First-Principles Study on the Effect of Strain on Single-Layer Molybdenum Disulfide
Abstract
:1. Introduction
2. Calculation Model and Method
3. Results and Discussion
3.1. Energy Band
3.2. Density of States
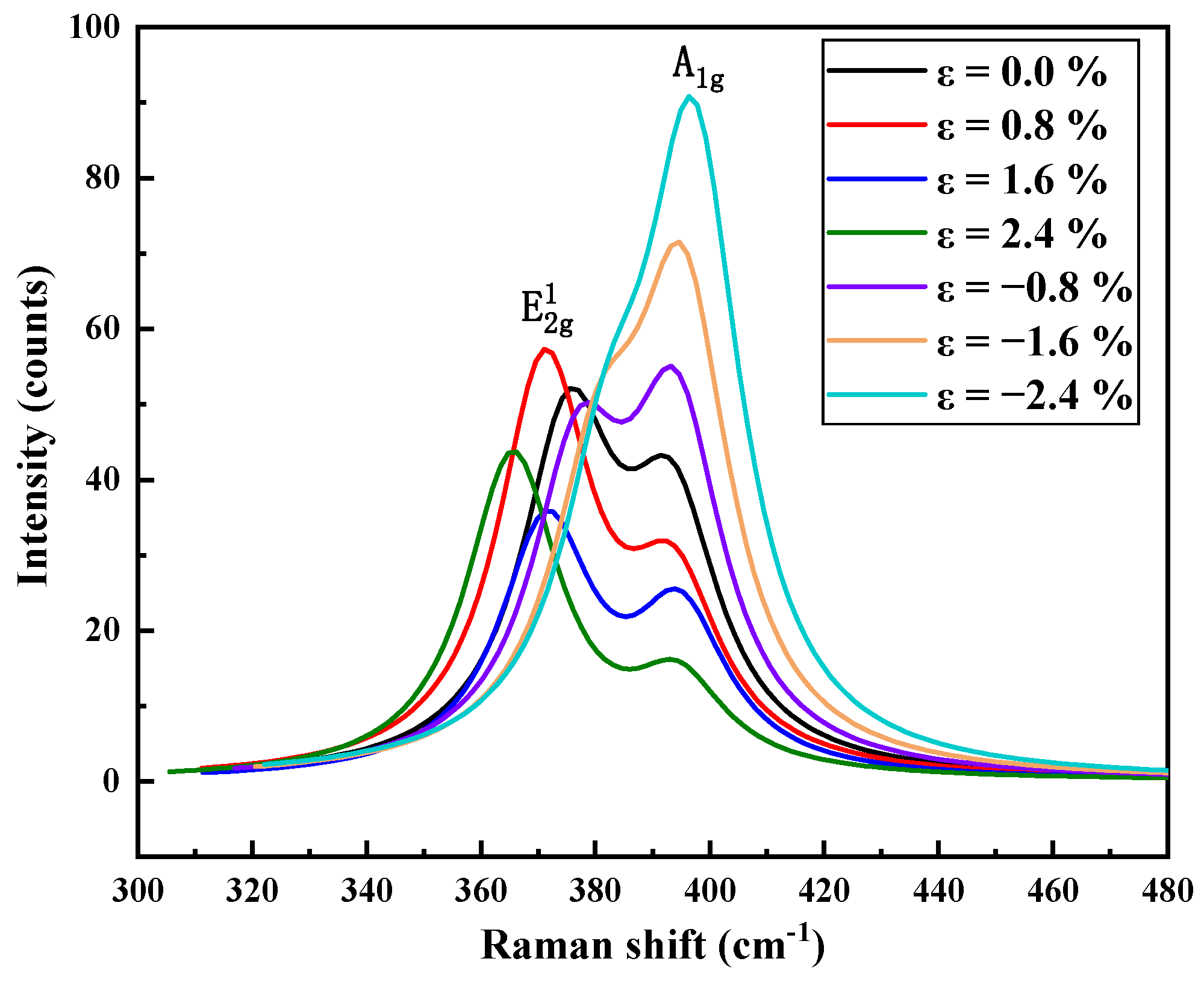
3.3. Thermodynamic Properties and Raman Spectrum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zeng, Z.; Zhang, H. Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2013, 42, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Ryzhii, V.; Otauji, T.; Ryzhii, M.; Shuur, M. Double graphene-layer plasma resonances terahertz detector. J. Phys. D Appl. Phys. 2012, 45, 302001. [Google Scholar] [CrossRef]
- Guang, G.; Zhang, S.; Liu, S.; Cai, Y.; Low, M.; Teng, C.P.; Phang, I.Y.; Cheng, Y.; Duei, K.L.; Srinivasan, B.M.; et al. Protein induces layer-by-layer exfoliation of transition metal dichalcogenides. J. Am. Chem. Soc. 2015, 137, 6152–6155. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Han, T.; Liu, H.; Wang, S.; Chen, S.; Xie, H.; Yang, K. Probing the Field-Effect Transistor with Monolayer MoS2 Prepared by APCVD. Nanomaterials 2019, 9, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Yan, X.; Song, X.; Bao, W.; Ding, S.; Zhang, D.W.; Zhou, P. WSe2/MoS2 and MoTe2/SnSe2 van der Waals heterostructure transistors with different band alignment. Nanotechnology 2017, 28, 415201. [Google Scholar] [CrossRef] [PubMed]
- Kadantsev, E.S.; Hawrylak, P. Electronic structure of a single MoS2 monolayer. Solid State Commun. 2012, 152, 909–913. [Google Scholar] [CrossRef]
- Lee, H.S.; Min, S.-W.; Chang, Y.-G.; Park, M.K.; Nam, T.; Kim, H.; Kim, J.H.; Ryu, S.; Im, S. MoS2 nanosheet phototransisitors with thickness-modulated optical energy gap. Nano Lett. 2012, 12, 3695–3700. [Google Scholar] [CrossRef]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.R.; Tenne, R. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar] [CrossRef]
- Kim, Y.; Huang, J.-L.; Lieber, C.M. Characterization of Nanometer Scale Wear and Oxidation of Transition Metal Dichalcogenide Lubricants by Atomic Force Microscopy. Appl. Phys. Lett. 1991, 59, 3404–3406. [Google Scholar] [CrossRef] [Green Version]
- Kamegawa, A.; Goto, Y.; Kataoka, R.; Takamura, H.; Okada, M. High-pressure synthesis of novel compounds in an Mg–Ni system. Renew. Energy 2007, 33, 221–225. [Google Scholar] [CrossRef]
- Heising, J.; Kanatzidis, M.G. Exfoliated and Restacked MoS2 and WS2: Ionic or Neutral Species Encapsulation and Ordering of Hard Electropositive Cations. J. Am. Chem. Soc. 1999, 121, 11720–11732. [Google Scholar] [CrossRef]
- Barakat, F.; Laref, A.; Alterary, S.; Faraji, S.; Alsalhi, M. Structural and optical behaviors of 2D-layered molybdenum disulfide thin film: Experimental and ab-initio insights. J. Mater. Res. Technol. 2021, 14, 780–796. [Google Scholar] [CrossRef]
- Chen, G.; Lu, B.; Cui, X.; Xia, J. Effects of Deposition and Annealing Temperature on the Structure and Optical Band Gap of MoS2 Films. Materials 2020, 13, 5515. [Google Scholar] [CrossRef]
- Tanabe, T.; Osak, J.; Miyajima, M.; Kitamura, K.; Oyama, Y. Raman and TEM characterization of 2D layered MoS2 crystals grown on non-metal surfaces by friction-induced synthesis. Appl. Surf. Sci. 2021, 561, 150016. [Google Scholar] [CrossRef]
- Dimakis, N.; Vadodaria, O.; Ruiz, K.; Gupta, S. Molybdenum disulfide monolayer electronic structure information as explored using density functional theory and quantum theory of atoms in molecules. Appl. Surf. Sci. 2021, 555, 149545. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Lee, Y.H.; Shi, Y.; Hsu, A.; Chin, M.L.; Li, L.-J.; Dubey, M.; Kong, J.; Palacios, T. Integrated circuits based on bilayer MoS2 transistors. Nano Lett. 2012, 12, 4674. [Google Scholar] [CrossRef] [Green Version]
- Kuc, A.; Zibouche, N.; Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfide TS2. Phys. Rev. B 2011, 83, 245213. [Google Scholar] [CrossRef] [Green Version]
- Brivio, J.; Alexander, D.T.L.; Kis, A. Ripples and Layers in Ultrathin MoS2 Membranes. Nano Lett. 2011, 11, 5148–5153. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verble, J.L.; Wieting, T.J. Lattice Mode Degeneracy in MoS2 and Other Layer Compounds. Phys. Rev. Lett. 1970, 25, 362–365. [Google Scholar] [CrossRef]
- He, X.; Li, H.; Zhu, Z.; Dai, Z.; Yang, Y.; Yang, P.; Zhang, Q.; Li, P.; Schwingenschlogl, U.; Zhang, X. Strain engineering in monolayer WS2, MoS2, and the WS2/MoS2 heterostructure. Appl. Phys. Lett. 2016, 109, 173105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Cheng, F.; Huang, J.; Yuan, H.; Wang, Q. Investigation of uniaxial strain in twisted few-layer MoS2. Phys. Lett. A 2021, 418, 127709. [Google Scholar] [CrossRef]
- Wang, Y.; Cong, C.; Qiu, C.; Yu, T. Raman Spectroscope study of lattice vibration and crystallographic orientation of monolayer MoS2 under uniaxial strain. Small 2013, 9, 2857–2867. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.G.; Lombardo, A.; Nair, R.R.; Bonetti, A.; Savini, G.; Jalil, R.; Bonini, N.; Basko, D.M.; Galiotis, C.; Marzari, N.; et al. Uniaxial strain in graphene by Raman spectroscopy: G peak splitting, Grüneisen parameters, and sample orientation. Phys. Rev. B 2009, 79, 205433. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Peng, J. Pressure effect on the structural, electronic, and elastic properties and Debye temperature of Rh3Nb: First-principles calculations. RSC Adv. 2016, 6, 78028–78035. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Application of the generalized gradientapproximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671. [Google Scholar] [CrossRef]
- Molina-Sanchez, A.; Wittz, L. Phonons in single-layer and few-layer MoS2 and WS2. Phys. Rev. B 2011, 84, 155413. [Google Scholar] [CrossRef] [Green Version]
- Terrones, H.; Lopez-Urias, F.; Terrones, M. Novel hetero-layered materials with tunable direct band gaps by sandwiching different metal disulfides and diselenides. Sci. Rep. 2013, 3, 1549. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.A.; Francisco, E.; Luaña, V. GIBBS: Isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput. Phys. Commun. 2004, 158, 57–72. [Google Scholar] [CrossRef]
- Baroni, S.; De Gironcoli, S.; Dal Corso, A. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef] [Green Version]
- Anderson, O.L. A Simplified method for calculating the Debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Xiao, B.; Feng, J.; Zhou, C.T. The elasticity, bond hardness and thermodynamic properties of X2B (X = Cr, Mn, Fe, Co, Ni, Mo, W) investigated by DFT theory. Phys. B Condens. Matter. 2010, 405, 1274–1278. [Google Scholar] [CrossRef]



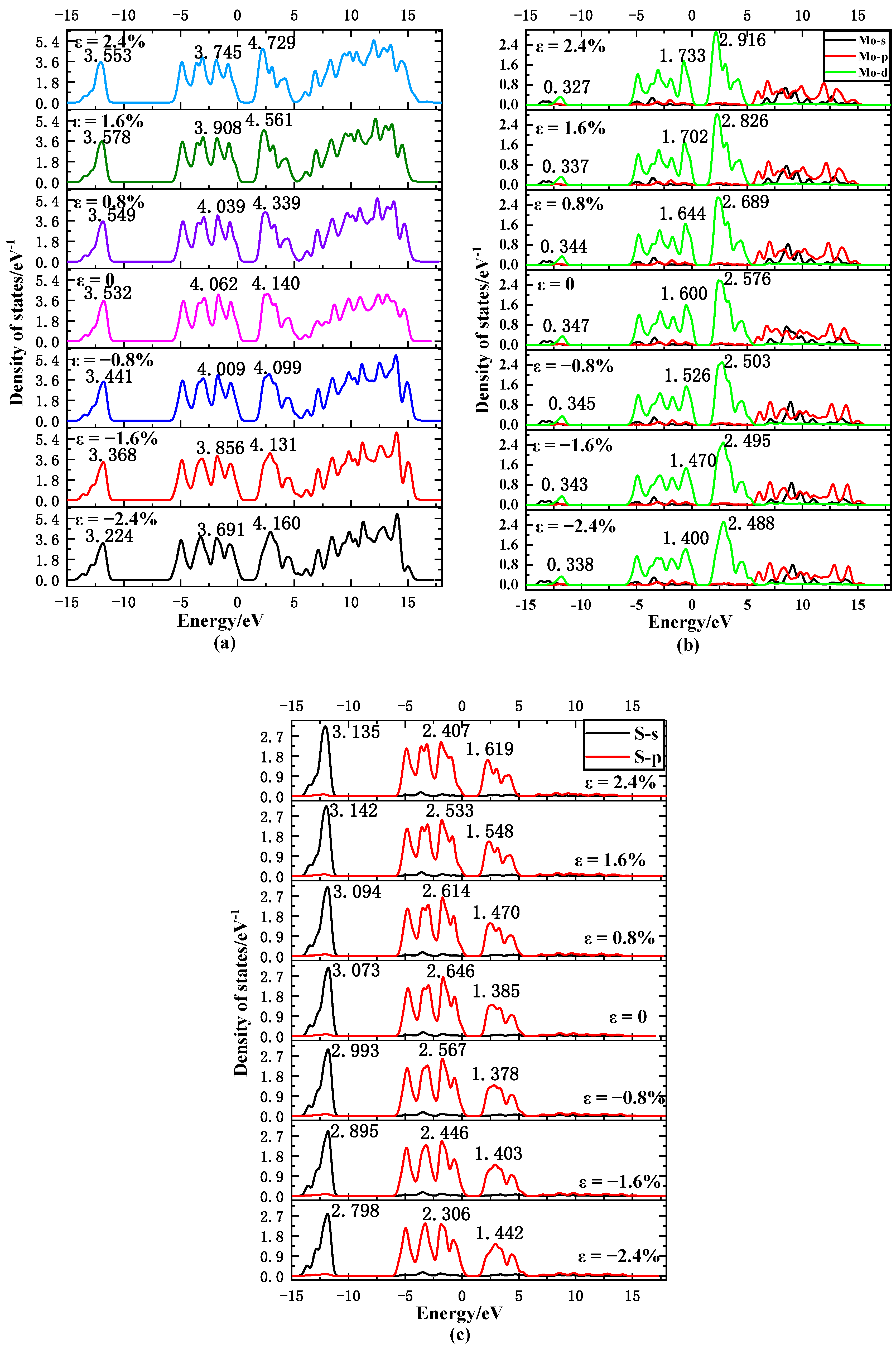
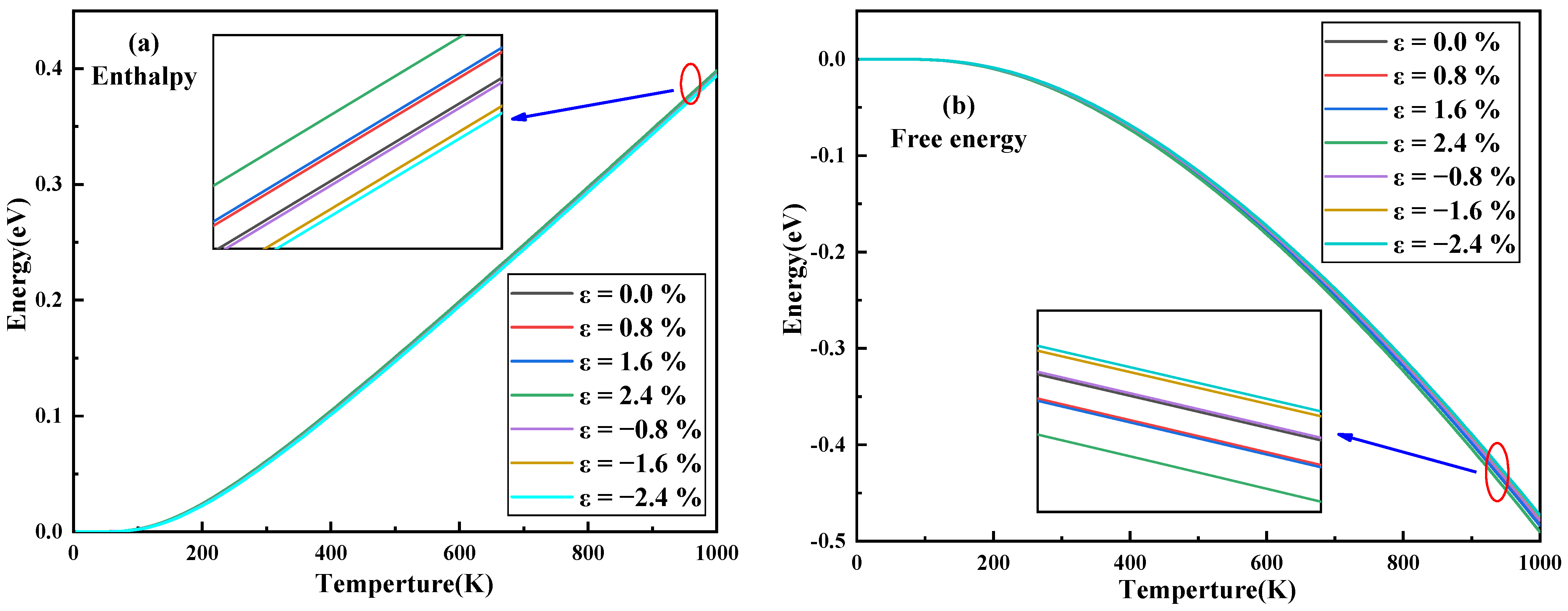
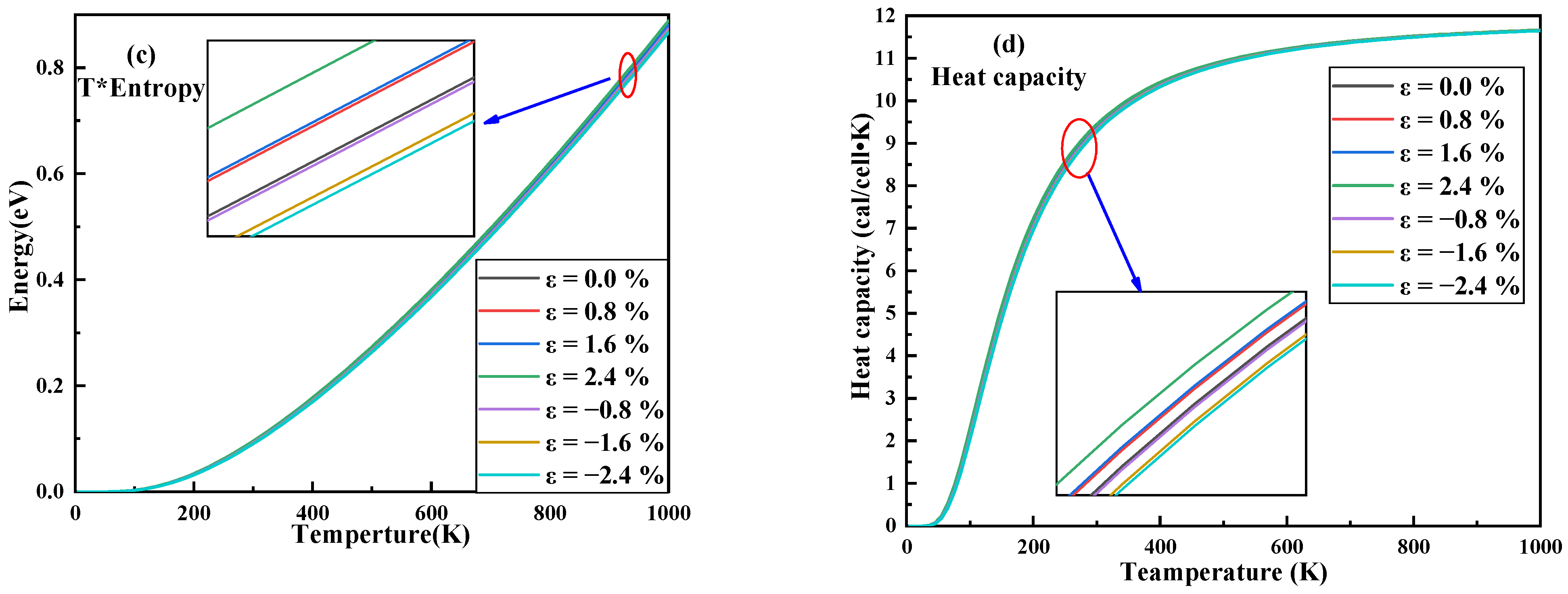
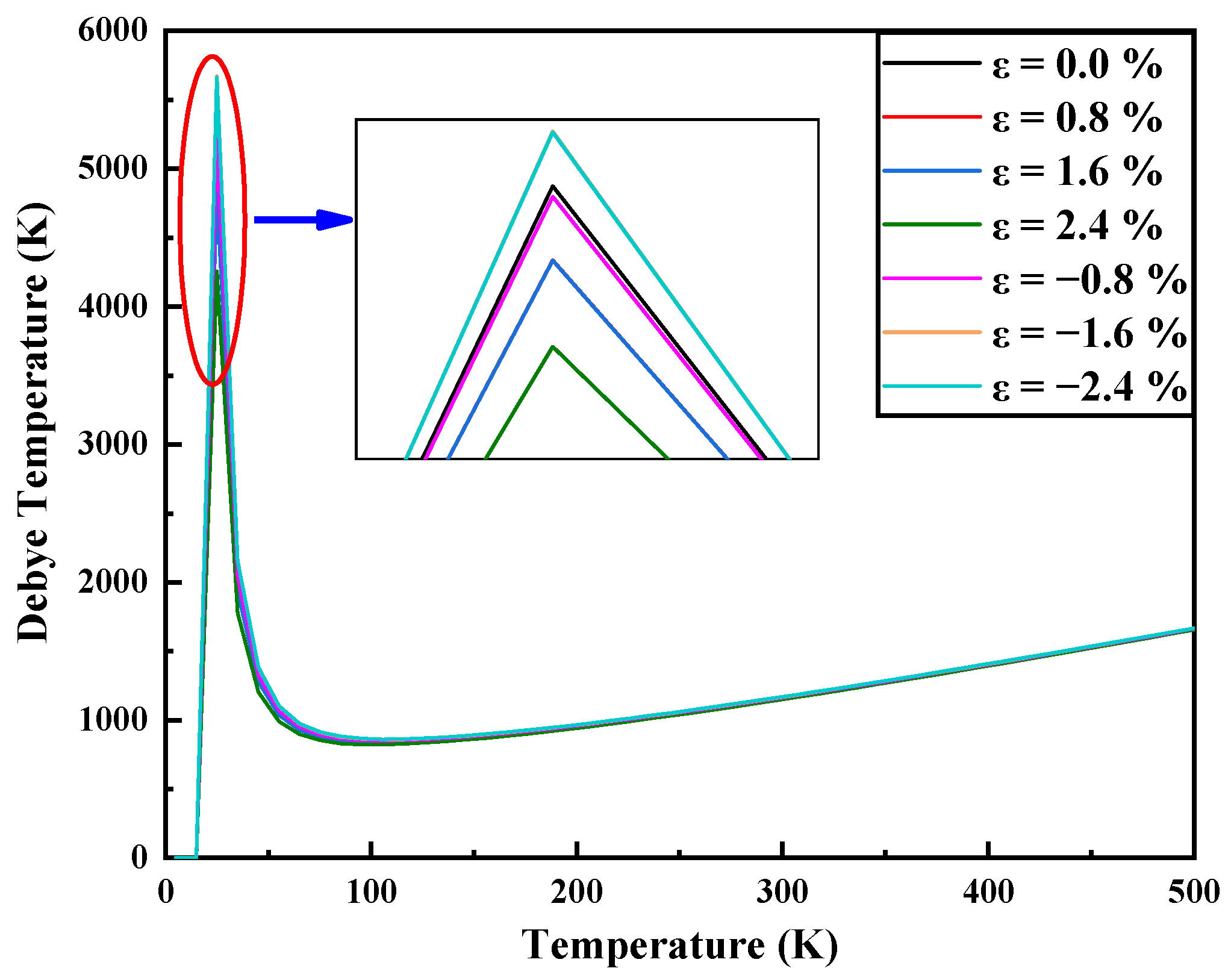
| Strain | −2.4% | −1.6% | −0.8% | 0 | 0.8% | 1.6% | 2.4% |
| Lattice constant/Å | 3.08416 | 3.10944 | 3.13472 | 3.160 | 3.18528 | 3.21056 | 3.23584 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, C.; Liu, H.; Wang, S.; Yang, K. First-Principles Study on the Effect of Strain on Single-Layer Molybdenum Disulfide. Nanomaterials 2021, 11, 3127. https://doi.org/10.3390/nano11113127
Chong C, Liu H, Wang S, Yang K. First-Principles Study on the Effect of Strain on Single-Layer Molybdenum Disulfide. Nanomaterials. 2021; 11(11):3127. https://doi.org/10.3390/nano11113127
Chicago/Turabian StyleChong, Chen, Hongxia Liu, Shulong Wang, and Kun Yang. 2021. "First-Principles Study on the Effect of Strain on Single-Layer Molybdenum Disulfide" Nanomaterials 11, no. 11: 3127. https://doi.org/10.3390/nano11113127
APA StyleChong, C., Liu, H., Wang, S., & Yang, K. (2021). First-Principles Study on the Effect of Strain on Single-Layer Molybdenum Disulfide. Nanomaterials, 11(11), 3127. https://doi.org/10.3390/nano11113127







