Treatment with Argovit® Silver Nanoparticles Induces Differentiated Postharvest Biosynthesis of Compounds with Pharmaceutical Interest in Carrot (Daucus carota L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Processing
2.3. Sample Preparation for Phytochemical Analyses
2.4. Total Phenolic Content and Determination of Antioxidant Capacity
2.5. Quantification of Individual Phenolic Compounds by HPLC
2.6. Analysis of Individual Phenolic Compounds by HPLC-MS
2.7. Quantification of Silver Content in Carrot Tissue after Exposure by ICP-OES
2.8. Statistical Analysis
3. Results
3.1. Total Phenolic Content and Antioxidant Capacity
3.2. Identification of Phenolic Compounds in Carrots
3.3. Quantification of Phenolic Compounds in Carrots and Selectivity
3.4. Quantification of Silver after Carrots Exposure to AgNPs Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. European Food and Nutrition Action Plan 2015–2020. 2014. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/activities/technical-support-to-member-states/promoting-fruit-and-vegetable-consumption (accessed on 10 October 2021).
- Antunes-Ricardo, M.; Rodríguez-Rodríguez, C.; Gutiérrez-Uribe, J.A.; Cepeda-Cañedo, E.; Serna-Saldívar, S.O. Bioaccessibility, intestinal permeability and plasma stability of isorhamnetin glycosides from Opuntia ficusindica (L.). Int. J. Mol. Sci. 2017, 18, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.; Serna-Saldívar, S.O. Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods Hum. Nutr. 2009, 64, 146–152. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa Has More Phenolic Phytochemicals and a Higher Antioxidant Capacity than Teas and Red Wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef] [PubMed]
- Averna, M.; Casazza, A.A.; Martines, A.; Pedrazzi, M.; Franchi, A.; De Tullio, R.; Perego, P.; Melloni, E. Cell protection from Ca2+-overloading by bioactive molecules extracted from olive pomace. Nat. Prod. Res. 2018, 6419, 1449–1455. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Liu, W.; Li, C. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Granados, O.; Torre-Villalvazo, I.; Serna-Saldivar, S.O.; Torres, N.; Palacios-González, B.; Tovar, A.R. Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br. J. Nutr. 2014, 112, 886–899. [Google Scholar] [CrossRef] [Green Version]
- British Broadcasting Corporation (BBC) Research. Plant-Derived Drugs: Global Markets. 2017. Available online: https://www.bccresearch.com/market-research/biotechnology/botanical-and-plant-derived-drugs-global-markets.html (accessed on 23 September 2021).
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled Abiotic Stresses Revisited: From Homeostasis through Hormesis to Extreme Stresses and the Impact on Nutraceuticals and Quality during Pre- and Postharvest Applications in Horticultural Crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) seed priming induced growth and biochemical changes in wheat under water deficit conditions. Biol. Trace Elem. Res. 2013, 151, 284–293. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Chavez-Santoscoy, R.A.; Lecona-Guzmán, C.A.; Bogdanchikova, N.; Salinas-Ruíz, J.; Gómez-Merino, F.C.; Pestryakov, A. Hormetic response by silver nanoparticles on in vitro multiplication of sugarcane (Saccharum spp. Cv. Mex 69-290) using a temporary immersion system. Dose-Response 2017, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, B.G.; Del, C.; Rodríguez, S.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Nair, V.; Serrano-Sandoval, S.N.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Wounding and UVB light synergistically induce the postharvest biosynthesis of indicaxanthin and betanin in red prickly pears. Postharvest Biol. Technol. 2020, 167, 111247. [Google Scholar] [CrossRef]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenumgraecum). Bull. Natl. Res. Cent. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Swamy, M.K.; Sinniah, U.R. (Eds.) Natural Bio-Active Compounds; Springer: Singapore, 2019. [Google Scholar]
- Giridhar, P.; Ravishankar, G.A. Efficient micropropagation of Vanilla planifolia Andr. under influence of thidiazuron, zeatin and coconut milk. Indian J. Biotechnol. 2004, 3, 113–118. [Google Scholar]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol. Plant. 2014, 37, 1–11. [Google Scholar] [CrossRef]
- Batool, S.U.; Javed, B.; Sohail; Zehra, S.S.; Mashwani, Z.-U.-R.; Raja, N.I.; Khan, T.; ALHaithloul, H.A.S.; Alghanem, S.M.; Al-Mushhin, A.A.M.; et al. Exogenous Applications of Bio-fabricated Silver Nanoparticles to Improve Biochemical, Antioxidant, Fatty Acid and Secondary Metabolite Contents of Sunflower. Nanomaterials 2021, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Baskar, V.; Venkatesh, J.; Park, S.W. Impact of biologically synthesized silver nanoparticles on the growth and physiological responses in Brassica rapa ssp. pekinensis. Environ. Sci. Pollut. Res. 2015, 22, 17672–17682. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Figueroa, F.; Arellano-García, M.E.; Leyva-Aguilera, C.; Ruíz-Ruíz, B.; Luna Vázquez-Gómez, R.; Radilla-Chávez, P.; Chávez-Santoscoy, R.A.; Pestryakov, A.; Toledano-Magaña, Y.; García-Ramos, J.C.; et al. Argovit™ Silver Nanoparticles Effects on Allium cepa: Plant Growth Promotion without Cyto Genotoxic Damage. Nanomaterials 2020, 10, 1386. [Google Scholar] [CrossRef]
- Luna-Vazquez-Gomez, R.; Arellano-Garcia, M.E.; Garcia-Ramos, J.C.; Radilla-Chavez, P.; Salas-Vargas, D.S.; Casillas-Figueroa, F.; Ruiz-Ruiz, B.; Bogdanchikova, N.; Pestryakov, A. Hemolysis of human erythrocytes by argovitTM AgNPs from healthy and diabetic donors: An in vitro study. Materials 2021, 14, 2792. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, B.; Arellano-García, M.E.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Toledano-Magaña, Y.; Casillas-Figueroa, F.; Luna-Vazquez-Gomez, R.; Pestryakov, A.; García-Ramos, J.C.; Bogdanchikova, N. Cytokinesis-Block Micronucleus Assay Using Human Lymphocytes as a Sensitive Tool for Cytotoxicity/Genotoxicity Evaluation of AgNPs. ACS Omega 2020, 5, 12005–12015. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramírez, O.U.; Valenzuela-Salas, L.M.; Blanco-Salazar, A.; Rodríguez-Arenas, J.A.; Mier-Maldonado, P.A.; García-Ramos, J.C.; Bogdanchikova, N.; Pestryakov, A.; Toledano-Magaña, Y. Antitumor Activity against Human Colorectal Adenocarcinoma of Silver Nanoparticles: Influence of [Ag]/[PVP] Ratio. Pharmaceutics 2021, 13, 1000. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Salas, L.; Girón-Vázquez, N.G.; García-Ramos, J.C.; Torres-Bugarín, O.; Gómez, C.; Pestryakov, A.; Villarreal-Gómez, L.J.; Toledano-Magaña, Y.; Bogdanchikova, N. Antiproliferative and Antitumour Effect of Nongenotoxic Silver Nanoparticles on Melanoma Models. Oxidative Med. Cell. Longev. 2019, 2019, 4528241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hamauzu, D. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). Int. J. Food Agric. Environ. 2004, 2, 332. [Google Scholar]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Ganzon, J.G.; Chen, L.G.; Wang, C.-C. 4-O-Caffeoylquinic acid as an antioxidant marker for mulberry leaves rich in phenolic compounds. J. Food Drug Anal. 2018, 26, 985–993. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Chen, G.; Almeida, M.C.; Soares, D.M.; de Souza, G.E.; Lopes, N.P.; Lantz, R.C. Effects of caffeoylquinic acid derivatives and C-flavonoid from Lychnophora ericoides on in vitro inflammatory mediator production. Nat. Prod. Commun. 2010, 5, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Farias-Pereira, R.; Oshiro, J.; Kim, K.-H.; Park, Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.; Seo, K.; Ki, S.H. 5-Caffeoylquinic acid ameliorates oxidative stress-mediated cell death via Nrf2 activation in hepatocytes. Pharm Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Misawa, K.; Nishimura, H.; Hirata, T.; Yamamoto, M.; Ota, N. 5-Caffeoylquinic Acid Ameliorates Cognitive Decline and Reduces Aβ Deposition by Modulating Aβ Clearance Pathways in APP/PS2 Transgenic Mice. Nutrients 2020, 12, 494. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, C.; Cittadini, M.C.; Soria, E.A. Pharmacological Activity of Quercetin and 5 Caffeoylquinic Acid Oral Intake in Male Balb/c Mice with Lung Adenocarcinoma. Arch. Med. Res. 2020, 51, 8–12. [Google Scholar] [CrossRef]
- Ghosh, S.; Basak, P.; Dutta, S.; Chowdhury, S.; Sil, P.C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem. Toxicol. 2017, 103, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, L.; Li, Q.; Yang, S.; Zhang, Y.; Wang, Y. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]

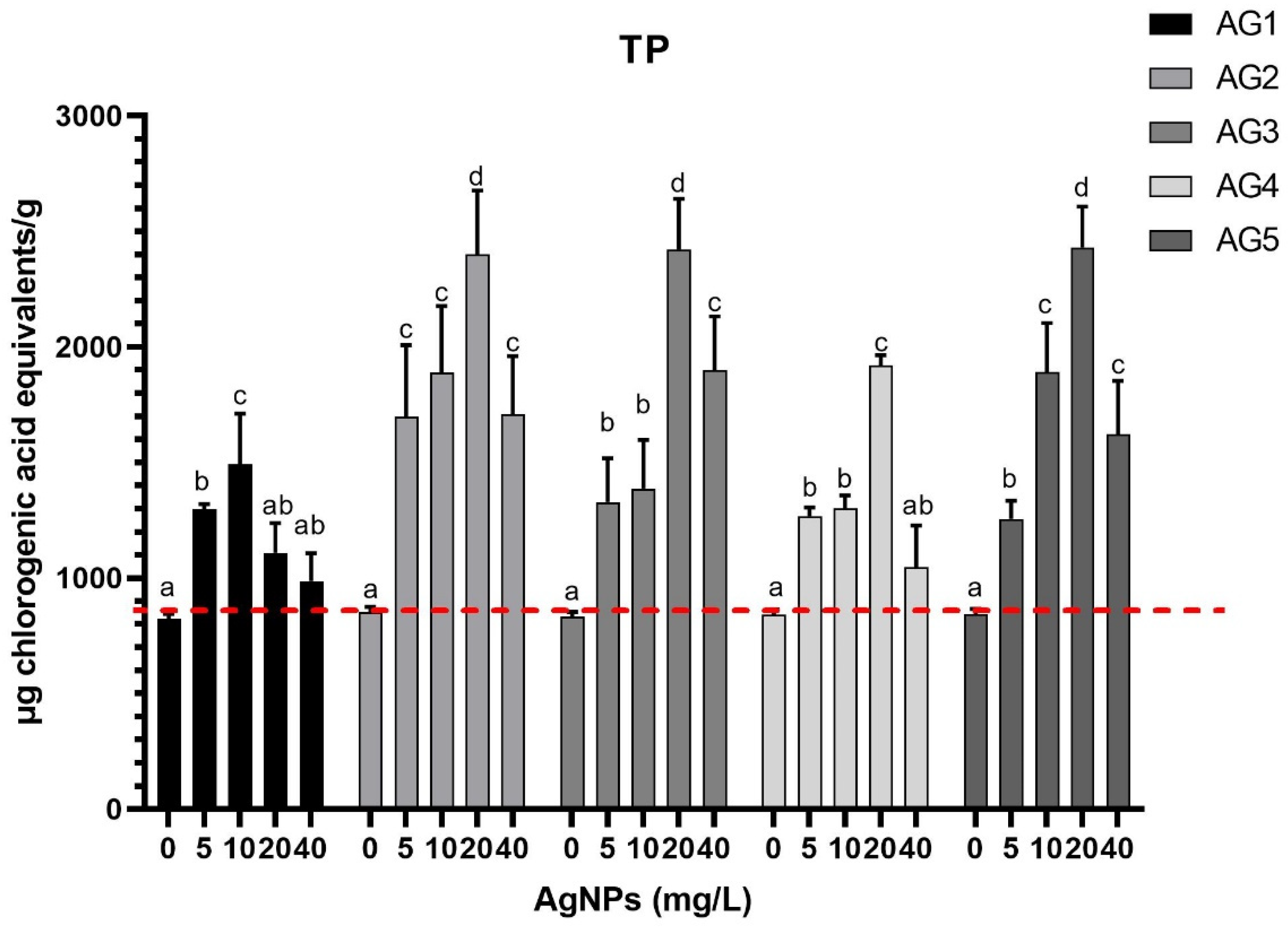
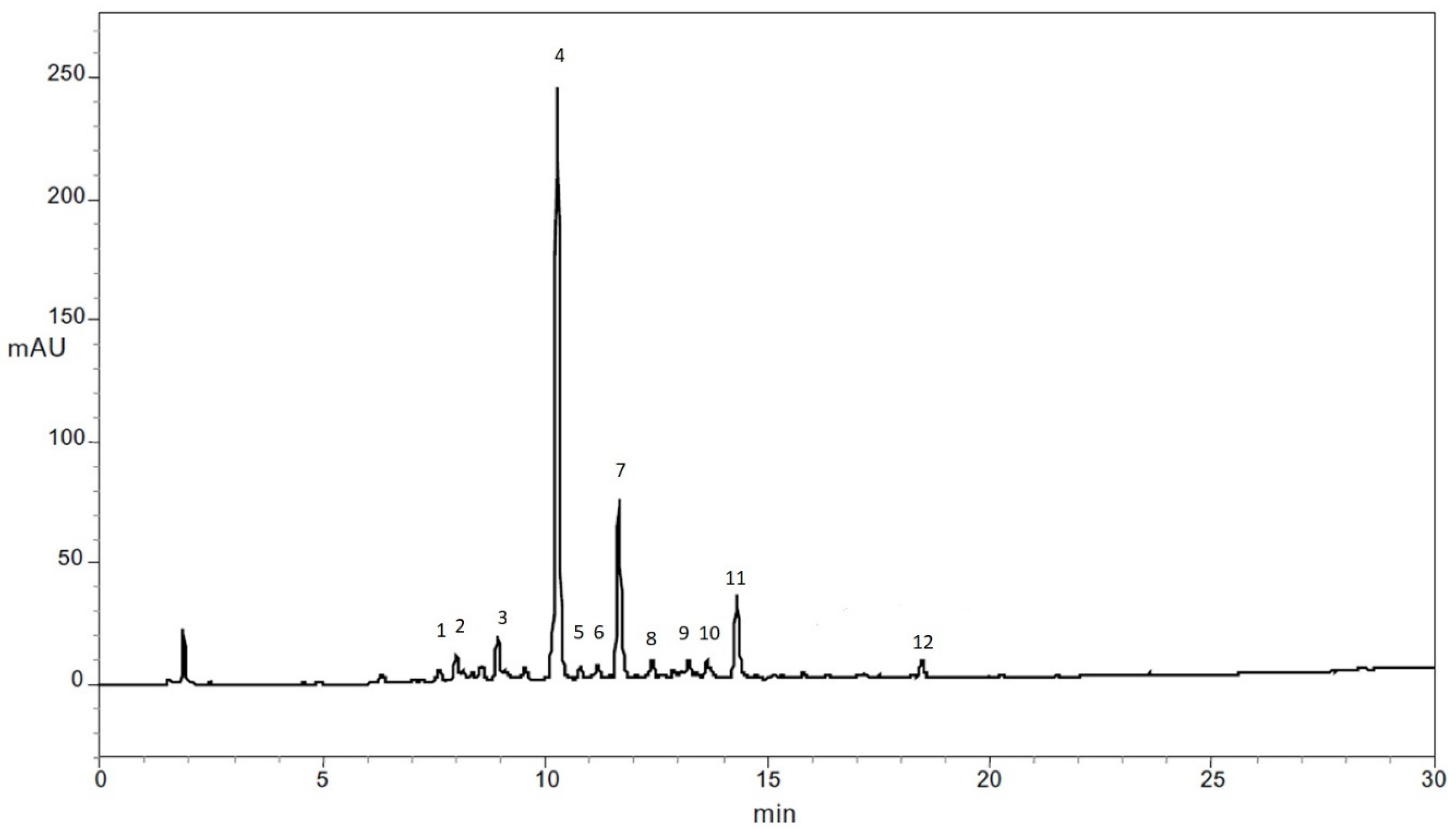

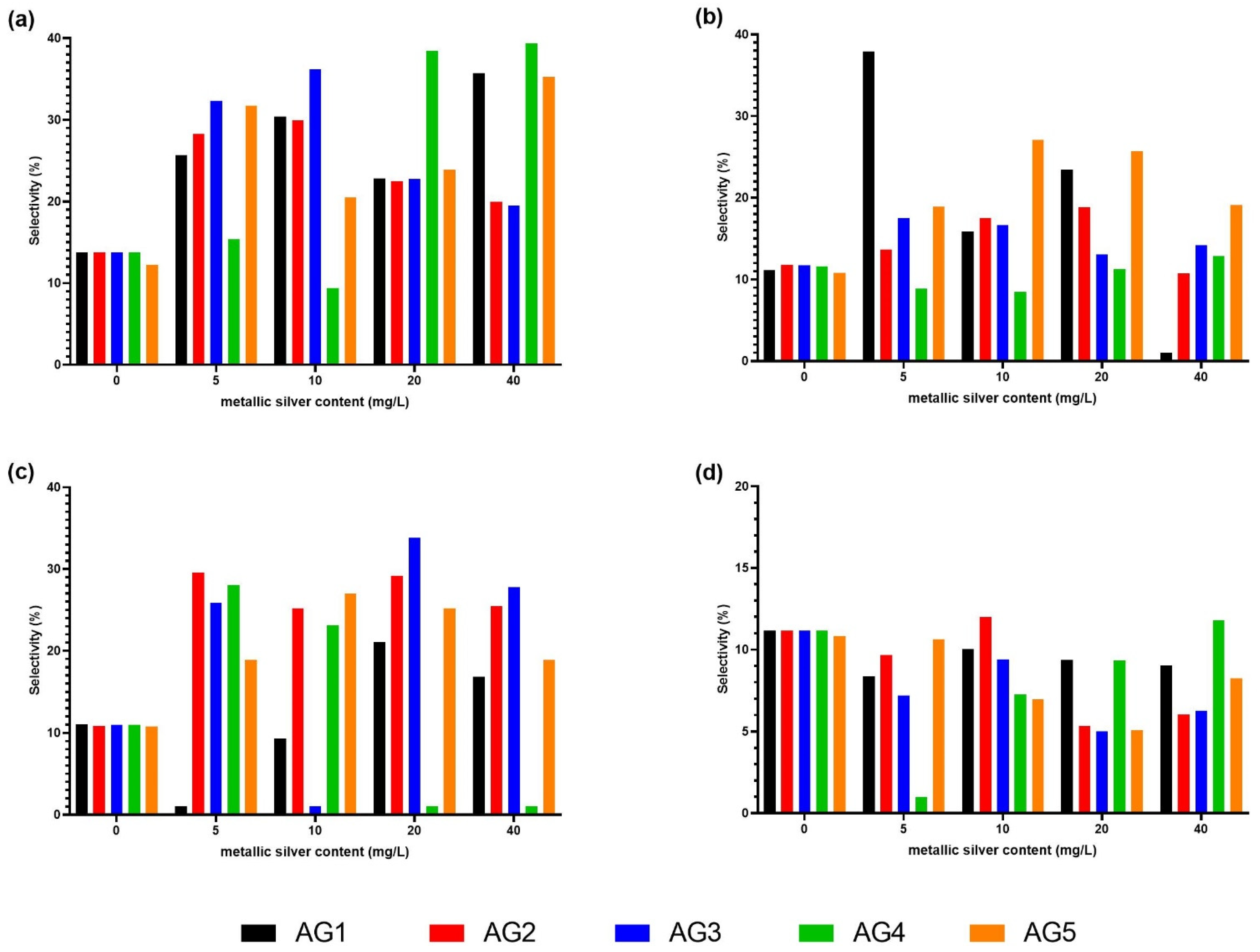
| ID | Compound | [M-H] (m/z) | Fragments |
|---|---|---|---|
| 1 | Chlorogenic Acid | 353 | MS2 [353]: 345, 255, 147 |
| 2 | 3-O-caffeoylquinic acid | 353 | MS2 [353]: 135, 179, 191 MS3 [353→191] |
| 3 | 5′-caffeoylquinic acid | 353 | MS2 [353]: 179, 191 MS3 [353→191] |
| 4 | Ferulic acid | 193 | |
| 5 | Caffeic acid | 179.9 | |
| 6 | Cis-5′-caffeoylquinic acid | 353 | MS2 [353]: 135, 179, 191 |
| 7 | 4′p-Coumaroylquinic acid | 337 | MS2 [337]: 191 |
| 8 | 3-O-Feruloylquinic acid | 367 | MS2 [367]: 173, 193 MS3 [367→173] |
| 9 | 5-O-Feruloylquinic acid | 367 | MS2 [367]: 191 MS3 [367→191] |
| 10 | Caffeic acid derivative | 367 | MS2 [367]: 135, 179, 191 MS3 [367→179] |
| 11 | 3′4′-Dicafferoylquinic acid | 527 | MS2 [527]: 203, 365 MS3 [527→365] |
| 12 | 3′5′-Dicafferoylquinic acid | 515 | MS2 [515]: 353 MS3 [515→353] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoscoy-Berber, L.S.; Antunes-Ricardo, M.; Gallegos-Granados, M.Z.; García-Ramos, J.C.; Pestryakov, A.; Toledano-Magaña, Y.; Bogdanchikova, N.; Chavez-Santoscoy, R.A. Treatment with Argovit® Silver Nanoparticles Induces Differentiated Postharvest Biosynthesis of Compounds with Pharmaceutical Interest in Carrot (Daucus carota L.). Nanomaterials 2021, 11, 3148. https://doi.org/10.3390/nano11113148
Santoscoy-Berber LS, Antunes-Ricardo M, Gallegos-Granados MZ, García-Ramos JC, Pestryakov A, Toledano-Magaña Y, Bogdanchikova N, Chavez-Santoscoy RA. Treatment with Argovit® Silver Nanoparticles Induces Differentiated Postharvest Biosynthesis of Compounds with Pharmaceutical Interest in Carrot (Daucus carota L.). Nanomaterials. 2021; 11(11):3148. https://doi.org/10.3390/nano11113148
Chicago/Turabian StyleSantoscoy-Berber, Laura Sofia, Marilena Antunes-Ricardo, Melissa Zulahi Gallegos-Granados, Juan Carlos García-Ramos, Alexey Pestryakov, Yanis Toledano-Magaña, Nina Bogdanchikova, and Rocio Alejandra Chavez-Santoscoy. 2021. "Treatment with Argovit® Silver Nanoparticles Induces Differentiated Postharvest Biosynthesis of Compounds with Pharmaceutical Interest in Carrot (Daucus carota L.)" Nanomaterials 11, no. 11: 3148. https://doi.org/10.3390/nano11113148
APA StyleSantoscoy-Berber, L. S., Antunes-Ricardo, M., Gallegos-Granados, M. Z., García-Ramos, J. C., Pestryakov, A., Toledano-Magaña, Y., Bogdanchikova, N., & Chavez-Santoscoy, R. A. (2021). Treatment with Argovit® Silver Nanoparticles Induces Differentiated Postharvest Biosynthesis of Compounds with Pharmaceutical Interest in Carrot (Daucus carota L.). Nanomaterials, 11(11), 3148. https://doi.org/10.3390/nano11113148









