Abstract
Since Dietl et al. predicted that Co-doped ZnO may show room-temperature ferromagnetism (RTFM) in 2000, researchers have focused on the investigation of ferromagnetic ZnO doped with various transition metals. However, after decades of exploration, it has been found that undoped ZnO nanostructures can also show RTFM, which in general is dependent on ZnO morphologies. Here, we will give an overall review on undoped ZnO nanomaterials with RTFM. The advanced strategies to achieve multidimensional (quasi-0D, 1D, 2D, and 3D) ferromagnetic ZnO nanostructures and the mechanisms behind RTFM are systematically presented. We have successfully prepared ferromagnetic nanostructures, including thin films, horizontal arrays and vertical arrays. The existing challenges, including open questions about quantum-bound ZnO nanostructures, are then discussed.
1. Introduction
In recent years, due to their potential applications in spintronic devices, diluted magnetic semiconductors have attracted the attention of researchers [1]. Many research groups have attempted to look for compounds with room-temperature ferromagnetism (RTFM) since 2000, which can be obtained with doping transition metal elements into metal oxides including ZnO [2,3,4,5,6], TiO2 [7,8,9,10], SnO2 [11,12,13,14,15,16], In2O3 [17,18] and HfO2 [10,19,20,21]. Since it was predicted based on the Zener model that Co-doped ZnO could exhibit RTFM [22], researchers have gradually focused on transition metal-doped ZnO systems. As an overview of the many publications on doped ZnO with RTFM, some review articles [4,6,23,24,25,26,27,28,29,30,31,32,33] are presented in Table 1.

Table 1.
Summary of research on ferromagnetic (FM) zinc oxide. RTFM (room-temperature ferromagnetism).
In fact, undoped ZnO can also exhibit ferromagnetic ordering. The spin state related with Zn vacancy (VZn) was identified first by its electron spin resonance (ESR) spectrum in electron-irradiated ZnO single crystals [34]. It is worthwhile noting that recent experimental results have shown that nano-engineering can also induce ferromagnetic behavior in non-ferromagnetic bulk materials [35,36,37]. The magnetic ordering obtained by the minimum number of vacancies was not successfully detected at that time. Amazingly, it was found later that the miniature of the bulk materials in nanoscale, such as nanoparticles (NPs), could exhibit novel magnetic behaviors in undoped ZnO. In 2006, RTFM (Ms, ~0.0005 emu/g) was observed in sol-gel ZnO NPs [38], which could be attributed to oxygen vacancies (VO). Subsequently, Banerjee et al. [39]. found that thermal annealing could induce RTFM in ZnO white powder, owing to the formation of VO clusters during the thermal treatment. Then, RTFM with a saturation magnetization up to 35.65 emu/g was also observed in undoped ZnO thin films (TFs) by pulsed laser deposition (PLD) and VZn located at the surface or the interface of the samples could be the source of RTFM [40]. More importantly, various ZnO nanostructures with RTFM were obtained by various approaches, such as ionic layer epitaxy [41], polymer-assisted deposition (PAD) [42,43,44], electrochemical deposition method [45], sol-gel [36,46,47], ball milling (BM) [48,49,50], and PLD [51], etc. Here, we will give an overview of the timeline of undoped ZnO nanostructures with RTFM (Figure 1).
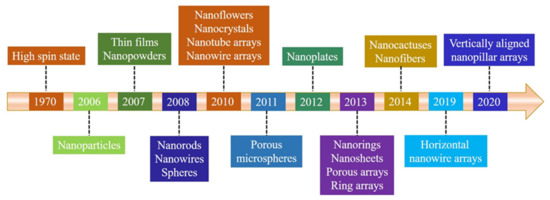
Figure 1.
Timeline showing key developments of ZnO nanostructures with RTFM.
2. Dimension Design
ZnO nanostructures can be divided into the following categories according to dimensions, such as: quasi-0D materials (quantum dots (QDs), and nanoparticles (NPs), 1D materials (nanorods (NRs), nanowires (NWs), nanostructured arrays, capped nanowires/nanotubes arrays, and other nanostructures), 2D materials (nanosheets, nanoplates, and thin films (TFs)), and 3D materials (single crystals, and porous microspheres).
2.1. Zero- and Quasi Zero-Dimensional Materials
Qusai-0D materials, such as Quantum dots (QDs), are crystalline particles with sizes less than 100 nm. ZnO QDs with RTFM (Figure 2) are commonly deposited by sol-gel (SOL) [36,47,52,53,54], solid-state reactions (SSR) [38], ball milling (BM) [49,55,56,57,58,59,60,61] pulsed laser deposition (PLD) [51], and coprecipitation (COP) [62,63] and so on. Previous studies have shown that RTFM of QDs can be controlled by synthetic temperature [52], milling time, annealing temperature or atmosphere [53,54], Zn source and capping [36,47]. As another kind of quasi zero dimensional material, nanoparticles (NPs) are about hundreds of nanometers in size. ZnO NPs with RTFM have been synthesized by BM [49,59], microwave plasma assisted spray (MPAS) [64], the miceller method (MIC) [39], COP [62] and the surfactant-free wet chemical method (SWCM) [65] (Figure 2). It has also been found that RTFM can be controlled by annealing parameters [39,59,62,65], particle size [64] and milling time [49]. The size-dependent RTFM in ZnO QDs/NPs (Figure 3) can be observed [52]. Ms is increased gradually by decreasing the diameters of ZnO NPs, and then RTFM will transform into paramagnetism (PM) or diamagnetism (DM).
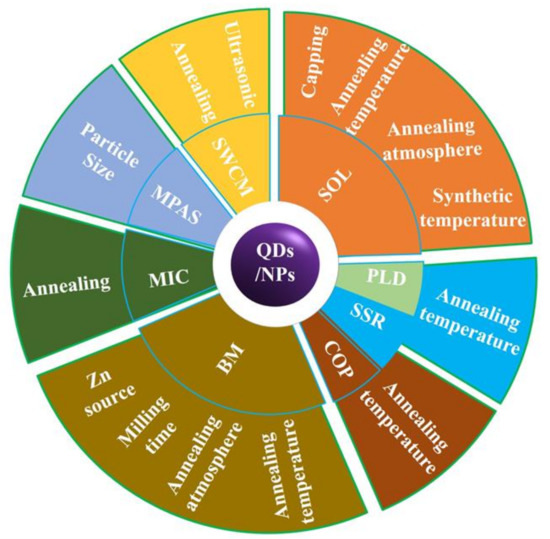
Figure 2.
Overview of currently available preparation strategies for fabricating ZnO QDs/NPs with RTFM. Sol-gel (SOL); Pulsed laser deposition (PLD); Solid-state reactions (SSR); Coprecipitation (COP); Ball milling (BM); Miceller method (MIC); Microwave plasma assisted spray (MPAS); Surfactant-free wet chemical method (SWCM).
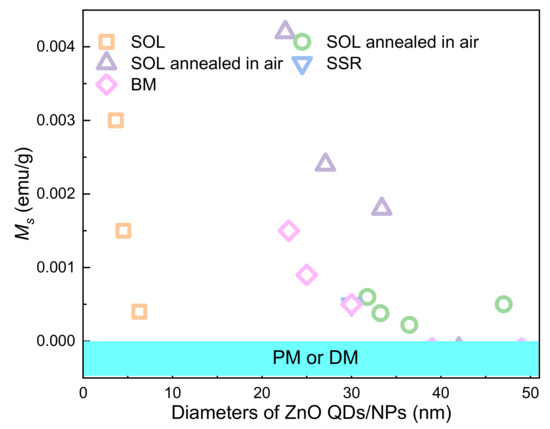
Figure 3.
The size-dependent RTFM in ZnO QDs/NPs.
2.2. One-Dimensional Materials
2.2.1. Nanowires/Nanorods
RTFM has been found in ZnO NWs [66] prepared by electro-deposition (ED). Due to the incomplete oxidation of zinc NWs, zinc clusters are formed in ZnO NWs, which induce room temperature ferromagnetism. Subsequently, ZnO NRs exhibiting RTFM have been obtained by hydrothermal method (HYT) [67] with Zn(Ac)2·2H2O and NaOH (Figure 4A). When the diameters of the NRs decreases, the Ms of the as-grown sample increases. Zni at the surface of ZnO NRs may be one of the reasons for RTFM.
In addition, VZn can also be produced in ZnO NRs with RFTM (Figure 4B), which were synthesized by COP [68]. The as-prepared NWs [69] (Figure 4C) show an obvious RTFM (Ms, ~0.007 emu/g). However, highly crystalline ZnO NWs [69] (Figure 4) prepared by the pulsed laser vaporization (PLV) process show no RTFM. X-ray fine structure spectroscopy study confirms that VZn can cause RTFM in ZnO NRs.
Furthermore, ZnO NRs have been obtained in aqueous conditions [70]. As-prepared NRs show RTFM behavior. Strangely, when the size of the NRs decrease, the magnetization of ZnO NRs increases. Singly charged oxygen vacancies (VO+) localized on the sample is the origin of RTFM. Single-crystalline ZnO NWs with RTFM (Ms, ~0.001 emu/g) (Figure 4D,E) have been obtained by the vapor transport method (VTM) [71]. RTFM related with VO can be modulated by selecting different catalysts and changing the growth temperature. Jana et al. [72] has reported ZnO NRs (Ms, ~0.059 emu/g) using hydrolysis of zinc acetate. Moreover, polycrystalline ZnO NRs with RTFM have also been prepared by a wet chemical method (WCM) [73]. RTFM in ZnO NRs (Figure 4) is attributed to VO+.
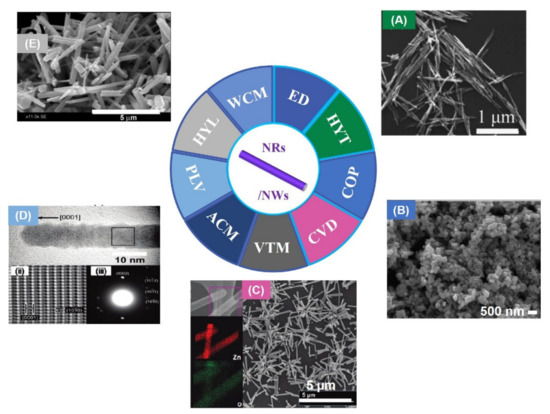
Figure 4.
ZnO NRs/NWs. (A) ZnO NRs obtained by hydrothermal method (HYT) (Reproduced with permission from [67]. Copyright 2008, American Institute of Physics). (B) ZnO NRs prepared by coprecipitation method (COP) (Reproduced with permission from [68]. Copyright 2009, Elsevier). (C) SEM images of ZnO NWs (Reproduced with permission from [69]. Copyright 2010, American Chemical Society). (D) HRTEM image of ZnO NRs (Reproduced with permission from [69]. Copyright 2010, American Chemical Society). (E) ZnO NRs synthesised by wet chemical method (WCM) (Reproduced with permission from [72]. Copyright 2018, Elsevier). Electro-deposition (ED); Chemical vapor deposition (CVD); Pulsed laser vaporization (PLV); Aqueous chemical method (ACM); Hydrolysis (HYL).
2.2.2. Nanostructure Arrays
ZnO nanostructure arrays (Figure 5) have been fabricated by various approaches, such as HYT [74], colloidal template method (CTM) [75], chemical vapor deposition (CVD) [76], chemical bath deposition (CBD) [76,77], and polymer-assisted deposition (PAD) [42,43,44]. ZnO NWs are usually synthesized by a seed method. Firstly, ZnO TFs are prepared by magnetron sputtering and used as seed layer, then ZnO NRs arrays are obtained by HYT [74]. The defect states are controlled by annealing in O2 and H2 atmosphere at 500 °C, respectively. It has been found that VO is closely related to RTFM by PL spectrum and vibrating sample magnetometer (VSM). Furthermore, vertically oriented NRs arrays (VNRA) and randomly oriented NRs arrays (RNRA) have been synthesized by CVD and CBD [76]. These results show that RTFM is originated from the VO+.
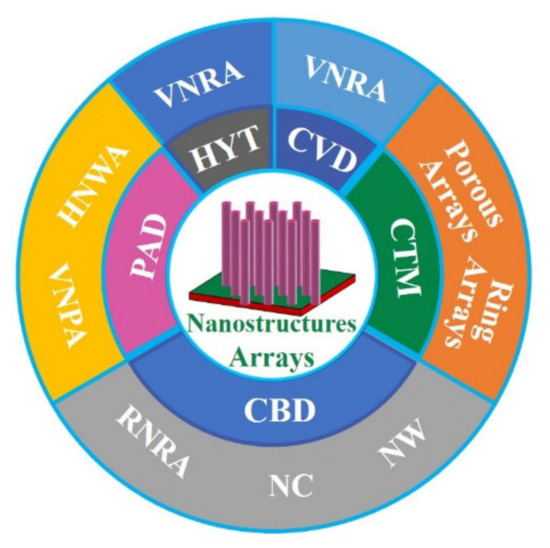
Figure 5.
ZnO nanostructure arrays.
Interestingly, ZnO porous arrays (Ms, ~5.7 emu/g) and ring arrays with RTFM (Ms, ~0.25 emu/g) have been fabricated by CTM [75] (Figure 5). When the grain size decreases to ~3 nm, the Ms can increase to ~5.7 emu/g. The results show that grain size and VO concentration can affect the RTFM. The presence of the defects in ZnO nanocactuses (Ms, ~3.0 emu/cm3) than in the ZnO NWs (Ms, ~2.3 emu/cm3) was observed [77] in Figure 5. The low temperature method can generate more defects, and then induce the higher Ms in nanocactuses. ZnO HNWA [43] and VNPA [44] have also been obtained by PAD in our lab. Our results show that more VZn can potentiate RTFM [43,44]. Thiol-capped ZnO NWs/NTs arrays can present RTFM [78]. The M-H loops of the thiol-capped samples reveal the height-dependent and morphology-dependent RTFM (Figure 6). Combing magnetic measurements and calculations show that the origin of ferromagnetism is mainly attributed to spin-polarized 3p electrons in S sites and, therefore, has a strong correlation with Zn–S bond anisotropy.
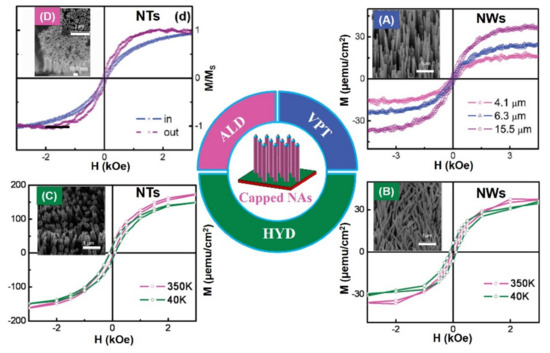
Figure 6.
RTFM of ZnO nanowire (NWs)/nanotube arrays (NTs) is modulated by capping with thiol. (A) M–H curves for ZnO NWs of different heights. (B) M–H curves for ZnO NW arrays of diameters ~150 nm. (C) M–H curves for ZnO NT arrays. (D) NT arrays (Reproduced with permission from [78]. Copyright 2009, Elsevier).
In the nanowire system, the change of diameter has no obvious effect on RTFM (Figure 7). However, the height-dependent RTFM has been observed in capped ZnO NWs (Figure 6A).
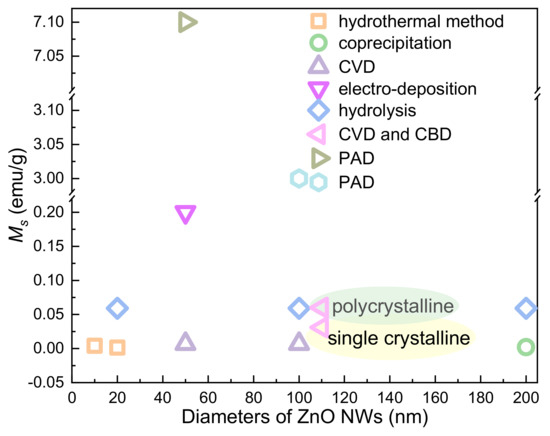
Figure 7.
The size-dependent RTFM in ZnO NWs.
2.2.3. Other Nanostructures
ZnO nanoflowers with RTFM (Ms, ~0.069 emu/g) have been obtained by HYT [79]. TEM images show that this flower sample is composed of closely packed NRs. XPS indicates that isolated vacancy clusters is closely related to the RTFM. In addition, microdiscs and porous nanoassembly of ZnO have been synthesized by soft-chemical approaches [80]. Furthermore, various types of ZnO nanostructures have been obtained by a microwave assisted HYT [81]. Spectrum measurements show that the defects may mediate RTFM [80,81]. The morphology-dependent RTFM is shown in Figure 8. ZnO HNWA has the highest Ms is ~7.1 emu/g.
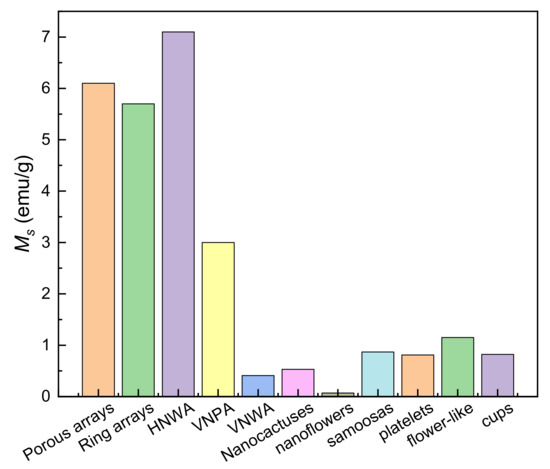
Figure 8.
Saturation magnetizations (Ms) of various ZnO nanostructures. Data from refs. [43,44,75,77,79,81].
2.3. Two-Dimensional Materials
2.3.1. Nanosheets/Nanoplates
Two-dimensional (2D) nanosheets/nanoplates have been synthesized by HYT [45,82,83] and ionic layer epitaxy (ILE) [41] in Figure 9. The average thickness of ZnO Nanoplates is estimated to be ~20 nm by the SEM images and XRD peak. Interestingly, it is only when the thickness is reduced to 5–8 nm, the samples exhibit RTFM. Interestingly, first principles calculation results show that when the thickness of the NSs decreases to a certain range, the magnetic moment will be generated; however, when the size of the NWs decreases along the a-axis and b-axis, the magnetic moment will not be generated. The distorted bands may be responsible for the RTFM in ZnO nanoplates.
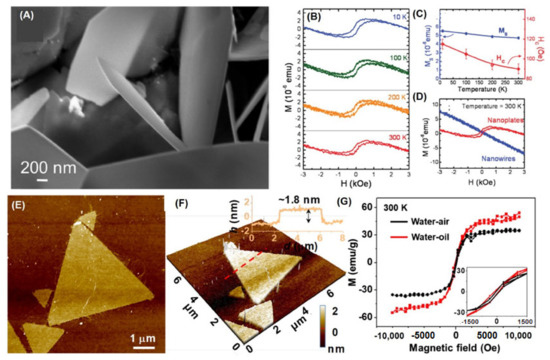
Figure 9.
(A) ZnO nanoplates synthesized by HYT. (B) M–H curves for ZnO nanoplates. (C) The corresponding Ms and coercivities Hc are plotted in (A). (D) M–H curves for ZnO nanowires and nanoplates (Reproduced with permission from [82]. Copyright 2012, American Chemical Society). (E,F) AFM images of two-dimensional (2D) ZnO nanosheets by ILE. (G) M-H curves for 2D ZnO nanosheets (Reproduced with permission from [41]. Copyright 2019, American Chemical Society).
ZnO single-crystalline nanosheets with RTFM have been obtained [45]. TEM images present that ZnO sheets and dodecyl sulfate bilayers compose the lamellar structure. The results show the cluster of spin-polarized defects induce RTFM in single-crystalline ZnO nanosheets.
Two-dimensional ZnO nanosheets with high VZn concentration have been grown by ILE [41] (Figure 9E,G). The nanosheets annealed in Ar at 400 °C for 1h show Ms of 50.9 emu/g in Figure 9G. Significantly, DFT calculations and experimental results suggest that VZn could be associated with the RTFM.
By contrast with 2D materials, ZnO nanosheets do not show obvious thickness dependence. However, ZnO nanosheets with saturation magnetization up to 50.9 emu/g have been obtained recently by ILE (Figure 10).
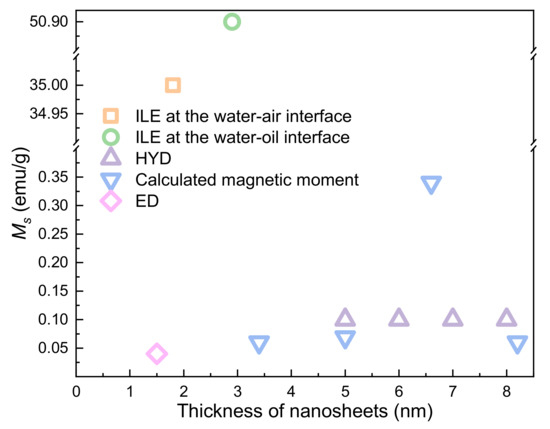
Figure 10.
The thickness-dependent RTFM in ZnO nanosheets.
2.3.2. Thin Films
In 2007, RTFM has been first observed in undoped ZnO TFs by PLD [40]. The results suggest that VZn can generate RTFM, where the ferromagnetism is related to the thickness of ZnO TFs. Soon after, calculations [84] not only showed that undoped ZnO TFs and NWs can be ferromagnetic but also indicated that VZn is responsible for RTFM in low-dimensional magnetic ZnO nanostructures. Similar to other nanostructures [38,40,67,80,81], VZn prefers to reside on ZnO NWs, resulting in stronger RTFM.
The thickness-dependent ferromagnetism in ZnO TFs has been investigated by Kapilashrami et al. [85]. With increasing the film thickness, the ferromagnetism first increases; when the film thickness exceeds 480 nm (Ms, ~0.62 emu/g), the ferromagnetism then decreases; with increasing of film thickness, the ferromagnetism will transform into paramagnetism (PM) or even diamagnetism (DM). The defect is mainly responsible for the observed RTFM.
Grain boundaries can also mediate RTFM. ZnO TFs were also obtained by liquid ceramics method [86]. The HRTEM images show that ZnO grains is surrounded by the amorphous area. Furthermore, RTFM origins from the amorphous regions related the defects. By decreasing the grain size, the magnetic volume fractions will increase [87]. Tietze et al. [87]. built a magnetic shell model related to the grain boundaries, which clarified the origin of RTFM in ZnO TFs.
In the ZnO TFs system, VZn [40,84,88,89,90] can also induce the RTFM. The existence of VZn is often confirmed by photoluminescence and positron annihilation spectrum. Xing et al. [88] has found that the sol-gel derived samples contain more VZn, compared with the MBE-ZnO TFs, which shows much stronger RTFM. Similarly, VZn is also the origin of RTFM in polycrystalline ZnO TFs prepared by PAD in our lab [89,90].
Additionally, undoped ZnO TFs [91,92] have been fabricated with CVD [93]. Interestingly, a nanosized structure can induce RTFM in ZnO TFs. Similar to ZnO NRs [71,76], VO can generate RTFM in undoped ZnO TFs obtained by PED [94] and undoped ZnO NPs prepared by an electrospinning method [95]. Moreover, Zhang et al. [96] indicated that RFTM in pure ZnO TFs should be related to Zni. The shallow donor caused by Zni defects might modify the electronic structure of ZnO TFs, leading to the RTFM.
The thickness dependence of RTFM has been observed in films prepared by different experimental methods (Figure 11). The RTFM of ZnO TFs is affected by the defects on the surface of the sample or at the interface between the sample and the substrate.
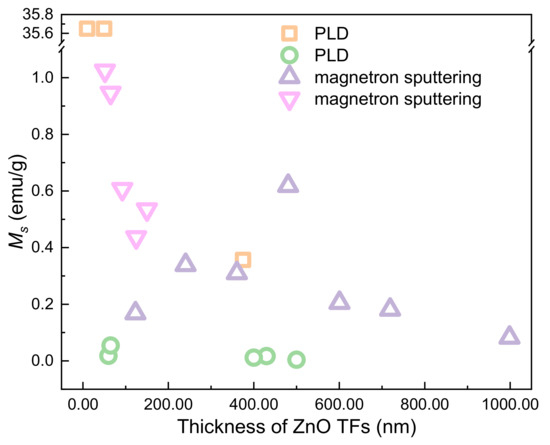
Figure 11.
The thickness-dependent RTFM in ZnO TFs.
2.4. Three-Dimensional Materials
In 1970, Galland et al. [34] irradiated ZnO single crystals with 3MeV electrons. ESR spectra showed that VZn can generate the high spin state. Furthermore, the intrinsic defect just like Zni and VO may give rise to the RTFM in single crystalline ZnO [97].
Interestingly, the metal–insulator transition has been found in the ZnO supercells adsorbed with hydrogen atoms by first-principles calculations [98]. This phase transition can affect the magnetic transformation. Experimentally, ZnO single crystals have been obtained by HTY [99]. The hydrogen penetration depth is confirmed at 20 nm by stopping and range of ions in matter (SRIM) simulation. SQUID measurements indicate that the Ms increases with the treatment time and the concentration of hydrogen. Subsequently, hydrogen atoms may trigger the RTFM in ZnO single crystals in Khalid et al. [100]. The Ms is up to ~4 emu/g. Strangely, the vacancies defects and the interstitial Zn atoms cannot induce the RTFM in ZnO monocrystal [101].
HYT [102] has been developed to grow Zn5(OH)8Ac2·2H2O microspheres. In fact, the microspheres are grown from the nanosheets, which are curved and connected to each other. ZnO porous microspheres [103] have been obtained from Zn5(OH)8Ac2·2H2O microspheres annealing at 500 °C. The RTFM in pure ZnO microspheres origins from VZn and shallows donors.
3. Ferromagnetism of Undoped ZnO Nanostructures
3.1. Influence of Precursor Selection on Room-Temperature Ferromagnetism (RTFM)
The preparation of materials has always been optimized by selecting more suitable precursors. In 2019, Zn(NO3)2·6H2O and ZnCl2 were selected as zinc sources to prepare ZnO NPs [104]. The difference of Ms was nearly four times, which may have been due to VO in the samples produced by selecting different precursors.
3.2. Substrate Effects on Ferromagnetism
Substrates have played an important role in material synthesis [4,93,94,105,106,107,108]. ZnO TFs have been grown on single-crystalline substrates by MOCVD [93]. M–H curves show that ZnO TFs on sapphire substrate are non-ferromagnetic (Figure 12A,B). However, the structural defect located on the substrate–film interface can cause RTFM in ZnO TFs. Interestingly, different substrates have been used to fabricate ZnO TFs with the same thickness by PED [94], and their ferromagnetic behaviors are very different. The ZnO TFs on silicon substrate show stronger ferromagnetism than the others, because more VO can be created in the TFs [94]. The origin of RTFM may be VO.
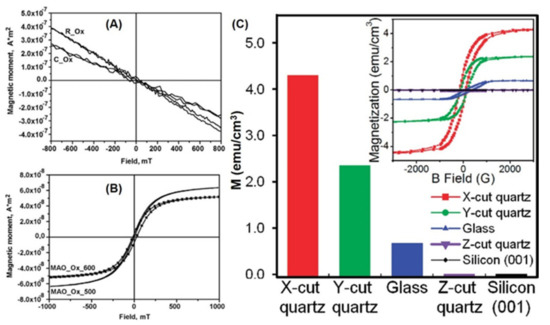
Figure 12.
Substrate effects on RTFM. (A) M-H loops of ZnO TFs prepared by CVD on sapphire substrates (Reproduced with permission from [93]. Copyright 2019, Elsevier). (B) M-H loops of ZnO TFs deposited by CVD on (111) MgAl2O4 substrates (Reproduced with permission from [93]. Copyright 2019, Elsevier). (C) Ms of ZnO TFs deposited on different substrates (Reproduced with permission from [106]. Copyright 2009, American Chemical Society).
ZnO TFs grown on sapphire substrates have been synthesized by PLD [105]. Only the samples on R-plane sapphire substrate exhibit RTFM. Furthermore, the results suggest that VZn is probably the reason for the RTFM. In addition, quartz, glass, and silicon have been selected as substrates to prepare ZnO TFs by PLD, and the RTFM in the samples may come from the coupling of unpaired electron spins induced by the oxygen 2p orbitals around the VZn and are enhanced by the in-plane compressive strain [106].
The modulation of RTFM by changing the substrate temperature has also been studied in ZnO TFs [107]. However, ZnO films grown at low substrate temperatures exhibit a larger Ms. Ms decreases with increasing the temperature. A similar result is found by Xu et al. [108]. Notably, the effect is different from that in TM-doped ZnO TFs [4].
3.3. Effect of Growth Conditions on RTFM
The RTFM can also be mediated by growth temperature. In 2012, Xu et al. [52] found that ZnO QDs with different sizes exhibited an obvious RTFM. Furthermore, the surface-volume ratio was closely related with the size-dependent ferromagnetism.
The size of QDs can also be controlled by milling time, which affects RTFM of QDs. Furthermore, it has been found that milling time is a superior way to mediate RTFM [49,57,58]. The grain size does not change monotonously with milling time, but Ms will increase monotonously in a certain range. In fact, VZn can induce RTFM in ZnO QDs.
Similarly, the grain size can be changed by milling time, as reported by Kisan et al. [57]. Interestingly, the transition from PM to FM is realized by this strategy. UV-vis spectra [57] and positron annihilation spectra [109] show that VO can affect the RTFM.
3.4. Influence of Post-Annealing on RTFM
Zhan et al. [110]. observed that RTFM can be produced by annealing in Ar. The magnetic field strength will affect the observed RTFM. When a magnetic field of 7T is applied, Ms is as high as 2.7 emu/g. Furthermore, PL spectra indicate that RTFM originates from VO+.
Undoped ZnO TFs with RTFM have been obtained by PAD [89]. The magnetization of the sample can potentiate by annealing in O2 or H2 gas. Positron annihilation spectroscopy indicates that VZn can induce the RTFM. In addition, we have also fabricated ferromagnetic Zn0.97Co0.03O TFs by PAD [90]. The Oi and VZn can be mediated with the annealing. The PL spectra show that the RTFM is closely related to Oi and VZn.
Changing annealing temperature is another strategy to mediate RTFM [53,54] The Hc, Mr and Ms increase with decreasing the annealing temperature of the samples. PL spectra show that Zni and VO related defects are closely related to the RTFM.
Interestingly, we have found that as-grown ZnO TFs can be transformed into centimeter-scale ZnO horizontal NWs arrays [43] by annealing in O2. With the extension of annealing time, the length becomes longer. After ZnO NWs arrays have been produced, the magnetic properties of the films transform PM into FM.
3.5. Dopping with Non-Magnetic Atoms
Notably, doping is also an effective way to modulate the RFTM of ZnO. Many research groups have attempted to look for compounds with RTFM since 2000, which can be obtained with doping transition metal elements into metal oxides such as Cr [4,111,112,113], Mn [2,3,4], Fe [114], Ni [115], Co [90,116,117,118], Sc [4,119,120], Ti [120], V [120], and Cu [120,121], and codoping such as CoFe [122], and MnCo [123]. However, experimental studies on TM-doped ZnO have produced inconsistent results and the mechanism of RTFM remains unclear. This promoted search for DMS based on alternative dopants. Manifestation of RTFM has also been undertaken by doping with non-magnetic dopants such as H [26,98,99,100,101,102,103,104,105,106,107,108,109,110,124,125], N [126,127], C [128,129,130,131], and S [47,78]. Interestingly, the observation of RTFM strongly suggests the presence of a spin-split band with a non-zero spin-orbit coupling in H-ZnO single crystals [100]. In addition, incorporation of N at 60° in ZnO induces the stronger FM, higher band gap reduction, larger reduction in surface roughness and higher reduction in the grains at RT [126]. Carbon-doped ZnO films show an intrinsic RTFM, which originates from the Zn-C system in the ZnO environment [128]. Meanwhile, thiol-capped nanotube arrays have exhibited a higher Ms than the nanowire arrays due to its larger surface-to-volume ratio and thus higher density of Zn–S bond spins. The observed dependence of the magnetic anisotropy of ZnO NTs haves suggested that the change in the ordering of Zn–S spins on the crystal facets can affect the extent of magnetic ordering and alter the preferred magnetization direction [78].
3.6. Capping
Capping organic molecules is a common method to mediate RTFM of non-magnetic materials. In 2007, Garcia et al. [36] controlled the ferromagnetism by capping ZnO NPs with three different organic molecules. The band structure was mediated with capping molecules, and then the RTFM was induced from DM.
4. Conclusions and Outlook
The present literature analysis reveals that the amount of research focused on un-doped ZnO nanostructures with RFTM has been increasing over the past two decades. We have successfully prepared ferromagnetic nanostructures, including thin films, horizontal arrays and vertical arrays. RTFM could be readily modulated by non-doping means including precursor selection, substrate effects, growth conditions, post-annealing, and capping. Some novel ways to drive the transition from DM to FM in undoped ZnO nanostructures have delivered interesting results, as shown in Figure 12.
The size-dependent RTFM in ZnO QDs/NPs (Figure 3) can be observed [52]. Ms is increased gradually by decreasing the diameters of ZnO QDs/NPs. However, in the nanowire system, the change of diameter has no obvious effect on RTFM (Figure 7). Interestingly, RTFM can be induced from non-magnetic ZnO nanosheets and TFs by reducing the thickness (Figure 10 and Figure 12). Notably, the magnetic anisotropy of ZnO nanostructures should be further studied.
Through theoretical calculation, it has been found that there is a driving force of phase transformation in ZnO. Therefore, fabrication of ultrathin (thickness < 2 nm) freestanding ZnO structures remains challenging. Amazingly, Taniguchi et al. [45] have reported that organic layers could allow the formation of ZnO nanosheets (~1.5 nm) with RTFM. In addition, Zhao et al. [132]. have presented the phase transitions of quantum-confined ZnO NWs by in-site TEM. Experimentally, it is still a great challenge to study RTFM in quantum-bound ZnO nanostructures.
Even though a variety of magnetic nanostructures have been prepared and studied, there are still many problems worthy of further study. (1) Development of new ferromagnetic nanostructures, such as nano-octagonal stars [133]. In the past, the morphology characterization mainly focused on 2D characterization. Recently, 3D electron tomography has emerged to analyze the 3D structure of nanostructures. In addition, the self-assembly of nanostructures and the evolution of ferromagnetism were further studied by an in situ method [133,134]. (2) Development of some emerging methods to prepare and study the nanostructure. (3) New characterization methods have been employed to investigate the nanostructures with RTFM [135,136]. The crystal nucleation process of FePt NPs in four dimensions [135] has been studied by atomic electron tomography (AET) [135]. In addition, electron tomography (ET) has revealed FeCo nano-octopods with RTFM. (4) The mechanism of undoped ZnO with RTFM needs further study.
Author Contributions
Writing—original draft preparation, H.R.; writing—review and editing, H.R. and G.X.; supervision, G.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Plan (Grant No. 2017YFB0405702), the National Natural Science Foundation of China (Grant No. 51671137), the Shandong Province Natural Science Foundation (Grant No. ZR2021MA042) and the Doctoral Scientific Research Foundation of Liaocheng University (Grant No. 318052054).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; von Molnár, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A Spin-Based Electronics Vision for the Future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Gupta, A.; Rao, K.V.; Owens, F.J.; Sharma, R.; Ahuja, R.; Osorio-Guillén, J.; Johansson, B.; Gehring, G.A. Ferromagnetism above room temperature in bulk and transparent thin films of Mn-doped ZnO. Nat. Mater. 2003, 2, 673–677. [Google Scholar] [CrossRef]
- Kundaliya, D.C.; Ogale, S.B.; Lofland, S.; Dhar, S.; Metting, C.J.; Shinde, S.R.; Ma, Z.; Varughese, B.; Ramanujachary, K.; Salamanca-Riba, L.; et al. On the origin of high-temperature ferromagnetism in the low-temperature-processed Mn–Zn–O system. Nat. Mater. 2004, 3, 709–714. [Google Scholar] [CrossRef]
- Pan, F.; Song, C.; Liu, X.J.; Yang, Y.C.; Zeng, F. Ferromagnetism and possible application in spintronics of transition-metal-doped ZnO films. Mat. Sci. Eng. R. 2008, 62, 1–35. [Google Scholar] [CrossRef]
- Herng, T.S.; Qi, D.; Berlijn, T.; Yi, J.; Yang, K.; Dai, Y.; Feng, Y.P.; Santoso, I.; Sánchez-Hanke, C.; Gao, X.Y.; et al. Room-Temperature Ferromagnetism of Cu-Doped ZnO Films Probed by Soft X-ray Magnetic Circular Dichroism. Phys. Rev. Lett. 2010, 105, 207201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, S.; Zhan, P.; Xie, Q.; Wang, W.; Zhang, Z. Defects-Driven Ferromagnetism in Undoped Dilute Magnetic Oxides: A Review. J. Mater. Sci. Technol. 2015, 31, 969–978. [Google Scholar] [CrossRef]
- Hong, N.H.; Sakai, J.; Prellier, W.; Hassini, A.; Ruyter, A.; Gervais, F. Ferromagnetism in transition-metal-doped TiO2 thin films. Phys. Rev. B. 2004, 70, 195204. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Murakami, M.; Shono, T.; Hasegawa, T.; Fukumura, T.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koshihara, S.-Y.; Koinuma, H. Room-Temperature Ferromagnetism in Transparent Transition Metal-Doped Titanium Dioxide. Science 2001, 291, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Blythe, H.J.; Ziese, M.; Behan, A.J.; Neal, J.R.; Mokhtari, A.; Ibrahim, R.M.; Fox, A.M.; Gehring, G.A. Carrier-induced ferromagnetism in n-type ZnMnAlO and ZnCoAlO thin films at room temperature. New J. Phys. 2006, 8, 135. [Google Scholar] [CrossRef]
- Yoon, S.D.; Chen, Y.J.; Yang, A.; Goodrich, T.L.; Zuo, X.; Arena, D.A.; Ziemer, K.; Vittoria, C.; Harriset, V.G. Oxygen-defect-induced magnetism to 880 K in semiconducting anatase TiO2−δ films. J. Phys.-Condens. Mat. 2006, 18, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Ogale, S.B.; Choudhary, R.J.; Buban, J.P.; Lofland, S.E.; Shinde, S.R.; Kale, S.N.; Kulkarni, V.N.; Higgins, J.; Lanci, C.; Simpson, J.R.; et al. High temperature ferromagnetism with a giant magnetic moment in transparent Co-doped SnO2. Phys. Rev. Lett. 2003, 91, 077205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coey, J.M.D.; Douvalis, A.P.; Fitzgerald, C.B.; Venkatesan, M. Ferromagnetism in Fe-doped SnO2 thin films. Appl. Phys. Lett. 2004, 84, 1332–1334. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Hou, Q.Y.; Liu, Y. Effect of Fe Doping and Point Defects (VO and VSn) on the Magnetic Properties of SnO2. J. Supercond. Nov. Magn. 2019, 32, 2877–2884. [Google Scholar] [CrossRef]
- Lin, L.; Wang, P.; Huang, J.; Yu, W.; Tao, H.; Zhu, L.; Zhang, Z. Investigation on Electronic Structures and Magnetic Properties of (Mn, Ga) Co-doped SnO2. J. Supercond. Nov. Magn. 2019, 32, 3601–3607. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, M.; Zhang, Y.; Hao, W.; Sun, L.; Cao, E.; Yang, Z. Effects of Oxygen Vacancy on the Magnetic Properties of Ni-Doped SnO2 Nanoparticles. J. Supercond. Nov. Magn. 2019, 32, 3509–3516. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mendes, G.M.S.L.; Ribeiro, T.S.; Silva, M.R.; Vasconcelos, I.F. Influence of Thermal-Treatment Effects on the Structural and Magnetic Properties of Sn1−xFexO2 Nanopowders Produced by Mechanical Milling. J. Supercond. Nov. Magn. 2020, 33, 1721–1728. [Google Scholar] [CrossRef]
- He, J.; Xu, S.F.; Yoo, Y.K.; Xue, Q.Z.; Lee, H.C.; Cheng, S.F.; Xiang, X.D.; Dionne, G.F.; Takeuchiet, I. Room temperature ferromagnetic n-type semiconductor in (In1−xFex)2O3−σ. Appl. Phys. Lett. 2005, 86, 052503. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.-X.; Xu, X.-H.; Zhang, J.; Fan, X.-C.; Wu, H.-S.; Gehring, G.A. Role of carrier and spin in tuning ferromagnetism in Mn and Cr-doped In2O3 thin films. Appl. Phys. Lett. 2010, 96, 52503. [Google Scholar] [CrossRef]
- Venkatesan, M.; Fitzgerald, C.B.; Coey, J.M.D. Unexpected magnetism in a dielectric oxide. Nature 2004, 430, 630. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Venkatesan, M.; Stamenov, P.; Fitzgerald, C.B.; Dorneles, L.S. Magnetism in hafniumk dioxide. Phys. Rev. B 2005, 72, 024450. [Google Scholar] [CrossRef] [Green Version]
- Hong, N.H.; Poirot, N.; Sakai, J. Evidence for magnetism due to oxygen vacancies in Fe-doped HfO2 thin films. Appl. Phys. Lett. 2006, 89, 042503. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener model description of ferromagnetism in zinc-blende magnetic semico,,nductors. Science 2000, 287, 1019. [Google Scholar] [CrossRef] [Green Version]
- Coey, J.M.D. d0 ferromagnetism. Solid State Sci. 2005, 7, 660–667. [Google Scholar] [CrossRef]
- Avrutin, V.; Izyumskaya, N.; Üzgür, O.; Silversmith, D.J.; Morkoç, H. Ferromagnetism in ZnO- and GaN-based diluted magnetic Semiconductors: Achievements and challenges. Proc. IEEE 2010, 98, 1288–1301. [Google Scholar] [CrossRef]
- Hernando, A.; Crespo, P.; Garcia, M.A.; Coey, J.M.D.; Ayuela, A.; Echenique, P.M. Revisiting magnetism of capped Au and ZnO nanoparticles: Surface band structure and atomic orbital with giant magnetic moment. Phys. Status Solidi B 2011, 248, 2352–2360. [Google Scholar] [CrossRef]
- Esquinazi, P.; Hergert, W.; Spemann, D.; Setzer, A.; Ernst, A. Defect-Induced Magnetism in Solids. IEEE Trans. Magn. 2013, 49, 4668–4674. [Google Scholar] [CrossRef]
- Singh, R. Unexpected magnetism in nanomaterials. J. Magn. Magn. Mater. 2013, 346, 58–73. [Google Scholar] [CrossRef]
- Opel, M.; Goennenwein, S.T.B.; Althammer, M.; Nielsen, K.-W.; Karrer-Müller, E.-M.; Bauer, S.; Senn, K.; Schwark, C.; Weier, C.; Güntherodt, G.; et al. Zinc oxide -From dilute magnetic doping to spin transport. Phys. Status Solidi B 2014, 251, 1700–1709. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yu, L.; Song, Q.; Du, Y. Tunable surface and/or interface ferromagnetism of ZnO nanoparticles. Ann. Phys. 2015, 358, 159–171. [Google Scholar] [CrossRef]
- Semisalova, A.S.; Orlov, A.; Smekhova, A.; Gan’shina, E.; Perov, N.; Anwand, W.; Potzger, K.; Lähderanta, E.; Granovsky, A. Above room temperature ferromagnetism in dilute magnetic oxide semiconductors. In Novel Functional Magnetic Materials: Fundamentals and Applications; Zhukov, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 187–219. [Google Scholar]
- Qi, B.; Ólafsson, S.; Gíslason, H. Vacancy defect-induced d0 ferromagnetism in undoped ZnO nanostructures: Controversial origin and challenges. Prog. Mater. Sci. 2017, 90, 45–74. [Google Scholar] [CrossRef]
- Aravind, A.; Jayaraj, M.K. ZnO-based dilute magnetic semiconductors. In Nanostructured Metal Oxides and Devices: Optical and Electrical Properties; Jayaraj, M.K., Ed.; Springer: Singapore, 2020; pp. 233–269. [Google Scholar]
- Li, X.-L.; Xu, X.-H. Homogeneous and inhomogeneous magnetic oxide semiconductors. Chin. Phys. B 2019, 28, 098506. [Google Scholar] [CrossRef]
- Galland, D.; Herve, A. ESR spectra of the zinc vacancy in ZnO. Phys. Lett. A 1970, 33, 1–2. [Google Scholar] [CrossRef]
- Potzger, K.; Zhou, S.Q.; Grenzer, J.; Helm, M.; Fassbender, J. An easy mechanical way to create ferromagnetic defective ZnO. Appl. Phys. Lett. 2008, 92, 182504. [Google Scholar] [CrossRef]
- Garcia, M.A.; Merino, J.M.; Fernández, P.E.; Quesada, A.; De la Venta, J.; Ruíz, G.M.L.; Hernando, A. Magnetic properties of ZnO nanoparticles. Nano Lett. 2007, 7, 1489–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köseoğlu, Y. A simple microwave-assisted combustion synthesis and structural, optical and magnetic characterization of ZnO nanoplatelets. Ceram. Int. 2014, 40, 4673–4679. [Google Scholar] [CrossRef]
- Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C.N.R. Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 2006, 74, 161306. [Google Scholar] [CrossRef]
- Banerjee, S.; Mandal, M.; Gayathri, N.; Sardar, M. Enhancement of ferromagnetism upon thermal annealing in pure ZnO. Appl. Phys. Lett. 2007, 91, 182501. [Google Scholar] [CrossRef] [Green Version]
- Hong, N.H.; Sakai, J.; Brizé, V. Observation of ferromagnetism at room temperature in ZnO thin films. J. Phy. Condens. Matter 2007, 19. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Y.Z.; Jacobs, R.; Shi, Y.Q.; Szlufarska, I.; Morgan, D.; Wang, X.D. Massive vacancy concentration yields strong room-temperature ferromagnetism in two-dimensional ZnO. Nano Lett. 2019, 19, 7085–7092. [Google Scholar] [CrossRef]
- Ren, H.; Liu, Y.; Zhang, L.; Liu, K. Synthesis, properties, and applications of large-scale two-dimensional materials by polymer-assisted deposition. J. Semicond. 2019, 40. [Google Scholar] [CrossRef]
- Ren, H.; Xiang, G.; Luo, J.; Yang, D.; Zhang, X. Direct catalyst-free self-assembly of large area of horizontal ferromagnetic ZnO nanowire arrays. Mater. Lett. 2018, 234, 384–387. [Google Scholar] [CrossRef]
- Luo, J.; Ren, H.; Zhang, X.; Xiang, G. Fabrication of vertically aligned ferromagnetic ZnO nanopillar arrays on sapphire substrates by polymer-assisted deposition. AIP Adv. 2020, 10, 015337. [Google Scholar] [CrossRef]
- Taniguchi, T.; Yamaguchi, K.; Shigeta, A.; Matsuda, Y.; Hayami, S.; Shimizu, T.; Matsui, T.; Yamazaki, T.; Funatstu, A.; Makinose, Y.; et al. Enhanced and Engineered d0Ferromagnetism in Molecularly-Thin Zinc Oxide Nanosheets. Adv. Funct. Mater. 2013, 23, 3140–3145. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, S.; Schmidt, H. Magnetic properties of ZnO nanopowders. J. Alloys Compd. 2009, 487, 665–667. [Google Scholar] [CrossRef]
- Chaboy, J.; Boada, R.; Piquer, C.; Marco, M.A.L.; García-Hernández, M.; Carmona, N.; Llopis, J.; Gonzalez, M.L.R.; Gonzalez-Calbet, J.M.; Fernandez, J.; et al. Evidence of intrinsic magnetism in capped ZnO nanoparticles. Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Wen, Z.; Zhang, H.; Qi, X.; Zhong, W.; Xu, L.; Wu, D.; Shen, K.; Xu, M. Room temperature ferromagnetism in ZnO prepared by microemulsion. AIP Adv. 2011, 1, 032127. [Google Scholar] [CrossRef]
- Phan, T.-L.; Zhang, Y.D.; Yang, D.S.; Nghia, N.X.; Thanh, T.D.; Yu, S.C. Defect-induced ferromagnetism in ZnO nanoparticles prepared by mechanical milling. Appl. Phys. Lett. 2013, 102, 072408. [Google Scholar] [CrossRef]
- Lemine, O.M. Induced Room-Temperature Ferromagnetism in Un-doped Nanocrystalline Metal Oxide Powders Obtained by Mechanical Milling: A Review. J. Supercond. Nov. Magn. 2016, 30, 271–274. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, Y.; Abiade, J.T. Ferromagnetic ZnO nanoparticles prepared by pulsed laser deposition in liquid. Mater. Lett. 2012, 85, 164–167. [Google Scholar] [CrossRef]
- Xu, X.; Xu, C.; Dai, J.; Hu, J.; Li, F.; Zhang, S. Size Dependence of Defect-Induced Room Temperature Ferromagnetism in Undoped ZnO Nanoparticles. J. Phys. Chem. C 2012, 116, 8813–8818. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, E. Nature of room-temperature ferromagnetism from undoped ZnO nanoparticles. Appl. Phys. A 2010, 99, 955–960. [Google Scholar] [CrossRef]
- Naji Aljawfi, R.; Rahman, F.; Shukla, D.K. Effect of the annealing temperature on the structural and magnetic properties of ZnO nanoparticles. Mater. Lett. 2013, 99, 18–20. [Google Scholar] [CrossRef]
- Ghose, S.; Gogurla, N.; Ranganathan, R.; Jana, D. The simultaneous emergence of free exciton emission and d0 ferromagnetism for undoped ZnO nanoparticles. RSC Adv. 2016, 6, 83909–83915. [Google Scholar] [CrossRef]
- Ghose, S.; Rakshit, T.; Ranganathan, R.; Jana, D. Role of Zn-interstitial defect states on d0 ferromagnetism of mechanically milled ZnO nanoparticles. RSC Adv. 2015, 5, 99766–99774. [Google Scholar] [CrossRef]
- Kisan, B.; Alagarsamy, P. Room temperature ferromagnetism in finite sized ZnO nanoparticles. Phys. B Condens. Matter 2014, 448, 115–119. [Google Scholar] [CrossRef]
- Kisan, B.; Kumar, J.; Alagarsamy, P. Experimental and first-principles study of defect-induced electronic and magnetic properties of ZnO nanocrystals. J. Phys. Chem. Solids 2020, 146, 109580. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.Q.; Wang, D.D.; Qi, N.; Gong, J.; Cao, C.Y.; Tang, Z. Positron annihilation study of the interfacial defects in ZnO nanocrystals: Correlation with ferromagnetism. J. Appl. Phys. 2010, 107, 023524. [Google Scholar] [CrossRef]
- Ghose, S.; Sarkar, A.; Chattopadhyay, S.; Chakrabarti, M.; Das, D.; Rakshit, T.; Ray, S.K.; Jana, D. Surface defects induced ferromagnetism in mechanically milled nanocrystalline ZnO. J. Appl. Phys. 2013, 114, 073516. [Google Scholar] [CrossRef]
- Xue, X.; Liu, L.; Wang, Z.; Wu, Y. Room-temperature ferromagnetism in hydrogenated ZnO nanoparticles. J. Appl. Phys. 2014, 115, 33902. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Z.; Fu, J.; Xu, Y.; Qi, J.; Xue, D. Room temperature ferromagnetism of pure ZnO nanoparticles. J. Appl. Phys. 2009, 105, 113928. [Google Scholar] [CrossRef]
- Pazhanivelu, V.; Blessington Selvadurai, A.P.; Murugaraj, R. Unexpected ferromagnetism in Ist group elements doped ZnO based DMS nanoparticles. Mater. Lett. 2015, 151, 112–114. [Google Scholar] [CrossRef]
- Wangensteen, T.; Dhakal, T.; Merlak, M.; Mukherjee, P.; Phan, M.; Chandra, S.; Srikanth, H.; Witanachchi, S. Growth of uniform ZnO nanoparticles by a microwave plasma process. J. Alloys Compd. 2011, 509, 6859–6863. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, Y.; Wang, D.; Wang, J.; Gao, Z.; Song, T. Surfactant-Free Fabrication of ZnO Spheres and Pseudospherical Structures. J. Phys. Chem. C 2008, 112, 9219–9222. [Google Scholar] [CrossRef]
- Yi, J.B.; Pan, H.; Lin, J.Y.; Ding, J.; Feng, Y.P.; Thongmee, S.; Liu, T.; Gong, H.; Wang, L. Ferromagnetism in ZnO Nanowires Derived from Electro-deposition on AAO Template and Subsequent Oxidation. Adv. Mater. 2008, 20, 1170–1174. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, Y.; Wang, D.; Wang, J.; Gao, Z.; Wang, L.; Yu, P.; Song, T. Impact of annealing on morphology and ferromagnetism of ZnO nanorods. Appl. Phys. Lett. 2008, 92, 081911. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kim, Y.; Koo, B.; Gautam, S.; Chae, K.H.; Kumar, R.; Lee, C. Room temperature ferromagnetism in chemically synthesized ZnO rods. Mater. Lett. 2009, 63, 194–196. [Google Scholar] [CrossRef] [Green Version]
- Podila, R.; Queen, W.; Nath, A.; Arantes, J.T.; Schoenhalz, A.L.; Fazzio, A.; Dalpian, G.M.; He, J.; Hwu, S.J.; Skove, M.J.; et al. Origin of FM Ordering in Pristine Micro- and Nanostructured ZnO. Nano Lett. 2010, 10, 1383–1386. [Google Scholar] [CrossRef]
- Panigrahy, B.; Aslam, M.; Misra, D.S.; Ghosh, M.; Bahadur, D. Defect-Related Emissions and Magnetization Properties of ZnO Nanorods. Adv. Funct. Mater. 2010, 20, 1161–1165. [Google Scholar] [CrossRef]
- Xing, G.Z.; Wang, D.D.; Yi, J.B.; Yang, L.L.; Gao, M.; He, M.; Yang, J.H.; Ding, J.; Sum, T.C.; Wu, T. Correlated d0 ferromagnetism and photoluminescence in undoped ZnO nanowires. Appl. Phys. Lett. 2010, 96, 112511. [Google Scholar] [CrossRef] [Green Version]
- Jana, A.; Sujatha Devi, P.; Mitra, A.; Bandyopadhyay, N.R. Synthesis of blue emitting ZnO nanorods exhibiting room temperature ferromagnetism. Mater. Chem. Phys. 2013, 139, 431–436. [Google Scholar] [CrossRef]
- Ghosh, B.; Ray, S.C.; Pontsho, M.; Sarma, S.; Mishra, D.K.; Wang, Y.F.; Pong, W.F.; Strydom, A.M. Defect induced room temperature ferromagnetism in single crystal, poly-crystal, and nanorod ZnO: A comparative study. J. Appl. Phys. 2018, 123, 161507. [Google Scholar] [CrossRef]
- Xu, X.Y.; Xu, C.X.; Lin, Y.; Ding, T.; Fang, S.J.; Shi, Z.L.; Xia, W.W.; Hu, J.G. Surface photoluminescence and magnetism in hydrothermally grown undoped ZnO nanorod arrays. Appl. Phys. Lett. 2012, 100, 172401. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, W.; Li, X.; Zeng, H.; Wang, G.; Wang, W.; Yang, Z.; Zhang, Y. Strong room-temperature ferromagnetism of pure ZnO nanostructure arrays via colloidal template. J. Mater. Chem. C 2013, 1, 6807–6812. [Google Scholar] [CrossRef]
- Xu, X.; Xu, C.; Lin, Y.; Li, J.; Hu, J. Comparison on Photoluminescence and Magnetism between Two Kinds of Undoped ZnO Nanorods. J. Phys. Chem. C 2013, 117, 24549–24553. [Google Scholar] [CrossRef]
- Singh, S.B.; Wang, Y.-F.; Shao, Y.-C.; Lai, H.-Y.; Hsieh, S.-H.; Limaye, M.V.; Chuang, C.-H.; Hsueh, H.-C.; Wang, H.; Chiou, J.-W.; et al. Observation of the origin of d0magnetism in ZnO nanostructures using X-ray-based microscopic and spectroscopic techniques. Nanoscale 2014, 6, 9166–9176. [Google Scholar] [CrossRef]
- Deng, S.Z.; Fan, H.M.; Wang, M.; Zheng, M.R.; Yi, J.B.; Wu, R.Q.; Tan, H.R.; Sow, C.H.; Ding, J.; Loh, Y.P.K.P. Thiol-capped ZnO nanowire/nanotube arrays with tunable magnetic properties at toom temperature. ACS Nano 2010, 4, 495–505. [Google Scholar] [CrossRef]
- Bie, X.; Wang, C.; Ehrenberg, H.; Wei, Y.; Chen, G.; Meng, X.; Zou, G.; Du, F. Room-temperature ferromagnetism in pure ZnO nanoflowers. Solid State Sci. 2010, 12, 1364–1367. [Google Scholar] [CrossRef]
- Gupta, J.; Bhargava, P.; Bahadur, D. Morphology dependent photocatalytic and magnetic properties of ZnO nanostructures. Phys. B Condens. Matter 2014, 448, 16–19. [Google Scholar] [CrossRef]
- Motaung, D.E.; Mhlongo, G.H.; Nkosi, S.S.; Malgas, G.F.; Mwakikunga, B.W.; Coetsee, E.; Swart, H.C.; Abdallah, H.M.I.; Moyo, T.; Ray, S.S. Shape-Selective Dependence of Room Temperature Ferromagnetism Induced by Hierarchical ZnO Nanostructures. ACS Appl. Mater. Interfaces 2014, 6, 8981–8995. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-I.; Choi, J.; Jang, S.S.; Gu, J.; Chang, Y.; Wortman, G.; Snyder, R.L.; Wang, Z.L. Magnetism in Dopant-Free ZnO Nanoplates. Nano Lett. 2012, 12, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-F.; Tang, L.-Z.; Sun, Q.; Sun, L.; Li, Z.-H.; Ren, S.-X. Ferromagnetism in High-Surface-Area ZnO Nanosheets Prepared by a Template-Assisted Hydrothermal Method. Chin. Phys. Lett. 2018, 35. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Chen, G.; Kawazoe, Y.; Jena, P. Vacancy-induced magnetism in ZnO thin films and nanowires. Phys. Rev. B 2008, 77, 205411. [Google Scholar] [CrossRef] [Green Version]
- Kapilashrami, M.; Xu, J.; Ström, V.; Rao, K.V.; Belova, L. Transition from ferromagnetism to diamagnetism in undoped ZnO thin films. Appl. Phys. Lett. 2009, 95, 033104. [Google Scholar] [CrossRef]
- Straumal, B.; Mazilkin, A.; Protasova, S.; Myatiev, A.; Goering, E.; Baretzky, B. Amorphous grain boundary layers in the ferromagnetic nanograined ZnO films. Thin Solid Films 2011, 520, 1192–1194. [Google Scholar] [CrossRef]
- Tietze, T.; Audehm, P.; Chen, Y.C.; Schütz, G.; Straumal, B.B.; Protasova, S.G.; Mazilkin, A.A.; Straumal, P.B.; Prokscha, T.; Luetkens, H.; et al. Interfacial dominated ferromagnetism in nanograined ZnO: A μSR and DFT study. Sci. Rep. 2015, 5, 8871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, G.Z.; Lu, Y.H.; Tian, Y.F.; Yi, J.B.; Lim, C.C.; Li, Y.F.; Li, G.P.; Wang, D.D.; Yao, B.; Ding, J.; et al. Defect-induced magnetism in undoped wide band gap oxides: Zinc vacancies in ZnO as an example. AIP Adv. 2011, 1, 022152. [Google Scholar] [CrossRef]
- Ren, H.T.; Xiang, G.; Gu, G.; Wang, W.J.; Zhang, P.; Wang, B.Y.; Cao, X.Z. Zinc Vacancy-induced room-temperature ferromagnetism in undoped ZnO thin films. J. Nanomat. 2012, 2012, 295358. [Google Scholar] [CrossRef]
- Ren, H.T.; Xiang, G.; Gu, G.X.; Zhang, X. Enhancement of ferromagnetism of ZnO:Co nanocrystals by post-annealing treatment: The role of oxygen interstitials and zinc vacancies. Mater. Lett. 2014, 122, 256–260. [Google Scholar] [CrossRef]
- Mal, S.; Narayan, J.; Nori, S.; Prater, J.; Kumar, D. Defect-mediated room temperature ferromagnetism in zinc oxide. Solid State Commun. 2010, 150, 1660–1664. [Google Scholar] [CrossRef]
- Kapilashrami, M.; Xu, J.; Biswas, A.; Tamaki, T.; Sharma, P.; Rao, K.; Belova, L. Coexistence of ultraviolet photo-response and room-temperature ferromagnetism in polycrystalline ZnO thin films. Mater. Lett. 2010, 64, 1291–1294. [Google Scholar] [CrossRef]
- Burova, L.I.; Perov, N.S.; Semisalova, A.S.; Kulbachinskii, V.A.; Vladimir, G.; Kytin, V.G.; Roddatis, V.V.; Alexander, L.; Vasiliev, A.L.; Andrey, R.; et al. Effect of the nanostructure on room temperature ferromagnetism and resistivity of undoped ZnO thin films grown by chemical vapor deposition. Thin Solid Films 2012, 520, 4580–4585. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, W.; Xie, Z.; Li, Z.; Zhang, Z.; Zhang, P.; Wang, B.; Cao, X. Substrate effect on the room-temperature ferromagnetism in un-doped ZnO films. Appl. Phys. Lett. 2012, 101, 031913. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zhang, W.Y.; An, X.Y.; Liu, Z.J.; Xie, E.Q.; Yang, C.; Chen, L.L. Room-temperature ferromagnetism in ZnO nanoparticles by electrospinning. Nanosci. Nanotech. Lett. 2014, 6, 446–449. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Zhang, X.; Xu, X.; Meng, F.; Tang, C.C. Defects Induced Room Temperature Ferromagnetism in ZnO Thin Films. Adv. Condens. Matter Phys. 2014, 2014, 806327. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.; Kumar, P.; Sharma, M.K.; Das, J.; Singh, S.; Roul, B.; Varma, S.; Chatterjee, R.; Srinivasu, V.; Kanjilal, D. Ferromagnetism in ZnO single crystal. Phys. B Condens. Matter 2010, 405, 2659–2663. [Google Scholar] [CrossRef]
- Sanchez, N.; Gallego, S.; Cerdá, J.; Muñoz, M.C. Tuning surface metallicity and ferromagnetism by hydrogen adsorption at the polar ZnO(0001) surface. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Esquinazi, P.; Spemann, D.; Anwand, W.; Bräuer, G. Hydrogen-mediated ferromagnetism in ZnO single crystals. New J. Phys. 2011, 13, 063017. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Esquinazi, P.D. Hydrogen-induced ferromagnetism in ZnO single crystals investigated by magnetotransport. Phys. Rev. B 2012, 85. [Google Scholar] [CrossRef] [Green Version]
- Khazanov, E.N.; Taranov, A.V.; Salamatov, E.I.; Shevchenko, E.V.; Charnaya, E.V. Features of defects of the crystal structure and magnetic properties of an undoped ZnO monocrystal. J. Commun. Technol. Electron. 2017, 62, 406–409. [Google Scholar] [CrossRef]
- Xia, Z.B.; Sha, J.; Fang, Y.J.; Wan, Y.T.; Wang, Z.L.; Wang, Y.W. Purposed built ZnO/Zn5(OH)8Ac2·2H2O architectures by hydrothermal synthesis. Cryst. Growth Des. 2010, 10, 2759–2765. [Google Scholar] [CrossRef]
- Xia, Z.B.; Wang, Y.W.; Fang, Y.J.; Wan, Y.T.; Xia, W.; Sha, J. Understanding the origin of ferromagnetism in ZnO porous microspheres by systematic investigations of the thermal decomposition of Zn5(OH)8Ac2·2H2O to ZnO. J. Phys. Chem. C 2011, 115, 14576–14582. [Google Scholar] [CrossRef]
- Tóthová, E.; Senna, M.; Yermakov, A.; Kováč, J.; Dutková, E.; Hegedüs, M.; Kaňuchová, M.; Baláž, M.; Bujňáková, Z.L.; Briančin, J.; et al. Zn source-dependent magnetic properties of undoped ZnO nanoparticles from mechanochemically derived hydrozincite. J. Alloys Compd. 2019, 787, 1249–1259. [Google Scholar] [CrossRef]
- Khalid, M.; Ziese, M.; Setzer, A.P.; Esquinazi, P.M.; Lorenz, M.H.; Hochmuth, H.; Grundmann, M.; Spemann, D.; Butz, T.; Brauer, G.; et al. Defect-induced magnetic order in pure ZnO films. Phys. Rev. B 2009, 80, 035331. [Google Scholar] [CrossRef] [Green Version]
- Ong, C.S.; Herng, T.S.; Huang, X.L.; Feng, Y.P.; Ding, J. Strain-Induced ZnO Spinterfaces. J. Phys. Chem. C 2011, 116, 610–617. [Google Scholar] [CrossRef]
- Mal, S.; Nori, S.; Narayan, J.; Prater, J.T. Defect-mediated ferromagnetism and controlled switching characteristics in ZnO. J. Mater. Res. 2011, 26, 1298–1308. [Google Scholar] [CrossRef]
- Xu, Q.; Wen, Z.; Xu, L.; Gao, J.; Wu, D.; Shen, K.; Qiu, T.; Tang, S.; Xu, M. Room temperature ferromagnetic pure ZnO. Phys. B Condens. Matter 2011, 406, 19–23. [Google Scholar] [CrossRef]
- Sanyal, D.; Chakrabarti, M.; Roy, T.K.; Chakrabarti, A. The origin of ferromagnetism and defect-magnetization correlation in nanocrystalline ZnO. Phys. Lett. A 2007, 371, 482–485. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, W.; Xie, Q.; Li, Z.; Zhang, Z. Enhanced room-temperature ferromagnetism in un-doped ZnO thin films by thermal annealing in a strong magnetic field. J. Appl. Phys. 2012, 111, 103524. [Google Scholar] [CrossRef]
- Hong, N.H.; Sakai, J.; Huong, N.T.; Poirot, N.; Ruyter, A. Role of defects in tuning ferromagnetism in diluted magnetic oxide thin films. Phys. Rev. B 2005, 72, 045336. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Li, L.; Wang, Y.X.; Gao, K.H.; Li, Z.Q.; Zheng, R.; Ringer, S.; Zhang, B. Role of point defects in room-temperature ferromagnetism of Cr-doped ZnO. Appl. Phys. Lett. 2007, 91, 72511. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Jena, P.; Kawazoe, Y. Magnetic coupling between Cr atoms doped at bulk and surface sites of ZnO. Appl. Phys. Lett. 2005, 87, 162509. [Google Scholar] [CrossRef] [Green Version]
- Thurber, A.P.; Beausoleil II, G.L.; Alanko, G.A.; Anghel, J.J.; Jones, M.S.; Johnson, L.M.; Zhang, J.H.; Hanna, C.B.; Tenne, D.A.; Punnoose, A. Magnetism of ZnO nanoparticles: Dependence on crystallite size and surfactant coating. J. Appl. Phys. 2011, 109, 07C305. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.K.; Kumar, Y.; Rajput, P.; Jha, S.N.; Bhattacharyya, D.; Shirage, P.M. Search for origin of room temperature ferromagnetism properties in Ni-doped ZnO nanostructure. ACS Appl. Mater. Inter. 2017, 9, 7691–7700. [Google Scholar] [CrossRef] [Green Version]
- Wojnarowicz, J.; Kusnieruk, S.; Chudoba, T.; Gierlotka, S.; Lojkowski, W.; Knoff, W.; Lukasiewicz, M.I.; Witkowski, B.; Wolska, A.; Klepka, M.; et al. Paramagnetism of cobalt-doped ZnO nanoparticles obtained by microwave solvothermal synthesis. Beilstein J. Nanotechnol. 2015, 6, 1957–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacManus-Driscoll, J.L.; Khare, N.; Liu, Y.; Vickers, M.E. Structural Evidence for Zn Intersititials in Ferromagnetic Zn1–xCoxO Films. Adv. Mater. 2007, 19, 2925–2929. [Google Scholar] [CrossRef]
- Chen, R.; Luo, F.C.; Liu, Y.Z.; Song, Y.; Dong, Y.; Wu, S.; Cao, J.H.; Yang, F.Y.; N’Diaye, A.; Shafer, P.; et al. Tunable room-temperature ferromagnetism in Co-doped two-dimensional van der Waals ZnO. Nat. Commun. 2021, 12, 3953. [Google Scholar] [CrossRef]
- Coey, J.; Venkatesan, M.; Fitzgerald, C.; Dorneles, L.; Stamenov, P.; Lunney, J. Anisotropy of the magnetization of a dilute magnetic oxide. J. Magn. Magn. Mater. 2004, 290–291, 1405–1407. [Google Scholar] [CrossRef]
- Venkatesan, M.; Fitzgerald, C.B.; Lunney, J.G.; Coey, J.M.D. Anisotropic Ferromagnetism in Substituted Zinc Oxide. Phys. Rev. Lett. 2004, 93, 177206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchholz, D.B.; Chang, R.P.H.; Song, J.H.; Ketterson, J.B. Room-temperature Ferromagnetism in Cu-doped ZnO Thin Films. Appl. Phys. Lett. 2005, 87, 082504. [Google Scholar] [CrossRef]
- Huang, J.C.A.; Hsu, H.S. Inspection of magnetic semiconductor and clustering structure in CoFe-doped ZnO films by bias-dependent impedance spectroscopy. Appl. Phys. Lett. 2005, 87, 132503. [Google Scholar] [CrossRef]
- Du, C.L.; Gu, Z.B.; Lu, M.H.; Wang, J.; Zhang, S.T.; Zhao, J.; Cheng, G.X.; Heng, H.; Chen, Y.F. Raman spectroscopy of (Mn, Co)-codoped ZnO films. J. Appl. Phys. 2006, 99, 123515. [Google Scholar] [CrossRef]
- Robaina, O.V.; Cabrera, A.F.; Meyer, M.; Romano, R.M.; Cruz, A.F.; Torres, C.E.R. Room-Temperature Ferromagnetism Induced by High-Pressure Hydrogenation of ZnO. J. Phys. Chem. C 2019, 123, 19851–19861. [Google Scholar] [CrossRef]
- Salimian, A.; Abul, H.A.; Aminishahsavarani, A.; Upadhyaya, H. Hypothesis on the influence of the magnetic behaviour of hydrogen doped Zinc oxide during its plasma sputtering process. Coatings 2021, 11, 222. [Google Scholar] [CrossRef]
- Hariwal, R.V.; Malik, H.K.; Negi, A.; Kandasami, A. Controlling room temperature ferromagnetism and band gap in ZnO nanostructured thin films by varying angle of implantation. RSC Adv. 2018, 8, 6278–6287. [Google Scholar] [CrossRef] [Green Version]
- Jindal, K.; Tomar, M.; Katiyar, R.; Gupta, V. Transition from diamagnetic to ferromagnetic state in laser ablated nitrogen doped ZnO thin films. AIP Adv. 2015, 5, 027117. [Google Scholar] [CrossRef]
- Pan, H.; Yi, J.B.; Shen, L.; Wu, R.Q.; Yang, J.H.; Lin, J.Y.; Feng, Y.P.; Ding, J.; Van, L.H.; Yin, J.H. Room-temperature ferromagnetism in Carbon-doped ZnO. Phys. Rev. Lett. 2007, 99, 127201. [Google Scholar] [CrossRef] [Green Version]
- Nagare, B.J.; Chacko, S.; Kanhere, D.G. Ferromagnetism in Carbon-Doped Zinc Oxide Systems. J. Phys. Chem. A 2010, 114, 2689–2696. [Google Scholar] [CrossRef] [Green Version]
- Pham, A.; Assadi, M.H.N.; Zhang, Y.B.; Yu, A.B.; Li, S. Weak d0 magnetism in C and N doped ZnO. J. Appl. Phys. 2011, 110, 123917. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Malik, H.K.; Asokan, K.; Kandasami, A. Tuning of optical bandgap and magnetization of C-implanted ZnO thin films. EPL Europhys. Lett. 2015, 110, 67006. [Google Scholar] [CrossRef]
- Zhao, P.; Guan, X.; Zheng, H.; Jia, S.; Li, L.; Liu, H.; Zhao, L.; Sheng, H.; Meng, W.; Zhuang, Y.; et al. Surface- and Strain-Mediated Reversible Phase Transformation in Quantum-Confined ZnO Nanowires. Phys. Rev. Lett. 2019, 123, 216101. [Google Scholar] [CrossRef]
- Garnero, C.; Pierrot, A.; Gatel, C.; Marcelot, C.; Arenal, R.; Florea, I.; Mantel, A.B.; Soulantica, K.; Poveda, P.; Chaudret, B.; et al. Single-crystalline body centered FeCo nano-octopods: From one-pot chemical growth to a complex 3D magnetic configuration. Nano Lett. 2021, 21, 3664–3670. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Chen, Z.; Ha, Y.-C.; Wiley, B.J. Real-Time Visualization of Diffusion-Controlled Nanowire Growth in Solution. Nano Lett. 2014, 14, 4671–4676. [Google Scholar] [CrossRef]
- Zhou, J.H.; Yang, Y.S.; Yang, Y.; Kim, D.S.; Yuan, A.; Tian, X.Z.; Ophus, C.; Sun, F.; Schmid, A.K.; Nathanson, M.; et al. Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 2019, 570, 500–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, R.E.; Houben, L.; Wolf, S.G.; Leitus, G.; Lang, Z.-L.; Carbó, J.J.; Poblet, Z.-L.L.J.J.C.J.M.; Neumann, R.E.S.R. Real-time molecular scale observation of crystal formation. Nat. Chem. 2016, 9, 369–373. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).