Configuration of Multifunctional Polyimide/Graphene/Fe3O4 Hybrid Aerogel-Based Phase-Change Composite Films for Electromagnetic and Infrared Bi-Stealth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PI/Graphene/Fe3O4 Hybrid Aerogel Films
2.3. Preparation of PI/Graphene/Fe3O4 Aerogel/PEG Composite Films
2.4. Characterizations and Measurements
3. Results and Discussion
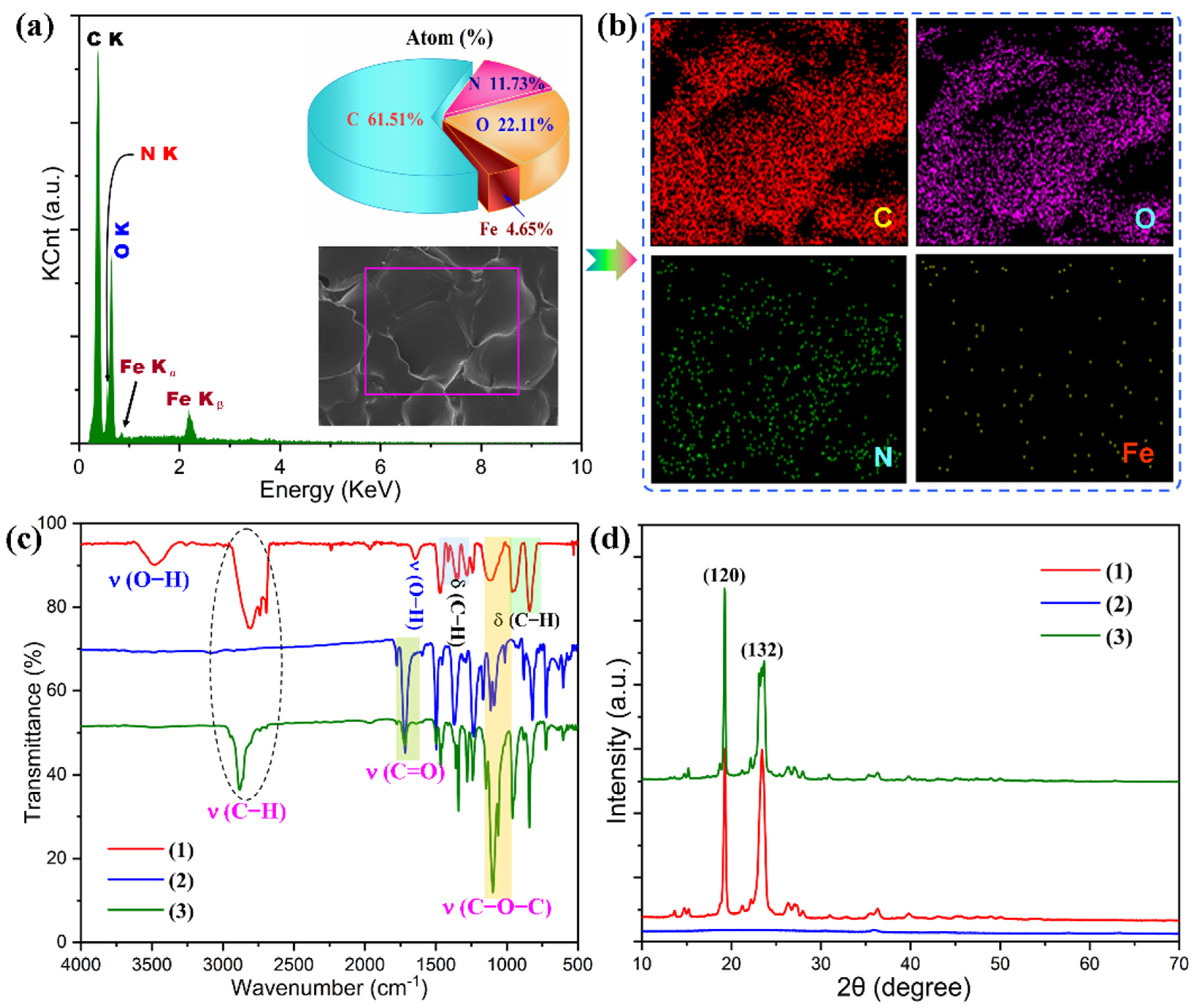
3.1. Structural Characterizations of Graphene/Fe3O4 Hybrid
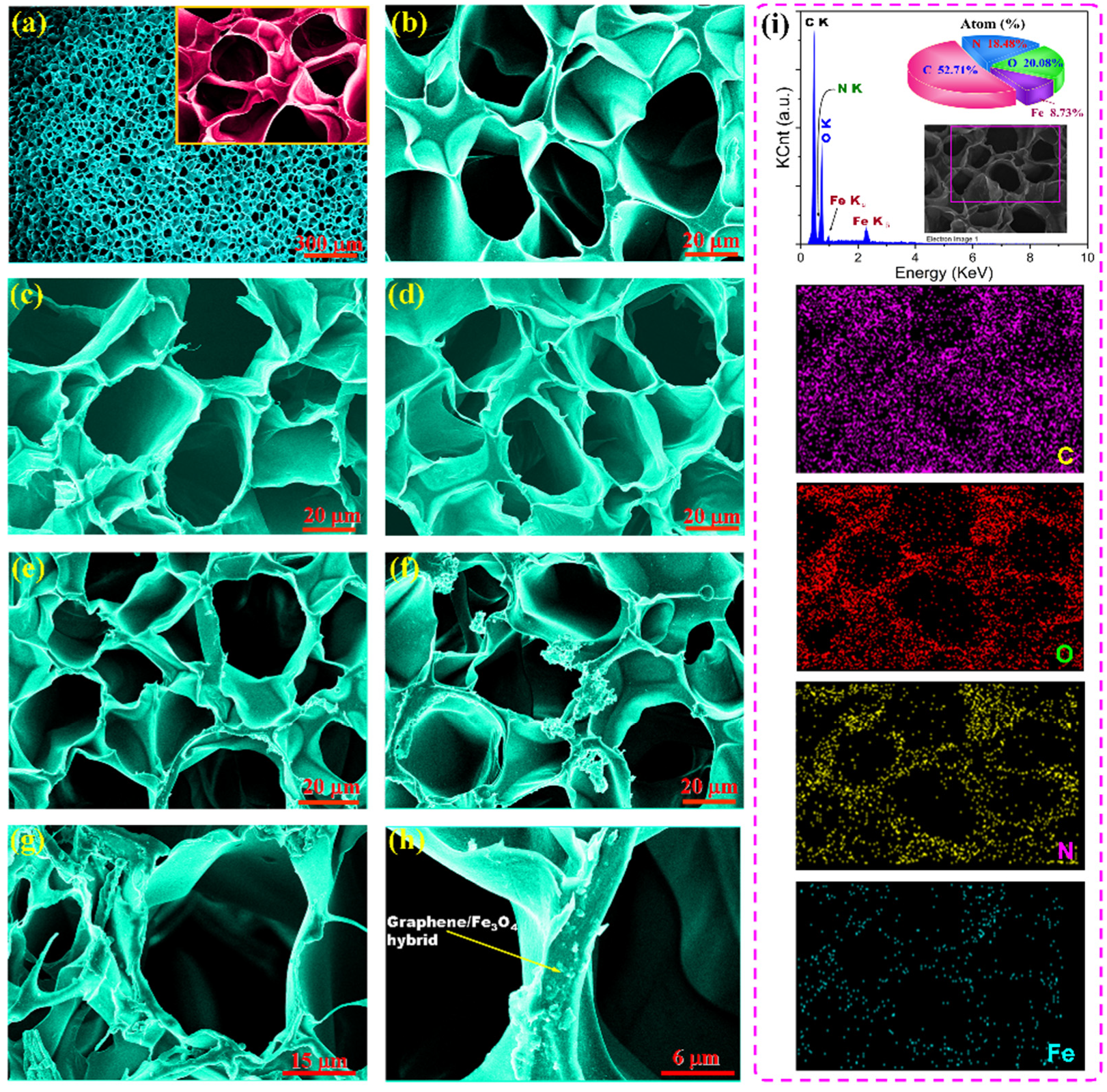
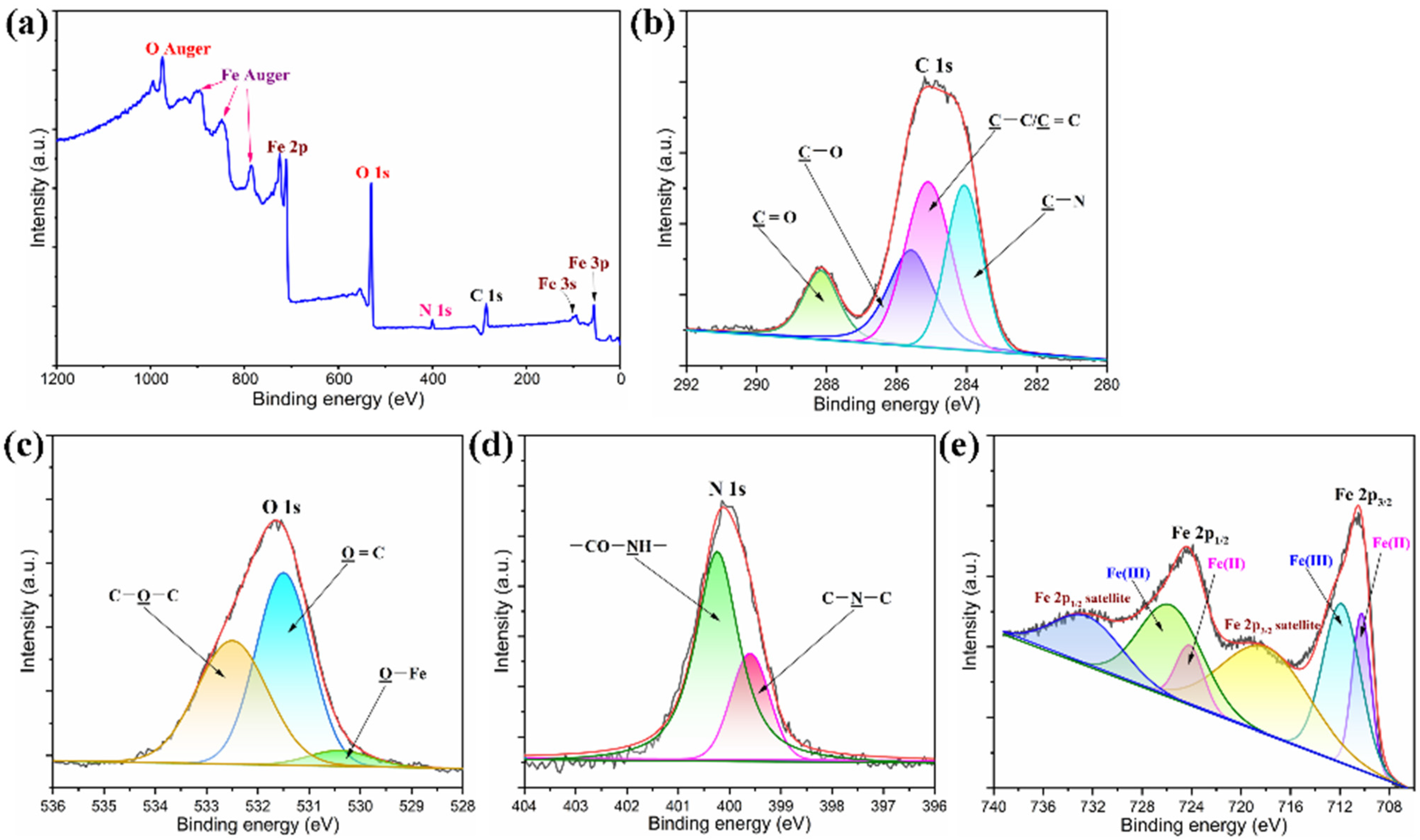
3.2. Microstructure and Chemical Characterizations of PI/Graphene/Fe3O4 Hybrid Aerogel Films
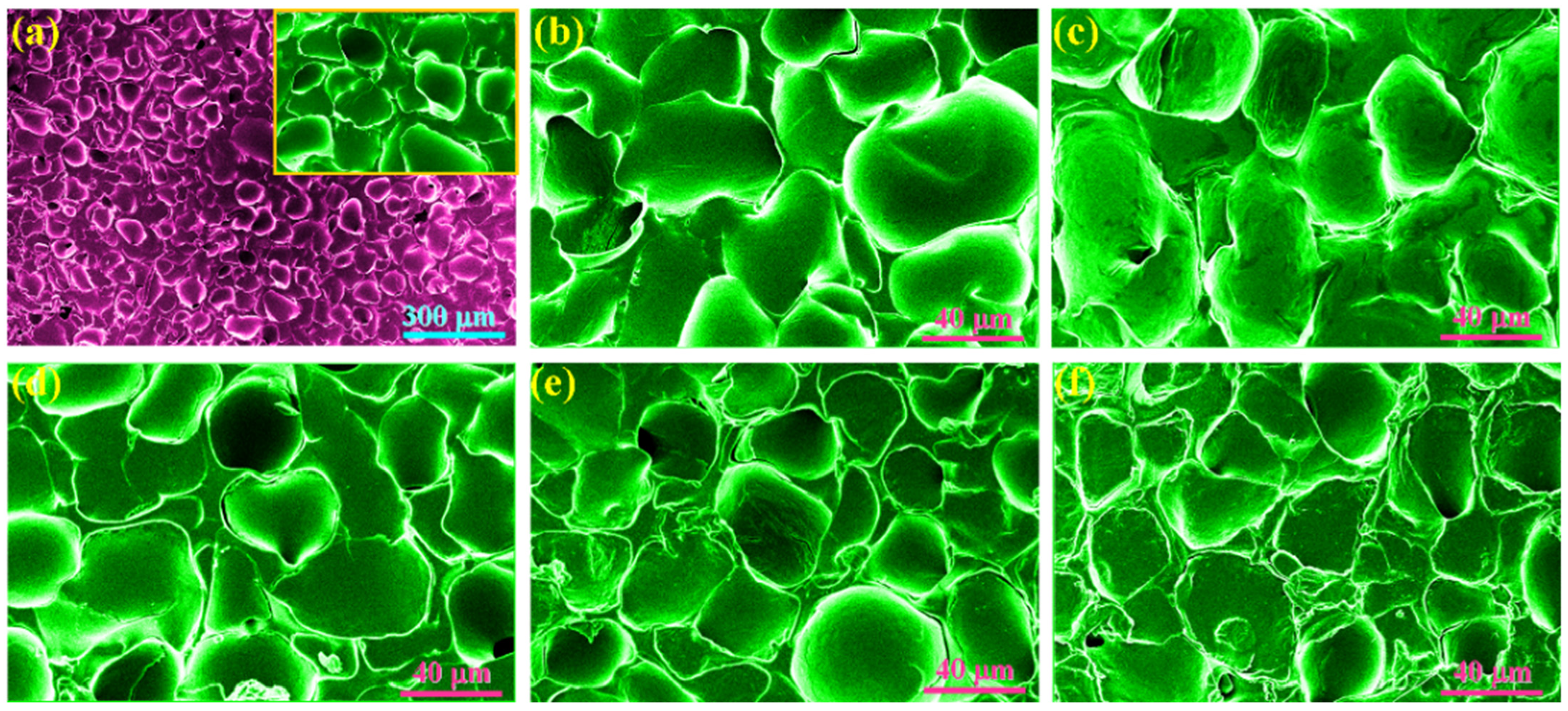
3.3. Morphology and Chemical Characterizations of PI/Graphene/Fe3O4 Aerogel/PEG Composite Films
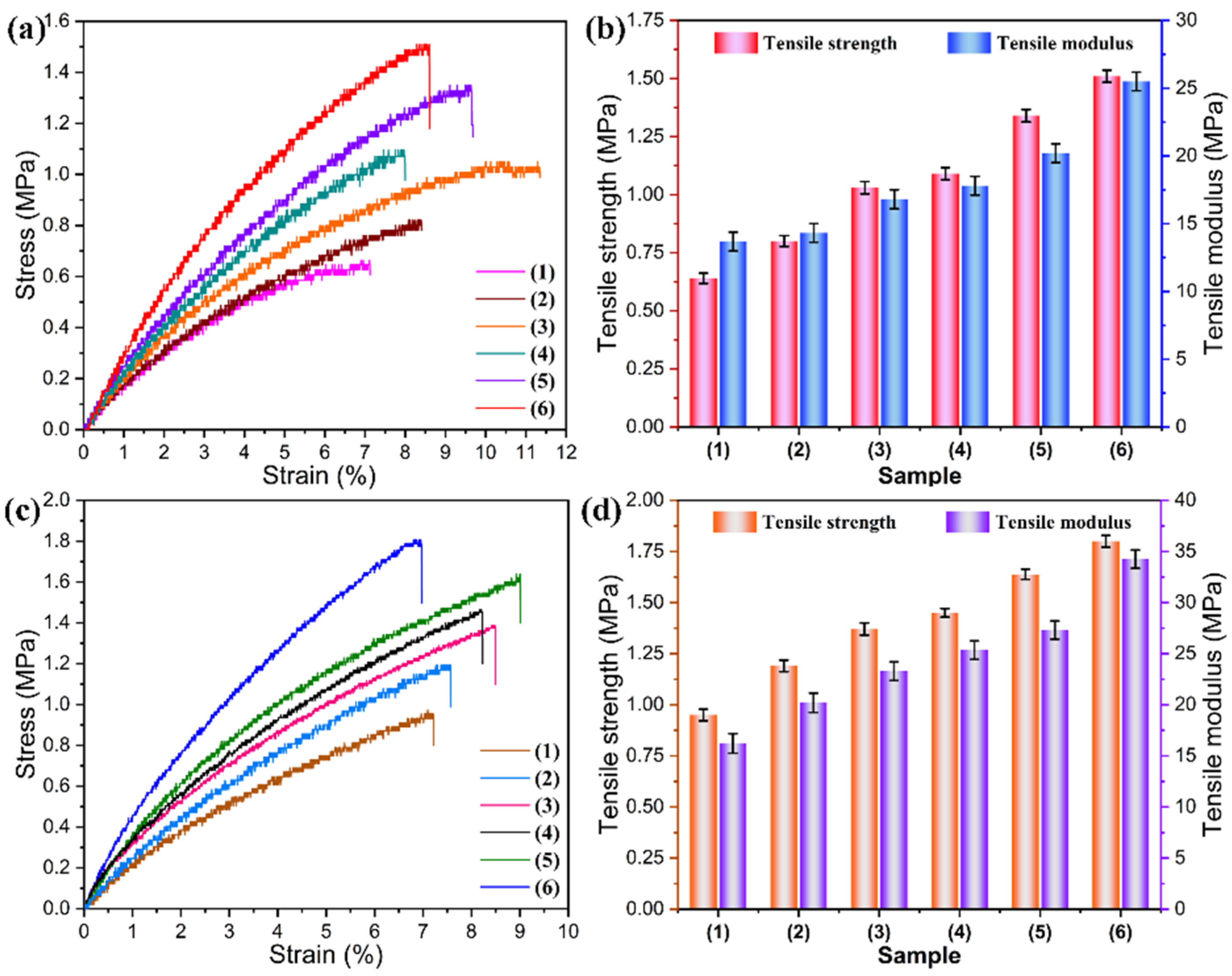
3.4. Mechanical Performance
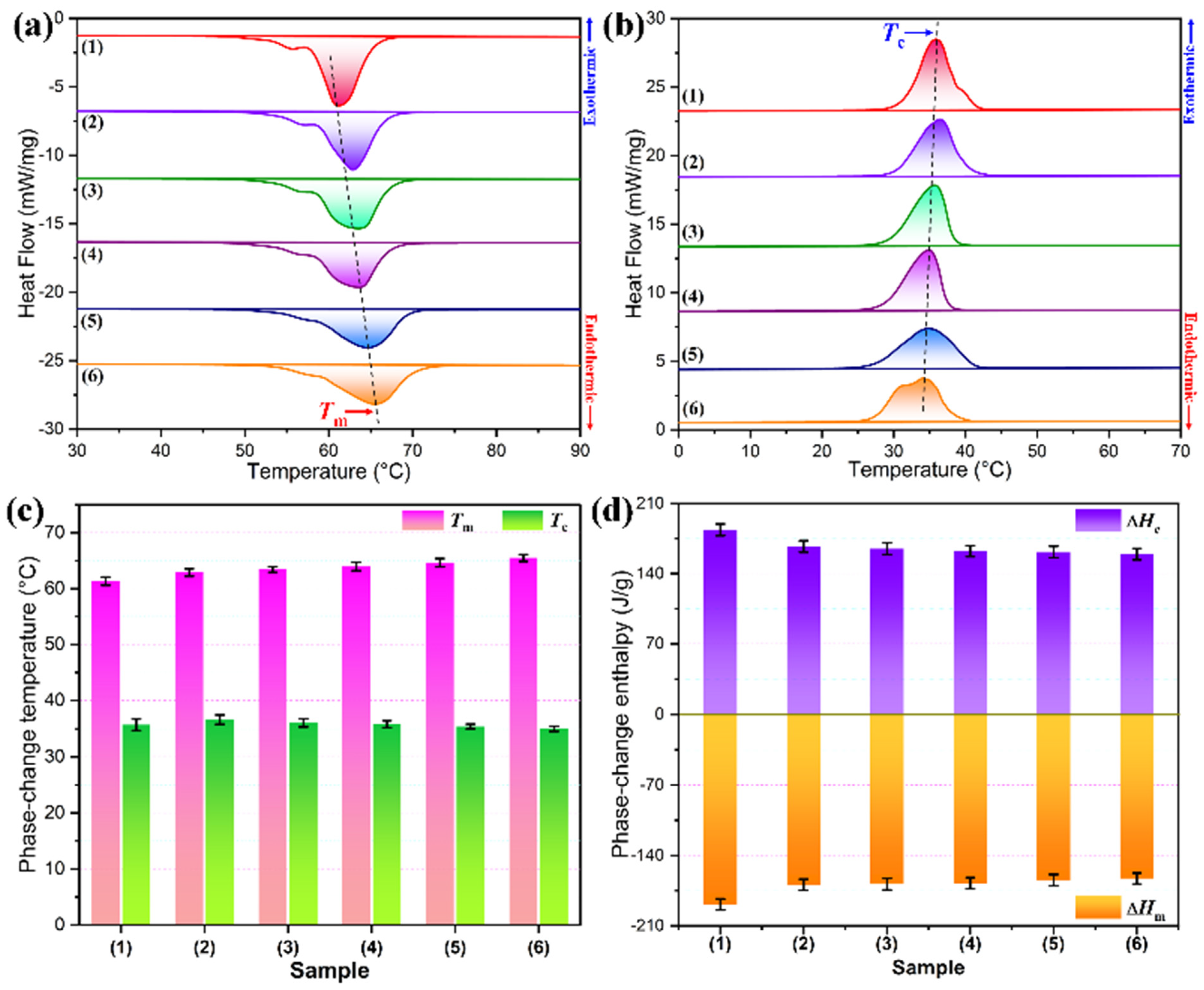
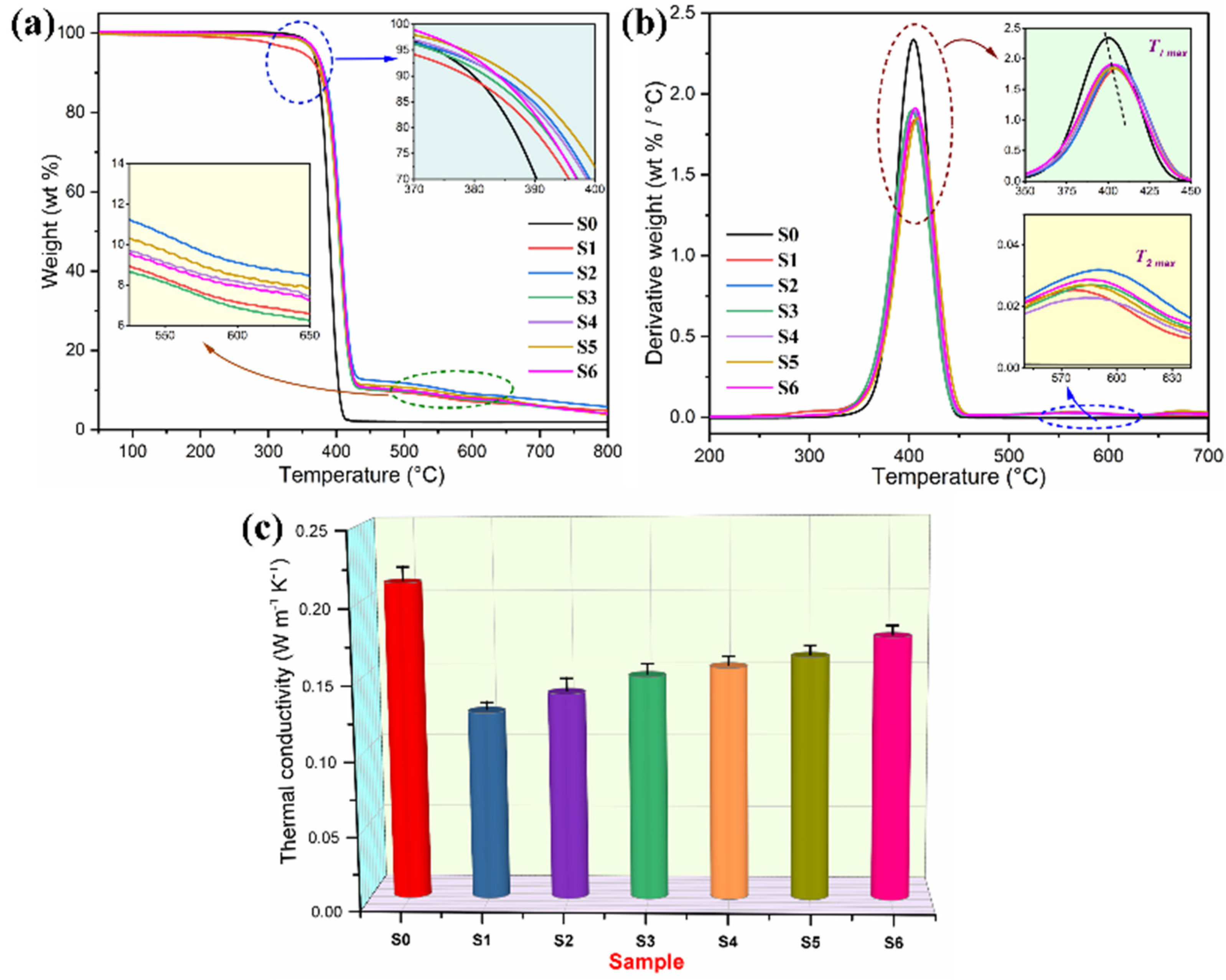
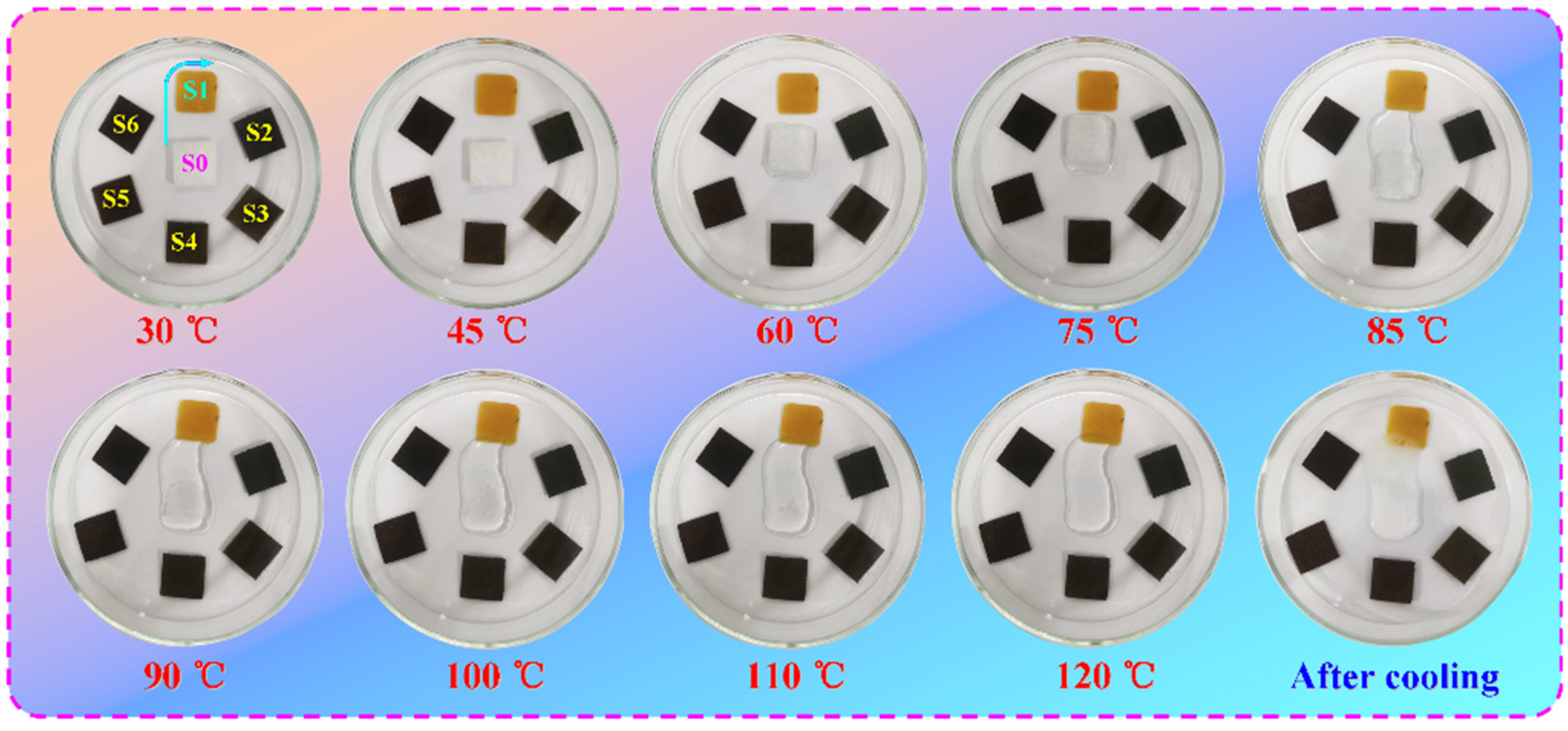
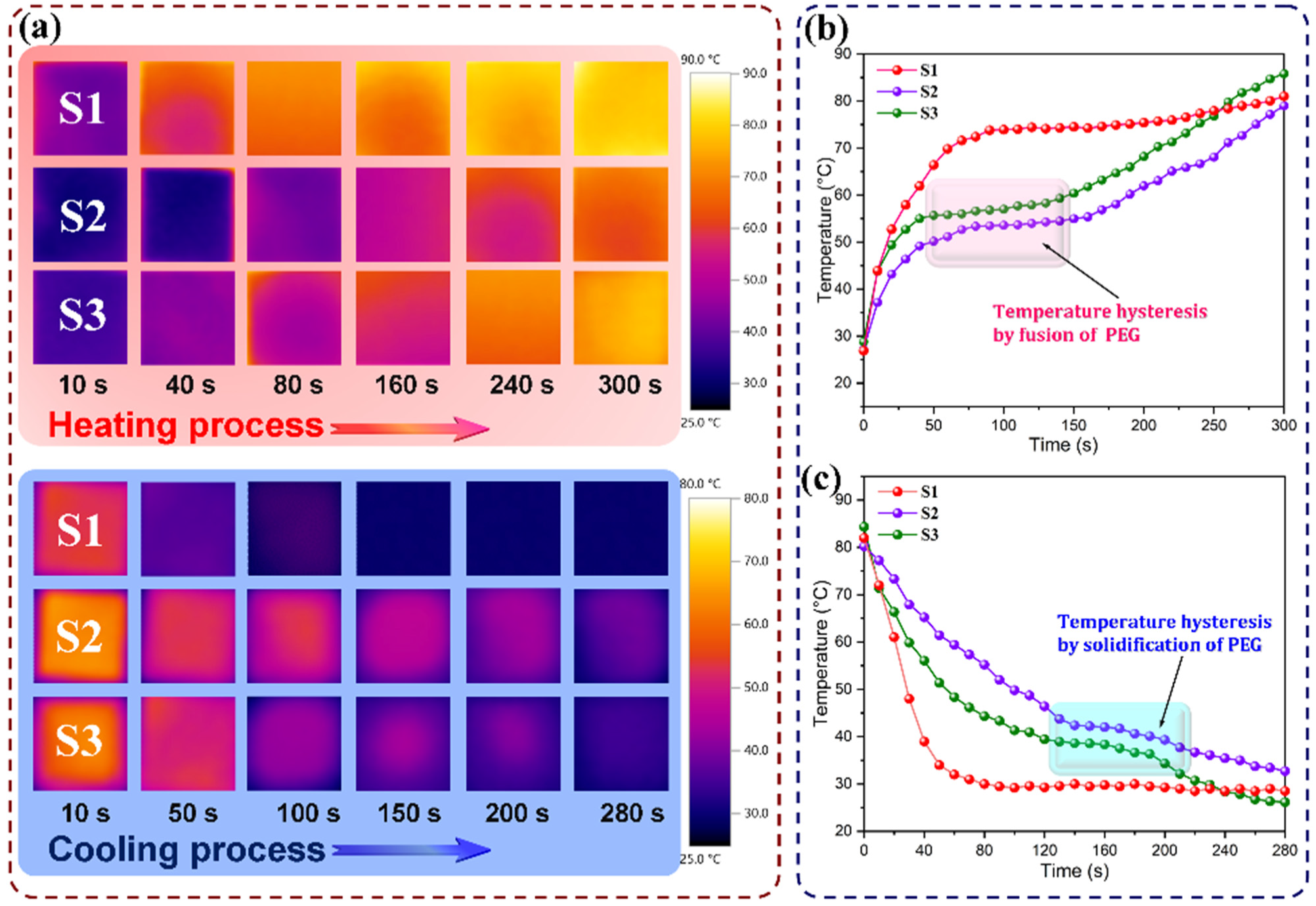
3.5. Phase-Change Behavior and Thermal Performance
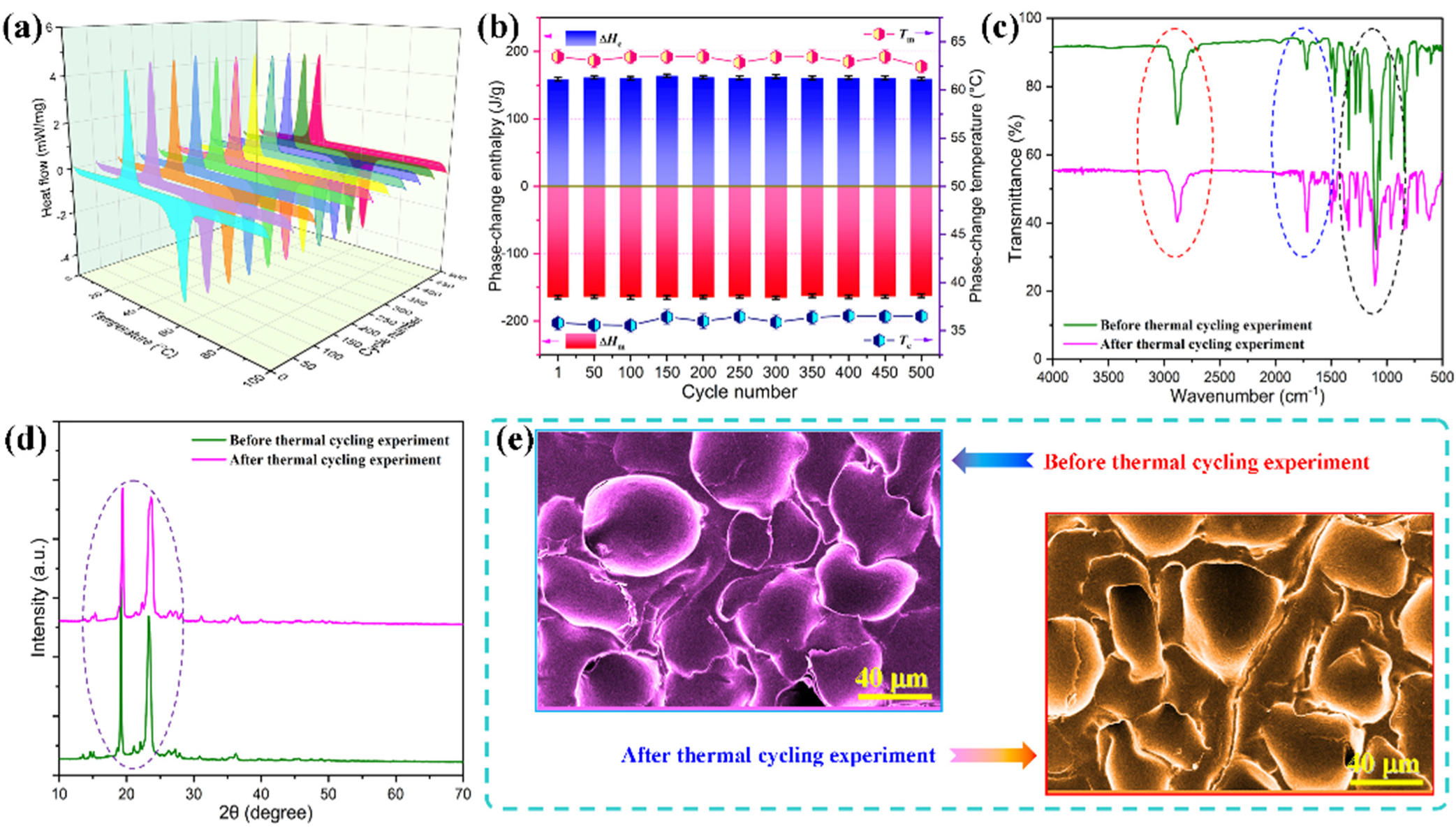
3.6. Thermal Stability and Heat-Impact Resistance
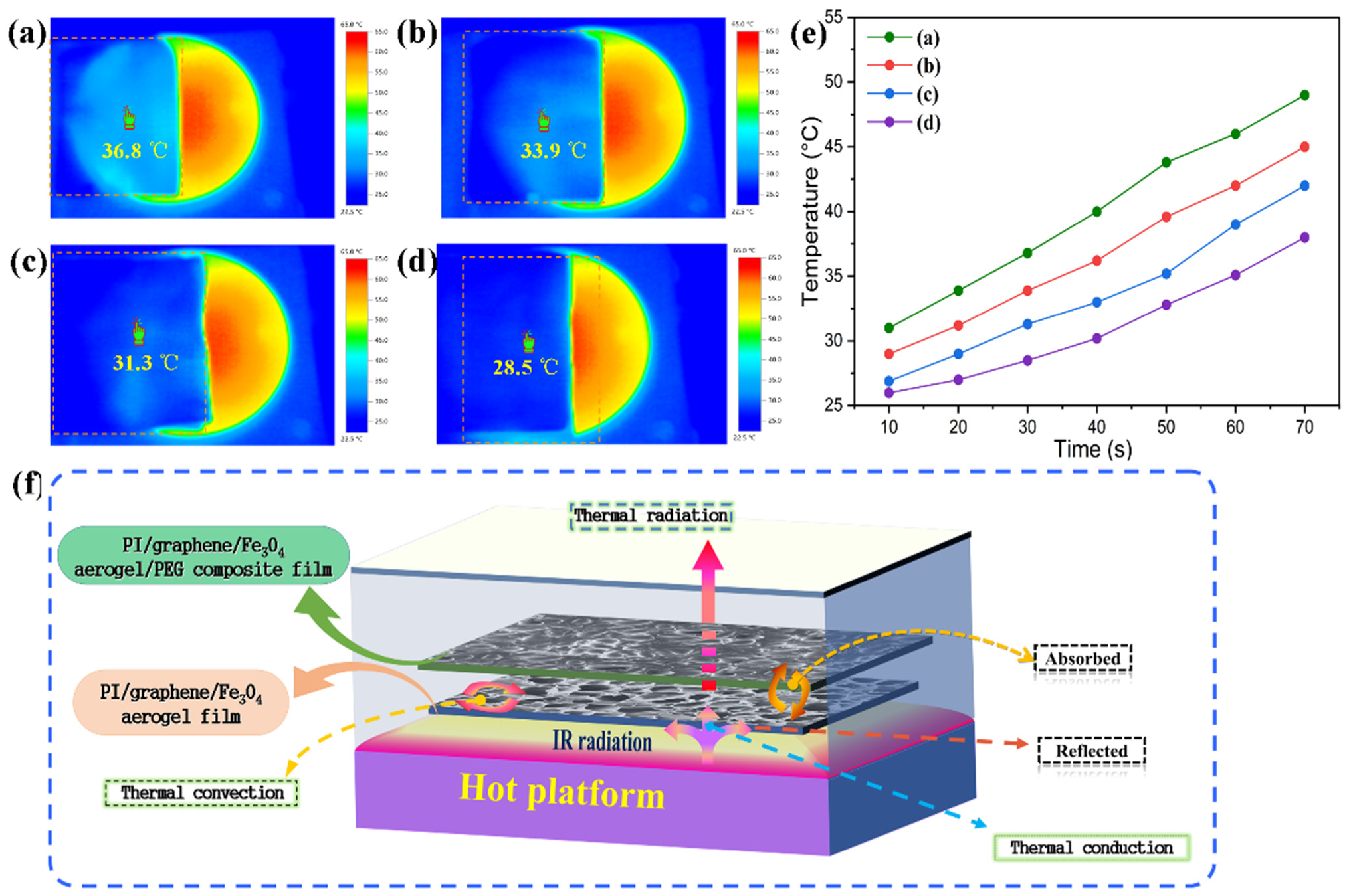
3.7. IR Stealth and Thermal Camouflage Behaviors
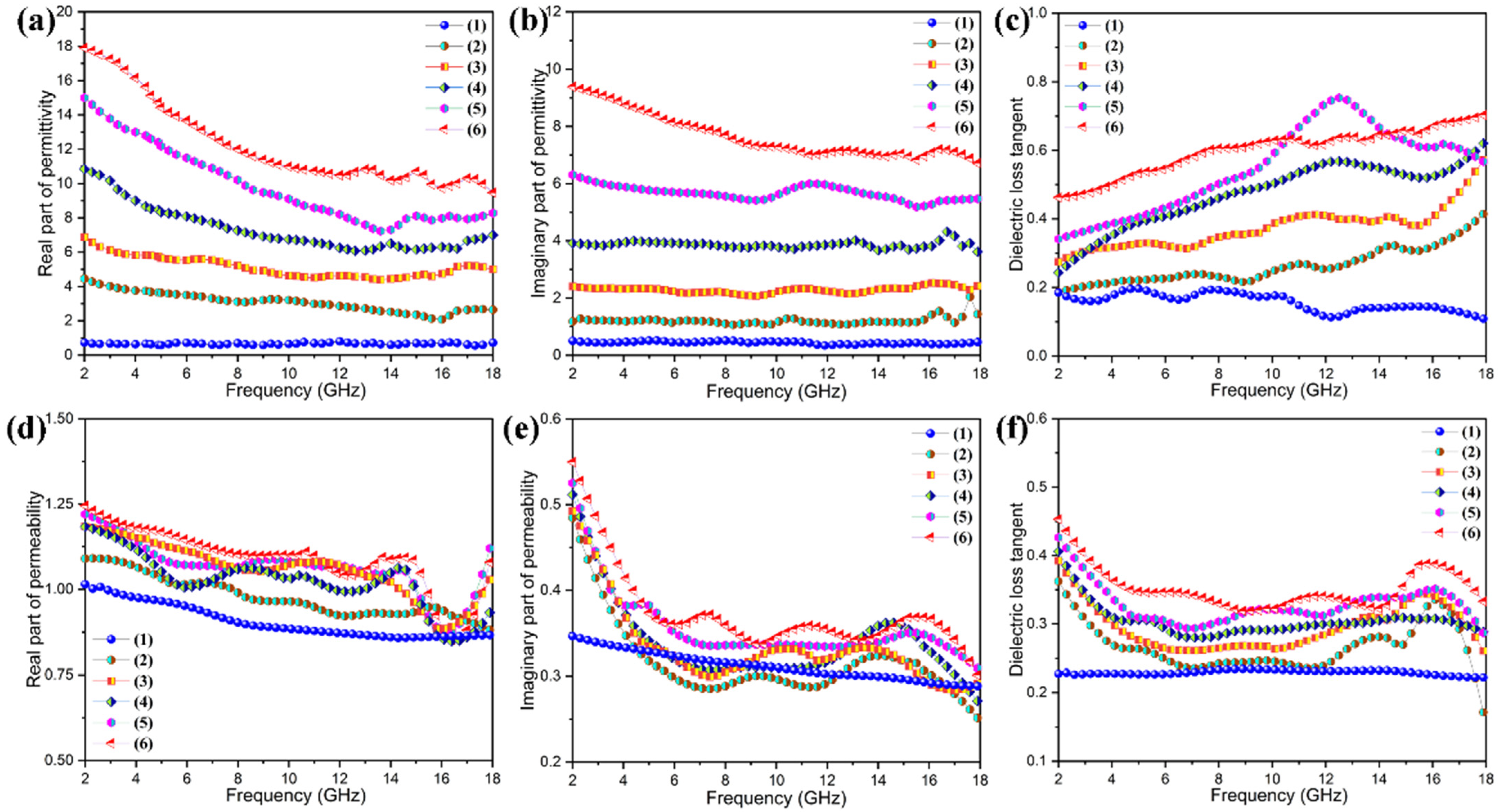
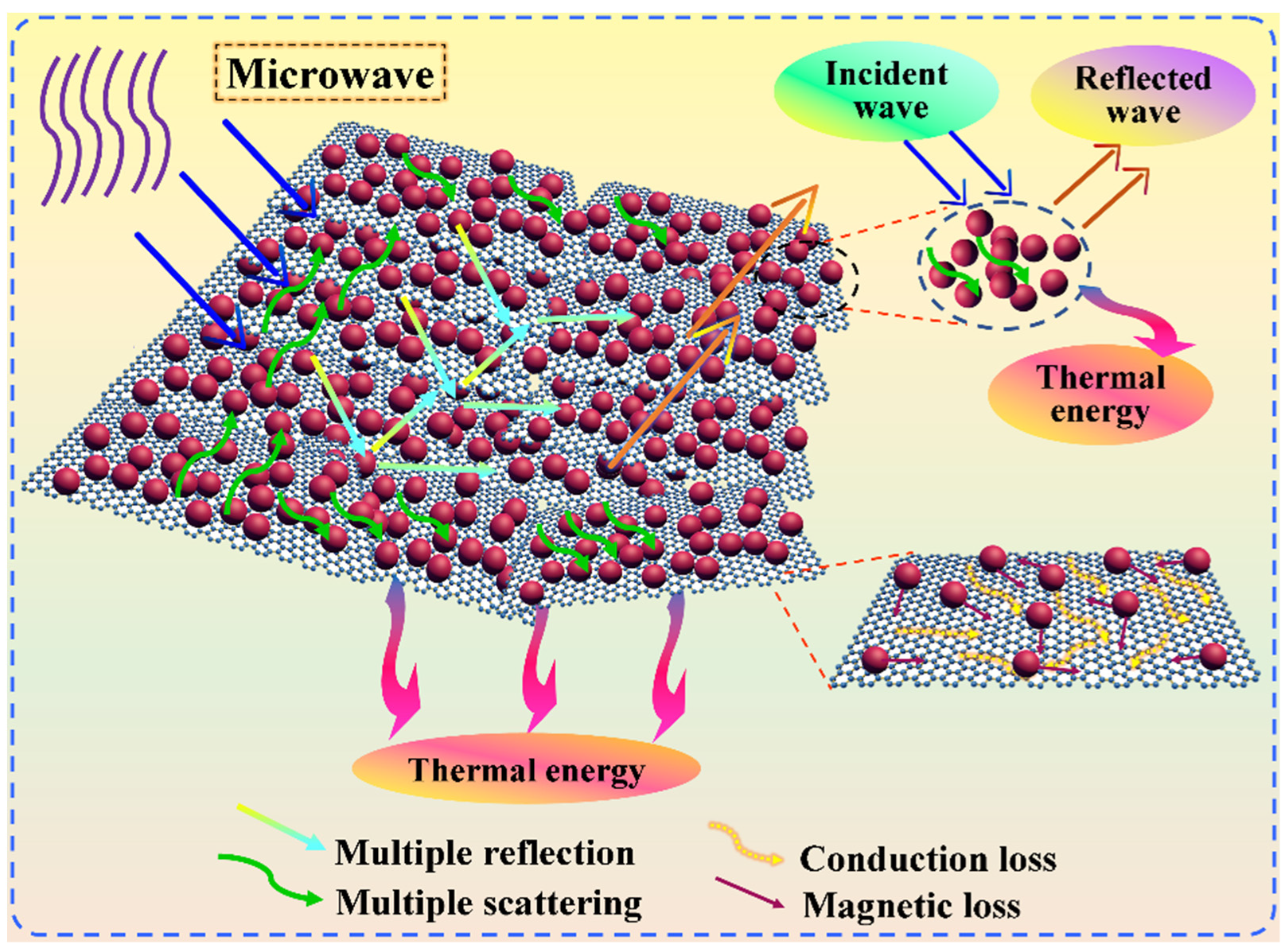
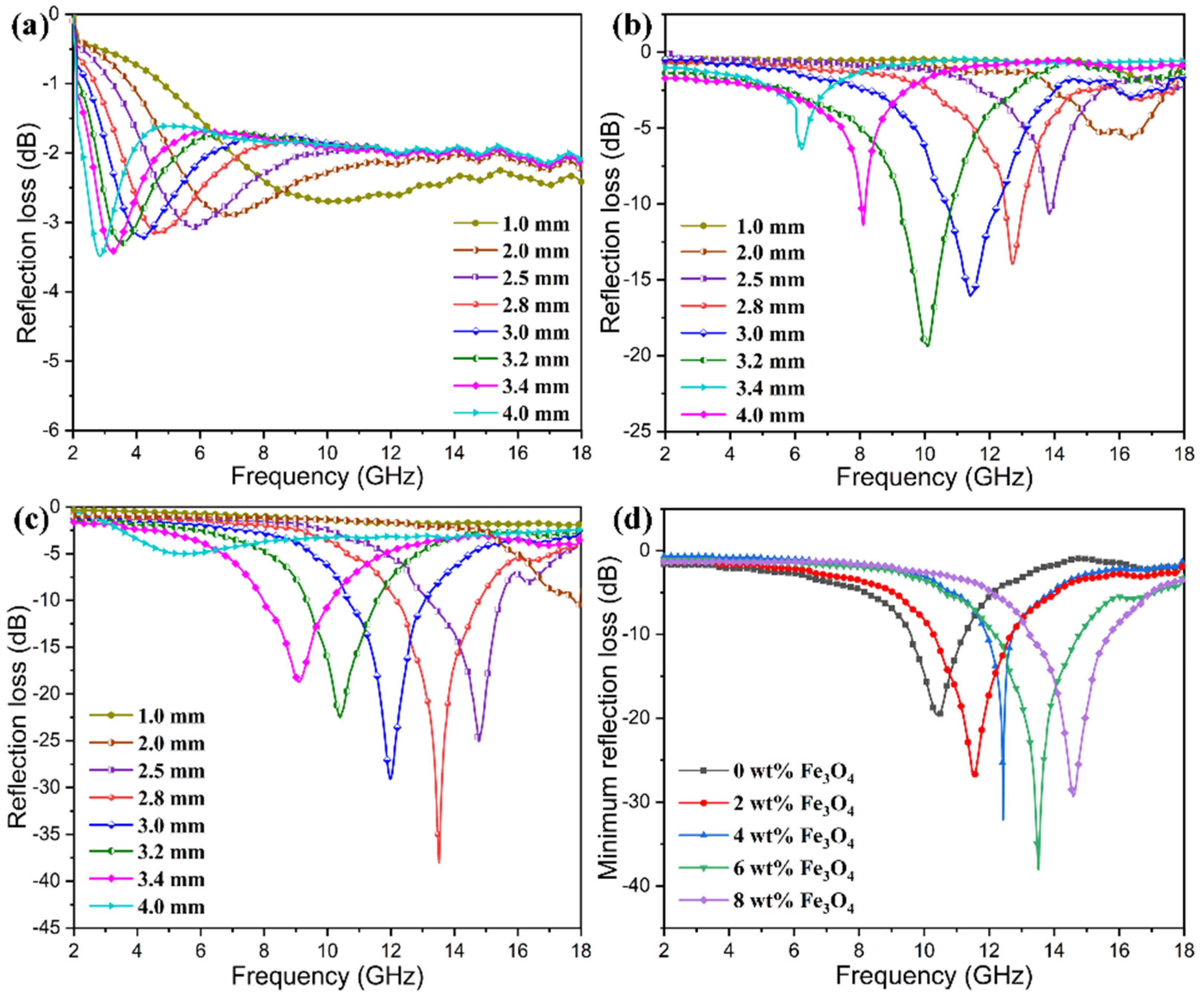
3.8. Microwave Absorption and EM Stealth Behaviors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Niu, S.; Chang, H.; Wang, Y.; Feng, Y.; Zhang, Y.; Li, G.; Chen, S.; Qu, Y.; Xiao, L. Thermal Infrared and Broadband Microwave Stealth Glass Windows Based on Multi-Band Optimization. Opt. Express 2021, 29, 13610–13623. [Google Scholar] [CrossRef]
- Baranwal, N.; Mahulikar, S.P. Infrared Signature of Aircraft Engine with Choked Converging Nozzle. J. Thermophys. Heat Tr. 2016, 30, 854–862. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Lv, H.; Shi, T.; Ji, G.; Hou, Y. Lightweight and Flexible Cotton Aerogel Composites for Electromagnetic Absorption and Shielding Applications. Adv. Electron. Mater. 2019, 6, 1900796. [Google Scholar] [CrossRef]
- Chen, H.; Ma, W.; Huang, Z.; Zhang, Y.; Huang, Y.; Chen, Y. Graphene-Based Materials toward Microwave and Terahertz Absorbing Stealth Technologies. Adv. Opt. Mater. 2019, 7, 1801318. [Google Scholar] [CrossRef]
- Savi, P.; Giorcelli, M.; Quaranta, S. Multi-Walled Carbon Nanotubes Composites for Microwave Absorbing Applications. Appl. Sci. 2019, 9, 851. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Tian, C.; Xu, C.; Wang, J.; Huang, S.; Li, X.; Yang, B.; Fan, Q.; Qu, S. Multi-Spectral Functional Metasurface Simultaneously with Visible Transparency, Low Infrared Emissivity and Wideband Microwave Absorption. Infrared Phys. Technol. 2020, 110, 103469. [Google Scholar] [CrossRef]
- Zhong, S.; Wu, L.; Liu, T.; Huang, J.; Jiang, W.; Ma, Y. Transparent Transmission-Selective Radar-Infrared Bi-Stealth Structure. Opt. Express 2018, 26, 16466–16476. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Xiong, Y.; Zhang, L.; Xu, W.; Duan, G.; Mei, C.; Jiang, S.; Rui, Z.; Zhang, K. Porous Aerogel and Sponge Composites: Assisted by Novel Nanomaterials for Electromagnetic Interference Shielding. Nano Today 2021, 38, 101204. [Google Scholar] [CrossRef]
- Fang, F.; Jing, W.; Yang, W. High Performance Electrospinning Fiberous Membranes for Infrared Stealth Camouflage. Infrared Phys. Techn. 2018, 93, 130–135. [Google Scholar] [CrossRef]
- Phan, L.; Walkup, W.G.T.; Ordinario, D.D.; Karshalev, E.; Jocson, J.M.; Burke, A.M.; Gorodetsky, A.A. Reconfigurable Infrared Camouflage Coatings from a Cephalopod Protein. Adv. Mater. 2013, 25, 5621–5625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Ren, Z.; Liu, X.; Wang, K.; Wang, Q. Infrared-Visible Compatible Stealth Based on Al-SiO2 Nanoparticle Composite Film. Opt. Commun. 2021, 482, 126608. [Google Scholar] [CrossRef]
- Chen, L.; Lu, C.; Fang, Z.; Lu, Y.; Ni, Y.; Xu, Z. Infrared Emissivity and Microwave Absorption Property of Sm0.5Sr0.5CoO3 Perovskites Decorated with Carbon Nanotubes. Mater. Lett. 2013, 93, 308–311. [Google Scholar] [CrossRef]
- Chu, H.; Zhang, Z.; Liu, Y.; Leng, J. Silver Particles Modified Carbon Nanotube Paper/Glassfiber Reinforced Polymer Composite Material for High Temperature Infrared Stealth Camouflage. Carbon 2016, 98, 557–566. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.; Huang, C.; Peng, J.; Ji, C.; Yang, J.; Huang, Y.; Guo, Y.; Luo, X. Flexible and Transparent Microwave-Infrared Bistealth Structure. Adv. Mater. Technol. 2019, 4, 1900063. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.; Pang, Y.; Wang, J.; Yan, M.; Wang, W.; Wang, A.; Jiang, J.; Qu, S. Hybrid Metasurfaces for Infrared-Multiband Radar Stealth-Compatible Materials Applications. IEEE Access 2019, 7, 147586–147595. [Google Scholar] [CrossRef]
- Gu, W.; Tan, J.; Chen, J.; Zhang, Z.; Zhao, Y.; Yu, J.; Ji, G. Multifunctional Bulk Hybrid Foam for Infrared Stealth, Thermal Insulation, and Microwave Absorption. ACS Appl. Mater. Interfaces 2020, 12, 28727–28737. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Chang, S.J.; Kim, K.-H.; Dong, W.; Kim, S. A Novel Enhancement of Shape/Thermal Stability and Energy-Storage Capacity of Phase Change Materials Through the Formation of Composites with 3D Porous (3,6)-Connected Metal-Organic Framework. Chem. Eng. J. 2020, 389, 124430. [Google Scholar] [CrossRef]
- Minea, A.A. State of the Art in PEG-Based Heat Transfer Fluids and Their Suspensions with Nanoparticles. Nanomaterials 2021, 11, 86. [Google Scholar] [CrossRef]
- Wang, W.; Cai, Y.; Du, M.; Hou, X.; Liu, J.; Ke, H.; Wei, Q. Ultralight and Flexible Carbon Foam-Based Phase Change Composites with High Latent-Heat Capacity and Photothermal Conversion Capability. ACS Appl. Mater. Interfaces 2019, 11, 31997–32007. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Zhang, J.; Li, Z.; Chen, Z.; Deng, X. Infrared Stealth Performance Analysis of Photonic Crystal with High Heat Dissipation. Opt. Mater. 2021, 111, 110689–110694. [Google Scholar] [CrossRef]
- Qu, Y.; Li, Q.; Cai, L.; Pan, M.; Ghosh, P.; Du, K.; Qiu, M. Thermal Camouflage Based on the Phase—Changing Material GST. Light Sci. Appl. 2018, 7, 26–36. [Google Scholar] [CrossRef]
- Shen, B.; Zhai, W.; Tao, M.; Ling, J.; Zheng, W. Lightweight, Multifunctional Polyetherimide/Graphene@Fe3O4 Composite Foams for Shielding of Electromagnetic Pollution. ACS Appl. Mater. Interfaces 2013, 5, 11383–11391. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, X.; Zheng, Y.; Zhang, D.; Zhao, Y.; Wang, C.; Pan, C.; Liu, C.; Shen, C. Lightweight, Superelastic, and Hydrophobic Polyimide Nanofiber /MXene Composite Aerogel for Wearable Piezoresistive Sensor and Oil/Water Separation Applications. Adv. Funct. Mater. 2021, 31, 2008006. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Zhang, Y.; Zhang, Y.; Liu, T. Lightweight, Strong, and Super-Thermal Insulating Polyimide Composite Aerogels Under High Temperature. Compos. Sci. Technol. 2019, 173, 47–52. [Google Scholar] [CrossRef]
- Manikandan, A.; Vijaya, J.J.; Mary, J.A.; Kennedy, L.J.; Dinesh, A. Structural, Optical and Magnetic Properties of Fe3O4 Nanoparticles Prepared by a Facile Microwave Combustion Method. J. Ind. Eng. Chem. 2014, 20, 2077–2085. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Wei, S.; Zhang, B.; Yu, M.; Zhao, W.; Liu, J. Fabrication of Porous Graphene-Fe3O4 Hybrid Composites with Outstanding Microwave Absorption Performance. Compos. Part A Appl. Sci. Manuf. 2017, 95, 237–247. [Google Scholar] [CrossRef]
- Giarola, M.; Mariotto, G.; Ajò, D. Micro-Raman Investigations on Inclusions of Unusual Habit in a Commercial Tanzanite Gemstone. J. Raman Spectrosc. 2012, 43, 556–558. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Wang, Z.; Wang, J.; Huang, T.; Dong, J.; Zhang, Q. Flexible PEDOT:PSS/Polyimide Aerogels with Linearly Responsive and Stable Properties for Piezoresistive Sensor Applications. Chem. Eng. J. 2020, 395, 125115. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Z.; Kang, X.; Cheng, C.; Mao, X.; Li, R.; Lin, H.; Zhang, H. Electro-Enhanced Heterogeneous Activation of Peroxymonosulfate via Acceleration of Fe(III)/Fe(II) Redox Cycle on Fe-B Catalyst. Electrochim. Acta 2021, 377, 138073. [Google Scholar] [CrossRef]
- Yang, H.; Li, Z.; Zou, H.; Liu, P. Preparation of Porous Polyimide/In-Situ reduced Graphene Oxide Composite Films for Electromagnetic Interference Shielding. Polym. Advan. Technol. 2017, 28, 233–242. [Google Scholar] [CrossRef]
- Lourenco, M.J.; Alexandre, J.; Huisman, C.; Paredes, X.; de Castro, C.N. The Balance between Energy, Environmental Security, and Technical Performance: The Regulatory Challenge of Nanofluids. Nanomaterials 2021, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel Strategies and Supporting Materials Applied to Shape-Stabilize Organic Phase Change Materials for Thermal Energy Storage—A Review. Appl. Energy. 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Li, B.; Shu, D.; Wang, R.; Zhai, L.; Chai, Y.; Lan, Y.; Cao, H.; Zou, C. Polyethylene Glycol/Silica (PEG@SiO2) Composite Inspired by the Synthesis of Mesoporous Materials as Shape-Stabilized Phase Change Material for Energy Storage. Renew. Energ. 2020, 145, 84–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Sheng, D.; Liu, X.; Lin, C.; Ji, F.; Dong, L.; Xu, S.; Yang, Y. Synthesis and Properties of Crosslinking Halloysite Nanotubes/Polyurethane-Based Solid-Solid Phase Change Materials. Sol. Energy Mater. Sol. Cells 2018, 174, 84–93. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Liu, Y.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Hybrid Graphene Aerogels/Phase Change Material Composites: Thermal Conductivity, Shape-Stabilization and Light-to-Thermal Energy Storage. Carbon 2016, 100, 693–702. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Liaghat, G.; Vahid, S.; Spacie, C.; Paton, K.R.; Sainsbury, T. Improving the Fracture Toughness Properties of Epoxy Using Graphene Nanoplatelets at Low Filler Content. Nanocomposites 2017, 3, 85–96. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, T.; Zhang, Q.; Zhu, Y.; Zhang, H.; Xu, F.; Sun, L.; Wang, X.; Xia, Y.; Lin, X. Biomass Homogeneity Reinforced Carbon Aerogels Derived Functional Phase-Change Materials for Solar-Thermal Energy Conversion and Storage. Energy Environ. Mater. 2021. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Hamze, S.; Fal, J.; Marcos, M.A.; Estelle, P.; Zyla, G. Thermal and Physical Characterization of PEG Phase Change Materials Enhanced by Carbon-Based Nanoparticles. Nanomaterials 2020, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, W.; Wang, Z.; Peng, K.; Pan, W.; Xie, Q. Novel Form Stable Phase Change Materials Based on the Composites of Polyethylene Glycol/Polymeric Solid—Solid Phase Change Material. Sol. Energy Mater. Sol. Cells 2015, 134, 80–88. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Modified Phase Change Microcapsules with Calcium Carbonate and Graphene Oxide Shells for Enhanced Energy Storage and Leakage Prevention. ACS Sustain. Chem. Eng. 2018, 6, 5182–5191. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Q.; Ji, R.; Zhang, H.; Xu, F.; Huang, P.; Zou, Y.; Chu, H.; Lin, X.; Sun, L. Multielement Synergetic Effect of Boron Nitride and Multiwalled Carbon Nanotubes for the Fabrication of Novel Shape-Stabilized Phase-Change Composites with Enhanced Thermal Conductivity. ACS Appl. Mater. Interfaces 2020, 12, 41398–41409. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C. Preparation and Characterization of GO/PEG Photo-Thermal Conversion Form-Stable Composite Phase Change Materials. Renew. Energ. 2019, 141, 1005–1012. [Google Scholar] [CrossRef]
- Bu, H.; Xu, G.; Liu, C.; Guo, T.; Tan, S. The Self–Regulated Infrared Emissivity Properties of BCG/MgCl2/PEG-g-CDA Powders for Tunable IR Signals. Infrared Phys. Technol. 2018, 94, 280–285. [Google Scholar] [CrossRef]
- Gu, W.; Wang, G.; Zhou, M.; Zhang, T.; Ji, G. Polyimide-Based Foams: Fabrication and Multifunctional Applications. ACS Appl. Mater. Interfaces. 2020, 12, 48246–48258. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Zhai, Y.; Wu, L.; Zhang, Y. Magnetic Aligned Fe3O4-Reduced Graphene Oxide/Waterborne Polyurethane Composites with Controllable Structure for High Microwave Absorption Capacity. Carbon 2019, 152, 661–670. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhang, T.; Chang, H.; Xiao, P.; Chen, H.; Huang, Z.; Chen, Y. Broadband and Tunable High-Performance Microwave Absorption of an Ultralight and Highly Compressible Graphene Foam. Adv. Mater. 2015, 27, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, X.; Li, X.; Yang, B.; Yu, R. Hydrothermal Synthesis of Magnetic Fe3O4/Graphene Composites with Good Electromagnetic Microwave Absorbing Performances. J. Magn. Magn. Mater. 2017, 426, 114–120. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Li, Y.; Li, Z.; Zhao, H.; Ji, G.; Du, Y. The Outside-In Approach to Construct Fe3O4 Nanocrystals/Mesoporous Carbon Hollow Spheres Core-Shell Hybrids toward Microwave Absorption. ACS Sustain. Chem. Eng. 2018, 6, 1427–1435. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Y.; Wang, W.; Yu, D. Robust Magnetic and Electromagnetic Wave Absorption Performance of Reduced Graphene Oxide Loaded Magnetic Metal Nanoparticle Composites. Adv. Powder Technol. 2021, 32, 194–203. [Google Scholar] [CrossRef]
- Zhan, Y.; Meng, F.; Lei, Y.; Zhao, R.; Zhong, J.; Liu, X. One-Pot Solvothermal Synthesis of Sandwich-Like Graphene Nanosheets/Fe3O4 Hybrid Material and Its Microwave Electromagnetic Properties. Mater. Lett. 2011, 65, 1737–1740. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z. Eggplant-Derived SiC Aerogels with High-Performance Electromagnetic Wave Absorption and Thermal Insulation Properties. Chem. Eng. J. 2019, 373, 598–605. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Z.; Jiang, W. Size-Controllable Synthesis of Fe3O4 Nanospheres Decorated Graphene for Electromagnetic Wave Absorber. J. Mater. Sci. Mater. Electron. 2016, 27, 6010–6019. [Google Scholar] [CrossRef]
- Zong, M.; Huang, Y.; Zhao, Y.; Sun, X.; Qu, C.; Luo, D.; Zheng, J. Facile Preparation, High Microwave Absorption and Microwave Absorbing Mechanism of RGO-Fe3O4 Composites. RSC Adv. 2013, 3, 23638–23648. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Nie, X.; Yang, W.; Wang, Y.; Yu, R.; Shui, J. Multifunctional Organic-Inorganic Hybrid Aerogel for Self-Cleaning, Heat-Insulating, and Highly Efficient Microwave Absorbing Material. Adv. Funct. Mater. 2019, 29, 1807624. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Y.; Zong, M.; Ding, X.; Li, S.; Wang, M. Coupling CoFe2O4 and SnS2 Nanoparticles with Reduced Graphene Oxide as a High-Performance Electromagnetic Wave Absorber. Ceram. Int. 2016, 42, 15701–15708. [Google Scholar] [CrossRef]
- Fang, J.; Liu, T.; Chen, Z.; Wang, Y.; Wei, W.; Yue, X.; Jiang, Z. A Wormhole-Like Porous Carbon/Magnetic Particles Composite as an Efficient Broadband Electromagnetic Wave Absorber. Nanoscale 2016, 8, 8899–8909. [Google Scholar] [CrossRef] [PubMed]




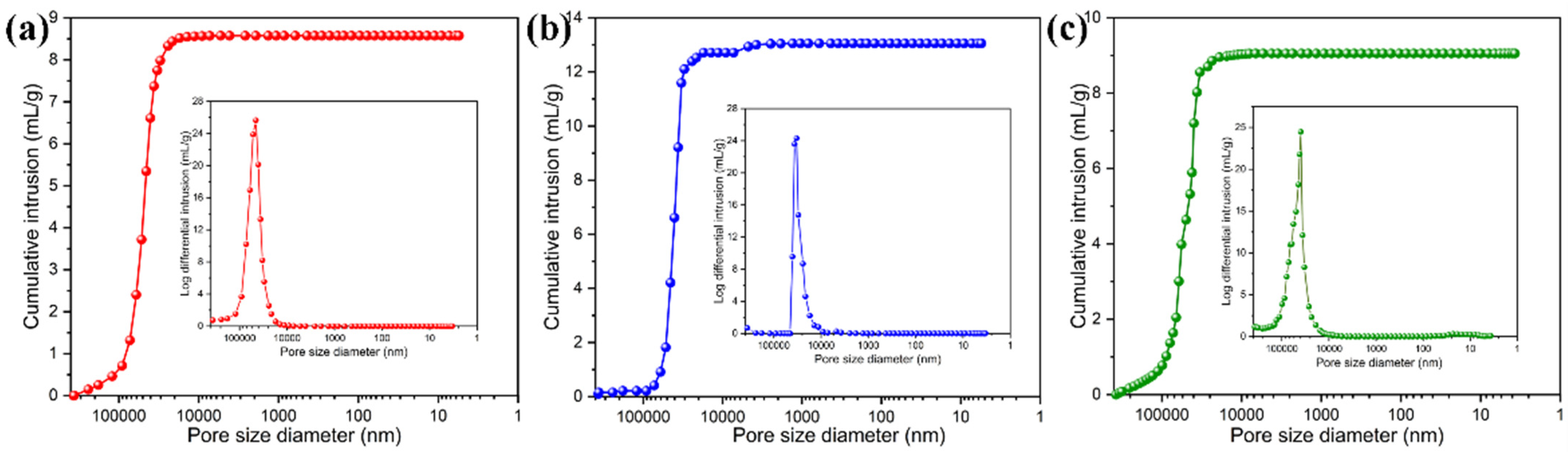
| PI/Graphene/Fe3O4 Hybrid Aerogel Films | Average Pore Diameter (μm) | Specific Surface Area (m2/g) | Total Immersion Volume (mL/g) | Porosity (%) |
|---|---|---|---|---|
| Pure PI | 36.37 | 1.14 | 8.77 | 89.24 |
| PI/graphene/Fe3O4 (0 wt%) | 34.21 | 1.28 | 12.92 | 90.45 |
| PI/graphene/Fe3O4 (2 wt%) | 33.48 | 1.71 | 9.64 | 88.71 |
| PI/graphene/Fe3O4 (4 wt%) | 33.05 | 2.42 | 10.81 | 90.40 |
| PI/graphene/Fe3O4 (6 wt%) | 31.82 | 3.24 | 9.44 | 89.48 |
| PI/graphene/Fe3O4 (8 wt%) | 29.02 | 5.43 | 8.11 | 91.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Zheng, Z.; Liu, H.; Wu, D.; Wang, X. Configuration of Multifunctional Polyimide/Graphene/Fe3O4 Hybrid Aerogel-Based Phase-Change Composite Films for Electromagnetic and Infrared Bi-Stealth. Nanomaterials 2021, 11, 3038. https://doi.org/10.3390/nano11113038
Shi T, Zheng Z, Liu H, Wu D, Wang X. Configuration of Multifunctional Polyimide/Graphene/Fe3O4 Hybrid Aerogel-Based Phase-Change Composite Films for Electromagnetic and Infrared Bi-Stealth. Nanomaterials. 2021; 11(11):3038. https://doi.org/10.3390/nano11113038
Chicago/Turabian StyleShi, Tao, Zhiheng Zheng, Huan Liu, Dezhen Wu, and Xiaodong Wang. 2021. "Configuration of Multifunctional Polyimide/Graphene/Fe3O4 Hybrid Aerogel-Based Phase-Change Composite Films for Electromagnetic and Infrared Bi-Stealth" Nanomaterials 11, no. 11: 3038. https://doi.org/10.3390/nano11113038
APA StyleShi, T., Zheng, Z., Liu, H., Wu, D., & Wang, X. (2021). Configuration of Multifunctional Polyimide/Graphene/Fe3O4 Hybrid Aerogel-Based Phase-Change Composite Films for Electromagnetic and Infrared Bi-Stealth. Nanomaterials, 11(11), 3038. https://doi.org/10.3390/nano11113038









