Preparation of Quantum Dot-Embedded Photonic Crystal Hydrogel and Its Application as Fluorescence Sensor for the Detection of Nitrite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
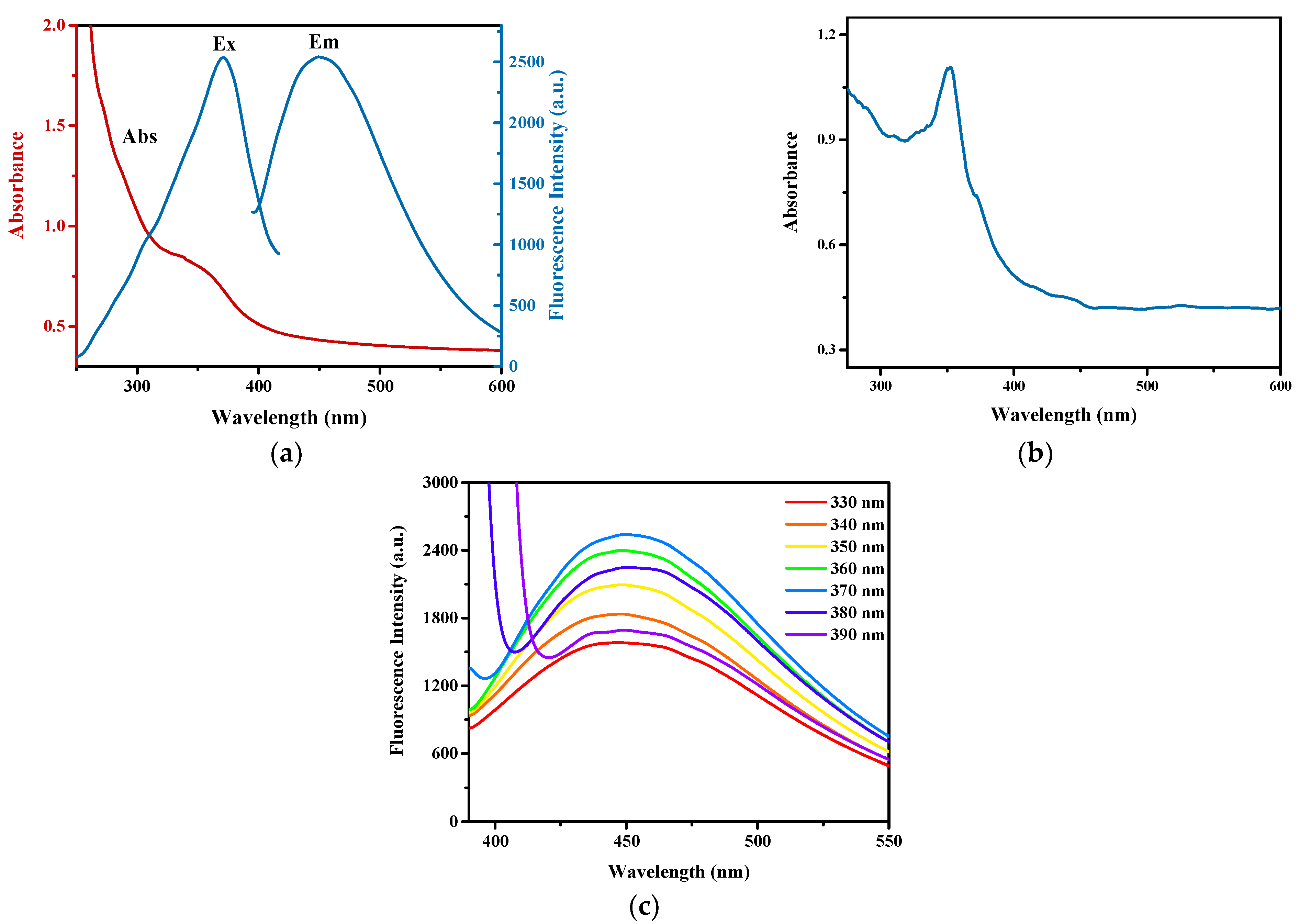
2.3. Synthesis of PEI-CdS QDs
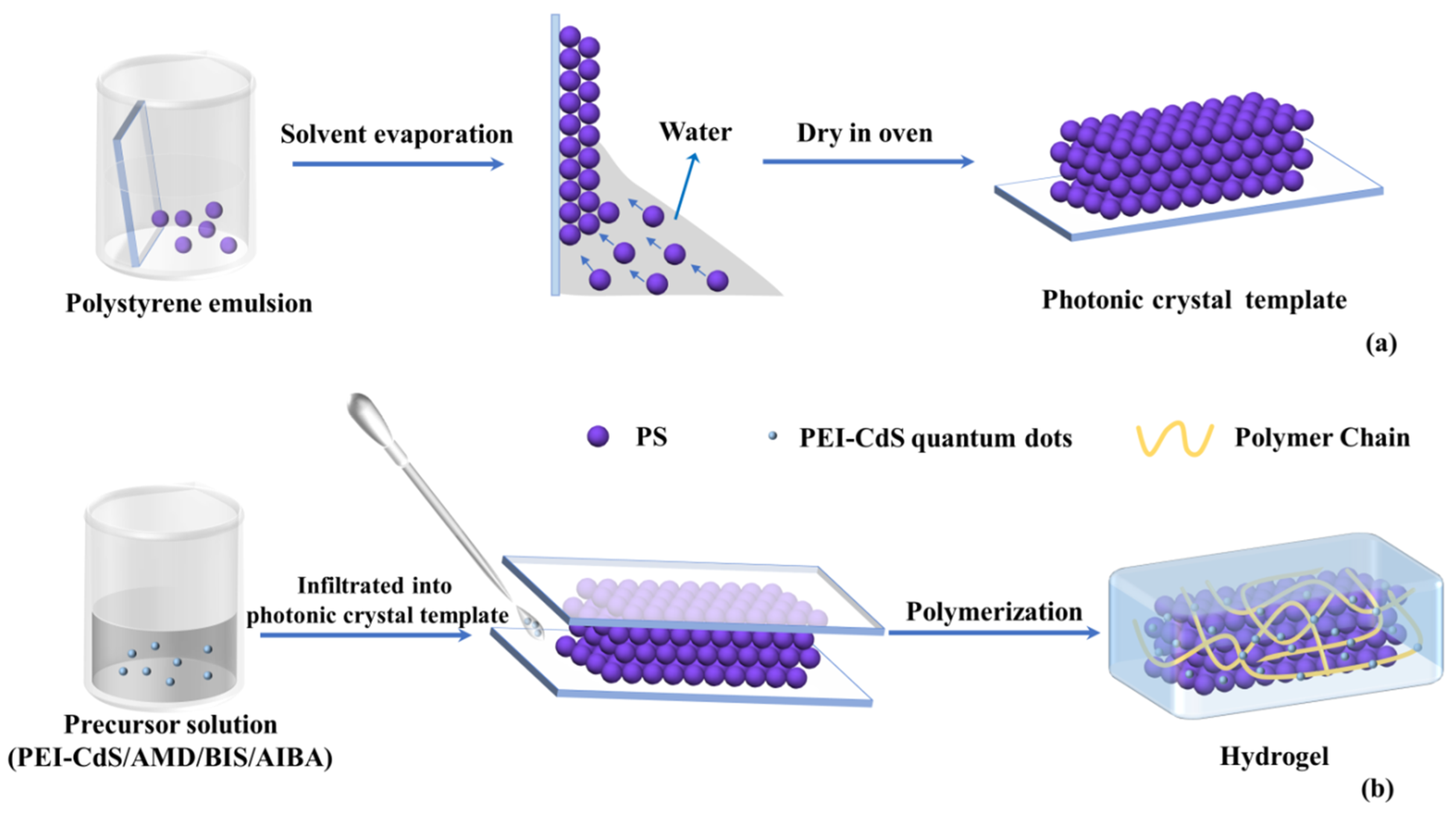
2.4. Preparation of Photonic Crystal Templates
2.5. Preparation of QD-Embedded Photonic Crystal Hydrogel
2.6. Sensing of Nitrite
3. Results and Discussion
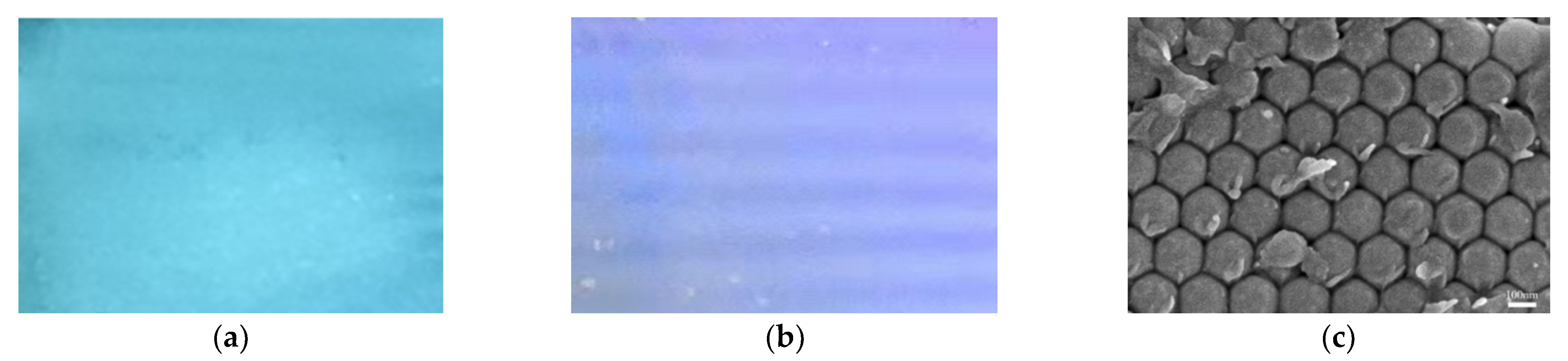
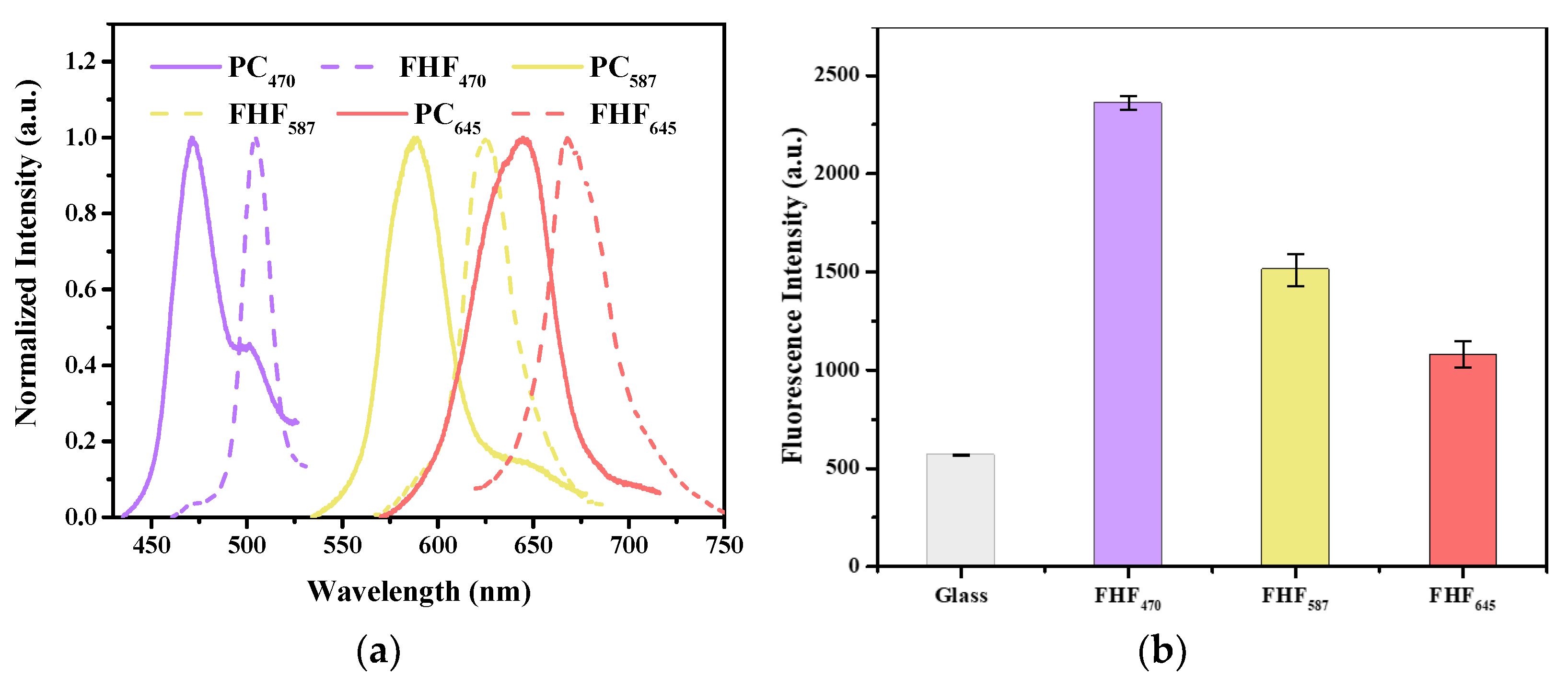
3.1. Preparation of QD-Embedded Photonic Crystal Hydrogel
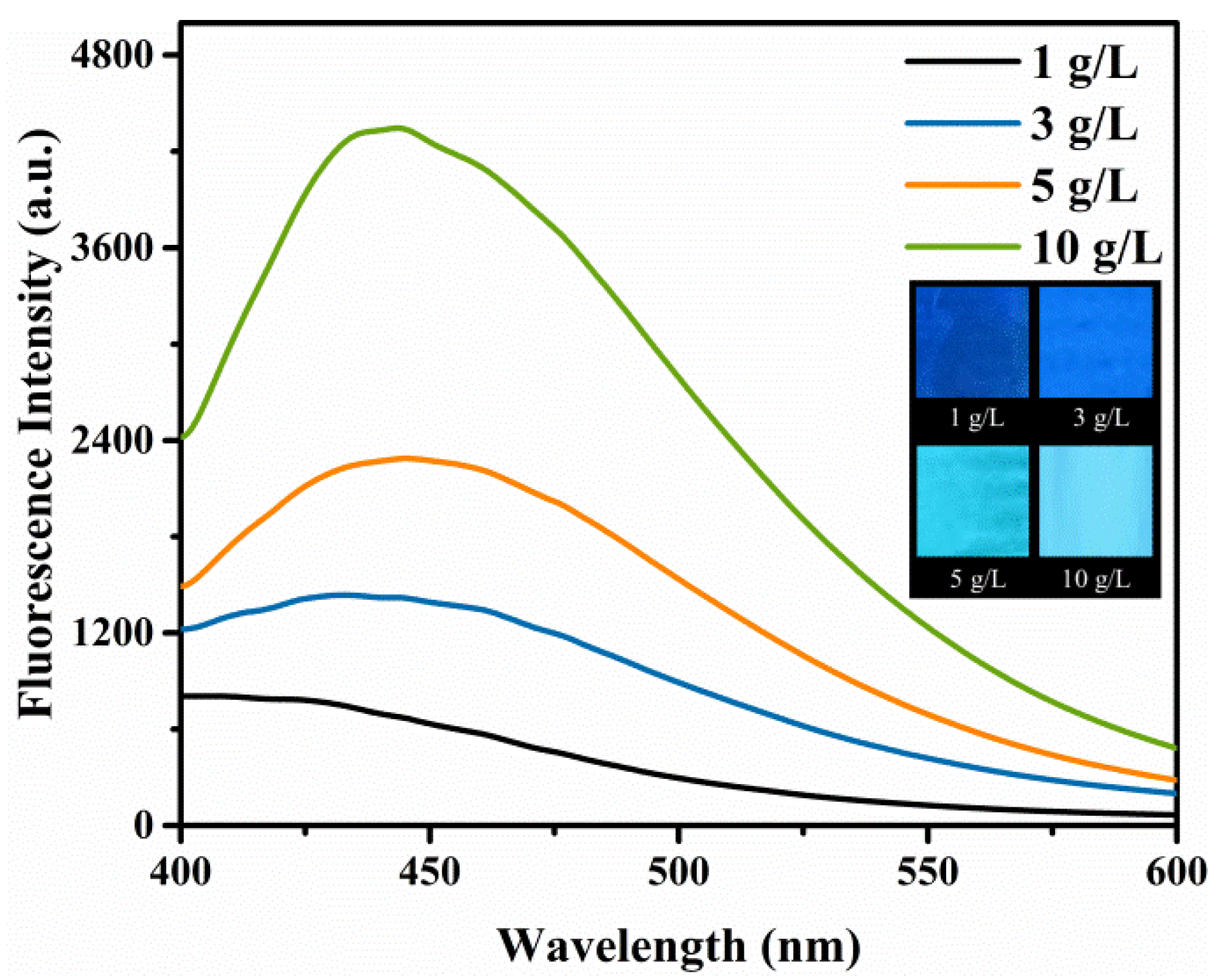
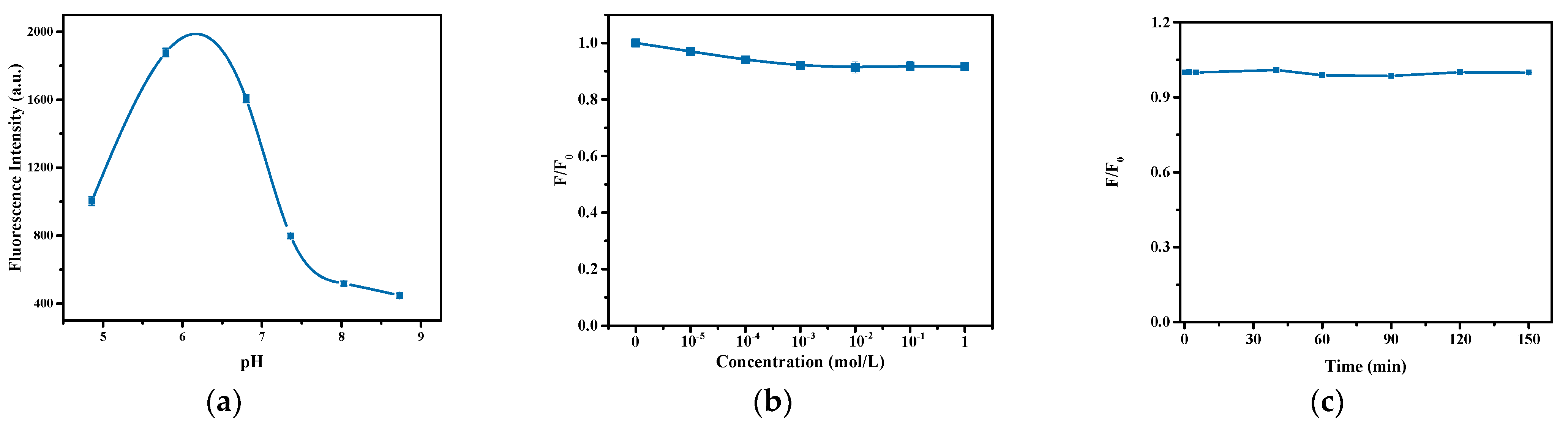
3.2. Optimization of the Fluorescence Property of the Hydrogel
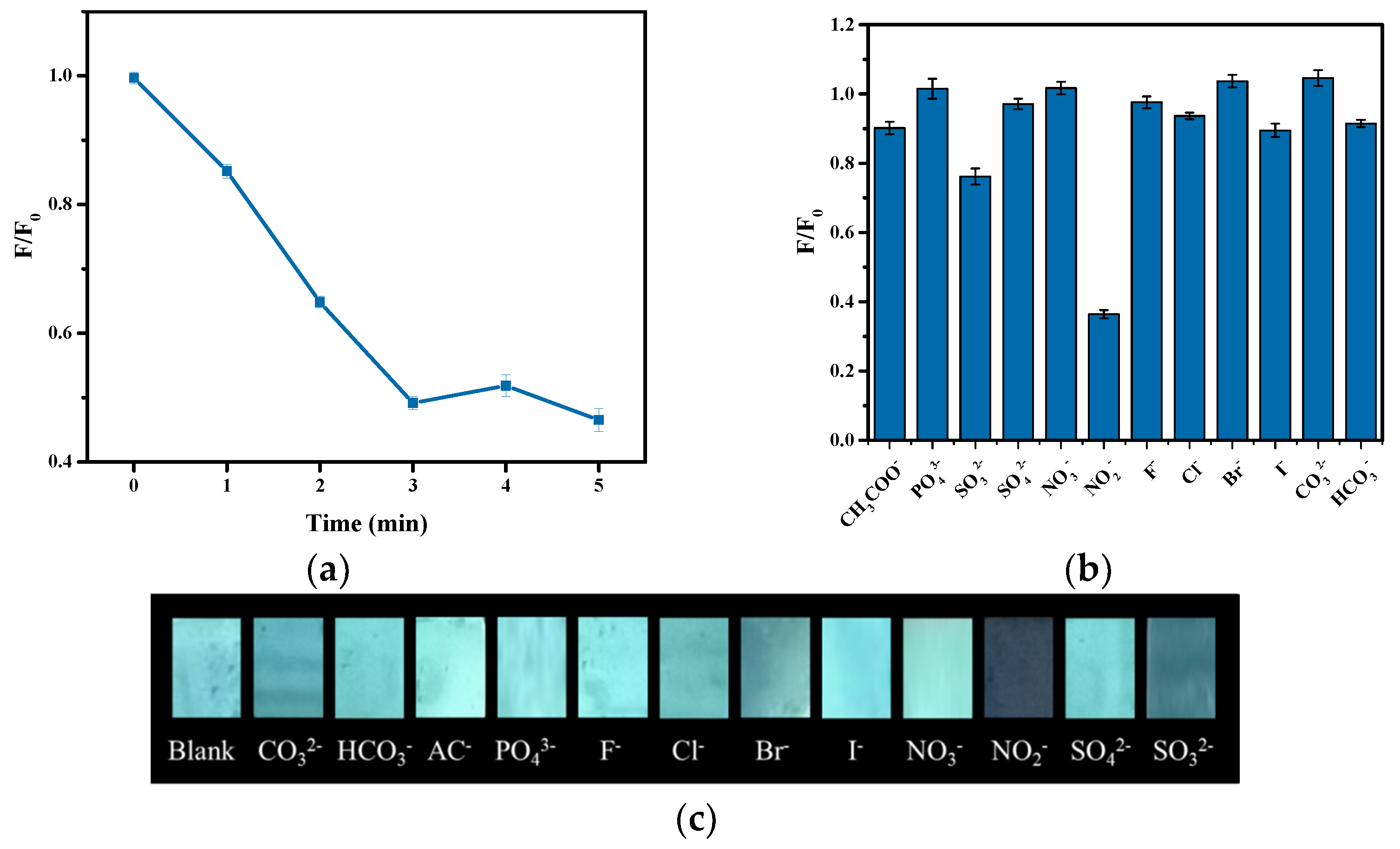
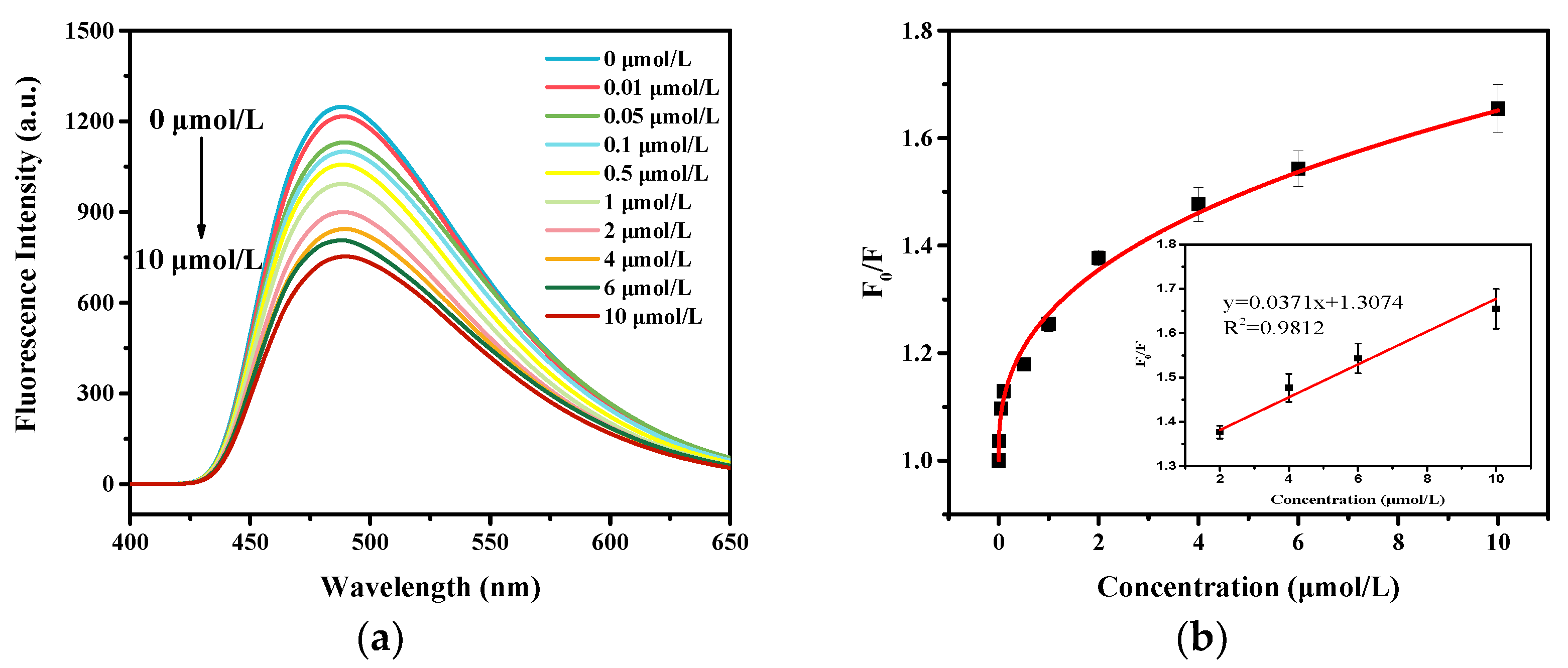
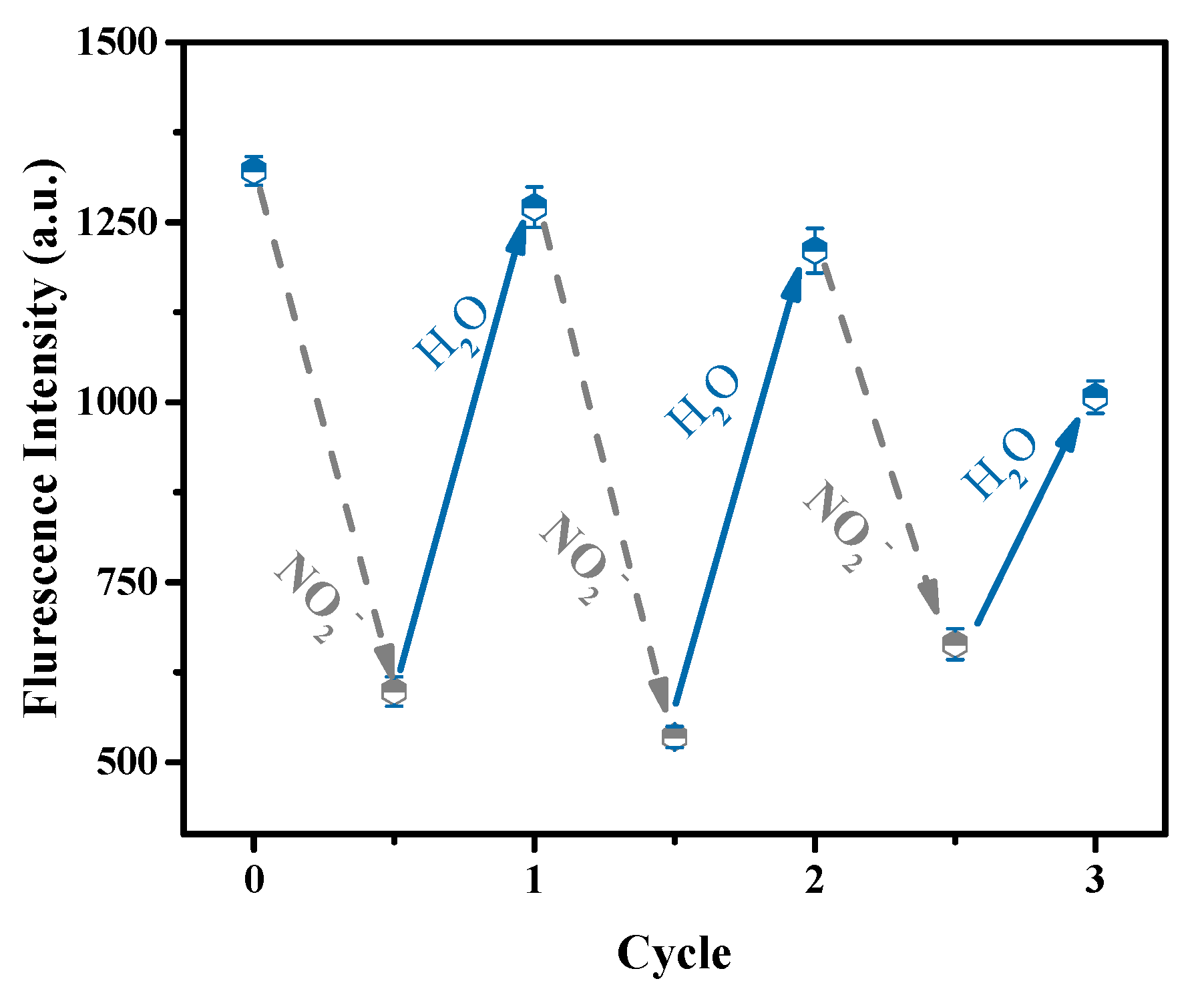
3.3. Sensing Application of the Hydrogel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fernando, K.A.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interf. 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Current advances in quantum-dots-based photoelectrochemical immunoassays. Chem. Asian J. 2017, 12, 2780–2789. [Google Scholar] [CrossRef]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-based quantum dots for Supercapacitors: Recent advances and future challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Tsuruda, Y.; Furukawa, T.; Negishi, L.; Imura, Y.; Sakuda, S.; Yoshimura, E.; Suzuki, M. Synthesis of CdSe quantum dots using fusarium oxysporum. Materials 2016, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Cang, Y.; Zhang, R.; Shi, G.; Zhang, J.; Liu, L.; Hou, X.; Yu, Z.; Fang, D.; Guo, X. Immobilized CdS quantum dots in spherical polyelectrolyte brushes: Fabrication, characterization and optical properties. J. Mater. Chem. C 2015, 3, 3745–3751. [Google Scholar] [CrossRef]
- Mandal, A.; Saha, J.; De, G. Stable CdS QDs with intense broadband photoluminescence and high quantum yields. Opt. Mater. 2011, 34, 6–11. [Google Scholar] [CrossRef]
- Ali, S.; Aslam, M.; Farooq, W.; Fatehmulla, A.; Atif, M. Assembly of CdS quantum dots onto hierarchical TiO2 structure for quantum dots sensitized solar cell applications. Materials 2015, 8, 2376–2386. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhou, X.-Q.; Lin, J.-M.; Liu, S.-Y.; Lu, C.-H.; Liu, L.-J.; Chen, Y.; Yu, L.-P. Concentration-modulated dual-excitation fluorescence of carbon dots used for ratiometric sensing of Fe3+. Microchem. J. 2021, 164, 106028. [Google Scholar] [CrossRef]
- Wang, B.; Chen, P.-Y.; Zhao, R.-X.; Zhang, L.; Chen, Y.; Yu, L.-P. Carbon-dot modified polyacrylonitrile fibers: Recyclable materials capable of selectively and reversibly adsorbing small-sized anionic dyes. Chem. Eng. J. 2020, 391, 123484. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.; Yang, M. CdS QDs amplified electrochemiluminescence of N,S co-doped graphene quantum dots and its application for Pb(II) determination. Chem. Lett. 2018, 47, 44–47. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.-J.; Chen, Y. A biocompatible poly(N-vinylimidazole)-dot with both strong luminescence and good catalytic activity. RSC Adv. 2016, 6, 2141–2148. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Song, Y.; Zhang, G.; Zhang, H.; Yang, B. Fluorescent nanocomposite based on PVA polymer dots. Acta Chim. Sin. 2012, 70, 2311–2315. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Cao, L.; LeCroy, G.E.; Wang, P.; Meziani, M.J.; Dong, Y.; Liu, Y.; Luo, P.G.; Sun, Y.P. Carbon “quantum” dots for fluorescence labeling of cells. ACS Appl. Mater. Interfaces 2015, 7, 19439–19445. [Google Scholar] [CrossRef] [PubMed]
- Martina, P.; Jiri, M.; Sofiane, K.; Monika, D.; Vit, M.; Vladimir, V. Synthesis of ZnO quantum dots with enhanced fluorescence quantum yield. J. Nanosci. Nanotechnol. 2016, 16, 7622–7629. [Google Scholar] [CrossRef]
- Bian, F.; Sun, L.; Cai, L.; Wang, Y.; Zhao, Y. Quantum dots from microfluidics for nanomedical application. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1567. [Google Scholar] [CrossRef]
- Liu, K.; Gao, G.-B.; Sun, T.-L. β-HgS quantum dots: Preparation, properties and applications. Prog. Chem. 2017, 29, 776–784. [Google Scholar]
- Campuzano, S.; Yanez-Sedeno, P.; Pingarron, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Ren, J.; Stagi, L.; Innocenzi, P. Fluorescent carbon dots in solid-state: From nanostructures to functional devices. Prog. Solid State Chem. 2021, 62, 100295. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, M.; Liu, Y. Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect. Nanoscale 2020, 12, 1826–1832. [Google Scholar] [CrossRef]
- Truskewycz, A.; Beker, S.A.; Ball, A.S.; Murdoch, B.; Cole, I. Incorporation of quantum carbon dots into a PVP/ZnO hydrogel for use as an effective hexavalent chromium sensing platform. Anal. Chim. Acta 2020, 1099, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef]
- Jiao, Q.; Cao, L.; Zhao, Z.; Zhang, H.; Li, J.; Wei, Y. Zwitterionic hydrogel with high transparency, ultrastretchability, and remarkable freezing resistance for wearable strain sensors. Biomacromolecules 2021, 22, 1220–1230. [Google Scholar] [CrossRef]
- Chen, S.; Tang, F.; Tang, L.; Li, L. Synthesis of Cu-nanoparticle hydrogel with self-healing and photothermal properties. ACS Appl. Mater. Interfaces 2017, 9, 20895–20903. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, X.; Wang, G.Y.; Duo, Y.R.; Zhang, X.Y.; Hu, X.H.; Zhang, X.J. Double network self-healing graphene hydrogel by two step method for anticancer drug delivery. Mater. Technol. 2014, 29, 210–213. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zhao, Z.; Sun, L.; Zou, M.; Ren, J.; Zhao, Y. Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci. China Mater. 2018, 61, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Huang, X.; Luo, Y.; Yuan, H.; Ren, T.; Li, X.; Xu, D.; Guo, X.; Wu, Y. Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr(VI). Chem. Eng. J. 2021, 407, 127050. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wan, J.; Jiang, S.; Wu, Y.; Wang, W.; Gong, X.; Wang, H. Quantum dots-based hydrogels for sensing applications. Chem. Eng. J. 2021, 408, 127351. [Google Scholar] [CrossRef]
- Hwang, Y.; Park, J.Y.; Kwon, O.S.; Joo, S.; Lee, C.S.; Bae, J. Incorporation of hydrogel as a sensing medium for recycle of sensing material in chemical sensors. Appl. Surf. Sci. 2018, 429, 258–263. [Google Scholar] [CrossRef]
- Yan, X.; Rahman, S.; Rostami, M.; Tabasi, Z.A.; Khan, F.; Alodhayb, A.; Zhang, Y. Carbon quantum dot-incorporated chitosan hydrogel for selective sensing of Hg2+ ions: Synthesis, characterization, and density functional theory calculation. ACS Omega 2021, 6, 23504–23514. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Grazon, C.; Sensharma, P.; Nguyen, T.T.; Feng, Y.; Chern, M.; Baer, R.C.; Varongchayakul, N.; Cook, K.; Lecommandoux, S.; et al. Hydrogel-embedded quantum dot-transcription factor sensors for quantitative progesterone detection. ACS Appl. Mater. Interfaces 2020, 12, 43513–43521. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Namgung, H.; Lee, T.S. Synthesis of a glucose oxidase-conjugated, polyacrylamide-based, fluorescent hydrogel for a reusable, ratiometric glucose sensor. Polym. Chem. 2016, 7, 6655–6661. [Google Scholar] [CrossRef]
- Xu, L.; Wang, R.; Kelso, L.C.; Ying, Y.; Li, Y. A target-responsive and size-dependent hydrogel aptasensor embedded with QD fluorescent reporters for rapid detection of avian influenza virus H5N1. Sens. Actuators B Chem. 2016, 234, 98–108. [Google Scholar] [CrossRef]
- Ren, H.H.; Fan, Y.; Wang, B.; Yu, L.P. Polyethylenimine-capped CdS quantum dots for sensitive and selective detection of Nitrite in vegetables and water. J. Agric. Food Chem. 2018, 66, 8851–8858. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Wang, J.-X.; Ji, Z.-Y.; Hu, W.-P.; Jiang, L.; Song, Y.-L.; Zhu, D.-B. Solid-state fluorescence enhancement of organic dyes by photonic crystals. J. Mater. Chem. 2007, 17, 90–94. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, Y.; Buddhudu, S.; Ng, S.L.; Lam, Y.L.; Kam, C.H. Photoluminescence of ZnS:Mn embedded in three-dimensional photonic crystals of submicron polymer spheres. Appl. Phys. Lett. 2000, 76, 3513–3515. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Wei, L.; Dai, G.; Zhu, X.; Zhu, Y.; Mei, T.; Li, J.; Wang, X. Synthesis of a novel kind of uniform fluorescent silica colloids and their assembled photonic film for sensitive detection of Cu2+ ions. Mater. Express 2017, 7, 351–360. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, L.; Wang, B.; Yu, L. Preparation of Quantum Dot-Embedded Photonic Crystal Hydrogel and Its Application as Fluorescence Sensor for the Detection of Nitrite. Nanomaterials 2021, 11, 3126. https://doi.org/10.3390/nano11113126
Li R, Li L, Wang B, Yu L. Preparation of Quantum Dot-Embedded Photonic Crystal Hydrogel and Its Application as Fluorescence Sensor for the Detection of Nitrite. Nanomaterials. 2021; 11(11):3126. https://doi.org/10.3390/nano11113126
Chicago/Turabian StyleLi, Rongzhen, Lian Li, Bin Wang, and Liping Yu. 2021. "Preparation of Quantum Dot-Embedded Photonic Crystal Hydrogel and Its Application as Fluorescence Sensor for the Detection of Nitrite" Nanomaterials 11, no. 11: 3126. https://doi.org/10.3390/nano11113126




