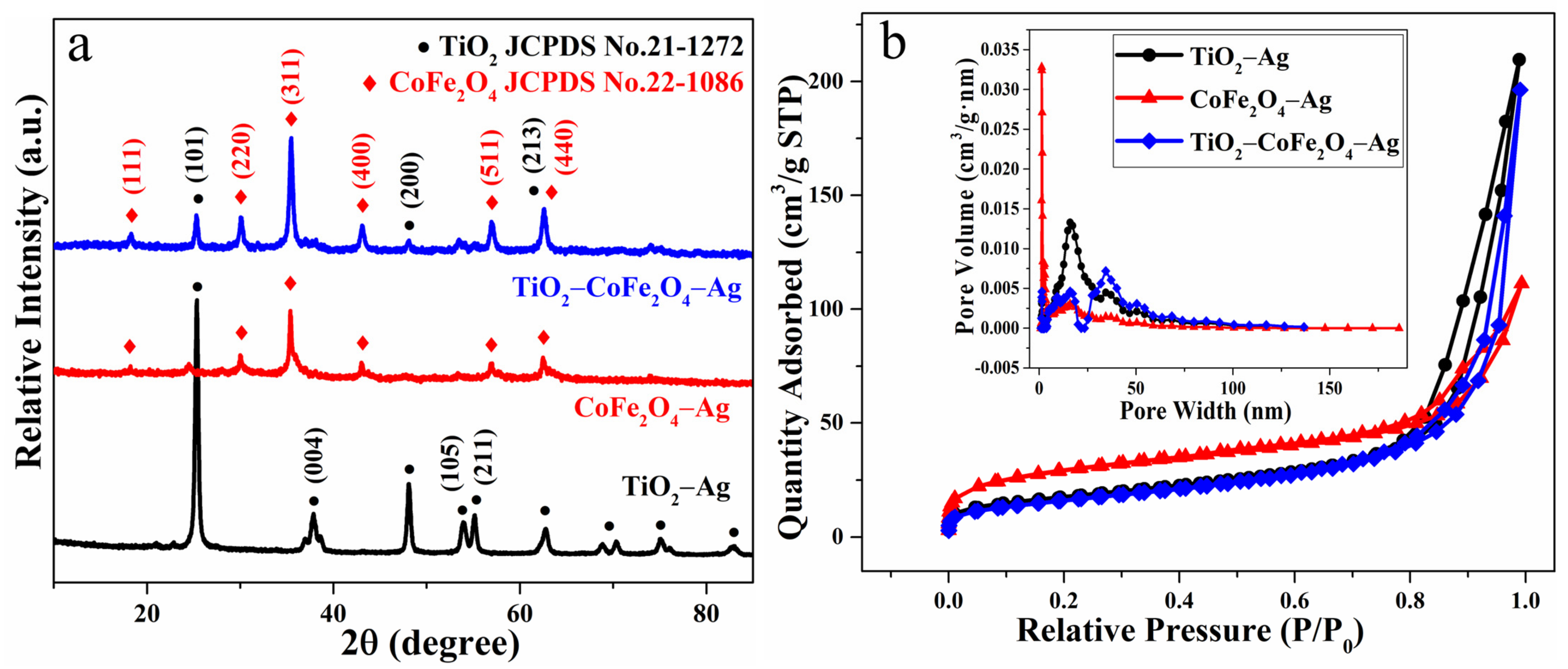
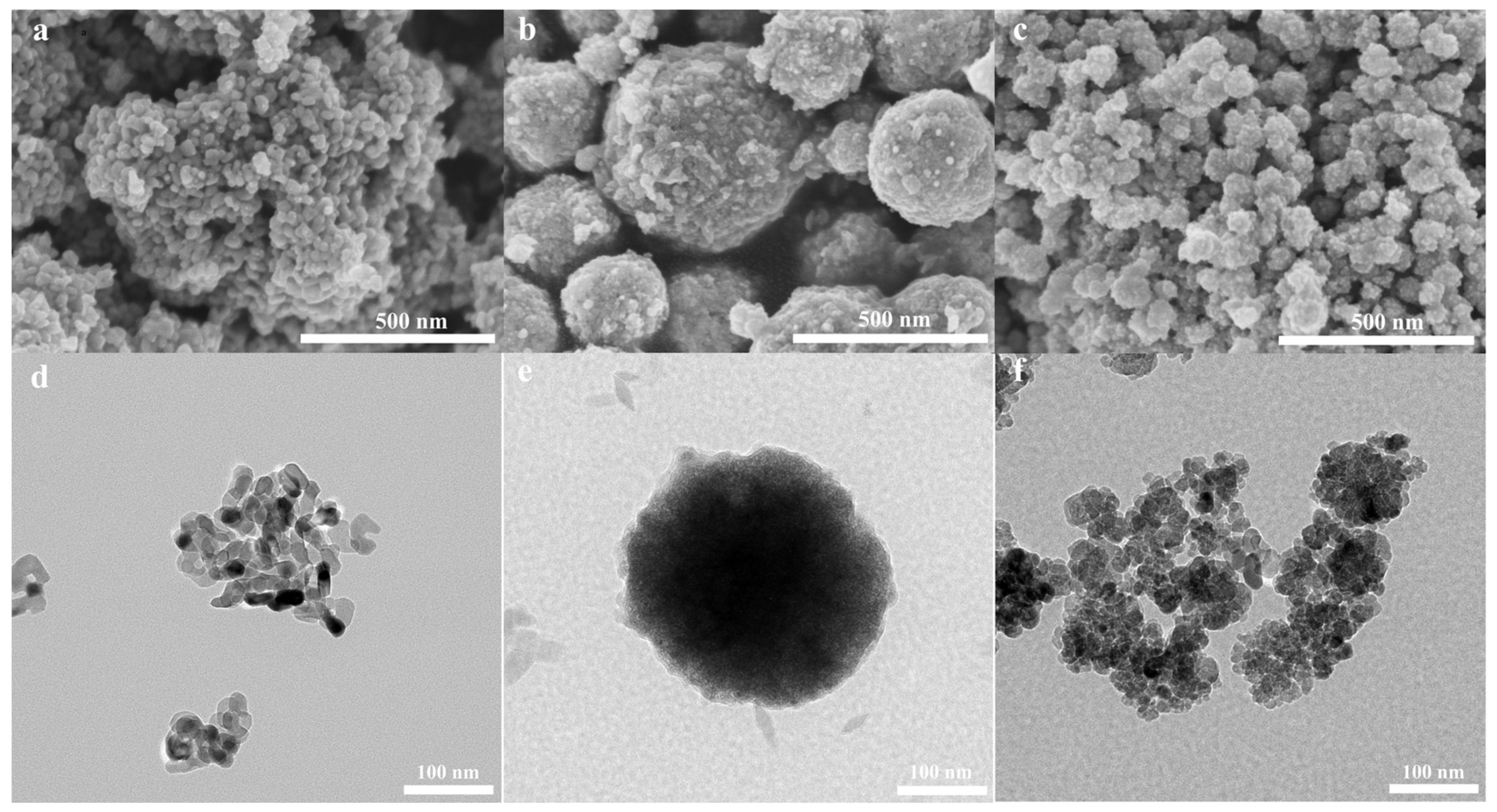
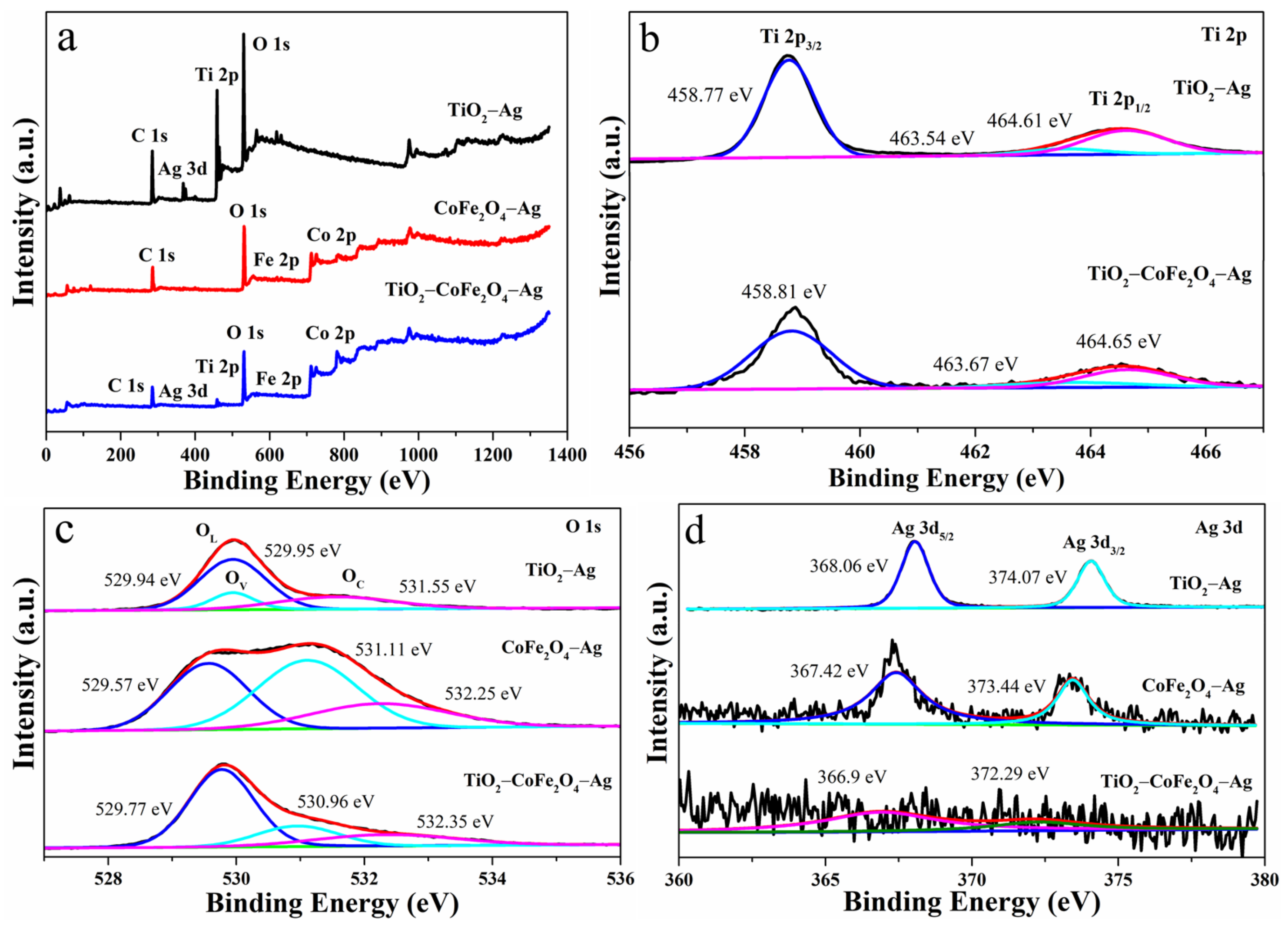
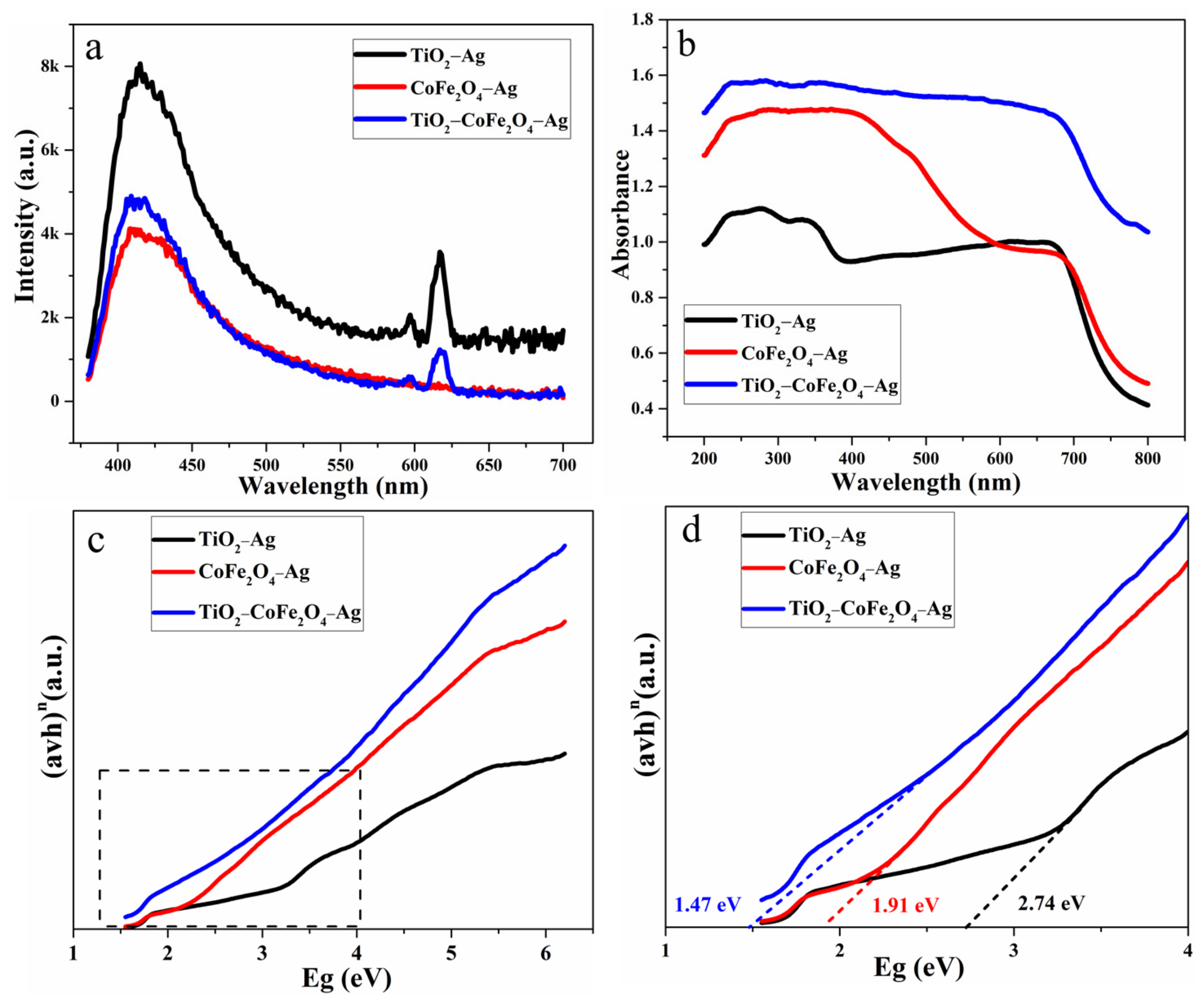
3.1. Structural and Morphological Characterization
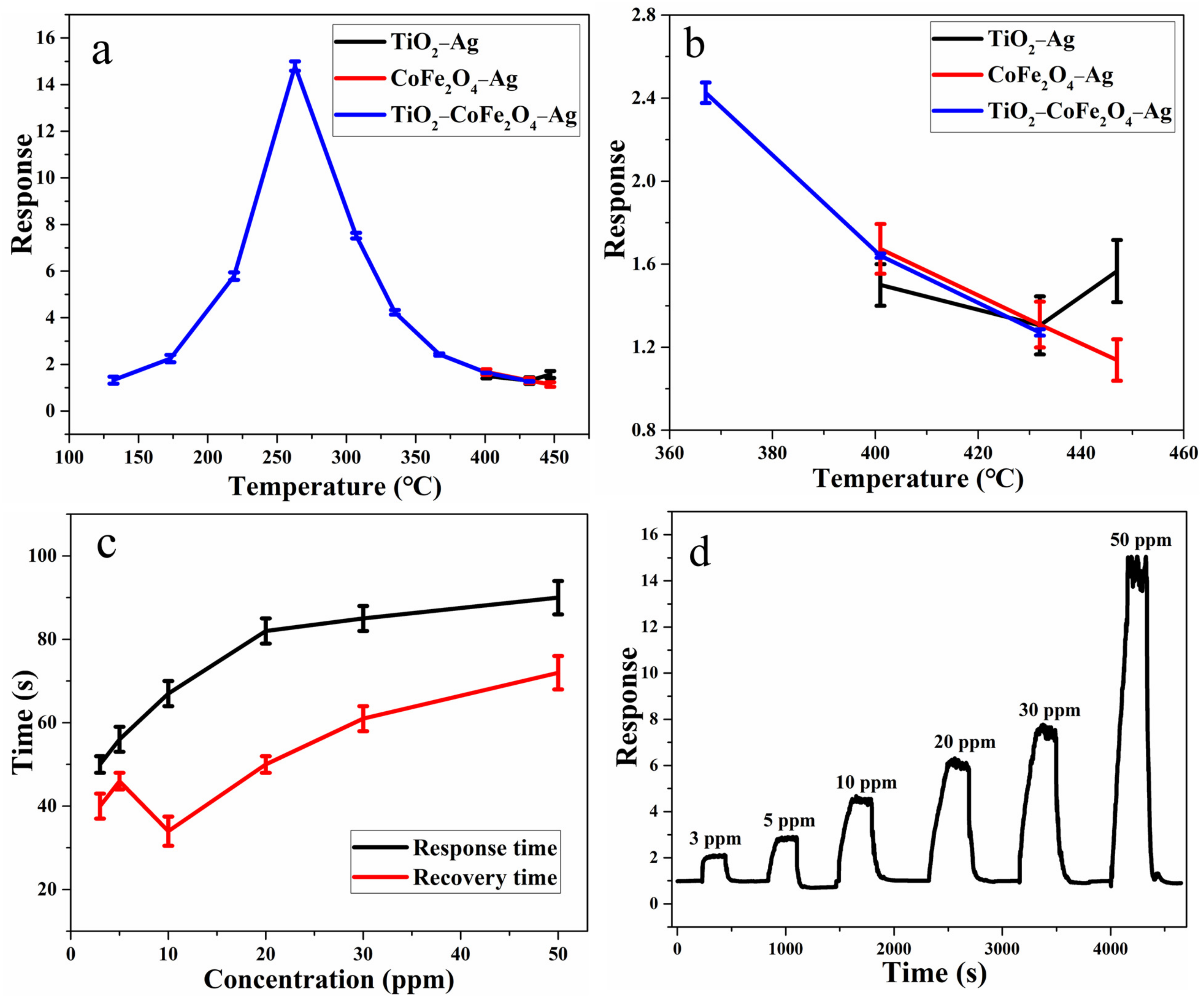
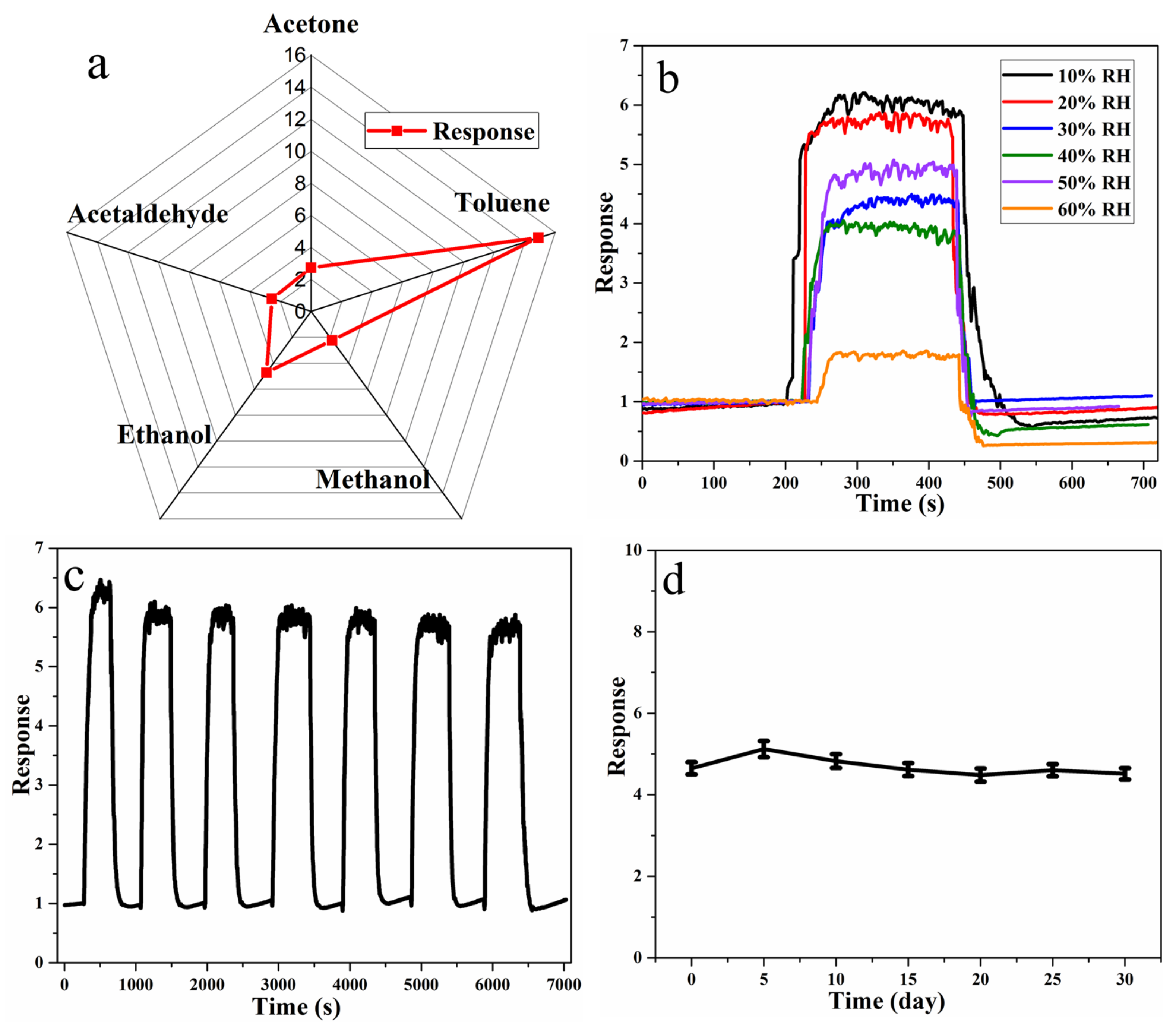
3.2. Gas-Sensing Characteristics
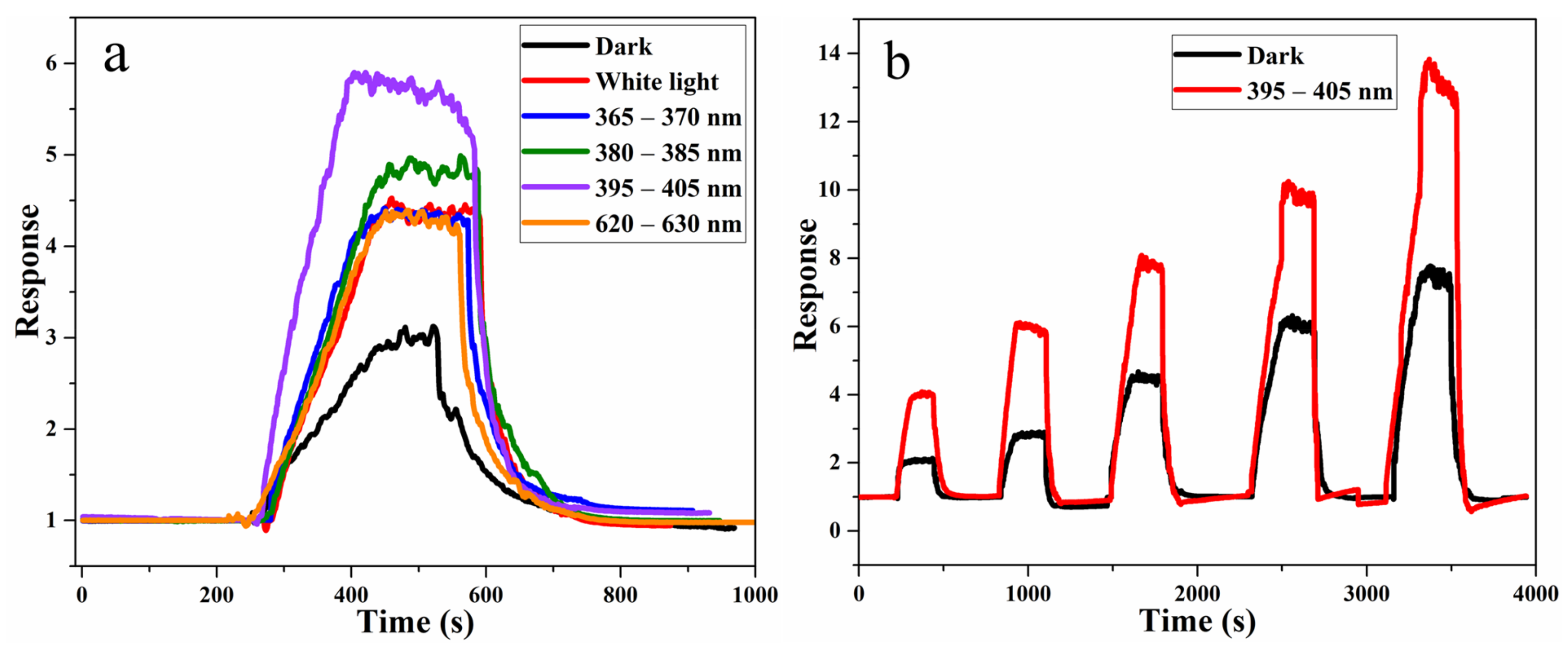
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.; Sun, Y.; Tan, Z.; Xu, X.; Fu, Y.; Feng, Z.; Zhu, J. Fabrication of cubic Co3O4-hexagonal ZnO disk/rGO as a two-phase benzaldehyde sensor via a sequential nucleation strategy. Sens. Actuators B 2021, 330, 129384–129396. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Chaudhary, V.; Dahiya, M.S.; Rana, P.S.; Nehra, S.P.; Duhan, S. Facile synthesis of hybridized mesoporous Au@TiO2/SnO2 as efficient photocatalyst and selective VOC sensor. ChemistrySelect 2016, 1, 3247–3258. [Google Scholar] [CrossRef]
- Fiedot-Tobola, M.; Suchorska-Wozniak, P.; Startek, K.; Rac-Rumijowska, O.; Szukiewicz, R.; Kwoka, M.; Teterycz, H. Correlation between microstructure and chemical composition of zinc oxide gas sensor layers and their gas-sensitive properties in chlorine atmosphere. Sensors 2020, 20, 6951. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jeong, S.Y.; Moon, Y.K.; Lee, J.H. Dual-mode gas sensor for ultrasensitive and highly selective detection of xylene and toluene using Nb-doped NiO hollow spheres. Sens. Actuators B 2019, 301, 127140–127178. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Gu, C.; Wang, W.; Geng, B.; Sun, Y.; Liu, J. Effective VOCs gas sensor based on porous SnO2 microcubes prepared via spontaneous phase segregation. Sens. Actuators B 2012, 173, 599–606. [Google Scholar] [CrossRef]
- Wang, X.; Ren, P.; Tian, H.; Fan, H.; Cai, C.; Liu, W. Enhanced gas sensing properties of SnO2: The role of the oxygen defects induced by quenching. J. Alloys Compd. 2016, 669, 29–37. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Xu, M.; Hu, X.; Zhang, H.; Wang, Y.; Huang, W. A Au-functionalized ZnO nanowire gas sensor for detection of benzene and toluene. Phys. Chem. Chem. Phys. 2013, 15, 17179–17186. [Google Scholar] [CrossRef]
- Peng, C.; Guo, J.; Yang, W.; Shi, C.; Liu, M.; Zheng, Y.; Xu, J.; Chen, P.; Huang, T.; Yang, Y. Synthesis of three-dimensional flower-like hierarchical ZnO nanostructure and its enhanced acetone gas sensing properties. J. Alloys Compd. 2016, 654, 371–378. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, X.; Wang, J.; Gaskov, A.M.; Akbar, S.A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators B 2016, 230, 330–336. [Google Scholar] [CrossRef]
- Fomekong, R.L.; Kelm, K.; Saruhan, B. High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method. Sensors 2020, 20, 5992. [Google Scholar] [CrossRef]
- Ma, J.; Ren, Y.; Zhou, X.; Liu, L.; Zhu, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: Gas sensing performance and mechanism study. Adv. Funct. Mater. 2018, 1705268–1705280. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Song, L.; Gong, H.; Niu, Q. Ionic liquid-assisted synthesis of WO3 particles with enhanced gas sensing properties. J. Mater. Chem. A 2013, 1, 15377–15382. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, H.; Gao, X.; Wang, Y. Fabrication of porous α-Fe2O3 nanoshuttles and their application for toluene sensors. RSC Adv. 2014, 4, 30840–30850. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Luan, Y.; Li, J.; Song, A. In(OH)3 particles from an ionic liquid precursor and their conversion to porous In2O3 particles for enhanced gas sensing properties. CrystEngComm 2013, 15, 1659–1822. [Google Scholar]
- Naberezhnyi, D.; Rumyantseva, M.; Filatova, D.; Batuk, M.; Hadermann, J.; Baranchikov, A.; Khmelevsky, N.; Aksenenko, A.; Konstantinova, E.; Gaskov, A. Effects of Ag Additive in Low Temperature CO Detection with In2O3 Based Gas Sensors. Nanomaterials 2018, 8, 801. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Huang, B.; Zhou, J.; Xie, E. Synthesis of porous Co3O4 nanonetworks to detect toluene at low concentration. Phys. Chem. Chem. Phys. 2014, 16, 19327–19332. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yi, G.; Li, H.; Shi, C.; Sun, G.; Zhang, Z. Hydrothermal Synthesis of Co3O4/ZnO Hybrid Nanoparticles for Triethylamine Detection. Nanomaterials 2019, 9, 1599. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Xin, F.; Yin, X. Photocatalytic reduction of carbon dioxide over ZnFe2O4/TiO2 nanobelts heterostructure in cyclohexanol. J. Colloid Interface Sci. 2015, 442, 60–66. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.Q.; Chen, Z.; Chi, L. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef]
- Luan, V.H.; Tien, H.N.; Hur, S.H.; Han, J.H.; Lee, W. Three-Dimensional Porous Nitrogen-Doped NiO Nanostructures as Highly Sensitive NO2 Sensors. Nanomaterials 2017, 7, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Wang, C.; Liu, J.; Zhou, X.; Li, X.; Hu, X.; Lu, G. Hierarchical assembly of alpha-Fe2O3 nanosheets on SnO2 hollow nanospheres with enhanced ethanol sensing properties. ACS Appl. Mater. Inter. 2015, 7, 19119–19125. [Google Scholar] [CrossRef]
- Cui, S.; Wen, Z.; Huang, X.; Chang, J.; Chen, J. Stabilizing MoS2 nanosheets through SnO2 nanocrystal decoration for high-performance gas sensing in air. Small 2015, 11, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, Y.E.; Li, P.; Zhang, Y. Facile fabrication of MoS2-modified SnO2 hybrid nanocomposite for ultrasensitive humidity sensing. ACS Appl. Mater. Inter. 2016, 8, 14142–14182. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qiao, Y.; Huang, J.; Yao, H.; Zhang, Y.; Li, Y.; Du, J.; Fan, W. One-pot synthesis of mesoporous spherical SnO2@graphene for high-sensitivity formaldehyde gas sensors. RSC Adv. 2016, 6, 25198–25202. [Google Scholar] [CrossRef]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive room-temperature H2S gas sensors employing SnO2 quantum wire/reduced graphene oxide nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Huang, B.; Li, X. UV-activated formaldehyde sensing properties of hollow TiO2@SnO2 heterojunctions at room temperature. Sens. Actuators B 2020, 319, 128264–128274. [Google Scholar] [CrossRef]
- Platonov, V.; Rumyantseva, M.; Khmelevsky, N.; Gaskov, A. Electrospun ZnO/Pd nanofibers: CO sensing and humidity effect. Sensors 2020, 20, 7333. [Google Scholar] [CrossRef]
- Pan, F.; Lin, H.; Zhai, H.; Miao, Z.; Zhang, Y.; Xu, K.; Guan, B.; Huang, H.; Zhang, H. Pd-doped TiO2 film sensors prepared by premixed stagnation flames for CO and NH3 gas sensing. Sens. Actuators B 2018, 261, 451–459. [Google Scholar] [CrossRef]
- Tamgadge, R.M.; Shukla, A. Fluorine-doped anatase for improved supercapacitor electrode. Electrochim. Acta 2018, 289, 342–353. [Google Scholar] [CrossRef]
- Qiu, C.; Shen, J.; Lin, J.; Liu, D.; Li, D.; Zhang, J.; Zhang, Z.; Lin, H.; Wang, X.; Fu, X. Construction of the rutile/anatase micro-heterophase junction photocatalyst from anatase by liquid nitrogen quenching method. ACS Appl. Energy Mater. 2021, 4, 10172–10186. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Joshi, N.; Dankwort, T.; Lin, L.; Kienle, L. Au–TiO2-loaded cubic g-C3N4 nanohybrids for photocatalytic and volatile organic amine sensing applications. ACS Appl. Mater. Inter. 2018, 10, 34087–34097. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Song, X.; Li, T.; Liu, G.; Chen, Z.; Dong, Z.; Liu, Y. Sized dependence and microstructural defects on highly photocatalytic activity based on multisized CdTe quantum dots sensitized TiO2. Surf. Interface Anal. 2019, 51, 968–981. [Google Scholar] [CrossRef]
- Monamary, A.; Vijayalakshmi, K.; Jereil, S.D. Fe overlayered hybrid TiO2/ITO nanocomposite sensor for enhanced hydrogen sensing at room temperature by novel two step process. Sens. Actuators B 2019, 287, 278–289. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997–1902023. [Google Scholar] [CrossRef]
- Ullah, K.; Meng, Z.-D.; Ye, S.; Zhu, L.; Oh, W.-C. Synthesis and characterization of novel PbS–graphene/TiO2 composite with enhanced photocatalytic activity. J. Ind. Eng. Chem. 2014, 20, 1035–1042. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, S.; Li, K.; Han, B.; Chen, Y.; Pang, B.; Yu, L.; Dong, L. The favourable synergistic operation of photocatalysis and catalytic oxygen reduction reaction by a novel heterogeneous CoFe2O4-TiO2 nanocomposite. Appl. Surf. Sci. 2020, 516, 146142–146155. [Google Scholar] [CrossRef]
- Sagu, J.S.; Wijayantha, K.G.U.; Tahir, A.A. The pseudocapacitive nature of CoFe2O4 thin films. Electrochim. Acta 2017, 246, 870–878. [Google Scholar] [CrossRef] [Green Version]
- Thu Do, T.A.; Giang, H.T.; Van Huong, D.; Ngan, P.Q.; Thai, G.H.; Thu, D.T.; Lam, T.D. Correlation between photoluminescence spectra with gas sensing and photocatalytic activities in hierarchical ZnO nanostructures. RSC Adv. 2017, 7, 9826–9832. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Ag nanoparticles modified TiO2 spherical heterostructures with enhanced gas-sensing performance. Sens. Actuators B 2011, 155, 716–721. [Google Scholar] [CrossRef]
- Zhang, X.; Song, D.; Liu, Q.; Chen, R.; Hou, J.; Liu, J.; Zhang, H.; Yu, J.; Liu, P.; Wang, J. Designed synthesis of Ag-functionalized Ni-doped In2O3 nanorods with enhanced formaldehyde gas sensing properties. J. Mater. Chem. C 2019, 7, 7219–7229. [Google Scholar] [CrossRef]
- Nakarungsee, P.; Srirattanapibul, S.; Issro, C.; Tang, I.M.; Thongmee, S. High performance Cr doped ZnO by UV for NH3 gas sensor. Sens. Actuators A 2020, 314, 112230–112261. [Google Scholar] [CrossRef]
- Raghu, A.V.; Karuppanan, K.K.; Pullithadathil, B. Controlled carbon doping in anatase TiO2(101) facets: Superior trace-level ethanol gas sensor performance and adsorption kinetics. Adv. Mater. Interfaces 2019, 6, 1801714–1801726. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 nanomaterials: A review of recent advances. Chem. Eng. J. 2018, 297–395. [Google Scholar] [CrossRef]
- Šutka, A.; Käämbre, T.; Pärna, R.; Döbelin, N.; Vanags, M.; Smits, K.; Kisand, V. Ag sensitized TiO2 and NiFe2O4 three-component nanoheterostructures: Synthesis, electronic structure and strongly enhanced visible light photocatalytic activity. RSC Adv. 2016, 6, 18834–18842. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Jian, X.; Xu, J.; Gao, Z.; Song, Y.-Y. NIR light-driven photocatalysis on amphiphilic TiO2 nanotubes for controllable drug release. ACS Appl. Mater. Inter. 2020, 12, 23606–23616. [Google Scholar] [CrossRef]
- Shaikh, S.K.; Ganbavle, V.V.; Inamdar, S.I.; Rajpure, K.Y. Multifunctional zinc oxide thin films for high-performance UV photodetectors and nitrogen dioxide gas sensors. RSC Adv. 2016, 6, 25641–25650. [Google Scholar] [CrossRef]
- Lei, T.; Rao, Z.; Zhang, S.; Cai, S.; Xie, C. The irreversible R-T curves of metal oxide gas sensor under programmed temperature cycle. Sens. Actuators B 2016, 235, 481–491. [Google Scholar] [CrossRef]
- Tonezzer, M.; Le, D.T.T.; Iannotta, S.; Van Hieu, N. Selective discrimination of hazardous gases using one single metal oxide resistive sensor. Sens. Actuators B 2018, 277, 121–128. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Sun, S.; Wang, J.; Yan, W. One-dimensional nanomaterials in resistive gas sensor: From material design to application. Chemosensors 2021, 9, 198. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zeng, W. Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl. Surf. Sci. 2018, 427, 281–287. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Shimizu, F.M.; Mastelaro, V.R.; M’Peko, J.C.; Lin, L.; Oliveira, O.N., Jr. UV-assisted chemiresistors made with gold-modified ZnO nanorods to detect ozone gas at room temperature. Mikrochim. Acta 2019, 186, 418–427. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, S.; Fei, T.; Liu, S.; Zhang, T. Construction of ZnO/SnO2 heterostructure on reduced graphene oxide for enhanced nitrogen dioxide sensitive performances at room temperature. ACS Sens. 2019, 4, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Tian, K.; Fu, H.; Feng, Y.; Luo, R.; Li, D.; Chen, A.; Liu, C.C. Novel α-Fe2O3/BiVO4 heterojunctions for enhancing NO2 sensing properties. Sens. Actuators B 2018, 268, 136–143. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent advances in emerging 2D material-based gas sensors: Potential in disease diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329–1901356. [Google Scholar] [CrossRef]
- Noh, J.; Kwon, S.H.; Park, S.; Kim, K.K.; Yoon, Y.J. TiO2 nanorods and Pt nanoparticles under a UV-LED for an NO2 gas sensor at room temperature. Sensors 2021, 21, 1826. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Wu, Z.; Guo, J.; Jia, D. Ni-doped ZnS nanospheres decorated with Au nanoparticles for highly improved gas sensor performance. Sensors 2018, 18, 2882. [Google Scholar] [CrossRef] [Green Version]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: A combined experimental and first-principle study. Sens. Actuators B 2019, 296, 126710–126720. [Google Scholar] [CrossRef]
- Mirzaei, A.; Bang, J.H.; Oum, W.; Kwon, Y.J.; Kim, J.H.; Choi, S.W.; Kim, S.S.; Kim, H.W. Selective H2S-sensing performance of Si nanowires through the formation of ZnO shells with Au functionalization. Sens. Actuators B 2019, 289, 1–14. [Google Scholar]
- Horprathum, M.; Srichaiyaperk, T.; Samransuksamer, B.; Wisitsoraat, A.; Eiamchai, P.; Limwichean, S.; Chananonnawathorn, C.; Aiempanakit, K.; Nuntawong, N.; Patthanasettakul, V. Ultrasensitive hydrogen sensor based on Pt-decorated WO nanorods prepared by glancing-angle dc magnetron sputtering. ACS Appl. Mater. Inter. 2014, 6, 22051–22060. [Google Scholar] [CrossRef]
- Yang, X.; Fu, H.; Tian, Y.; Xie, Q.; Xiong, S.; Han, D.; Zhang, H.; An, X. Au decorated In2O3 hollow nanospheres: A novel sensing material toward amine. Sens. Actuators B 2019, 296, 126696–126706. [Google Scholar] [CrossRef]
- Yang, X.; Fu, H.; Zhang, L.; An, X.; Xiong, S.; Jiang, X.; Yu, A. Enhanced gas sensing performance based on the fabrication of polycrystalline Ag@TiO2 core-shell nanowires. Sens. Actuators B 2019, 286, 483–492. [Google Scholar] [CrossRef]
- Reddeppa, M.; Mitta, S.B.; Park, B.-G.; Kim, S.-G.; Park, S.H.; Kim, M.-D. DNA-CTMA functionalized GaN surfaces for NO2 gas sensor at room temperature under UV illumination. Org. Electron. 2019, 65, 334–340. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Liu, J.; Peng, Z.; Zhou, J.; Zhang, H.; Li, Y. Optoelectronic gas sensor based on few-layered InSe nanosheets for NO2 detection with ultrahigh antihumidity ability. Anal. Chem. 2020, 92, 11277–11287. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. 2D Nanostructures of CoFe2O4 and NiFe2O4: Efficient Oxygen Evolution Catalyst. Electrochim. Acta 2018, 273, 462–473. [Google Scholar] [CrossRef]

| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| TiO2−Ag | 63.21 | 0.324 | 20.21 |
| CoFe2O4−Ag | 102.75 | 0.155 | 8.84 |
| TiO2−CoFe2O4−Ag | 59.04 | 0.304 | 19.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, L.; Kang, Y.; Yu, F. Light-Excited Ag-Doped TiO2−CoFe2O4 Heterojunction Applied to Toluene Gas Detection. Nanomaterials 2021, 11, 3261. https://doi.org/10.3390/nano11123261
Wang W, Zhang L, Kang Y, Yu F. Light-Excited Ag-Doped TiO2−CoFe2O4 Heterojunction Applied to Toluene Gas Detection. Nanomaterials. 2021; 11(12):3261. https://doi.org/10.3390/nano11123261
Chicago/Turabian StyleWang, Wenhao, Lu Zhang, Yanli Kang, and Feng Yu. 2021. "Light-Excited Ag-Doped TiO2−CoFe2O4 Heterojunction Applied to Toluene Gas Detection" Nanomaterials 11, no. 12: 3261. https://doi.org/10.3390/nano11123261
APA StyleWang, W., Zhang, L., Kang, Y., & Yu, F. (2021). Light-Excited Ag-Doped TiO2−CoFe2O4 Heterojunction Applied to Toluene Gas Detection. Nanomaterials, 11(12), 3261. https://doi.org/10.3390/nano11123261







