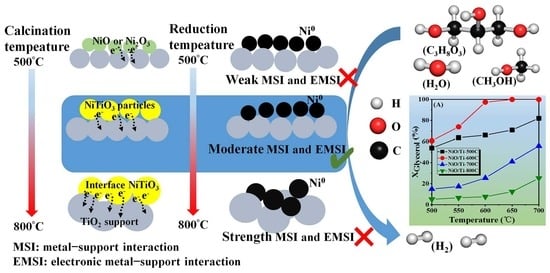
Study of the Metal–Support Interaction and Electronic Effect Induced by Calcination Temperature Regulation and Their Effect on the Catalytic Performance of Glycerol Steam Reforming for Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. GSR Catalyst Testing
2.3. Catalyst Characterization
3. Results and Discussion
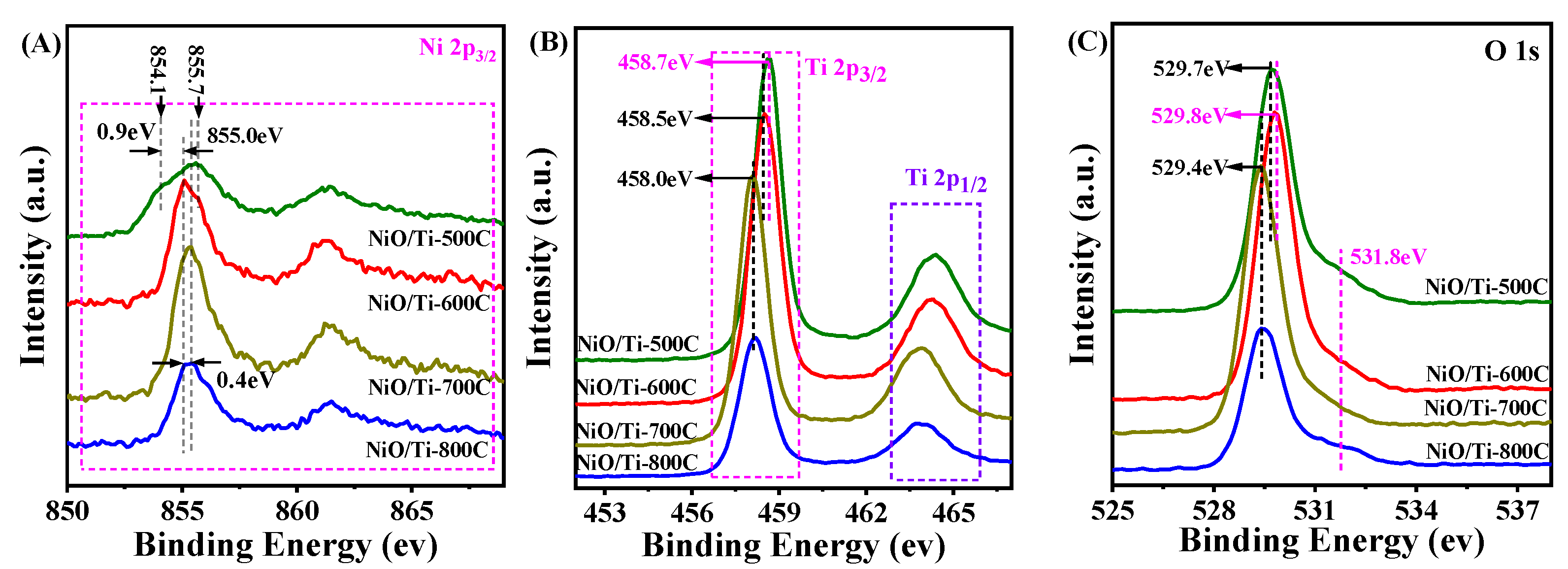
3.1. Physicochemical Properties of the Samples
3.2. Catalytic Performance Tests
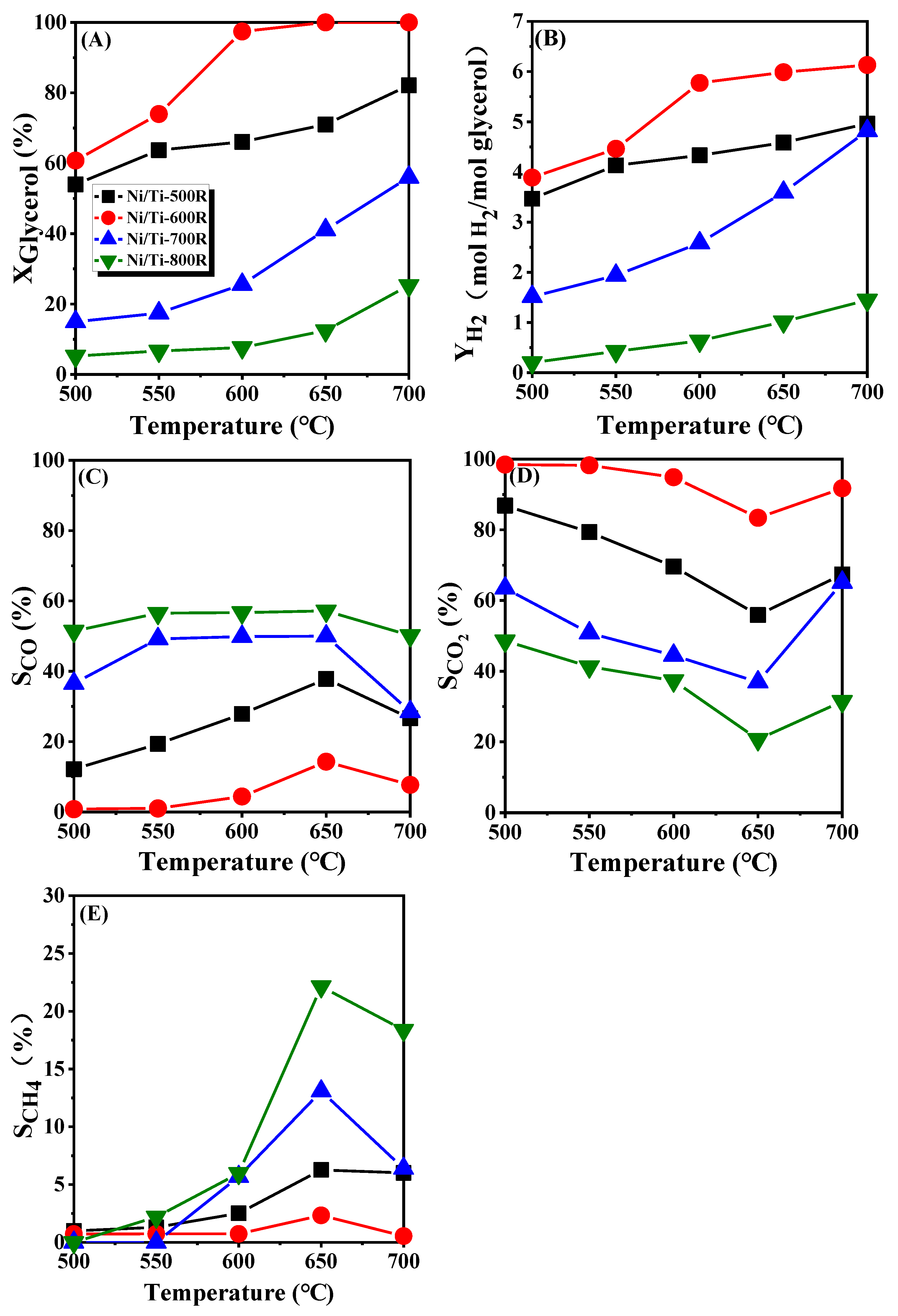
3.2.1. Catalytic Performance Tests of GSR
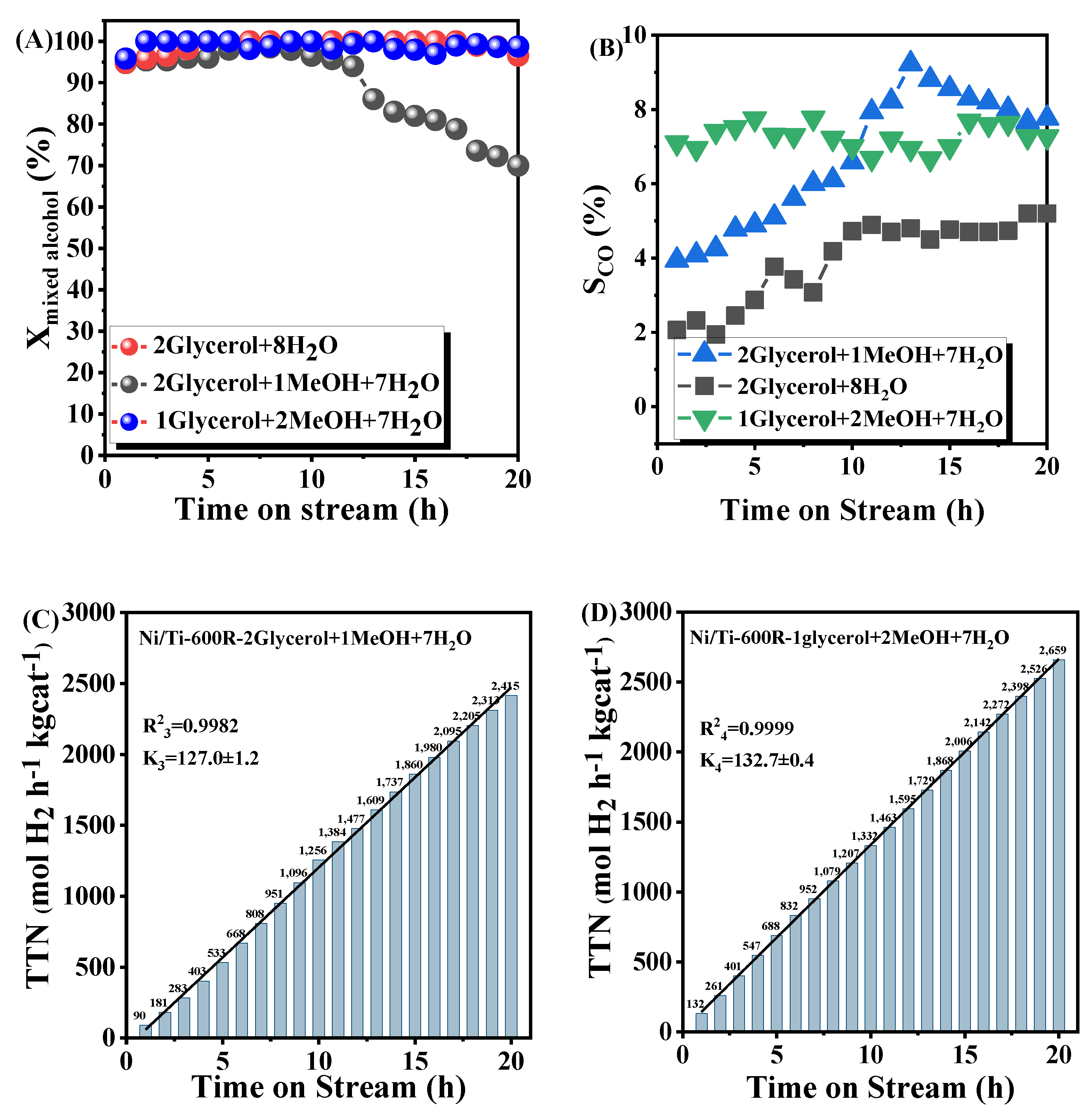
3.2.2. Catalytic Performance Tests of GMSR
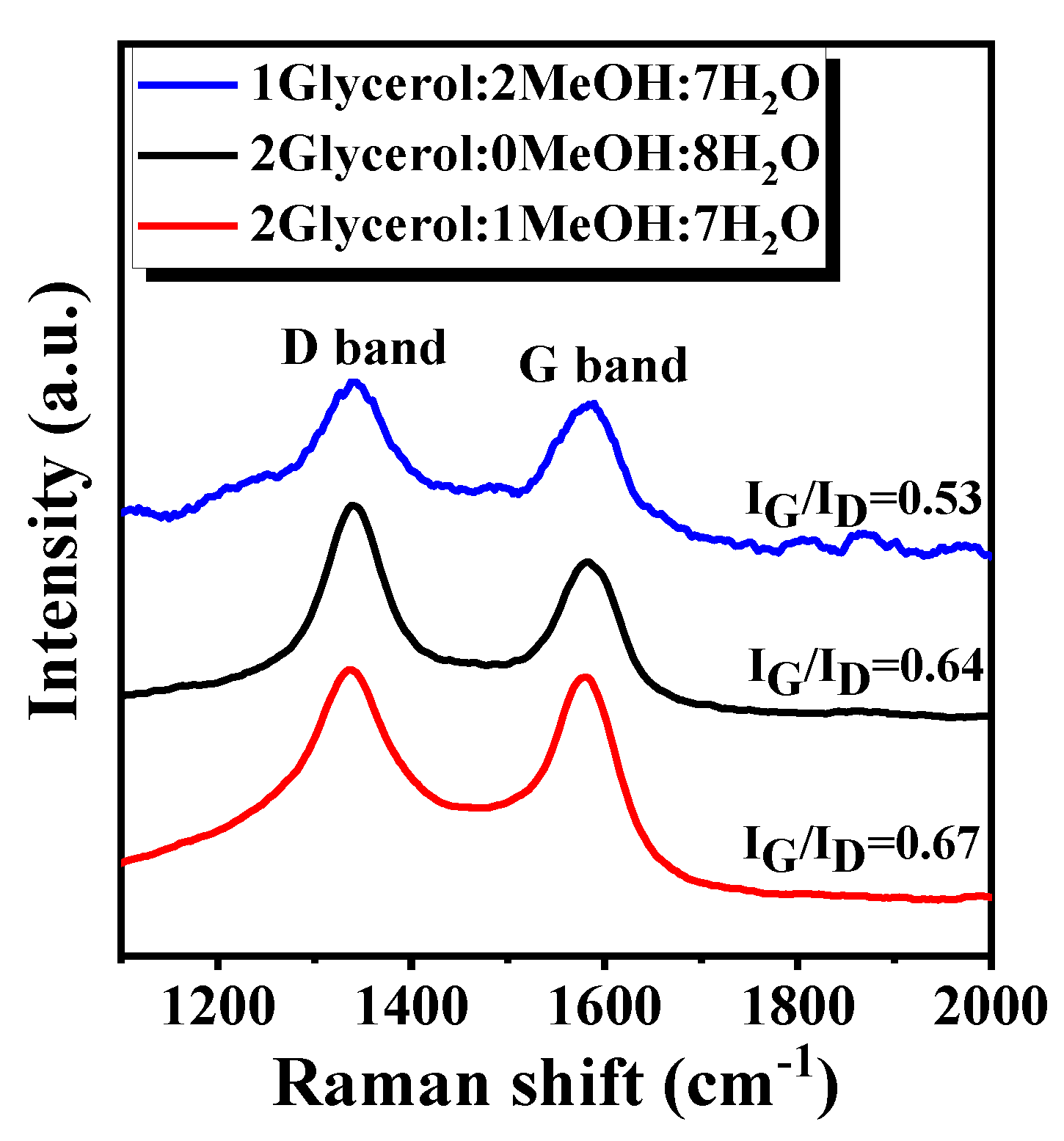
4. Mechanism Analysis of Catalyst Deactivation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, R.K. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Tlili, O.; Mansilla, C.; Frimat, D.; Perez, Y. Hydrogen market penetration feasibility assessment: Mobility and natural gas markets in the US, Europe, China and Japan. Int. J. Hydrogen Energy 2019, 44, 16048–16068. [Google Scholar] [CrossRef]
- Cohen, R.L.; Wernick, J.H. Hydrogen Storage Materials: Properties and Possibilites. Science 1981, 214, 1081–1087. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698–9725. [Google Scholar] [CrossRef]
- Chen, D.; He, L. Towards an Efficient Hydrogen Production from Biomass: A Review of Processes and Materials. ChemCatChem 2011, 3, 490–511. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, H.; Huang, Z.; Li, S.; Wu, G.; Ma, X.; Gong, J. Hydrogen Production via Steam Reforming of Ethanol on Phyllosilicate-Derived Ni/SiO2: Enhanced Metal–Support Interaction and Catalytic Stability. ACS Sustain. Chem. Eng. 2012, 1, 161–173. [Google Scholar] [CrossRef]
- Cui, Y.; Galvita, V.; Rihko-Struckmann, L.; Lorenz, H.; Sundmacher, K. Steam reforming of glycerol: The experimental activity of La1−xCexNiO3 catalyst in comparison to the thermodynamic reaction equilibrium. Appl. Catal. B Environ. 2009, 90, 29–37. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della, P.C. From glycerol to value-added products. Angew. Chem. Int. Ed. Engl. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, L.F.; Álvarez, A.; Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Montes, M.; Odriozola, J.A. Influence of the shape of Ni catalysts in the glycerol steam reforming. Appl. Catal. B Environ. 2012, 123–124, 379–390. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Schwengber, C.A.; Alves, H.J.; Schaffner, R.A.; da Silva, F.A.; Sequinel, R.; Bach, V.R.; Ferracin, R.J. Overview of glycerol reforming for hydrogen production. Renew. Sust. Energ. Rev. 2016, 58, 259–266. [Google Scholar] [CrossRef]
- Moraes, T.S.; Neto, R.C.R.; Ribeiro, M.C.; Mattos, L.V.; Kourtelesis, M.; Ladas, S.; Verykios, X.; Noronha, F.B. Ethanol conversion at low temperature over CeO2—Supported Ni-based catalysts. Effect of Pt addition to Ni catalyst. Appl. Catal. B Environ. 2016, 181, 754–768. [Google Scholar] [CrossRef]
- Pohar, A.; Hočevar, S.; Likozar, B.; Levec, J. Synthesis and characterization of gallium-promoted copper–ceria catalyst and its application for methanol steam reforming in a packed bed reactor. Catal. Today 2015, 256, 358–364. [Google Scholar] [CrossRef]
- Lotrič, A.; Sekavčnik, M.; Pohar, A.; Likozar, B.; Hočevar, S. Conceptual design of an integrated thermally self-sustained methanol steam reformer—High-temperature PEM fuel cell stack manportable power generator. Int. J. Hydrogen Energy 2017, 42, 16700–16713. [Google Scholar] [CrossRef]
- Rubin, K.; Pohar, A.; Dasireddy, V.D.B.C.; Likozar, B. Synthesis, characterization and activity of CuZnGaOx catalysts for the water–gas shift (WGS) reaction for H2 production and CO removal after reforming. Fuel Process. Technol. 2018, 169, 217–225. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Zou, Q.; He, D.; Zhang, L.; Xu, Z.; He, S.; Luo, Y. Unravelling the Nature of the Active Species as well as the Doping Effect over Cu/Ce-Based Catalyst for Carbon Monoxide Preferential Oxidation. ACS Catal. 2019, 9, 2177–2195. [Google Scholar] [CrossRef]
- Lu, J.; Lei, Y.; Wan, G.; Mei, Z.; Yu, J.; Zhao, Y.; He, S.; Luo, Y. Weakening the metal-support strong interaction to enhance catalytic performances of alumina supported Ni-based catalysts for producing hydrogen. Appl. Catal. B Environ. 2020, 263, 118177. [Google Scholar] [CrossRef]
- Gray, J.T.; Che, F.; McEwen, J.-S.; Ha, S. Field-assisted suppression of coke in the methane steam reforming reaction. Appl. Catal. B Environ. 2020, 260, 118132. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Hou, F.; Wu, C.; Pan, L.; Zou, J.; Wang, L.; Zhang, X.; Liu, G.; Li, G. Engineering oxygen vacancies and nickel dispersion on CeO2 by Pr doping for highly stable ethanol steam reforming. Appl. Catal. B Environ. 2019, 258, 117940. [Google Scholar] [CrossRef]
- Chen, S.; Pei, C.; Gong, J. Insights into interface engineering in steam reforming reactions for hydrogen production. Energy Environ. Sci. 2019, 12, 3473–3495. [Google Scholar] [CrossRef]
- Zhou, G.; Barrio, L.; Agnoli, S.; Senanayake, S.D.; Evans, J.; Kubacka, A.; Estrella, M.; Hanson, J.C.; Martinez-Arias, A.; Fernandez-Garcia, M.; et al. High activity of Ce(1-x)Ni(x)O(2-y) for H2 production through ethanol steam reforming: Tuning catalytic performance through metal-oxide interactions. Angew. Chem. Int. Ed. Engl. 2010, 49, 9680–9684. [Google Scholar] [CrossRef]
- Turczyniak, S.; Teschner, D.; Machocki, A.; Zafeiratos, S. Effect of the surface state on the catalytic performance of a Co/CeO2 ethanol steam-reforming catalyst. J. Catal. 2016, 340, 321–330. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Sanchez, M.C.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B Environ. 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Rossetti, I.; Lasso, J.; Finocchio, E.; Ramis, G.; Nichele, V.; Signoretto, M.; di Michele, A. TiO2-supported catalysts for the steam reforming of ethanol. Appl. Catal. A Gen. 2014, 477, 42–53. [Google Scholar] [CrossRef]
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Challenges and strategies for optimization of glycerol steam reforming process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhang, H.; Zhong, H.; Zhu, X.; Tian, Z.; Xu, D.; Yi, B. Preparation and characterization of carbon-supported PtRuIr catalyst with excellent CO-tolerant performance for proton-exchange membrane fuel cells. J. Catal. 2006, 238, 468–476. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. A review of steam reforming of glycerol. Chem. Pap. 2019, 73, 2619–2635. [Google Scholar] [CrossRef]
- Li, K.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Liu, R.; Gong, J. Dry reforming of methane over La2O2CO3-modified Ni/Al2O3 catalysts with moderate metal support interaction. Appl. Catal. B Environ. 2020, 264, 118448. [Google Scholar] [CrossRef]
- Xu, M.; He, S.; Chen, H.; Cui, G.; Zheng, L.; Wang, B.; Wei, M. TiO2–x-Modified Ni Nanocatalyst with Tunable Metal–Support Interaction for Water–Gas Shift Reaction. ACS Catal. 2017, 7, 7600–7609. [Google Scholar] [CrossRef]
- Xu, M.; Yao, S.; Rao, D.; Niu, Y.; Liu, N.; Peng, M.; Zhai, P.; Man, Y.; Zheng, L.; Wang, B.; et al. Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction. J. Am. Chem. Soc. 2018, 140, 11241–11251. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.J.A.; Prakash, M.G.; Mahalakshmy, R.; Elangovan, T.; Viswanathan, B. Selective hydrogenation of acetophenone over nickel supported on titania. Catal. Sci. Technol. 2012, 2, 1429–1436. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Louis, C.; Delannoy, L. Bimetallic Ni–Zn/TiO2 catalysts for selective hydrogenation of alkyne and alkadiene impurities from alkenes stream. Res. Chem. Intermediat. 2021, 47, 91–116. [Google Scholar] [CrossRef]
- De Korte, P.H.M.; Blasse, G. Water photoelectrolysis using nickel titanate and niobate as photoanodes. J. Solid State Chem. 1982, 44, 150–155. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chang, Y.-H.; Yang, W.-D.; Tsai, B.-S. Synthesis and characterization of ilmenite NiTiO3 and CoTiO3 prepared by a modified Pechini method. J. Non-Cryst. Solids 2006, 352, 789–794. [Google Scholar] [CrossRef]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Arya, A.; Ningthoujam, R.S.; Tewari, R.; Rao, R.; Chalke, B.; Ayyub, P.; Tripathi, A.K.; et al. pn Heterojunctions in NiO: TiO2 composites with type-II band alignment assisting sunlight driven photocatalytic H2 generation. Appl. Catal. B Environ. 2018, 221, 443–458. [Google Scholar] [CrossRef]
- Lopes, K.P.; Cavalcante, L.S.; Simões, A.Z.; Varela, J.A.; Longo, E.; Leite, E.R. NiTiO3 powders obtained by polymeric precursor method: Synthesis and characterization. J. Alloys Compd. 2009, 468, 327–332. [Google Scholar] [CrossRef]
- Ho, S.W.; Chu, C.Y.; Chen, S.G. Effect of thermal treatment on the nickel state and CO hydrogenation activity of titania-supported nickel catalysts. J. Catal. 1998, 178, 34–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wen, X.; Liu, Y. Partial oxidation of methane over Ni/Ce-Ti-O catalysts. Chem. Eng. J. 2006, 121, 115–123. [Google Scholar] [CrossRef]
- Lazaro, M.; Echegoyen, Y.; Alegre, C.; Suelves, I.; Moliner, R.; Palacios, J. TiO2 as textural promoter on high loaded Ni catalysts for methane decomposition. Int. J. Hydrogen Energy 2008, 33, 3320–3329. [Google Scholar] [CrossRef]
- De Sousa, F.F.; de Sousa, H.S.A.; Oliveira, A.C.; Junior, M.C.C.; Ayala, A.P.; Barros, E.B.; Viana, B.C.; Filho, J.M.; Oliveira, A.C. Nanostructured Ni-containing spinel oxides for the dry reforming of methane: Effect of the presence of cobalt and nickel on the deactivation behaviour of catalysts. Int. J. Hydrogen Energy 2012, 37, 3201–3212. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Surface charge of variable porosity Al2O3(s) and SiO2(s) adsorbents. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Chen, G.; Shang, L.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Ultrafine NiO Nanosheets Stabilized by TiO2 from Monolayer NiTi-LDH Precursors: An Active Water Oxidation Electrocatalyst. J. Am. Chem. Soc. 2016, 138, 6517–6524. [Google Scholar] [CrossRef]
- Sasi, B.; Gopchandran, K.G. Nanostructured mesoporous nickel oxide thin films. Nanotechnology 2007, 18, 115613. [Google Scholar] [CrossRef]
- Breeson, A.C.; Sankar, G.; Goh, G.K.L.; Palgrave, R.G. Phase quantification by X-ray photoemission valence band analysis applied to mixed phase TiO2 powders. Appl. Surf. Sci. 2017, 423, 205–209. [Google Scholar] [CrossRef]
- Alvarado, F.D.; Gracia, F. Oxidative steam reforming of glycerol for hydrogen production: Thermodynamic analysis including different carbon deposits representation and CO2 adsorption. Int. J. Hydrogen Energy 2012, 37, 14820–14830. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, J.; Du, Y.; Li, F.; Li, C.; Lu, J.; Wang, J.; Chen, Y. Hydrogen production by catalytic steam reforming of hydrocarbon fuels over Ni/Ce–Al2O3 bifunctional catalysts: Effects of SrO addition. Int. J. Hydrogen Energy 2016, 41, 13436–13447. [Google Scholar] [CrossRef]
- Feng, P.; Huang, K.; Xu, Q.; Qi, W.; Xin, S.; Wei, T.; Liao, L.; Yan, Y. Ni supported on the CaO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2020, 45, 8223–8233. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKetbi, M.; Polychronopoulou, K.; Goula, M.A. Ni supported on CaO-MgO-Al2O3 as a highly selective and stable catalyst for H2 production via the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2019, 44, 256–273. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Siakavelas, G.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The influence of SiO2 doping on the Ni/ZrO2 supported catalyst for hydrogen production through the glycerol steam reforming reaction. Catal. Today 2019, 319, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Zhang, C.; Li, S.; Han, Z.; Wang, T.; Ma, X.; Gong, J. Hydrogen Production via Glycerol Steam Reforming over Ni/Al2O3: Influence of Nickel Precursors. ACS Sustain. Chem. Eng. 2013, 1, 1052–1062. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, L.; Qin, Y. Study on the carbon deposition in steam reforming of ethanol over Co/CeO2 catalyst. Chem. Eng. J. 2008, 145, 25–31. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Liu, J.; Guo, C.; Wang, Y.; Zhang, J. Ethanol steam reforming reactions over Al2O3·SiO2-supported Ni–La catalysts. Fuel 2009, 88, 511–518. [Google Scholar] [CrossRef]
- Montero, C.; Ochoa, A.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Monitoring Ni0 and coke evolution during the deactivation of a Ni/La2O3–αAl2O3 catalyst in ethanol steam reforming in a fluidized bed. J. Catal. 2015, 331, 181–192. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.-J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B Environ. 2017, 202, 683–694. [Google Scholar] [CrossRef]

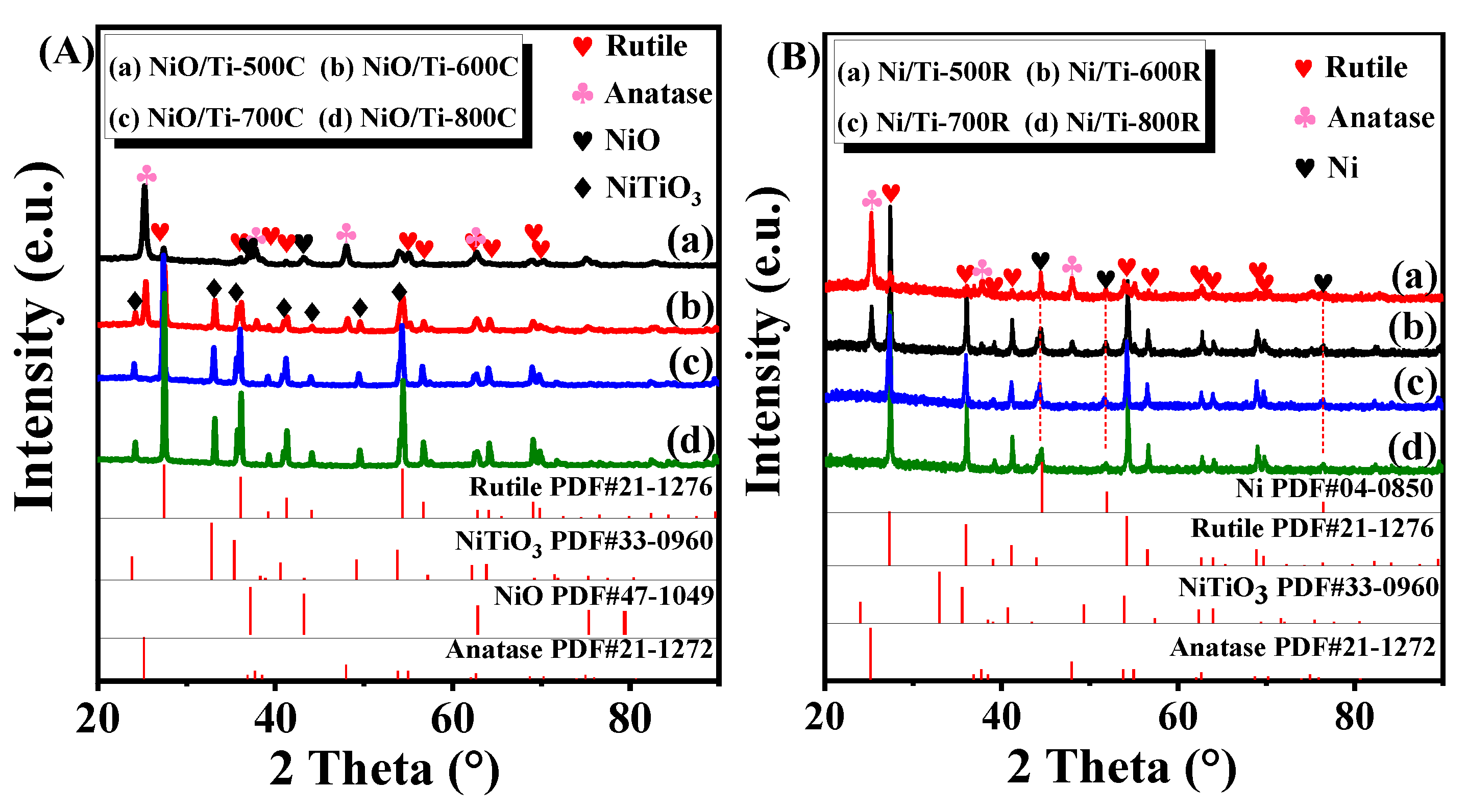
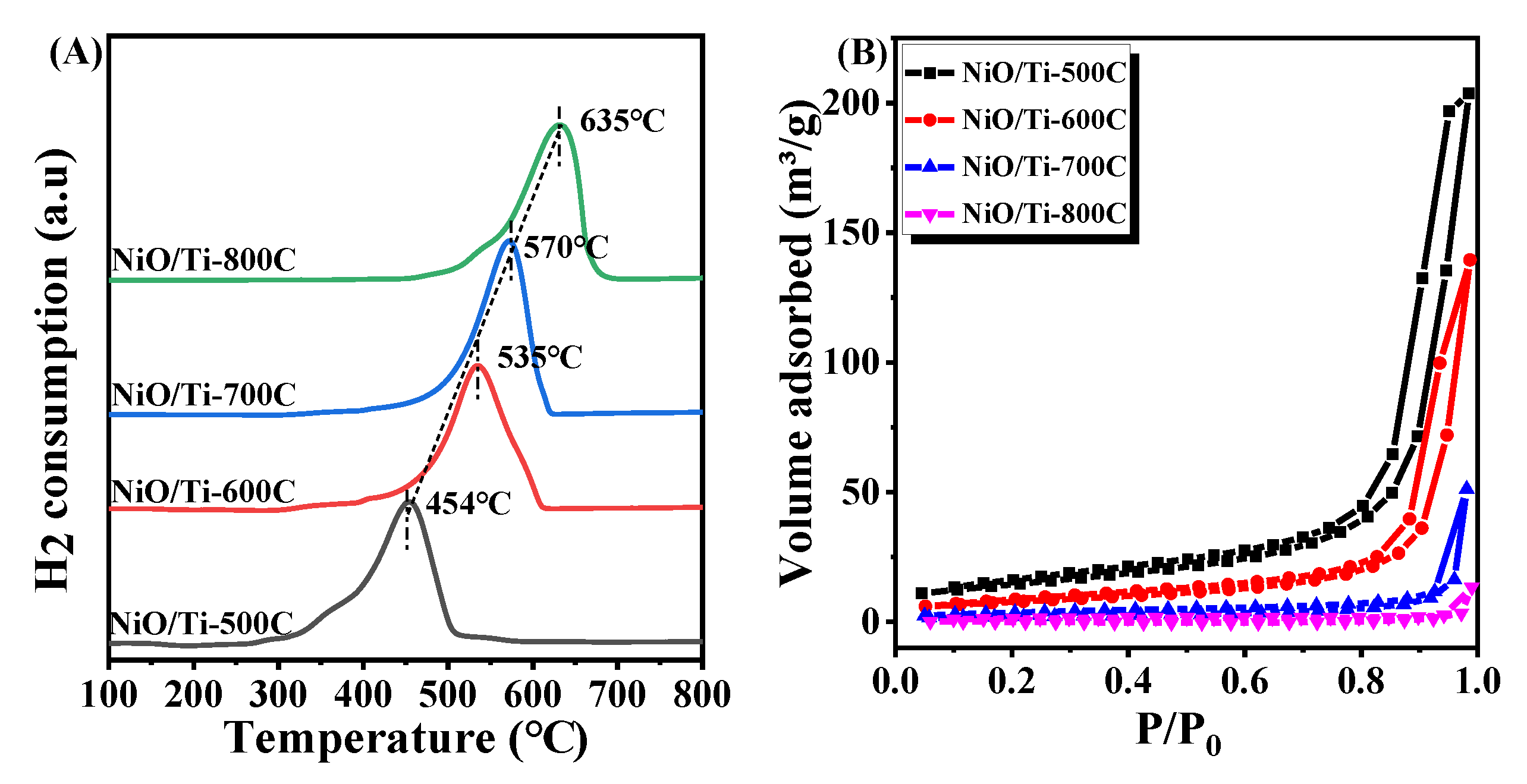



| Sample | The Proportion of Rutile (%) | Crystallite Size of Rutile [110] a (nm) | Crystallite Size of NiO [111] a (nm) | Crystallite Size of NiTiO3 [104] a (nm) | Crystallite Size of Ni [200] a (nm) | BET Surface Area (m2/g) b | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|---|---|---|---|---|
| NiO/Ti-500C | 11.5 | 27.3 | 14.97 | _ | _ | 64.56 ± 1 | 0.32 | 13.38 |
| NiO/Ti-600C | 54.0 | 36.2 | _ c | 33.70 | _ | 33.00 ± 0.5 | 0.22 | 15.31 |
| NiO/Ti-700C | 100.0 | 50.0 | _ | 46.50 | _ | 7.46 ± 0.5 | 0.08 | 3.25 |
| NiO/Ti-800C | 100.0 | 74.9 | _ | 67.10 | _ | 0.98 ± 0.3 | 0.02 | 3.92 |
| Ni/Ti-500R | 13.7 | 33.0 | _ | _ | 18.80 ± 1 | 41.38 ± 1 | 0.24 | 11.17 |
| Ni/Ti-600R | 71.2 | 44.3 | _ | _ | 19.77 ± 0.5 | 15.21 ± 0.5 | 0.11 | 14.56 |
| Ni/Ti-700R | 100.0 | 49.1 | _ | _ | 20.83 ± 1 | _ | _ | _ |
| Ni/Ti-800R | 100.0 | 75.0 | _ | _ | 23.25 ± 2 | 1.18 ± 0.2 | 0.01 | 4.28 |
| Samples | NH3 Desorbed (μmol/gcat) | H2 Uptake (μmolH2/gcat) | ||
|---|---|---|---|---|
| T < 550 °C | T > 550 °C | Total | ||
| TiO2 | 313.2 | 7.1 | 320.3 | - |
| Ni/Ti-500C | 265.2 | 80.1 | 345.3 | 74.4 |
| Ni/Ti-600C | 114.7 | 149.3 | 264.0 | 110.2 |
| Ni/Ti-700C | 47.8 | 127.6 | 175.4 | 35.6 |
| Ni/Ti-800C | 5.4 | 113.7 | 119.1 | 25.8 |
| Feed Glycerol/MeOH/H2O | Weight Loss (%) | Coke/Glycerol (mmol/mol) | Coke Formation Rate mol/gcat/s | Carbon Balance (%) | IG/ID |
|---|---|---|---|---|---|
| 1/2/7 | 5.1 | 0.25 | 5.9 × 10−8 | 99.1 | 0.53 |
| 2/0/8 | 9.4 | 0.37 | 1.1 × 10−7 | 99.1 | 0.64 |
| 2/1/7 | 12.8 | 0.44 | 1.5 × 10−7 | 89.2 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Wang, Y.; Lu, J.; Lu, H.; He, S.; Song, D.; Luo, Y.; Liu, J. Study of the Metal–Support Interaction and Electronic Effect Induced by Calcination Temperature Regulation and Their Effect on the Catalytic Performance of Glycerol Steam Reforming for Hydrogen Production. Nanomaterials 2021, 11, 3149. https://doi.org/10.3390/nano11113149
Zhu S, Wang Y, Lu J, Lu H, He S, Song D, Luo Y, Liu J. Study of the Metal–Support Interaction and Electronic Effect Induced by Calcination Temperature Regulation and Their Effect on the Catalytic Performance of Glycerol Steam Reforming for Hydrogen Production. Nanomaterials. 2021; 11(11):3149. https://doi.org/10.3390/nano11113149
Chicago/Turabian StyleZhu, Songshan, Yunzhu Wang, Jichang Lu, Huihui Lu, Sufang He, Di Song, Yongming Luo, and Jiangping Liu. 2021. "Study of the Metal–Support Interaction and Electronic Effect Induced by Calcination Temperature Regulation and Their Effect on the Catalytic Performance of Glycerol Steam Reforming for Hydrogen Production" Nanomaterials 11, no. 11: 3149. https://doi.org/10.3390/nano11113149
APA StyleZhu, S., Wang, Y., Lu, J., Lu, H., He, S., Song, D., Luo, Y., & Liu, J. (2021). Study of the Metal–Support Interaction and Electronic Effect Induced by Calcination Temperature Regulation and Their Effect on the Catalytic Performance of Glycerol Steam Reforming for Hydrogen Production. Nanomaterials, 11(11), 3149. https://doi.org/10.3390/nano11113149