Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements
Abstract
:1. Antimicrobial Surface Protection—Global Problem and Related Challenges
2. Smart Coatings—A General Concept
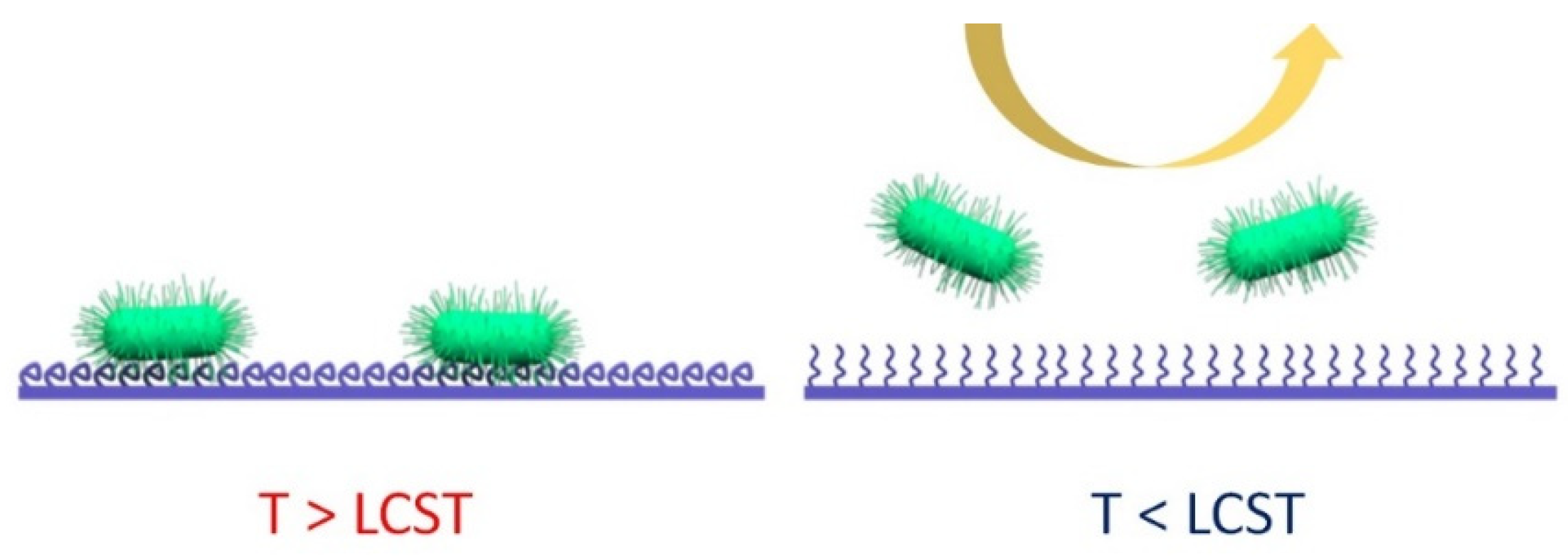
2.1. Temperature-Induced Surface Protection
2.1.1. Surface Antifouling Approach
2.1.2. Temperature Governed Antimicrobial Release
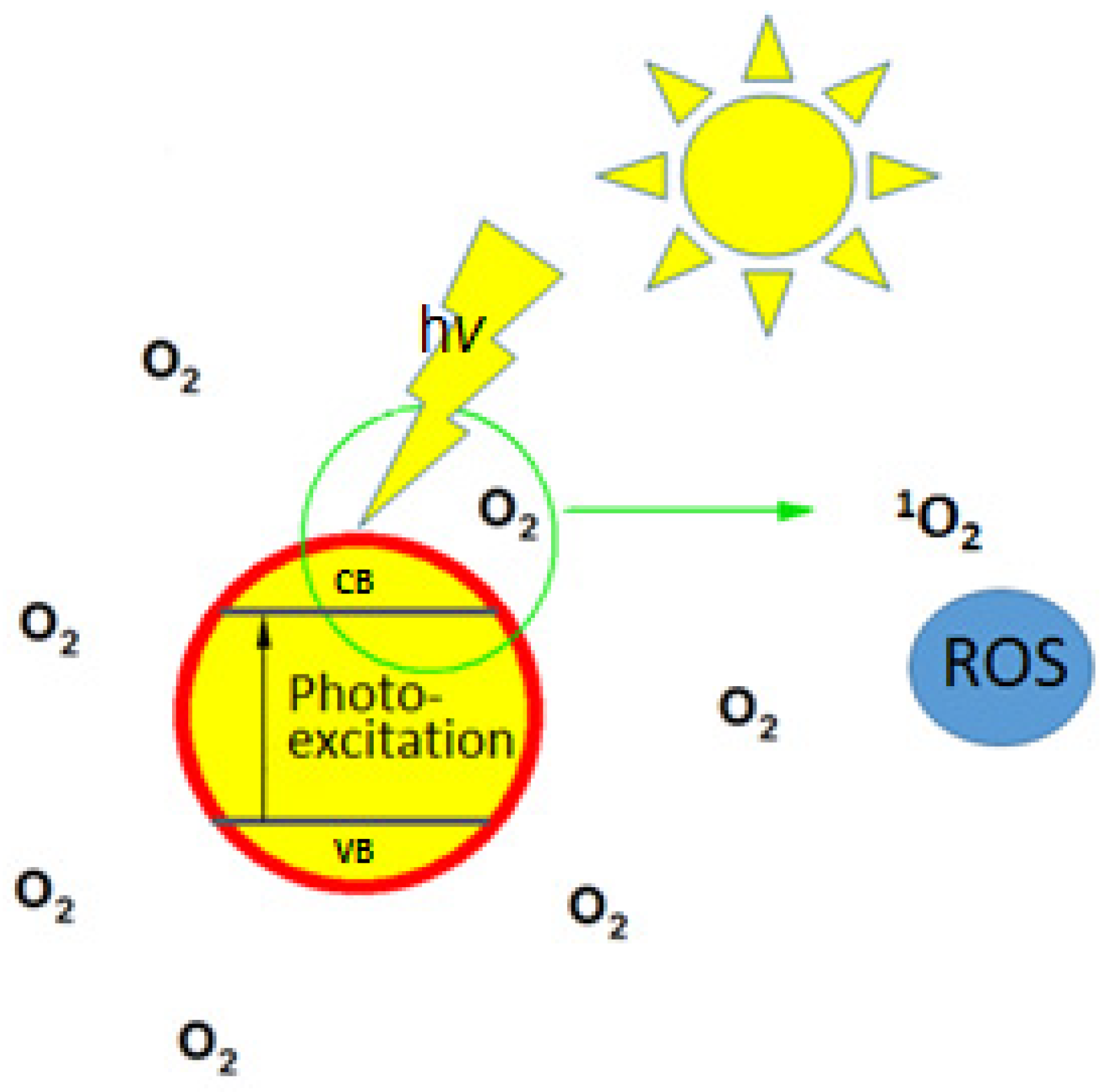
2.2. Light-Activated Antimicrobial Coatings
2.2.1. Reactive Oxygen Species-Based Approaches
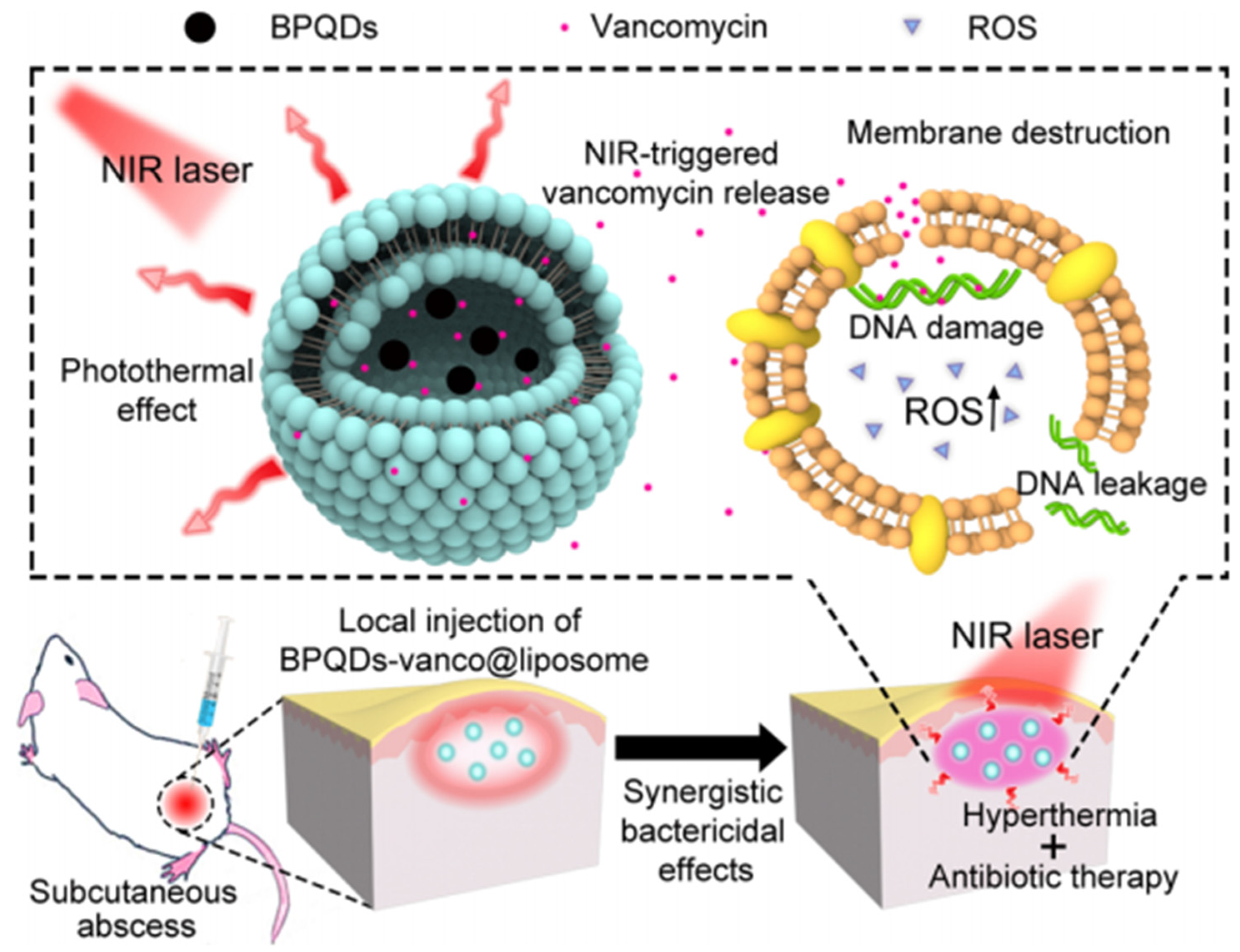
2.2.2. Light-to Heat Conversion
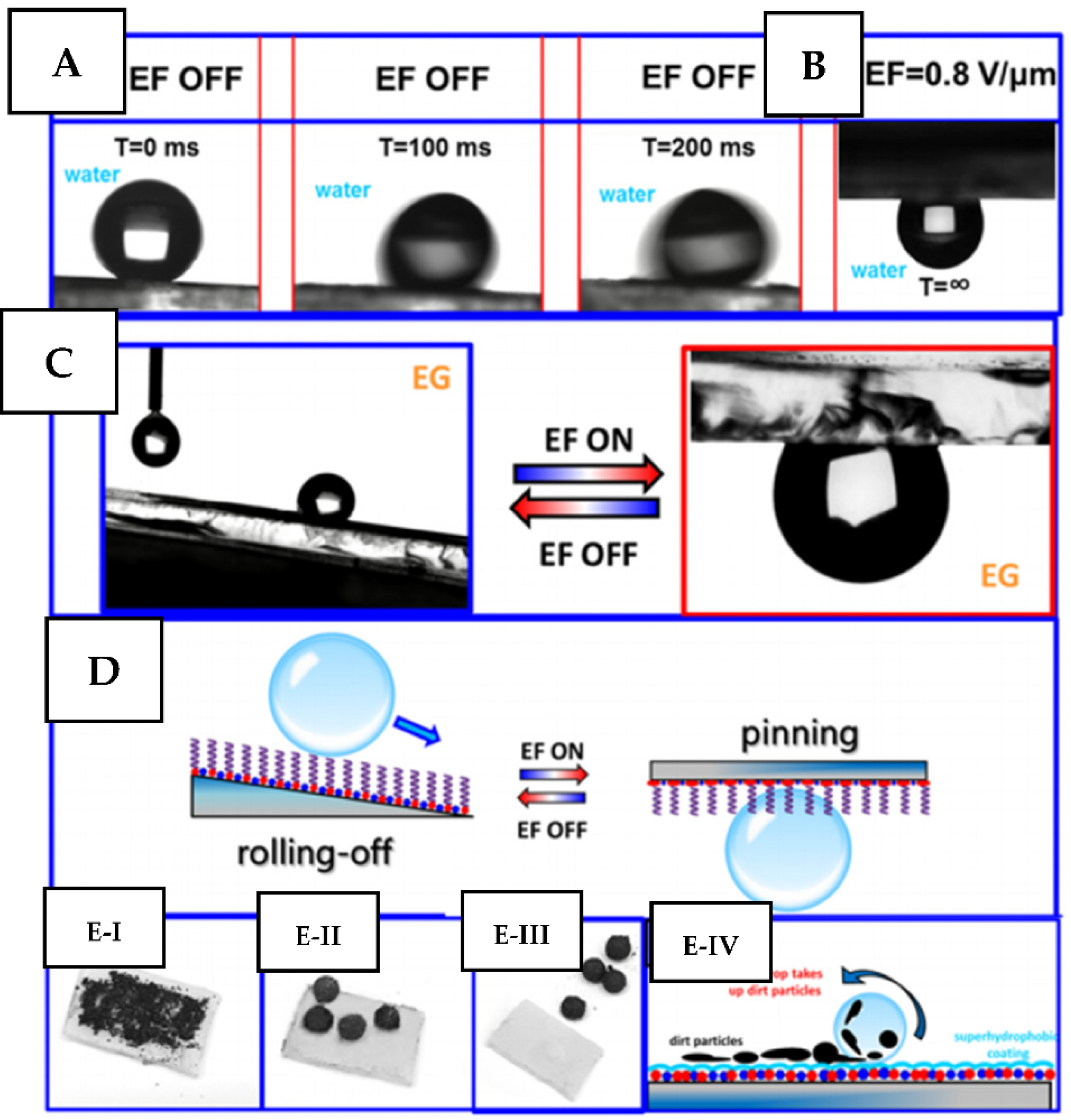
2.3. Electrical or Magneto-Responsive Coatings
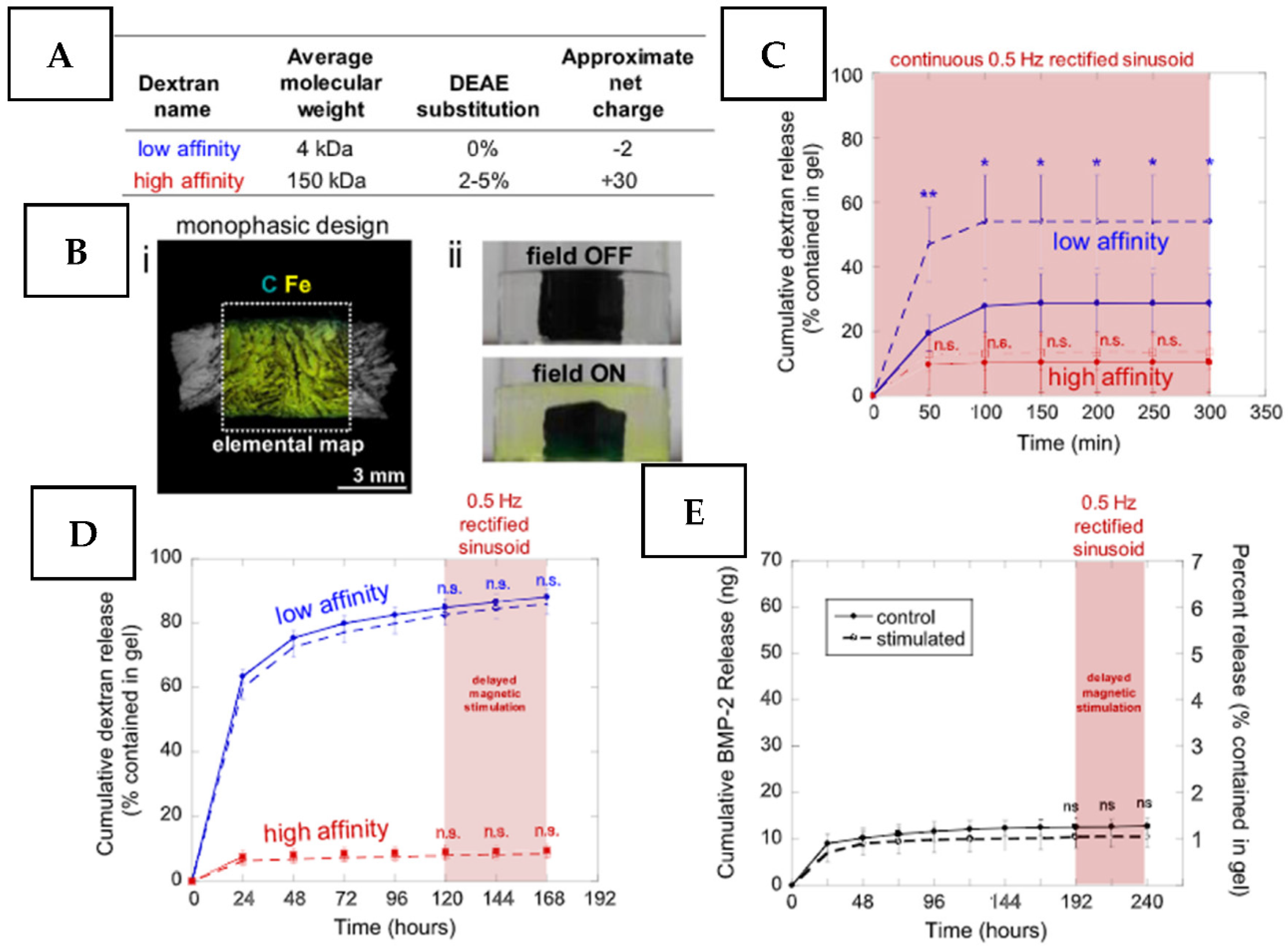
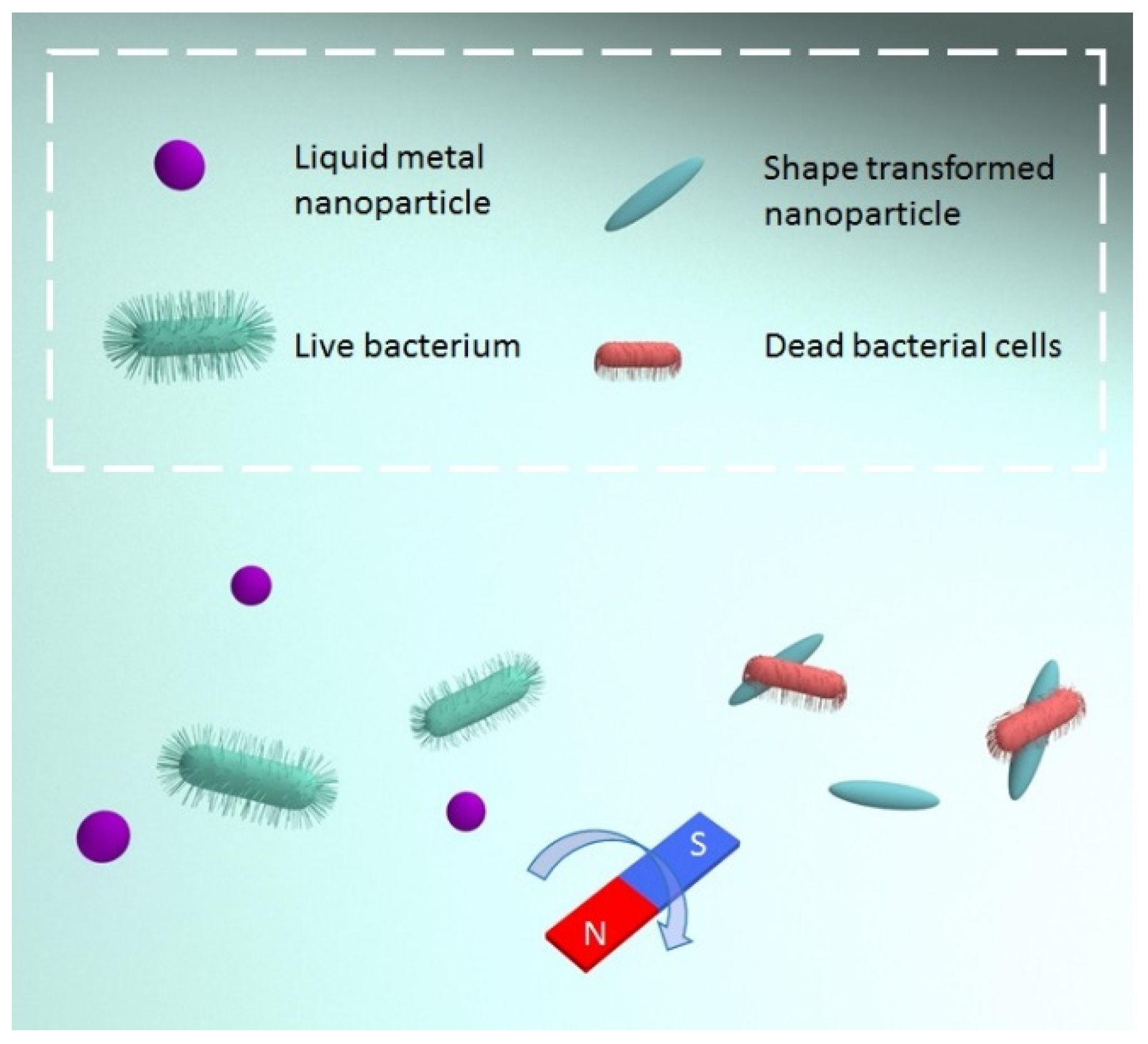
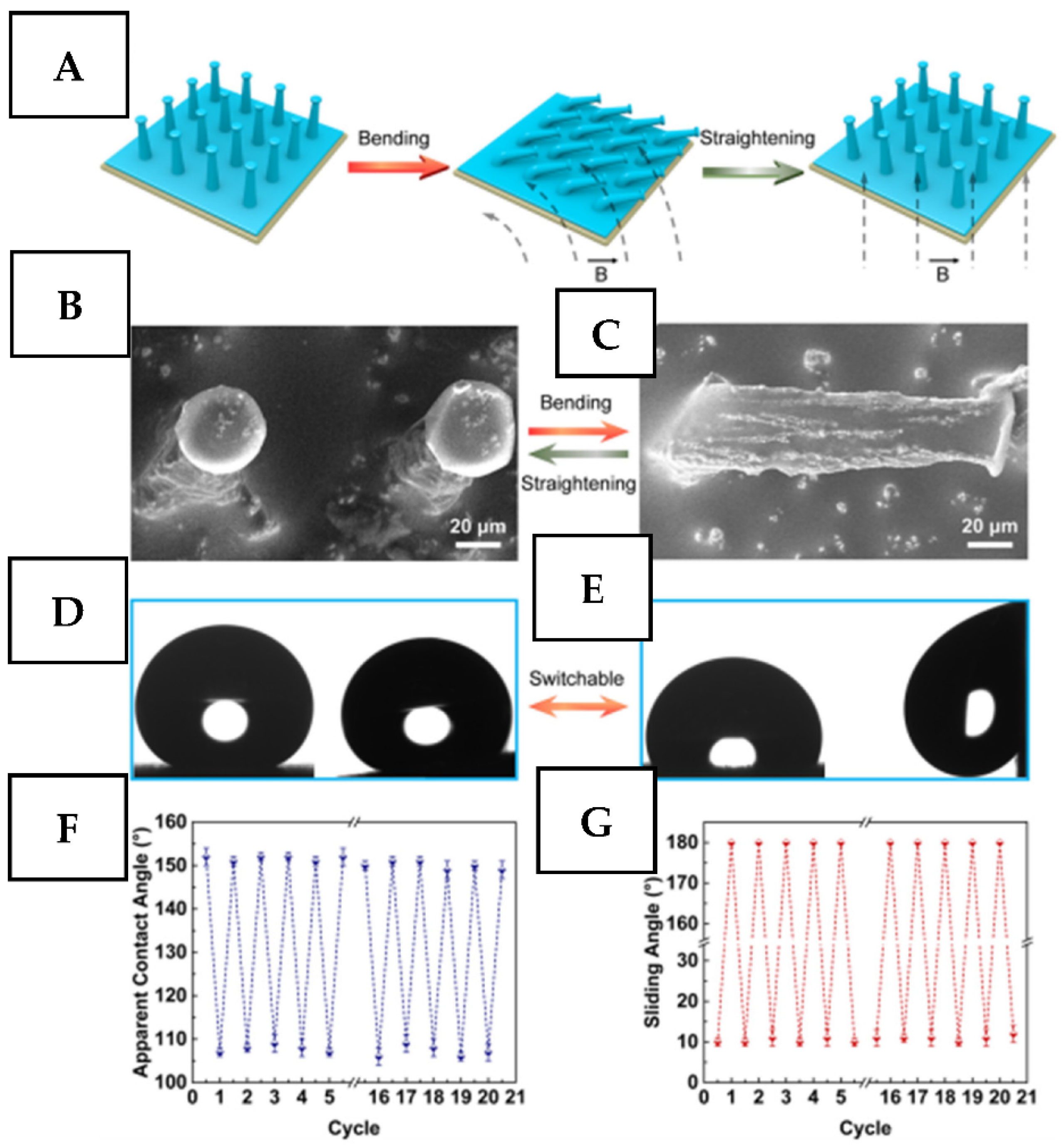
2.3.1. Magnetic Field
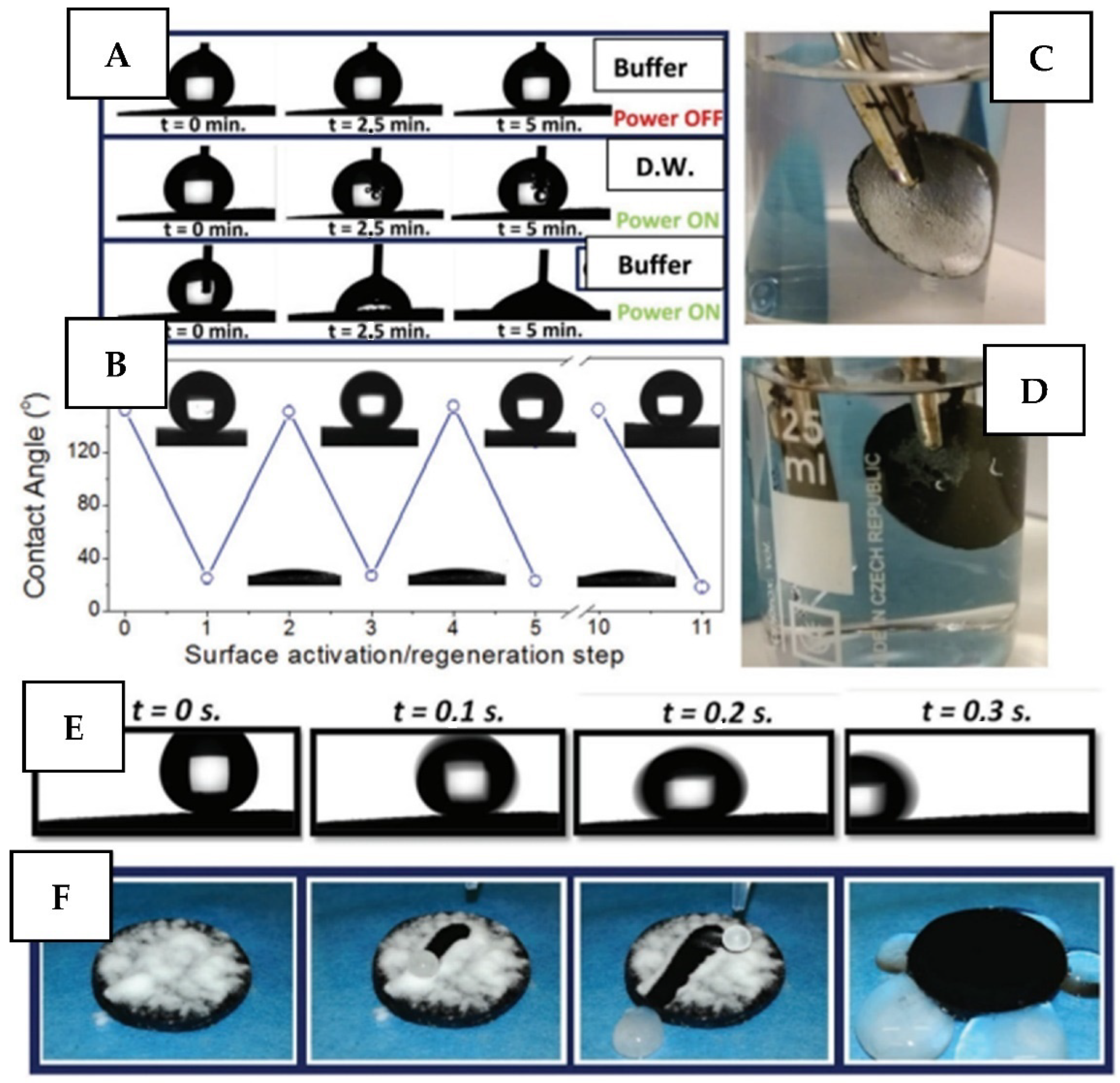
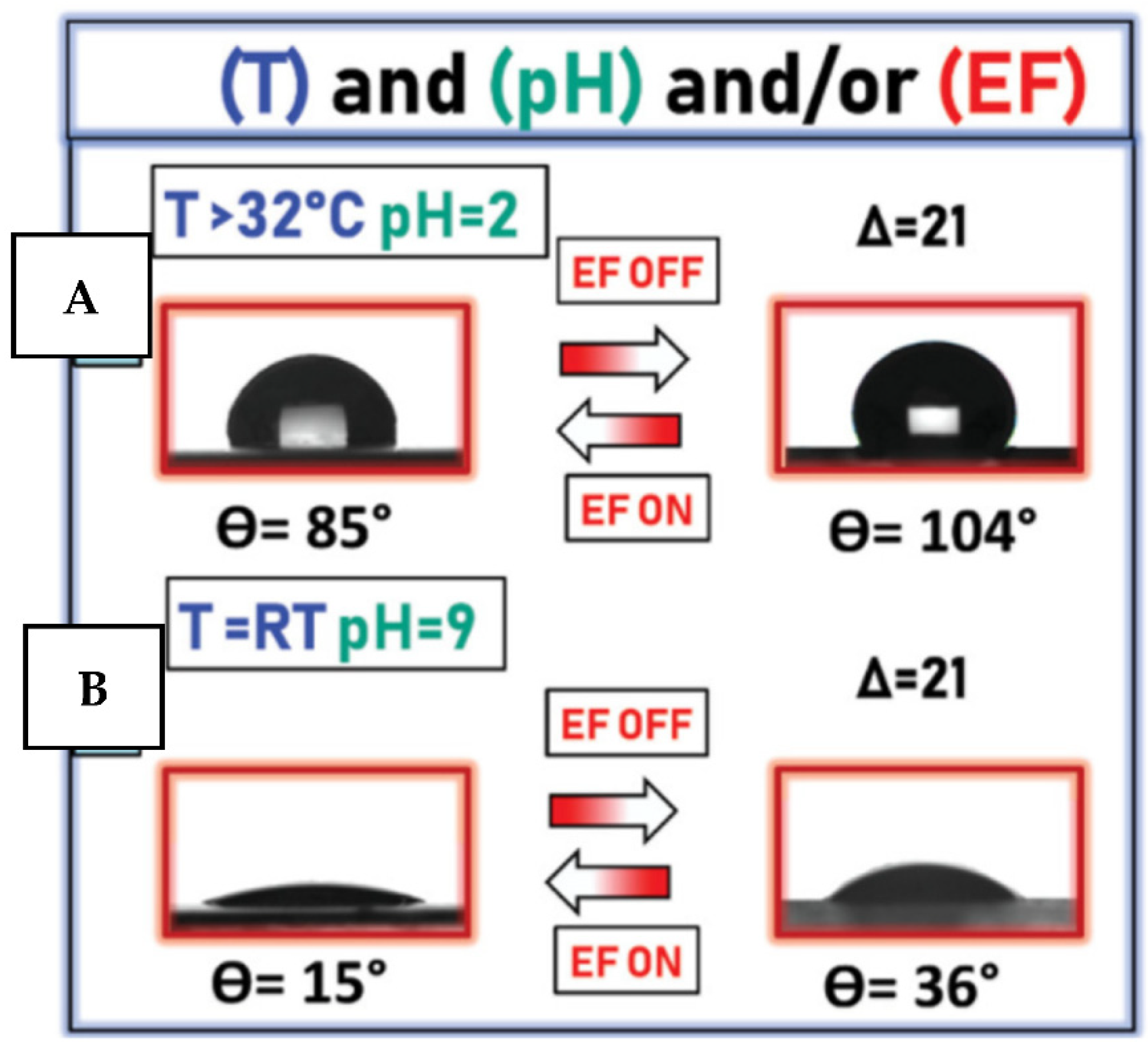
2.3.2. Electro-Responsive Coatings
2.4. Mechanically Responsive Coatings
2.5. Mixed Approaches
2.5.1. Physico-Chemical Stimulation
2.5.2. Physico-Physical Stimuli Combination
2.5.3. Multiresponsive Coatings
2.5.4. Multidrug Approach
2.5.5. Killing and Release
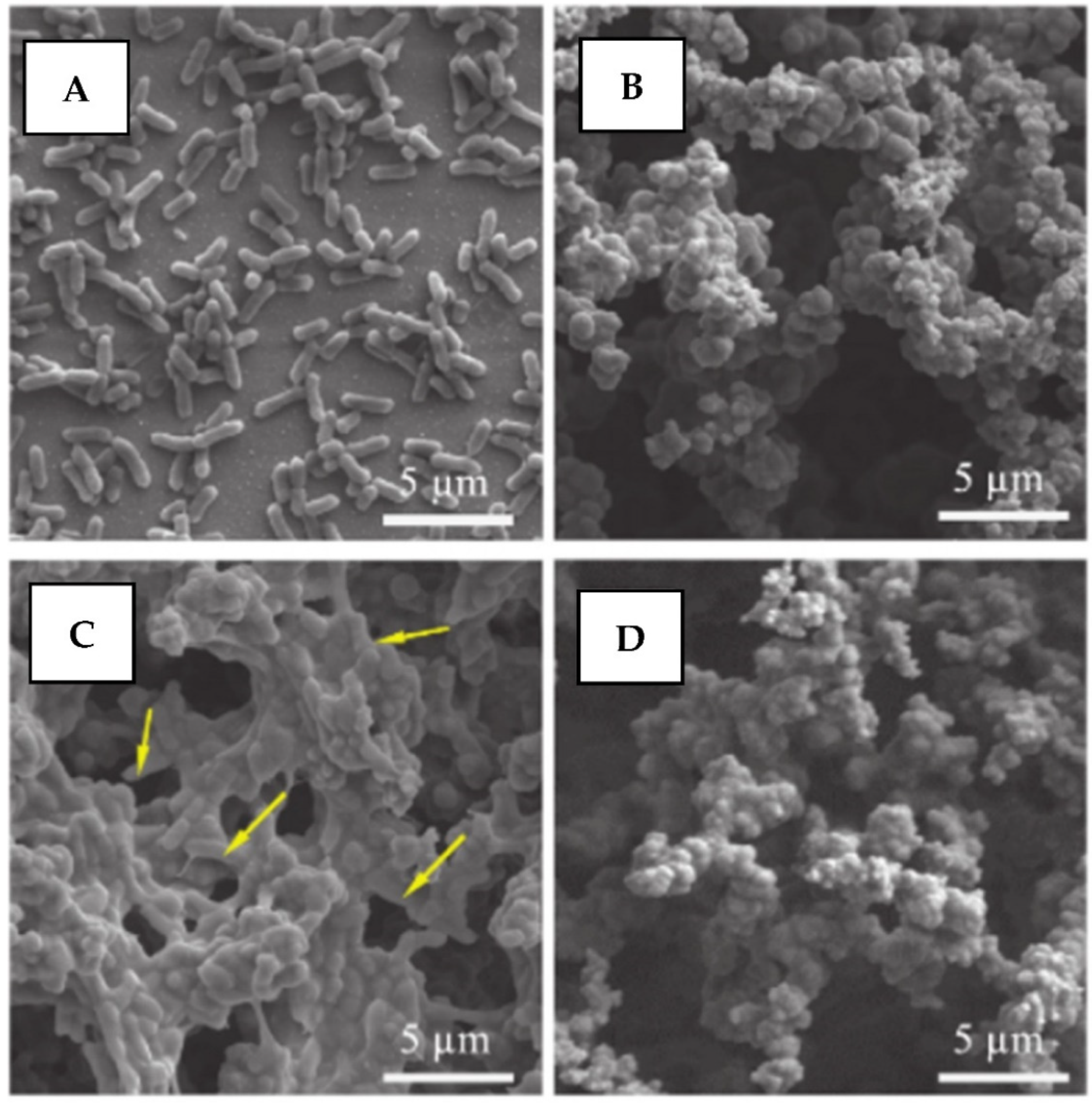
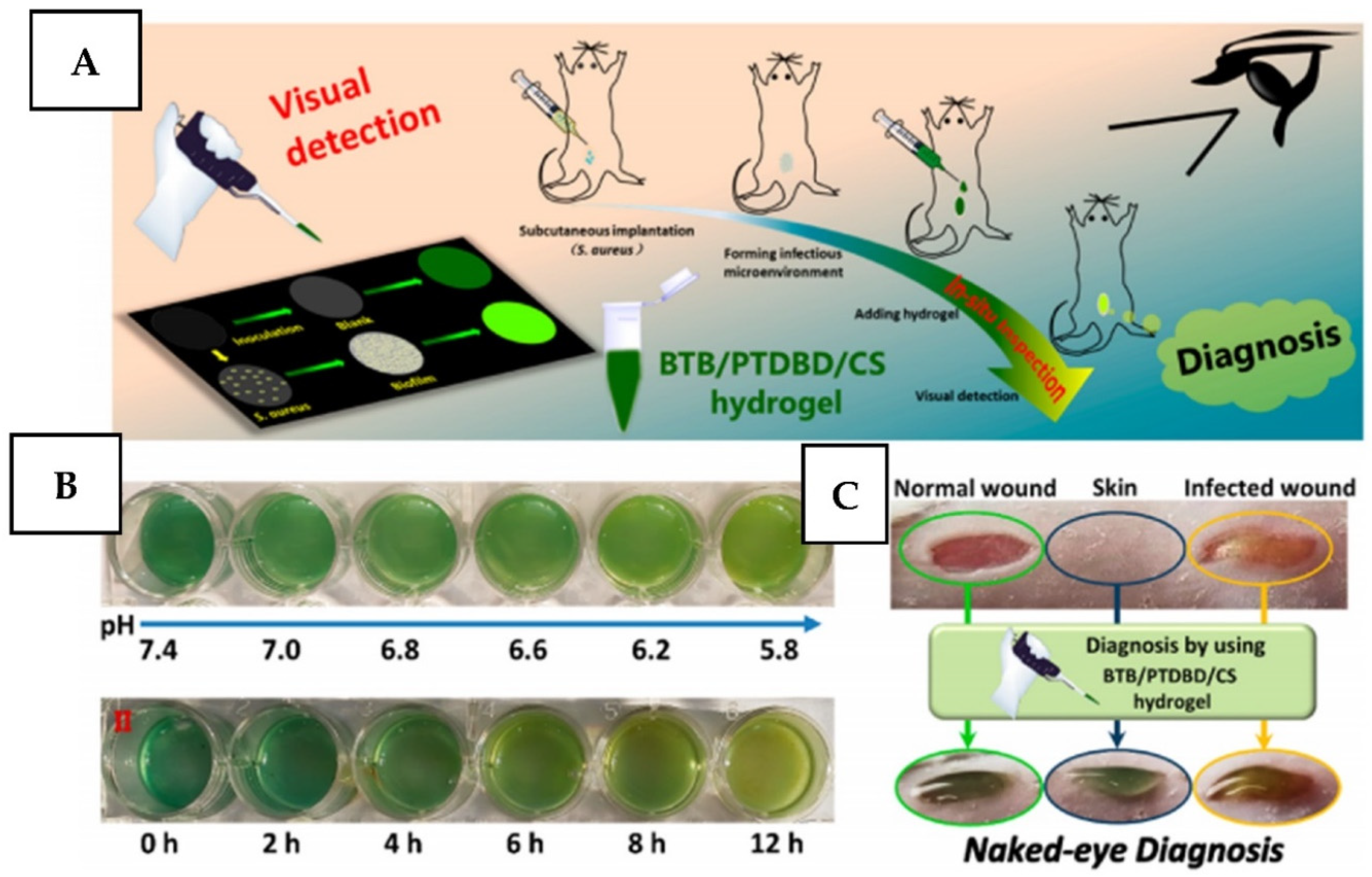
2.5.6. Detect and Kill
3. Outlook and Further Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| EF | Electric field |
| FITC | Fluorescein isothiocyanate |
| GLM | Gallium-based liquid metal |
| MRSA | Methicilin-resistant Staphylococcus aureus |
| NMP | Nitroxide-mediated polymerization |
| LCST | Lower critical solubility temperature |
| LED | Light-emitting diode |
| PEG | Polyethylene glycol |
| PMMA | Poly(methyl methacrylate) |
| PNIPAm | Poly(N-isopropylacrylamide) |
| PVDF | Polyvinylidene fluoride |
| QAS | Quaternary ammonium salts |
| RAFT | Reversible addition−fragmentation chain-transfer polymerization |
| ROS | Reactive oxygen species |
| TA | Tannic acid |
| TPP | Tetraphenylporphine |
References
- Pavithra, D.; Doble, M. Biofilm formation, bacterial adhesion and host response on polymeric implants--issues and prevention. Biomed. Mater. 2008, 3, 034003. [Google Scholar] [CrossRef]
- Qi, K.; Daoud, W.A.; Xin, J.H.; Mak, C.L.; Tang, W.; Cheung, W.P. Self-cleaning cotton. J. Mater. Chem. 2006, 16, 4567–4574. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.; Suh, H.; Choi, K.S. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Roosjen, A.; van der Mei, H.C.; Busscher, H.J.; Norde, W. Microbial adhesion to poly (ethylene oxide) brushes: Influence of polymer chain length and temperature. Langmuir 2004, 20, 10949–10955. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razatos, A.; Ong, Y.L.; Boulay, F.; Elbert, D.L.; Hubbell, J.A.; Sharma, M.M.; Georgiou, G. Force measurements between bacteria and poly(ethylene glycol)-coated surfaces. Langmuir 2000, 16, 9155–9158. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Liang, M.N.; Meluleni, G.; Pier, G.; Ingber, D.E.; Whitesides, G.M. Self-assembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir 2001, 17, 6336–6343. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mat. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata mechanical stiffness can regulate adhesion of viable bacteria. Biomacromolecules 2008, 9, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, K.A.; Verran, J. The effect of surface topography on the retention of microorganisms. Food Bioprod. Proc. 2006, 84, 253–259. [Google Scholar] [CrossRef]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; Lavalle, P. New smart antimicrobial hydrogels, nanomaterials, and coatings: Earlier action, more specific, better dosing? Adv. Healthc. Mater. 2021, 10, 2001199. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinanen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.; Zhang, E.; Han, X.; Zhu, H.; Shi, Y.; Cao, Z. Engineering and application perspectives on designing an antimicrobial surface. ACS Appl. Mater. Int. 2020, 12, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Hoch, M. Organotin compounds in the environment an overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Jin, Q.; Ji, J. Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 2020, 8, 6825–6839. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef]
- Stigter, M.; Bezemer, J.; De Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Mirobial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Bozja, J.; Sherrill, J.; Michielsen, S.; Stojiljkovic, I. Porphyrin-based, light-activated antimicrobial materials. J. Polym. Sci. Pol. Chem. 2003, 41, 2297–2303. [Google Scholar] [CrossRef]
- Hucknall, A.; Rangarajan, S.; Chilkoti, A. In pursuit of zero: Polymer brushes that resist the adsorption of proteins. Adv. Mat. 2009, 21, 2441–2446. [Google Scholar] [CrossRef]
- Statz, A.R.; Meagher, R.J.; Barron, A.E.; Messersmith, P.B. New peptidomimetic polymers for antifouling surfaces. J. Am. Chem. Soc. 2005, 127, 7972–7973. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Amiji, M.; Park, K. Surface modification of polymeric biomaterials with poly (ethylene oxide), albumin, and heparin for reduced thrombogenicity. J. Biomater. Sci.-Polym. Ed. 1993, 4, 217–234. [Google Scholar] [CrossRef] [Green Version]
- Prime, K.L.; Whitesides, G.M. Self-assembled organic monolayers: Model systems for studying adsorption of proteins at surfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Mrksich, M.; Whitesides, G.M. Self-assembled monolayers of alkanethiolates presenting tri (propylene sulfoxide) groups resist the adsorption of protein. J. Am. Chem. Soc. 1996, 118, 5136–5137. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P. Protein—surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Cheng, G.; Xue, H.; Zhang, Z.; Chen, S.; Jiang, S. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities. Angew. Chem. 2008, 120, 8963–8966. [Google Scholar] [CrossRef]
- Kurt, P.; Wood, L.; Ohman, D.E.; Wynne, K.J. Highly effective contact antimicrobial surfaces via polymer surface modifiers. Langmuir 2007, 23, 4719–4723. [Google Scholar] [CrossRef]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surface 2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef]
- Asuri, P.; Karajanagi, S.S.; Kane, R.S.; Dordick, J.S. Polymer–nanotube–enzyme composites as active antifouling films. Small 2007, 3, 50–53. [Google Scholar] [CrossRef]
- Kim, Y.D.; Dordick, J.S.; Clark, D.S. Siloxane-based biocatalytic films and paints for use as reactive coatings. Biotechnol. Bioeng. 2001, 72, 475–482. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Q.; Wang, J.; Ma, J. Preparation of large area double-walled carbon nanotube macro-films with self-cleaning properties. J. Mater. Sci. Technol. 2010, 26, 20–26. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. The “lotus effect” explained: Two reasons why two length scales of topography are important. Langmuir 2006, 22, 2966–2967. [Google Scholar] [CrossRef]
- Shang, H.M.; Wang, Y.; Limmer, S.J.; Chou, T.P.; Takahashi, K.; Cao, G.Z. Optically transparent superhydrophobic silica-based films. Thin Solid Films 2005, 472, 37–43. [Google Scholar] [CrossRef]
- Coulson, S.R.; Woodward, I.; Badyal, J.P.S.; Brewer, S.A.; Willis, C. Super-repellent composite fluoropolymer surfaces. J. Phys. Chem. B 2000, 104, 8836–8840. [Google Scholar] [CrossRef]
- Xiao, X.; Zhao, W.; Liang, J.; Sauer, K.; Libera, M. Self-defensive antimicrobial biomaterial surfaces. Colloids Surf. B Biointerfaces 2020, 192, 110989. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Urban, A.M.; Urban, M.W. Stimuli-Responsive Polymeric Films and Coatings, 1st ed.; American Chemical Society: Washington, DC, USA, 2005; pp. 107–121. [Google Scholar]
- Hao, X.; Chen, S.; Qin, D.; Zhang, M.; Li, W.; Fan, J.; Wang, C.; Dong, M.; Zhang, J.; Cheng, F.; et al. Antifouling and antibacterial behaviors of capsaicin-based pH responsive smart coatings in marine environments. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 108, 110361. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, R.; Wang, H.; Jiang, X.; Wang, H.; Yan, S.; Yin, J.; Luan, S. Bacterial activation of surface-tethered antimicrobial peptides for the facile construction of a surface with self-defense. Appl. Surf. Sci. 2019, 497, 143480. [Google Scholar] [CrossRef]
- Buchegger, S.; Kamenac, A.; Fuchs, S.; Herrmann, R.; Houdek, P.; Gorzelanny, C.; Obermeier, A.; Heller, S.; Burgkart, R.; Stritzker, B.; et al. Smart antimicrobial efficacy employing pH-sensitive ZnO-doped diamond-like carbon coatings. Sci. Rep. 2019, 9, 17246. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Jiang, Y.W.; Jia, H.R.; Wu, F.G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Edis, Z.; Bloukh, S.H. Facile synthesis of antimicrobial aloe vera-“smart” triiodide-PVP biomaterials. Biomimetics 2020, 5, 45. [Google Scholar] [CrossRef]
- Ista, L.K.; Pérez-Luna, V.H.; López, G.P. Surface-grafted, environmentally sensitive polymers for biofilm release. Appl. Environ. Microbiol. 1999, 65, 1603–1609. [Google Scholar] [CrossRef] [Green Version]
- Ista, L.K.; Mendez, S.; Lopez, G.P. Attachment and detachment of bacteria on surfaces with tunable and switchable wettability. Biofouling 2010, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Hong, X.; Wan, A.; Batteas, J.D.; Bergbreiter, D.E. Parallel effects of cations on PNIPAM graft wettability and PNIPAM solubility. ACS Appl. Mater. Int. 2010, 2, 452–458. [Google Scholar] [CrossRef]
- Guselnikova, O.; Marque, S.R.; Tretyakov, E.V.; Mares, D.; Jerabek, V.; Audran, G.; Joly, J.P.; Trusova, M.; Svorcik, V.; Lyutakov, O.; et al. Unprecedented plasmon-induced nitroxide-mediated polymerization (PI-NMP): A method for preparation of functional surfaces. J. Mater. Chem. A 2019, 7, 12414–12419. [Google Scholar] [CrossRef]
- Erzina, M.; Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Kolska, Z.; Miliutina, E.; Svorcik, V.; Lyutakov, O. Plasmon-polariton induced,“from surface” RAFT polymerization, as a way toward creation of grafted polymer films with thickness precisely controlled by self-limiting mechanism. Adv. Mater. Interfaces 2018, 5, 1801042. [Google Scholar] [CrossRef]
- Ista, L.K.; Mendez, S.; Pérez-Luna, V.H.; López, G.P. Synthesis of poly (N-isopropylacrylamide) on initiator-modified self-assembled monolayers. Langmuir 2001, 17, 2552–2555. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.; Kalachyova, Y.; Kolska, Z.; Libansky, M.; Zima, J.; Svorcik, V.; Lyutakov, O. Large-scale, ultrasensitive, highly reproducible and reusable smart SERS platform based on PNIPAm-grafted gold grating. ChemNanoMat 2017, 3, 135–144. [Google Scholar] [CrossRef]
- Guselnikova, O.; Svanda, J.; Postnikov, P.; Kalachyova, Y.; Svorcik, V.; Lyutakov, O. Fast and reproducible wettability switching on functionalized PVDF/PMMA surface controlled by external electric field. Adv. Mater. Interfaces 2017, 4, 1600886. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.; Sajdl, P.; Elashnikov, R.; Švorčík, V.; Lyutakov, O. Functional and switchable amphiphilic pmma surface prepared by 3D selective modification. Adv. Mater. Interfaces 2018, 5, 1701182. [Google Scholar] [CrossRef]
- Cunliffe, D.; de las Heras Alarcón, C.; Peters, V.; Smith, J.R.; Alexander, C. Thermoresponsive surface-grafted poly (N−isopropylacrylamide) copolymers: Effect of phase transitions on protein and bacterial attachment. Langmuir 2003, 19, 2888–2899. [Google Scholar] [CrossRef]
- De las Heras Alarcón, C.; Twaites, B.; Cunliffe, D.; Smith, J.R.; Alexander, C. Grafted thermo-and pH responsive co-polymers: Surface-properties and bacterial adsorption. Int. J. Pharm. 2005, 295, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Elashnikov, R.; Slepička, P.; Rimpelova, S.; Ulbrich, P.; Švorčík, V.; Lyutakov, O. Temperature-responsive PLLA/PNIPAM nanofibers for switchable release. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 72, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Elashnikov, R.; Lyutakov, O.; Kalachyova, Y.; Solovyev, A.; Svorcik, V. Tunable release of silver nanoparticles from temperature-responsive polymer blends. React. Funct. Polym. 2015, 93, 163–169. [Google Scholar] [CrossRef]
- Elashnikov, R.; Mares, D.; Podzimek, T.; Švorčík, V.; Lyutakov, O. Sandwiched gold/PNIPAm/gold microstructures for smart plasmonics application: Towards the high detection limit and Raman quantitative measurements. Analyst 2017, 142, 2974–2981. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aymonier, C.; Schlotterbeck, U.; Antonietti, L.; Zacharias, P.; Thomann, R.; Tiller, J.C.; Mecking, S. Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties. Chem. Commun. 2002, 2002, 3018–3019. [Google Scholar] [CrossRef] [Green Version]
- Lawson, M.C.; Shoemaker, R.; Hoth, K.B.; Bowman, C.N.; Anseth, K.S. Polymerizable vancomycin derivatives for bactericidal biomaterial surface modification: Structure—Function evaluation. Biomacromolecules 2009, 10, 2221–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Shen, D.; Zhou, L.; Li, W.; Li, X.; Yao, C.; Wang, R.; El-Toni, A.M.; Zhang, F.; Zhao, D. Spatially confined fabrication of core–shell gold nanocages@ mesoporous silica for near-infrared controlled photothermal drug release. Chem. Mater. 2013, 25, 3030–3037. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Zhu, H.; Cao, Z. Superdurable coating fabricated from a double-sided tape with long term “zero” bacterial adhesion. Adv. Mater. 2017, 29, 1606506. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Xie, J.; Zhu, H.; Cao, Z. A zwitterionic macro-crosslinker for durable non-fouling coatings. Chem. Commun. 2016, 52, 4671–4674. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Lin, W.; Wang, Z.; Zhang, J.; Ji, F.; Ma, G.; Yuan, Z.; Chen, S. Development of robust and recoverable ultralow-fouling coatings based on poly(carboxybetaine) ester analogue. ACS Appl. Mater. Interfaces 2015, 7, 16938–16945. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Yang, X.; Lei, K.; Wang, L. The innovative fabrication of nano-natural antimicrobial agent@ polymeric microgels-TiO2 hybrid films capable of absorbing UV and antibacterial on touch screen panel. Colloid Surf. B-Biointerfaces 2021, 197, 111410. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, Q.; Wang, D.; Wang, L.; Lin, F.; Wilson, P.; Haddleton, D.M. Bioinspired coating of TiO2 nanoparticles with antimicrobial polymers by Cu(0)-LRP: Grafting to vs. grafting from. Polym. Chem. 2017, 8, 6570–6580. [Google Scholar] [CrossRef]
- Page, K.; Wilson, M.; Parkin, I.P. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J. Polym. Sci. Pol. Chem. 2009, 19, 3819–3831. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube–TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Piccirillo, C.; Perni, S.; Gil-Thomas, J.; Prokopovich, P.; Wilson, M.; Pratten, J.; Parkin, I.P. Antimicrobial activity of methylene blue and toluidine blue O covalently bound to a modified silicone polymer surface. J. Mater. Chem. 2009, 19, 6167–6171. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Xie, R.; Imlay, J.A.; Shang, J.K. Visible-light-induced photocatalytic inactivation of bacteria by composite photocatalysts of palladium oxide and nitrogen-doped titanium oxide. Appl. Catal. B-Environ. 2009, 88, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Org. 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ullah, R.; Butt, A.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Wilson, M. Light-activated antimicrobial coating for the continuous disinfection of surfaces. Infect. Control. Hosp. Epidemiol. 2003, 24, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.; McCoy, C.P.; Gorman, S.P.; Jones, D.S.; Bell, S.E.; Brady, C.; McGlinchey, S.M. Anti-infective photodynamic biomaterials for the prevention of intraocular lens-associated infectious endophthalmitis. Biomaterials 2009, 30, 597–602. [Google Scholar] [CrossRef]
- Perni, S.; Piccirillo, C.; Pratten, J.; Prokopovich, P.; Chrzanowski, W.; Parkin, I.P.; Wilson, M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef]
- Prasad, G.K.; Agarwal, G.S.; Singh, B.; Rai, G.P.; Vijayaraghavan, R. Photocatalytic inactivation of Bacillus anthracis by titania nanomaterials. J. Hazard. Mater. 2009, 165, 506–510. [Google Scholar] [CrossRef]
- Chung, C.J.; Lin, H.I.; Chou, C.M.; Hsieh, P.Y.; Hsiao, C.H.; Shi, Z.Y.; He, J.L. Inactivation of Staphylococcus aureus and Escherichia coli under various light sources on photocatalytic titanium dioxide thin film. Surf. Coat. Technol. 2009, 203, 1081–1085. [Google Scholar] [CrossRef]
- Lyutakov, O.; Hejna, O.; Solovyev, A.; Kalachyova, Y.; Svorcik, V. Polymethylmethacrylate doped with porphyrin and silver nanoparticles as light-activated antimicrobial material. RSC Adv. 2014, 4, 50624–50630. [Google Scholar] [CrossRef]
- Gaufrès, E.; Tang, N.W.; Lapointe, F.; Cabana, J.; Nadon, M.A.; Cottenye, N.; Raymond, F.; Szkopek, T.; Martel, R. Giant Raman scattering from J-aggregated dyes inside carbon nanotubes for multispectral imaging. Nat. Photonics 2014, 8, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.W.; Yao, K.; Xu, Z.K. Nanomaterials with a photothermal effect for antibacterial activities: An overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.C.; Oh, J. Photothermal responsive porous membrane for treatment of infected wound. Polymers 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Sun, J.; Yu, Z.; Guo, Z.; Xu, C. Low-intensity near-infrared light-triggered spatiotemporal antibiotics release and hyperthermia by natural polysaccharide-based hybrid hydrogel for synergistic wound disinfection. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111530. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.S. Au–ZnO conjugated black phosphorus as a near-infrared light-triggering and recurrence-suppressing nanoantibiotic platform against Staphylococcus aureus. Pharmaceutics 2021, 13, 52. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Cao, Y.; Guo, Z.; Li, F.; Wang, L. Construction of chitosan-based hydrogel incorporated with antimonene nanosheets for rapid capture and elimination of bacteria. Adv. Funct. Mater. 2020, 30, 2003196. [Google Scholar] [CrossRef]
- Lin, J.; He, Z.; Liu, F.; Feng, J.; Huang, C.; Sun, X.; Deng, H. Hybrid hydrogels for synergistic periodontal antibacterial treatment with sustained drug release and NIR-responsive photothermal effect. Int. J. Nanomed. 2020, 15, 5377–5387. [Google Scholar] [CrossRef]
- Kalachyova, Y.; Olshtrem, A.; Guselnikova, O.A.; Postnikov, P.S.; Elashnikov, R.; Ulbrich, P.; Rimpelová, S.; Švorčík, V.; Lyutakov, O. Synthesis, characterization, and antimicrobial activity of near-IR photoactive functionalized gold multibranched nanoparticles. ChemistryOpen 2017, 6, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elashnikov, R.; Lyutakov, O.; Ulbrich, P.; Svorcik, V. Light-activated polymethylmethacrylate nanofibers with antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 64, 229–235. [Google Scholar] [CrossRef] [PubMed]
- <named-content content-type="background:white">Yu, H.; Peng, Y.; Yang, Y.; Li, Z.Y. Plasmon-enhanced light–matter interactions and applications. NPJ Computat. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Elashnikov, R.; Radocha, M.; Panov, I.; Rimpelova, S.; Ulbrich, P.; Michalcova, A.; Lyutakov, O. Porphyrin silver nanoparticles hybrids: Synthesis, characterization and antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 102, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Chandna, S.; Thakur, N.S.; Kaur, R.; Bhaumik, J. Lignin–bimetallic nanoconjugate doped pH-responsive hydrogels for laser-assisted antimicrobial photodynamic therapy. Biomacromolecules 2020, 21, 3216–3230. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wang, J.; Wang, Y.; Chen, A.; Wang, C.; Zhang, Y. Photon-responsive antibacterial nanoplatform for synergistic photothermal-/pharmaco-therapy of skin infection. ACS Appl. Mater. Interfaces 2019, 11, 300–310. [Google Scholar] [CrossRef]
- Jardim, K.V.; Palomec-Garfias, A.F.; Andrade, B.Y.G.; Chaker, J.A.; Báo, S.N.; Márquez-Beltrán, C.; Moya, S.E.; Parize, A.L.; Sousa, M.H. Novel magneto-responsive nanoplatforms based on MnFe2O4 nanoparticles layer-by-layer functionalized with chitosan and sodium alginate for magnetic controlled release of curcumin. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 92, 184–195. [Google Scholar] [CrossRef]
- Bigham, A.; Aghajanian, A.H.; Behzadzadeh, S.; Sokhani, Z.; Shojaei, S.; Kaviani, Y.; Hassanzadeh-Tabrizia, S.A. Nanostructured magnetic Mg2SiO4-CoFe2O4 composite scaffold with multiple capabilities for bone tissue regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 99, 83–95. [Google Scholar] [CrossRef]
- Kost, J.; Wolfrum, J.; Langer, R. Magnetically enhanced insulin release in diabetic rats. J. Biomed. Mater. Res. 1987, 21, 1367–1373. [Google Scholar] [CrossRef]
- Saslawski, O.; Weingarten, C.; Benoit, J.P.; Couvreur, P. Magnetically responsive microspheres for the pulsed delivery of insulin. Life Sci. 1988, 42, 1521–1528. [Google Scholar] [CrossRef]
- Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Controlled pulsatile drug release from a ferrogel by a high-frequency magnetic field. Macromolecules 2007, 40, 6786–6788. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.Y.; Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Hu, S.H.; Liu, K.H.; Liu, D.M.; Chen, S.Y. Preparation and characterization of smart magnetic hydrogels and its use for drug release. J. Magn. Magn. Mater. 2006, 304, e397–e399. [Google Scholar] [CrossRef]
- Fang, C.; Kievit, F.M.; Veiseh, O.; Stephen, Z.R.; Wang, T.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Fabrication of magnetic nanoparticles with controllable drug loading and release through a simple assembly approach. J. Control. Release 2012, 162, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, S.; Roco, C.; Déléris, A.; Spoerri, P.; Cezar, C.; Weaver, J.; Vandengburgh, H.; Mooney, D. Improved magnetic regulation of delivery profiles from ferrogels. Biomaterials 2018, 161, 179–189. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Mazzei, R.; Poerio, T.; Aimar, P.; Vankelecom, I.F.; Giorno, L. Pectinases immobilization on magnetic nanoparticles and their anti-fouling performance in a biocatalytic membrane reactor. RSC Adv. 2016, 6, 98737–98747. [Google Scholar] [CrossRef] [Green Version]
- Elbourne, A.; Cheeseman, S.; Atkin, P.; Truong, N.P.; Syed, N.; Mohiuddin, M.; Esrafilzadeh, D.; Cozzolino, D.; McConville, C.; Dickey, M.D.; et al. Antibacterial liquid metals: Biofilm treatment via magnetic activation. ACS Nano 2020, 14, 802–817. [Google Scholar] [CrossRef]
- Cheeseman, S.; Elbourne, A.; Kariuki, R.; Ramarao, A.V.; Zavabeti, A.; Syed, N.; Christofferson, A.J.; Kwon, K.Y.; Jung, W.; Dickey, M.D.; et al. Broad-spectrum treatment of bacterial biofilms using magneto-responsive liquid metal particles. J. Mat. Chem. B 2020, 8, 10776–10787. [Google Scholar] [CrossRef]
- Li, S.; Wei, C.; Lv, Y. Preparation and application of magnetic responsive materials in bone tissue engineering. Curr. Stem Cell Res. Ther. 2020, 15, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.A.; Lee, J.; Bharadwaj, N.A.; Ewoldt, R.H.; Kilian, K.A. Temporal modulation of stem cell activity using magnetoactive hydrogels. Adv. Healthc. Mater. 2016, 5, 2536–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbin, E.A.; Vite, A.; Peyster, E.G.; Bhoopalam, M.; Brandimarto, J.; Wang, X.; Bennett, A.I.; Clark, A.T.; Cheng, X.; Turner, K.T.; et al. Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS Appl. Mater. Interfaces 2019, 11, 20603–20614. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Liang, Y.; Li, L.; Duan, L.; Chen, J.; Zhu, F.; Lai, Y.; Zhu, W.; You, W.; et al. Preparation and biocompatibility of diphasic magnetic nanocomposite scaffold. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 87, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloid Surf. B-Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Durán-Guerrero, J.G.; Martínez-Rodríguez, M.A.; Garza-Navarro, M.A.; González-González, V.A.; Torres-Castro, A.; De La Rosa, J.R. Magnetic nanofibrous materials based on CMC/PVA polymeric blends. Carbohydr. Polym. 2018, 200, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, D.; Michel, J.; Suter, N.; de Aguiar, M.G.; Maas, M. Wet-spinning of magneto-responsive helical chitosan microfibers. Beilstein J. Nanotechnol. 2020, 11, 991–999. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Yin, Y.; Qi, H. A facile strategy to fabricate polysaccharide-based magnetic hydrogel based on enamine bond. Chin. J. Chem. 2020, 38, 1263–1268. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Wang, Z.; Liang, Y.; Cui, Z.; Zhao, J.; Li, X.; Ren, L. Multistimuli-responsive microstructured superamphiphobic surfaces with large-range, reversible switchable wettability for oil. ACS Appl. Mater. Interfaces 2019, 11, 28478–28486. [Google Scholar] [CrossRef]
- Yang, C.; Wu, L.; Li, G. Magnetically responsive superhydrophobic surface: In situ reversible switching of water droplet wettability and adhesion for droplet manipulation. ACS Appl. Mater. Interfaces 2018, 10, 20150–20158. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Han, G.; Guo, Z. A facile approach to achieve bioinspired PDMS@Fe3O4 fabric with switchable wettability for liquid transport and water collection. J. Mater. Chem. A 2018, 6, 22741–22748. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, Y.; Mao, S.; Nakajima, H.; Uchiyama, K. A reversibly electro-controllable polymer brush for electro-switchable friction. J. Mater. Chem. C 2017, 5, 5877–5881. [Google Scholar] [CrossRef]
- Drotlef, D.M.; Blümler, P.; Papadopoulos, P.; Del Campo, A. Magnetically actuated micropatterns for switchable wettability. ACS Appl. Mater. Interfaces 2014, 6, 8702–8707. [Google Scholar] [CrossRef] [PubMed]
- Grigoryev, A.; Tokarev, I.; Kornev, K.G.; Luzinov, I.; Minko, S. Superomniphobic magnetic microtextures with remote wetting control. J. Am. Chem. Soc. 2012, 134, 12916–12919. [Google Scholar] [CrossRef]
- Uppalapati, D.; Boyd, B.J.; Garg, S.; Travas-Sejdic, J.; Svirskis, D. Conducting polymers with defined micro-or nanostructures for drug delivery. Biomaterials 2016, 111, 149–162. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Seyfoddin, A.; Chan, A.; Chen, W.T.; Rupenthal, I.D.; Waterhouse, G.I.N.; Svirskis, D. Electro-responsive macroporous polypyrrole scaffolds for triggered dexamethasone delivery. Eur. J. Pharm. Biopharm. 2015, 94, 419–426. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Zhu, M.; Hao, Y.; Ma, X.; Feng, L.; Zhai, Y.; Ding, Y.; Cheng, G. Construction of a graphene/polypyrrole composite electrode as an electrochemically controlled release system. RSC Adv. 2019, 9, 12667–12674. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon nanotubes hybrid hydrogels for electrically tunable release of curcumin. Eur. Polym. J. 2017, 90, 1–12. [Google Scholar] [CrossRef]
- Servant, A.; Bussy, C.; Al-Jamal, K.; Kostarelos, K. Design, engineering and structural integrity of electro-responsive carbon nanotube-based hydrogels for pulsatile drug release. J. Mater. Chem. B 2013, 1, 4593–4600. [Google Scholar] [CrossRef]
- Bijukumar, D.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. An electro-conductive fluid as a responsive implant for the controlled stimuli-release of diclofenac sodium. Pharm. Dev. Technol. 2015, 21, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Mehrotra, R.; Margulis, K.; Zare, R.N. On-demand electrically controlled drug release from resorbable nanocomposite films. Nanoscale 2017, 9, 16429–16436. [Google Scholar] [CrossRef]
- Sutani, K.; Kaetsu, I.; Uchida, K.; Matsubara, Y. Stimulus responsive drug release from polymer gel.: Controlled release of ionic drug from polyampholyte gel. Radiat. Phys. Chem. 2002, 64, 331–336. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Firlak, M.; Berrow, S.R.; Halcovitch, N.R.; Baldock, S.J.; Yousafzai, B.M.; Hathout, R.M.; Hardy, J.G. Electrochemically enhanced drug delivery using polypyrrole films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.L.; Cardo, L.; Mancino, D.; Arnaiz, B.; Criado, A.; Prato, M. Electrochemically controlled cleavage of imine bonds on a graphene platform: Towards new electro-responsive hybrids for drug release. Nanoscale 2020, 12, 23824–23830. [Google Scholar] [CrossRef] [PubMed]
- Tohgha, U.N.; Alvino, E.L.; Jarnagin, C.C.; Iacono, S.T.; Godman, N.P. Electrowetting behavior and digital microfluidic applications of fluorescent, polymer-encapsulated quantum dot nanofluids. ACS Appl. Mater. Interfaces 2019, 11, 28487–28498. [Google Scholar] [CrossRef]
- Trick, J.L.; Song, C.; Wallace, E.J.; Sansom, M.S. Voltage gating of a biomimetic nanopore: Electrowetting of a hydrophobic barrier. ACS Nano 2017, 11, 1840–1847. [Google Scholar] [CrossRef]
- Han, Z.; Tay, B.; Tan, C.; Shakerzadeh, M.; Ostrikov, K. Electrowetting control of Cassie-to-Wenzel transitions in superhydrophobic carbon nanotube-based nanocomposites. ACS Nano 2009, 3, 3031–3036. [Google Scholar] [CrossRef]
- Sun, D.; Böhringer, K.F. Self-cleaning: From bio-inspired surface modification to MEMS/microfluidics system integration. Micromachines 2019, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Děkanovský, L.; Elashnikov, R.; Kubiková, M.; Vokatá, B.; Švorčík, V.; Lyutakov, O. Dual-action flexible antimicrobial material: Switchable self-cleaning, antifouling, and smart drug release. Adv. Funct. Mater. 2019, 29, 1901880. [Google Scholar] [CrossRef]
- Elashnikov, R.; Fitl, P.; Svorcik, V.; Lyutakov, O. Patterning of ultrathin polymethylmethacrylate films by in-situ photodirecting of the Marangoni flow. Appl. Surf. Sci. 2017, 394, 562–568. [Google Scholar] [CrossRef]
- Lyutakov, O.; Huttel, I.; Siegel, J.; Švorčík, V. Regular surface grating on doped polymer induced by laser scanning. Appl. Phys. Lett. 2009, 95, 173103. [Google Scholar] [CrossRef]
- Lyutakov, O.; Tůma, J.; Huttel, I.; Prajzler, V.; Siegel, J.; Švorčík, V. Polymer surface patterning by laser scanning. Appl. Phys. B-Lasers Opt. 2013, 110, 539–549. [Google Scholar] [CrossRef]
- Lyutakov, O.; Hüttel, I.; Prajzler, V.; Jeřábek, V.; Jančárek, A.; Hnatowicz, V.; Švorčík, V. Pattern formation in PMMA film induced by electric field. J. Polym. Sci. Pt. B-Polym. Phys. 2009, 47, 1131–1135. [Google Scholar] [CrossRef]
- Lyutakov, O.; Tuma, J.; Prajzler, V.; Huttel, I.; Hnatowicz, V.; Švorčík, V. Preparation of rib channel waveguides on polymer in electric field. Thin Solid Films 2010, 519, 1452–1457. [Google Scholar] [CrossRef]
- Lin, I.T.; Choi, Y.S.; Wojcik, C.; Wang, T.; Kar-Narayan, S.; Smoukov, S.K. Electro-responsive surfaces with controllable wrinkling patterns for switchable light reflection–diffusion–grating devices. Mater. Today 2020, 41, 51–61. [Google Scholar] [CrossRef]
- Idriss, H.; Elashnikov, R.; Guselnikova, O.; Postnikov, P.; Kolska, Z.; Lyutakov, O.; Švorčík, V. Reversible wettability switching of piezo-responsive nanostructured polymer fibers by electric field. Chem. Pap. 2021, 75, 191–196. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Svorcik, V.; Lyutakov, O. Multiresponsive wettability switching on polymer surface: Effect of surface chemistry and/or morphology tuning. Adv. Mater. Interfaces 2019, 6, 1801937. [Google Scholar] [CrossRef]
- Guselnikova, O.; Elashnikov, R.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Smart, piezo-responsive polyvinylidenefluoride/polymethylmethacrylate surface with triggerable water/oil wettability and adhesion. ACS Appl. Mater. Interfaces 2018, 10, 37461–37469. [Google Scholar] [CrossRef]
- Svanda, J.; Kalachyova, Y.; Slepicka, P.; Svorcik, V.; Lyutakov, O. Smart component for switching of plasmon resonance by external electric field. ACS Appl. Mater. Interfaces 2016, 8, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ilčíková, M.; Tkáč, J.; Kasák, P. Switchable materials containing polyzwitterion moieties. Polymers 2015, 7, 2344–2370. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.; Lamb, R.; Baulin, V.; Watson, G.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N.; Rubanov, S.; Hanssen, E.; Wandiyanto, J.; Truong, V.K.; et al. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Chai, M.; Yao, X.; Chen, W.; Chu, P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO2 nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2021, 6, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Hao, L.; Song, L.; Tian, L.; Fan, Y.; Zhao, J.; Liu, C.; Ming, W.; Ren, L. Lotus-leaf-inspired hierarchical structured surface with non-fouling and mechanical bactericidal performances. Chem. Eng. J. 2020, 398, 125609. [Google Scholar] [CrossRef]
- Caccavo, D.; Lamberti, G.; Barba, A.A. Mechanics and drug release from poroviscoelastic hydrogels: Experiments and modeling. Eur. J. Pharm. Biopharm. 2020, 152, 299–306. [Google Scholar] [CrossRef]
- Rajamanickam, R.; Kwon, K.; Tae, G. Soft and elastic hollow microcapsules embedded silicone elastomer films with enhanced water uptake and permeability for mechanical stimuli responsive drug delivery applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 111, 110789. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical force-triggered drug delivery. Chem. Rev. 2016, 116, 12536–12563. [Google Scholar] [CrossRef]
- Di, J.; Yao, S.; Ye, Y.; Cui, Z.; Yu, J.; Ghosh, T.K.; Zhu, Y.; Gu, Z. Stretch-triggered drug delivery from wearable elastomer films containing therapeutic depots. ACS Nano 2015, 9, 9407–9415. [Google Scholar] [CrossRef]
- Lee, K.Y.; Peters, M.C.; Mooney, D.J. Controlled drug delivery from polymers by mechanical signals. Adv. Mater. 2001, 13, 837–839. [Google Scholar] [CrossRef]
- Ballance, W.C.; Seo, Y.; Baek, K.; Chalifoux, M.; Kim, D.; Kong, H. Stretchable, anti-bacterial hydrogel activated by large mechanical deformation. J. Control. Release 2018, 275, 1–11. [Google Scholar] [CrossRef]
- Hyun, D.C.; Moon, G.D.; Park, C.J.; Kim, B.S.; Xia, Y.; Jeong, U. Strain-controlled release of molecules from arrayed microcapsules supported on an elastomer substrate. Angew. Chem. Int. Ed. 2011, 50, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Izawa, H.; Kawakami, K.; Sumita, M.; Tateyama, Y.; Hill, J.P.; Ariga, K. β-Cyclodextrin-crosslinked alginate gel for patient-controlled drug delivery systems: Regulation of host-guest interactions with mechanical stimuli. J. Mater. Chem. B 2013, 1, 2155–2161. [Google Scholar] [CrossRef]
- Larsen, M.B.; Boydston, A.J. Successive mechanochemical activation and small molecule release in an elastomeric material. J. Am. Chem. Soc. 2014, 136, 1276–1279. [Google Scholar] [CrossRef]
- Wang, J.; Colson, Y.L.; Grinstaff, M.W. Tension-activated delivery of small molecules and proteins from superhydrophobic composites. Adv. Healthc. Mater. 2018, 7, 1701096. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, M.; Kumar, A.; Powar, S.; Vaish, R. Rapid bacterial disinfection using low frequency piezocatalysis effect. J. Ind. Eng. Chem. 2019, 77, 355–364. [Google Scholar] [CrossRef]
- Feng, J.; Fu, Y.; Liu, X.; Tian, S.; Lan, S.; Xiong, Y. Significant improvement and mechanism of ultrasonic inactivation to Escherichia coli with piezoelectric effect of hydrothermally synthesized t-BaTiO3. ACS Sustain. Chem. Eng. 2018, 6, 6032–6041. [Google Scholar] [CrossRef]
- Biswas, A.; Saha, S.; Pal, S.; Jana, N.R. TiO2-templated BaTiO3 nanorod as a piezocatalyst for generating wireless cellular stress. ACS Appl. Mater. Interfaces 2020, 12, 48363–48370. [Google Scholar] [CrossRef]
- Vatlin, I.S.; Chernozem, R.V.; Timin, A.S.; Chernova, A.P.; Plotnikov, E.V.; Mukhortova, Y.R.; Surmeneva, M.A.; Surmenev, R.A. Bacteriostatic effect of piezoelectric poly-3-hydroxybutyrate and polyvinylidene fluoride polymer films under ultrasound treatment. Polymers 2020, 12, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Huo, L.; Wang, L.; Huang, X.; Li, J.; Guo, Z.; Hu, M.; Xue, H.; Gao, J. Superhydrophobic and multi-responsive fabric composite with excellent electro-photo-thermal effect and electromagnetic interference shielding performance. Chem. Eng. J. 2020, 391, 123537. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.Y.; Cheon, S.; Kim, S.; Kim, D.; Ryu, H. Stimuli-responsive magneto-/electro-chromatic color-tunable hydrophobic surface modified Fe3O4@SiO2 core–shell nanoparticles for reflective display approaches. RSC Adv. 2017, 7, 6988–6993. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.J.; Song, H.M.; Wang, G.; Zeng, Z.X.; Xue, Q.J. Dual stimuli-responsive polysulfone membranes with interconnected networks by a vapor-liquid induced phase separation strategy. J. Colloid Interface Sci. 2018, 531, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2020, 143, 297–304. [Google Scholar] [CrossRef]
- Elashnikov, R.; Radocha, M.; Rimpelova, S.; Švorčík, V.; Lyutakov, O. Thickness and substrate dependences of phase transition, drug release and antibacterial properties of PNIPAm-co-AAc films. RSC Adv. 2015, 5, 86825–86831. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloid Surf. B-Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- Ge, H.; Kong, Y.; Shou, D.; Deng, L. Communication—Three-dimensional electro-and pH-responsive polypyrrole/alginate hybrid for dual-controlled drug delivery. J. Electrochem. Soc. 2016, 163, G33. [Google Scholar] [CrossRef]
- Chandran, P.R.; Sandhyarani, N. An electric field responsive drug delivery system based on chitosan–gold nanocomposites for site specific and controlled delivery of 5-fluorouracil. RSC Adv. 2014, 4, 44922–44929. [Google Scholar] [CrossRef]
- Silva-Freitas, E.L.; Pontes, T.R.; Araújo-Neto, R.P.; Damasceno, Í.H.; Silva, K.L.; Carvalho, J.F.; Medeiros, A.C.; Silva, R.B.; Silva, A.K.A.; Morales, M.A.; et al. Design of magnetic polymeric particles as a stimulus-responsive system for gastric antimicrobial therapy. AAPS PharmSciTech 2017, 18, 2026–2036. [Google Scholar] [CrossRef]
- Zhang, Y.; Pi, Y.; Hua, Y.; Xie, J.; Wang, C.; Guo, K.; Zhao, Z.; Yong, Y. Bacteria responsive polyoxometalates nanocluster strategy to regulate biofilm microenvironments for enhanced synergetic antibiofilm activity and wound healing. Theranostics 2020, 10, 10031. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Y.; Wang, Y. Dual-responsive drug delivery system with real time tunable release behavior. Microporous Mesoporous Mater. 2014, 200, 46–51. [Google Scholar] [CrossRef]
- Akilo, O.D.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pradeep, P.; Modi, G.; Pillay, V. In situ thermo-co-electroresponsive mucogel for controlled release of bioactive agent. Int. J. Pharm. 2019, 559, 255–270. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Stansbury, J.W.; Zhu, X.; Wang, X.; Nie, J. Smart antibacterial surface made by photopolymerization. ACS Appl. Mater. Interfaces 2016, 8, 28047–28054. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, L.; Wang, L.; Xie, Z.; Gao, M.; Zhang, W. Fabrication of BaTiO3/Ni composite particles and their electro-magneto responsive properties. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 2017, 221, 54–62. [Google Scholar] [CrossRef]
- Priyanka; Kumar, A. Multistimulus-responsive supramolecular hydrogels derived by in situ coating of Ag nanoparticles on 5′-CMP-capped β-FeOOH binary nanohybrids with multifunctional features and applications. ACS Omega 2020, 5, 13672–13684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, P.; Zhang, Y.; Ma, Z.; Teng, K.; Hu, X.; Lu, L.; Zhang, Y.; Zhao, Y.; An, Q. Remarkably boosted molecular delivery triggered by combined thermal and flexoelectrical field dual stimuli. ChemistrySelect 2020, 5, 6715–6722. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Taylor, N.L.; Shi, S.; Wickremasinghe, N.C.; D’Souza, R.N.; Hartgerink, J.D. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials 2015, 52, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, D.; Tan, J.; Chang, Z.; Liu, X.; Ma, W.; Xu, Y. Near-infrared regulated nanozymatic/photothermal/photodynamic triple-therapy for combating multidrug-resistant bacterial infections via oxygen-vacancy molybdenum trioxide nanodots. Small 2021, 17, 2005739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Gao, F.; Wang, C.; Yang, Z.; Wu, H.; Li, C.; Cheng, L.; Peng, R. Facile preparation of Cu2Se nanosheets as dual-functional antibacterial agents. ACS Appl. Bio Mater. 2020, 3, 1418–1425. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lee, D.; Sheng, X.; Cohen, R.E.; Rubner, M.F. Two-level antibacterial coating with both release-killing and contact-killing capabilities. Langmuir 2006, 22, 9820–9823. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Kong, Q.; Lu, Y.; Zhang, B.; Wu, C.; Luo, R.; Wang, Y. Synergistic chemical and photodynamic antimicrobial therapy for enhanced wound healing mediated by multifunctional light-responsive nanoparticles. Biomacromolecules 2019, 20, 4581–4592. [Google Scholar] [CrossRef]
- Caires, C.S.; Farias, L.A.; Gomes, L.E.; Pinto, B.P.; Gonçalves, D.A.; Zagonel, L.F.; Nascimento, V.A.; Alves, D.C.B.; Colbeck, I.; Whitby, C.; et al. Effective killing of bacteria under blue-light irradiation promoted by green synthesized silver nanoparticles loaded on reduced graphene oxide sheets. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 113, 110984. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, H.L.; Ye, X.Q.; Cai, D.T.; Fang, J.T.; Sun, J.; Liu, Y.H. Synergistic potential of antimicrobial combinations against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1919. [Google Scholar] [CrossRef]
- Awad, M.; Yosri, M.; Abdel-Aziz, M.M.; Younis, A.M.; Sidkey, N.M. Assessment of the antibacterial potential of biosynthesized silver nanoparticles combined with vancomycin against methicillin-resistant Staphylococcus aureus–induced infection in rats. Biol. Trace Elem. Res. 2021, 199, 4225–4236. [Google Scholar] [CrossRef]
- Varisco, M.; Khanna, N.; Brunetto, P.S.; Fromm, K.M. New antimicrobial and biocompatible implant coating with synergic silver–vancomycin conjugate action. ChemMedChem 2014, 9, 1221–1230. [Google Scholar] [CrossRef]
- Ho, C.H.; Tobis, J.; Sprich, C.; Thomann, R.; Tiller, J.C. Nanoseparated polymeric networks with multiple antimicrobial properties. Adv. Mater. 2004, 16, 957–961. [Google Scholar] [CrossRef]
- Cai, H.; Wang, P.; Zhang, D. pH-responsive linkages-enabled layer-by-layer assembled antibacterial and antiadhesive multilayer films with polyelectrolyte nanocapsules as biocide delivery vehicles. J. Drug Deliv. Sci. Technol. 2019, 54, 101251. [Google Scholar] [CrossRef]
- Cao, Z.; Mi, L.; Mendiola, J.; Ella-Menye, J.R.; Zhang, L.; Xue, H.; Jiang, S. Reversibly switching the function of a surface between attacking and defending against bacteria. Angew. Chem. Int. Ed. 2012, 51, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Yang, C.; Huang, Y.; Ding, X.; Li, Y.; Fan, W.M.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial and antifouling hydrogels formed in situ from polycarbonate and poly(ethylene glycol) via Michael addition. Adv. Mater. 2012, 24, 6484–6489. [Google Scholar] [CrossRef] [PubMed]
- Lalani, R.; Liu, L. Electrospun zwitterionic poly(sulfobetaine methacrylate) for nonadherent, superabsorbent, and antimicrobial wound dressing applications. Biomacromolecules 2012, 13, 1853–1863. [Google Scholar] [CrossRef]
- Wei, T.; Zhan, W.; Yu, Q.; Chen, H. Smart biointerface with photoswitched functions between bactericidal activity and bacteria-releasing ability. ACS Appl. Mater. Interfaces 2017, 9, 25767–25774. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cho, J.; Shivapooja, P.; Ista, L.K.; Lopez, G.P. Nanopatterned smart polymer surfaces for controlled attachment, killing, and release of bacteria. ACS Appl. Mater. Interfaces 2013, 5, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, Y.; Huang, L.; Zhang, Y.; Ren, B.; Zhong, M.; Yang, J.; Zheng, J. Integration of antifouling and antibacterial properties in salt-responsive hydrogels with surface regeneration capacity. J. Mater. Chem. B 2018, 6, 950–960. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, C.J. Functionalization of polydopamine via the aza-Michael reaction for antimicrobial interfaces. Langmuir 2016, 32, 5019–5028. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Li, L.; Cheng, G.; Gong, X.; Zheng, J. Dual functionality of antimicrobial and antifouling of poly (N-hydroxyethylacrylamide)/salicylate hydrogels. Langmuir 2013, 29, 1517–1524. [Google Scholar] [CrossRef]
- Xu, G.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M. Switchable antimicrobial and antifouling coatings from tannic acid-scaffolded binary polymer brushes. ACS Sustain. Chem. Eng. 2020, 8, 2586–2595. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L. Novel light-responsive hydrogels with antimicrobial and antifouling capabilities. Langmuir 2018, 35, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, X.; Hu, L.; Zhang, Y.; Jiang, Y.; Mao, Z.; Xu, H.; Wang, B.; Feng, X.; Sui, X. A naked-eye detection polyvinyl alcohol/cellulose-based pH sensor for intelligent packaging. Carbohydr. Polym. 2020, 233, 115859. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.; Shariatzadeh, F.J.; Singh, H.; Logsetty, S.; Liu, S. Highly sensitive bacteria-responsive membranes consisting of core–shell polyurethane polyvinylpyrrolidone electrospun nanofibers for in situ detection of bacterial infections. ACS Appl. Mater. Interfaces 2020, 12, 45859–45872. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Nejad, M.A.F.; Parolo, C.; Shahrokhian, S.; Merkoçi, A. Smart chip for visual detection of bacteria using the electrochromic properties of polyaniline. Anal. Chem. 2019, 91, 14960–14966. [Google Scholar] [CrossRef]
- Hakovirta, M.; Aksoy, B.; Hakovirta, J. Self-assembled micro-structured sensors for food safety in paper based food packaging. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 53, 331–335. [Google Scholar] [CrossRef]
- Suaifan, G.A.; Al Nobani, S.W.; Shehadeh, M.B.; Darwish, R.M. Engineered colorimetric detection of Staphylococcus aureus extracellular proteases. Talanta 2019, 198, 30–38. [Google Scholar] [CrossRef]
- Kaur, K.; Chelangat, W.; Druzhinin, S.I.; Karuri, N.W.; Müller, M.; Schönherr, H. Quantitative E. coli enzyme detection in reporter hydrogel-coated paper using a smartphone camera. Biosensors 2021, 11, 25. [Google Scholar] [CrossRef]
- Alkekhia, D.; Safford, H.; Shukla, S.; Hopson, R.; Shukla, A. β-Lactamase triggered visual detection of bacteria using cephalosporin functionalized biomaterials. Chem. Commun. 2020, 56, 11098–11101. [Google Scholar] [CrossRef]
- Huang, T.W.; Lu, H.T.; Ho, Y.C.; Lu, K.Y.; Wang, P.; Mi, F.L. A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111396. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Guo, L.; Wang, Y.; Feng, L. Intelligent Hybrid Hydrogels for Rapid in Situ Detection and Photothermal Therapy of Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 39685–39694. [Google Scholar] [CrossRef]
- Hibbard, H.A.; Reynolds, M.M. Fluorescent nitric oxide donor for the detection and killing of Pseudomonas aeruginosa. J. Mater. Chem. B 2019, 7, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, J.; Liang, Y.; Yang, H.; Li, Q.; Li, Q.; Liao, D.; Liu, Y.; Liu, H.B. Development of an intelligent photosensitive antibacterial wound dressing: Simultaneous detection and treatment of bacterial infection for accelerated wound healing. ChemNanoMat 2020, 6, 516–523. [Google Scholar] [CrossRef]
- Chien, H.-W.; Chen, X.-Y.; Wen-Pei, T. Poly (methyl methacrylate)/titanium dioxide (PMMA/TiO2) nanocomposite with shark-skin structure for preventing biofilm formation. Mater. Lett. 2021, 285, 129098. [Google Scholar] [CrossRef]
- Dundar, A.F.; Kolewe, K.W.; Homyak, B.; Kurtz, I.S.; Schiffman, J.D.; Watkins, J.J. Bioinspired photocatalytic shark-skin surfaces with antibacterial and antifouling activity via nanoimprint lithography. ACS Appl. Mater. Interfaces 2018, 10, 20055–20063. [Google Scholar] [CrossRef] [PubMed]
- Zada, I.; Zhang, W.; Li, Y.; Sun, P.; Cai, N.; Gu, J.; Liu, Q.; Su, H.; Zhang, D. Angle dependent antireflection property of TiO2 inspired by cicada wings. Appl. Phys. Lett. 2016, 109, 153701. [Google Scholar] [CrossRef]









| Simple Methods of Surface Antimicrobial Response Activation | |
| Stimuli | Mechanism of Actions |
| Temperature | Temperature-governed antimicrobial releasePhase transformation of grafted polymers and repellence of attached bacteria or biomolecules |
| Light | ROS productionLight-to heat conversion and bacteria-killing |
| Electric field | Reversible antimicrobial entrapping/releaseExternal control of surface morphology and wettability (including slippery and bio-repellency) |
| Magnetic field | Remote triggering of drug releaseSurface shape control—mechanical killing |
| Mechanical forces | Mechano-induced antimicrobial agent(s) local extrusion |
| More Sophisticated Approaches and Recent Trends | |
| Approach | Advantages |
| Double and multi-stimuli control | Better control of antimicrobial agents release and activation; simultaneous utilization of several surface protection mechanisms |
| Killing and release | Prevention of surface deactivation due to killed bacteria residue or their metabolic products |
| Detect and kill | Ability to apply external physical triggering in the right place at the right time |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elashnikov, R.; Ulbrich, P.; Vokatá, B.; Pavlíčková, V.S.; Švorčík, V.; Lyutakov, O.; Rimpelová, S. Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements. Nanomaterials 2021, 11, 3083. https://doi.org/10.3390/nano11113083
Elashnikov R, Ulbrich P, Vokatá B, Pavlíčková VS, Švorčík V, Lyutakov O, Rimpelová S. Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements. Nanomaterials. 2021; 11(11):3083. https://doi.org/10.3390/nano11113083
Chicago/Turabian StyleElashnikov, Roman, Pavel Ulbrich, Barbora Vokatá, Vladimíra Svobodová Pavlíčková, Václav Švorčík, Oleksiy Lyutakov, and Silvie Rimpelová. 2021. "Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements" Nanomaterials 11, no. 11: 3083. https://doi.org/10.3390/nano11113083
APA StyleElashnikov, R., Ulbrich, P., Vokatá, B., Pavlíčková, V. S., Švorčík, V., Lyutakov, O., & Rimpelová, S. (2021). Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements. Nanomaterials, 11(11), 3083. https://doi.org/10.3390/nano11113083







