Thermal and Rheological Properties of Hydrophobic Nanosilica in Sunflower Oil Suspensions at High Pressures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Analysis
2.3. Calorimetric Analysis
2.4. Rheological Characterization
2.5. Aging Procedure
3. Results and Discussion
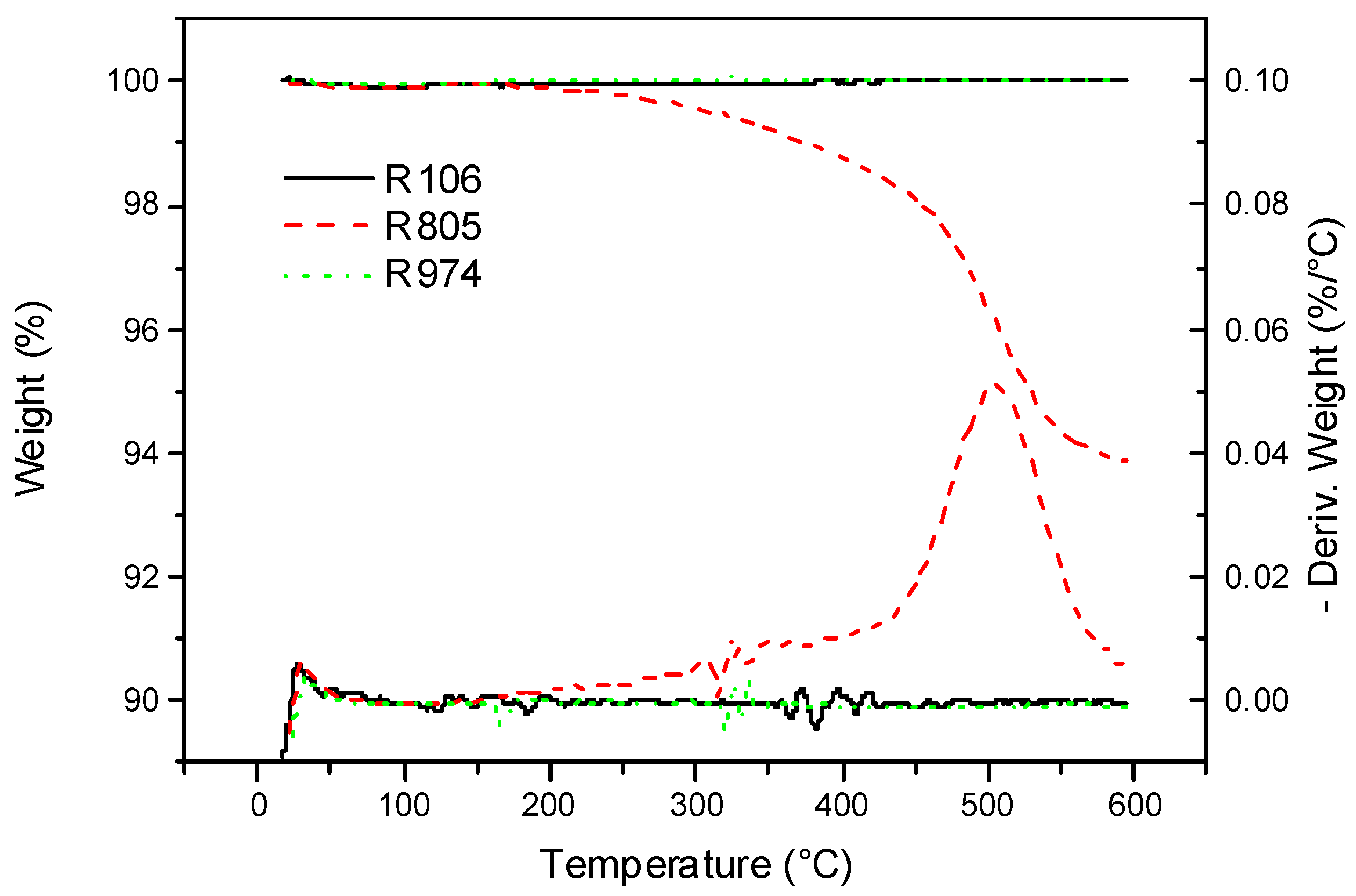
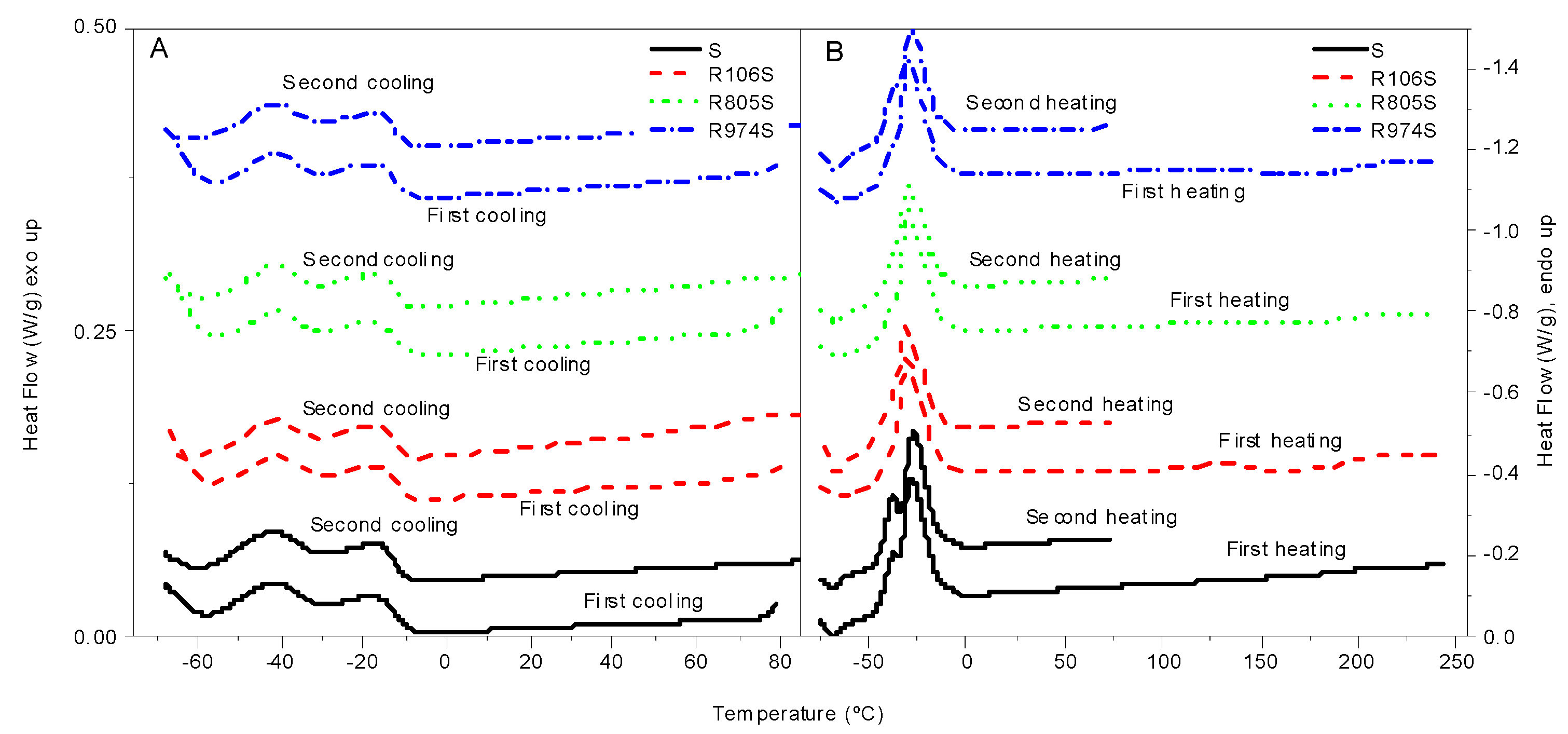
3.1. Thermal Characterization
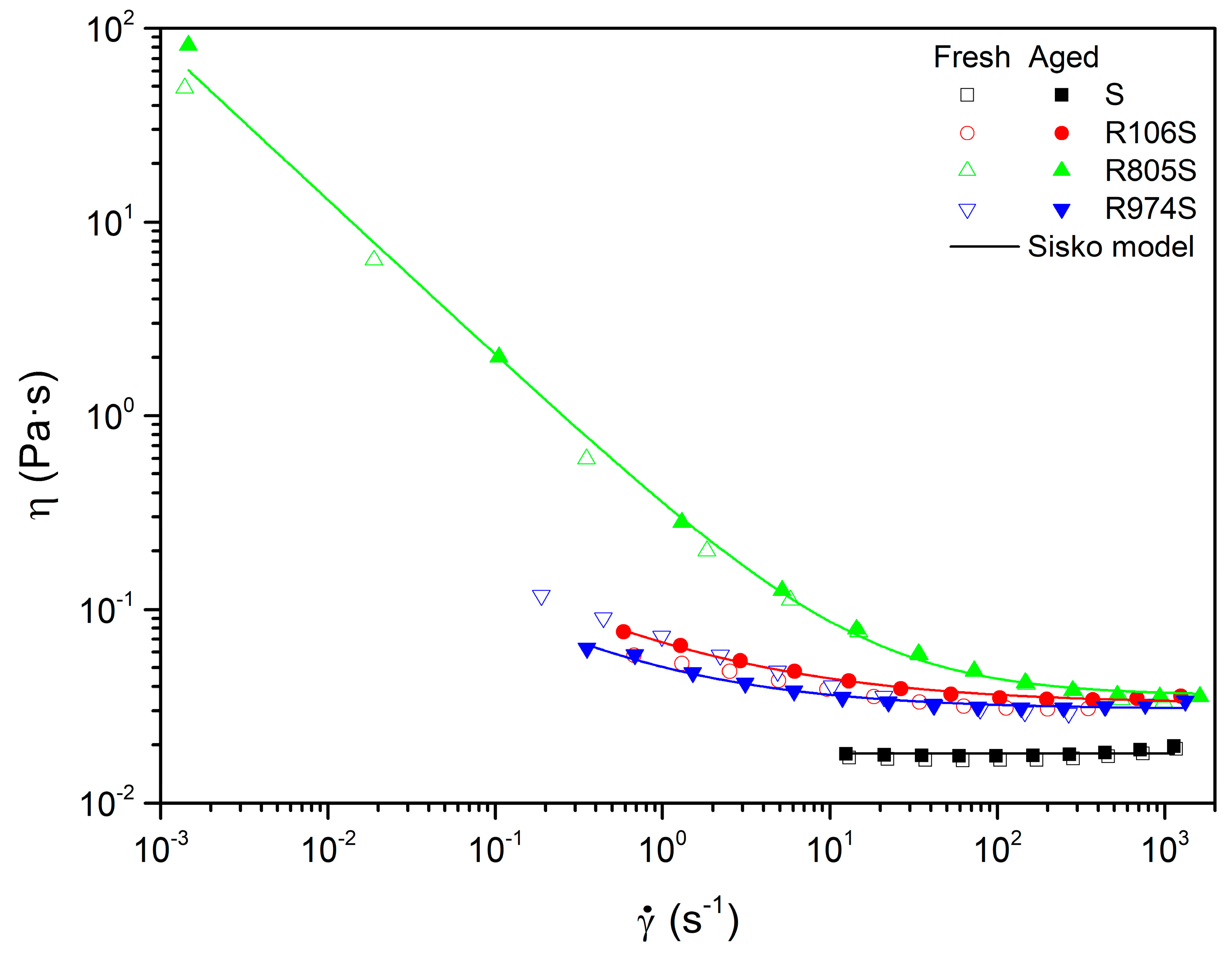
3.2. Rheological Characterization
3.3. Thermal Aging
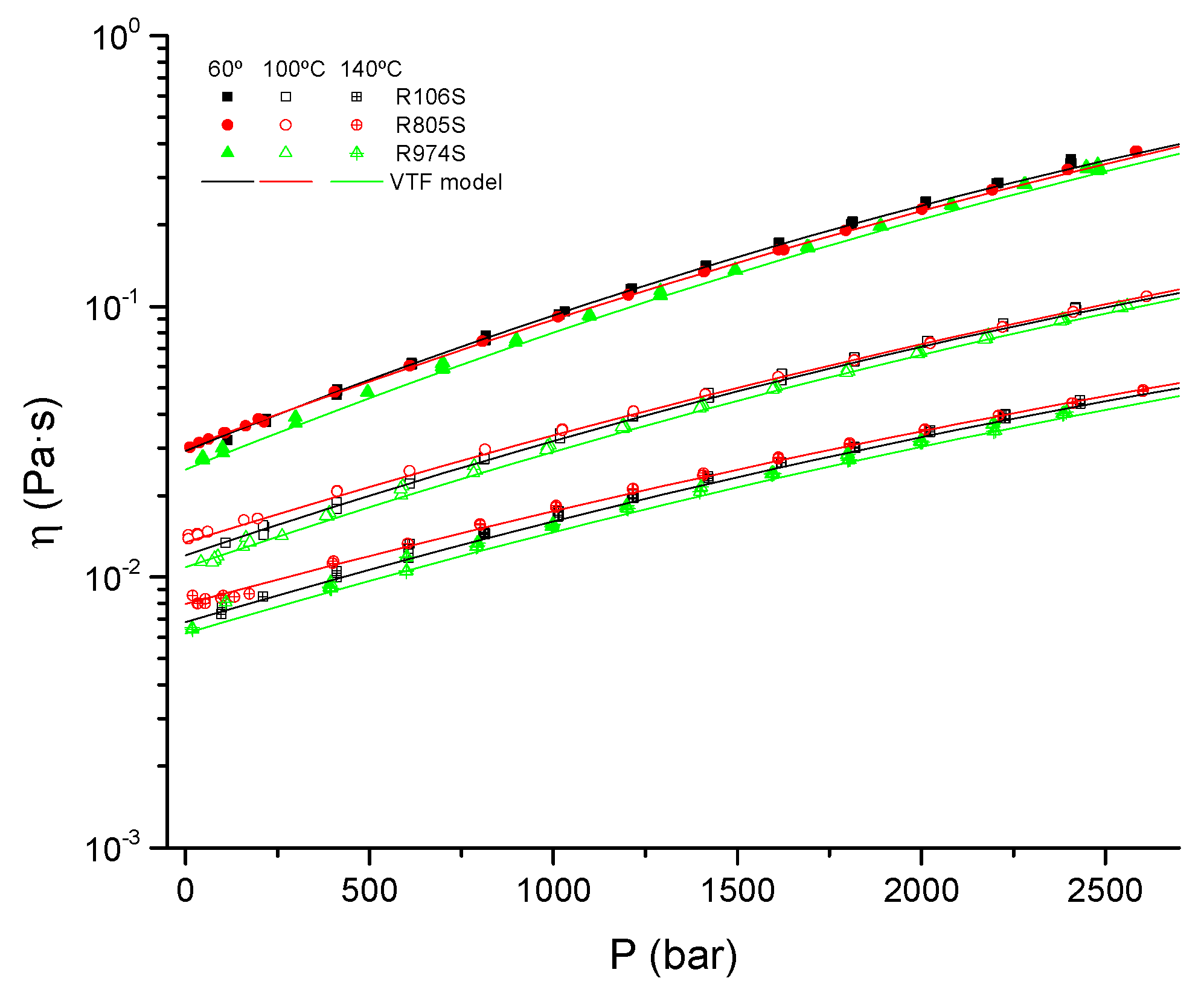
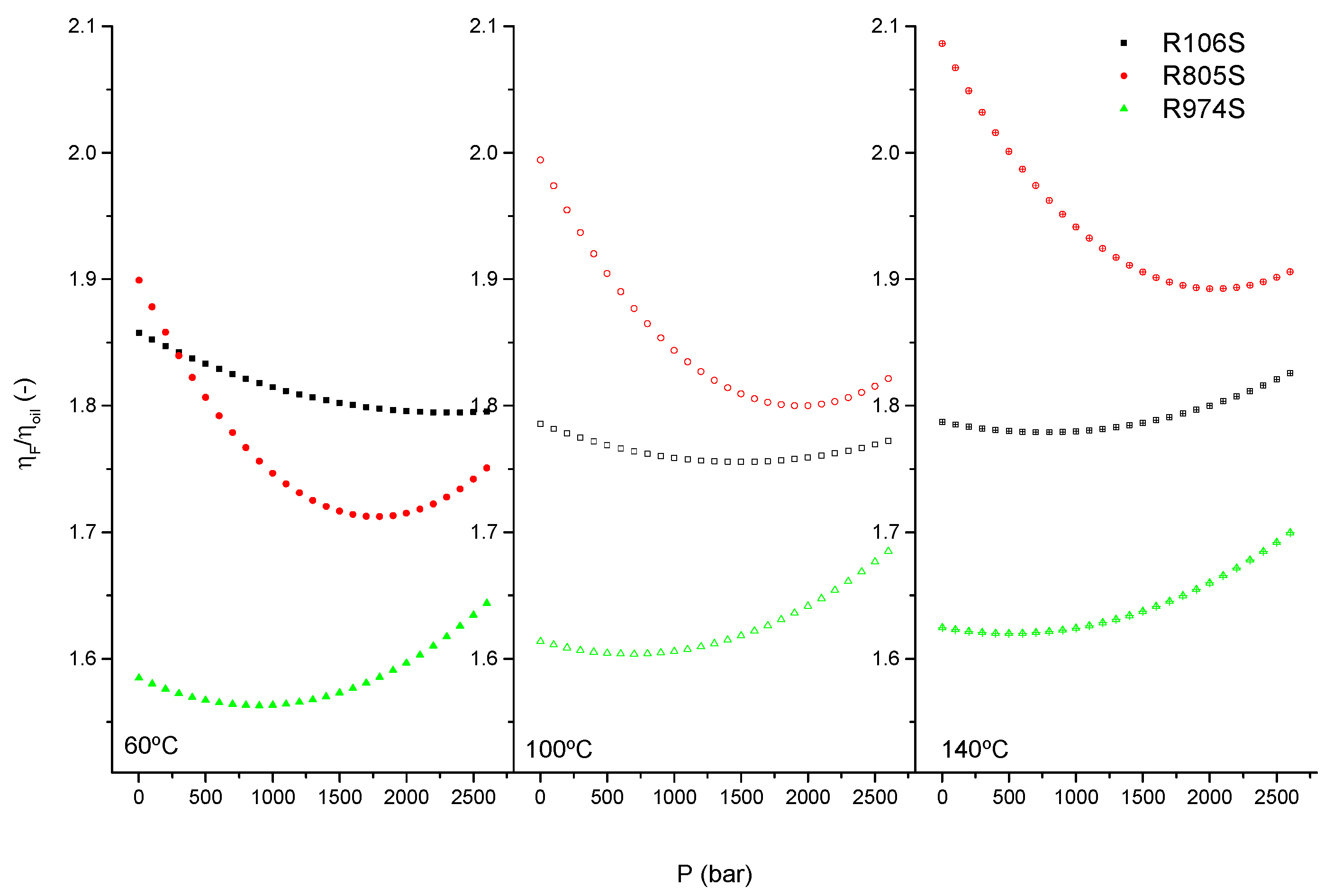
3.4. Pressure Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.E.; Al-Majed, A.A. Fundamentals of Sustainable Drilling Engineering; Wiley–Scrivener: Hoboken, NJ, USA, 2015. [Google Scholar]
- Fink, J. Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- Amani, M.; Al-Jubouri, M.; Shadravan, A. Comparative Study of Using Oil-Based Mud Versus Water-Based Mud in HPHT Fields. Adv. Pet. Explor. Dev. 2012, 4, 18–27. [Google Scholar] [CrossRef]
- Hermoso, J.; Martinez-Boza, F.; Gallegos, C. Influence of aqueous phase volume fraction, organoclay concentration and pressure on invert-emulsion oil muds rheology. J. Ind. Eng. Chem. 2015, 22, 341–349. [Google Scholar] [CrossRef]
- Reinoso, D.; Martín-Alfonso, M.; Luckham, P.; Martínez-Boza, F. Rheological characterisation of xanthan gum in brine solutions at high temperature. Carbohydr. Polym. 2019, 203, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, D.; Martín-Alfonso, M.; Luckham, P.; Martínez-Boza, F. Flow behavior and thermal resistance of xanthan gum in formate brine. J. Pet. Sci. Eng. 2020, 188, 106881. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.J.; Loaiza, J.M.; Delgado-Sánchez, C.; Martínez-Boza, F.J. Influence of Formate Concentration on the Rheology and Thermal Degradation of Xanthan Gum. Polymers 2021, 13, 3378. [Google Scholar] [CrossRef]
- Khodja, M.; Canselier, J.P.; Bergaya, F.; Fourar, K.; Khodja, M.; Cohaut, N.; Benmounah, A. Shale problems and water-based drilling fluid optimisation in the Hassi Messaoud Algerian oil field. Appl. Clay Sci. 2010, 49, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Razali, S.Z.; Yunus, R.; Rashid, S.A.; Lim, H.N.; Jan, B.M. Review of biodegradable synthetic-based drilling fluid: Progression, performance and future prospect. Renew. Sustain. Energy Rev. 2018, 90, 171–186. [Google Scholar] [CrossRef]
- Ahmed, A.; Elkatatny, S.; Al-Afnan, S. Applications of Biodiesel in Drilling Fluids. Geofluids 2021, 2021, 5565897. [Google Scholar] [CrossRef]
- Hermoso, J.; Martinez-Boza, F.; Gallegos, C. Influence of viscosity modifier nature and concentration on the viscous flow behaviour of oil-based drilling fluids at high pressure. Appl. Clay Sci. 2014, 87, 14–21. [Google Scholar] [CrossRef]
- Hermoso, J.; Martínez-Boza, F.; Gallegos, C. Combined Effect of Pressure and Temperature on the Viscous Behaviour of All-Oil Drilling Fluids. Oil Gas Sci. Technol. 2014, 69, 1283–1296. [Google Scholar] [CrossRef] [Green Version]
- Hermoso, J.; Martínez-Boza, F.; Gallegos, C. Organoclay influence on high pressure-high temperature volumetric properties of oil-based drilling fluids. J. Pet. Sci. Eng. 2017, 151, 13–23. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Jaber, M. Organoclays used as colloidal and rheological additives in oil-based drilling fluids: An overview. Appl. Clay Sci. 2019, 177, 63–81. [Google Scholar] [CrossRef] [Green Version]
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Composition and Properties of Drilling Fluids and Completion Fluids, 7th ed.; Gulf Professional Publishing: Houston, TX, USA, 2017. [Google Scholar]
- Tapavicza, S.V. Vegetable esters make drilling fluids more environmentally friendly. Oil Gas. J. 2005, 103, 22. [Google Scholar]
- Agwu, O.E.; Okon, A.N.; Udoh, F.D. A Comparative Study of Diesel Oil and Soybean Oil as Oil-Based Drilling Mud. J. Pet. Eng. 2015, 2015, 828451. [Google Scholar] [CrossRef]
- Ratkievicius, L.A.; Filho, F.J.V.D.C.; Neto, E.L.B.; Santanna, V.C. Modification of bentonite clay by a cationic surfactant to be used as a viscosity enhancer in vegetable-oil-based drilling fluid. Appl. Clay Sci. 2017, 135, 307–312. [Google Scholar] [CrossRef]
- Sulaimon, A.A.; Adeyemi, B.; Rahimi, M. Performance enhancement of selected vegetable oil as base fluid for drilling HPHT formation. J. Pet. Sci. Eng. 2017, 152, 49–59. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.; Mejía, A.; Martínez-Boza, F.; Martín-Alfonso, J. Rheological characterization of sepiolite-vegetable oil suspensions at high pressures. Appl. Clay Sci. 2021, 212, 106220. [Google Scholar] [CrossRef]
- Vryzas, Z.; Kelessidis, V.C. Nano-Based Drilling Fluids: A Review. Energies 2017, 10, 540. [Google Scholar] [CrossRef]
- Ali, M.; Jarni, H.H.; Aftab, A.; Ismail, A.R.; Saady, N.M.C.; Sahito, M.F.; Keshavarz, A.; Iglauer, S.; Sarmadivaleh, M. Nanomaterial-Based Drilling Fluids for Exploitation of Unconventional Reservoirs: A Review. Energies 2020, 13, 3417. [Google Scholar] [CrossRef]
- Alsaba, M.T.; Al Dushaishi, M.F.; Abbas, A.K. A comprehensive review of nanoparticles applications in the oil and gas industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Babaahmadi, M.; Lesaffer, A.; Dewettinck, K. Rheological profiling of organogels prepared at critical gelling concentrations of natural waxes in a triacylglycerol solvent. J. Agric. Food Chem. 2015, 63, 4862–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitby, C.P.; Krebsz, M.; Booty, S.J. Understanding the role of hydrogen bonding in the aggregation of fumed silica particles in triglyceride solvents. J. Colloid Interface Sci. 2018, 527, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Whitby, C.P. Structuring Edible Oils with Fumed Silica Particles. Front. Sustain. Food Syst. 2020, 4, 201. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.J.; Martinez-Boza, F.J.; Navarro Dominguez, F.J.; Partal, P. Device for Measuring Rheological Properties in Fluids. WO2020070359A1, 2021. [Google Scholar]
- Hermoso, J.; Jofore, B.D.; Martínez-Boza, F.J.; Gallegos, C. High Pressure Mixing Rheology of Drilling Fluids. Ind. Eng. Chem. Res. 2012, 51, 14399–14407. [Google Scholar] [CrossRef]
- Ou, C.-F.; Shen, P.-H. Thermal and Gas Barrier Properties of COC/SiO2 Nanocomposites. Polym. Compos. 2012, 20, 537–544. [Google Scholar] [CrossRef]
- Jayadas, N.H.; Nair, K.P.; Ajithkumar, G. Vegetable Oils as Base Oil for Industrial Lubricants: Evaluation Oxidative and Low Temperature Properties Using TGA, DTA and DSC. In Proceedings of the World Tribology Congress III, Volume 1, Washington, DC, USA, 12–16 September 2005; Volume 1, pp. 539–540. [Google Scholar] [CrossRef] [Green Version]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Artiaga, R. Effect of silica content on thermal stability of fumed silica/epoxy composites. Polym. Degrad. Stab. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Chapman, D. The polymorphism of glycerides. Chem. Rev. 1962, 62, 433. [Google Scholar] [CrossRef]
- Man, Y.B.C.; Tan, C.P. Comparative differential scanning calorimetric analysis of vegetable oils: II. Effects of cooling rate variation. Phytochem. Anal. 2002, 13, 142–151. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.; Perez, J. Wax appearance temperatures of vegetable oils determined by differential scanning calorimetry: Effect of triacylglycerol structure and its modification. Thermochim. Acta 2002, 395, 191–200. [Google Scholar] [CrossRef]
- Bode, R.; Ferch, H.; Fratzscher, H. Technical Bulletin Fine Particles. Basic Characteristics of AEROSIL® Fumed Silica; Number 11; Degussa AG: Frankfurt, Germany, 1967. [Google Scholar]
- Khan, S.A.; Zoeller, N.J. Dynamic rheological behavior of flocculated fumed silica suspensions. J. Rheol. 1993, 37, 1225–1235. [Google Scholar] [CrossRef]
- Chen, S.; Øye, G.; Sjöblom, J. Rheological Properties of Silica Particle Suspensions in Mineral Oil. J. Dispers. Sci. Technol. 2005, 26, 791–798. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kawaguchi, M. Stability and rheological properties of hydrophobic fumed silica suspensions in mineral oil. J. Dispers. Sci. Technol. 2018, 39, 1274–1279. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.J.; Martínez-Boza, F.; Partal, P.; Gallegos, C. Influence of pressure and temperature on the flow behaviour of heavy fuel oils. Rheol. Acta 2006, 45, 357–365. [Google Scholar] [CrossRef]
- Martínez-Boza, F.J.; Martín-Alfonso, M.J.; Gallegos, C.; Fernández, M. High-Pressure Behavior of Intermediate Fuel Oils. Energy Fuels 2011, 25, 5138–5144. [Google Scholar] [CrossRef]
- Hermoso, J.; Martínez-Boza, F.J.; Gallegos, C. Modeling Pressure-Viscosity Behavior of Oil-Based Drilling Fluids. Oil Gas Sci. Technol. Rev. De L’ifp 2017, 72, 18. [Google Scholar] [CrossRef] [Green Version]
- Agwu, O.E.; Akpabio, J.U.; Ekpenyong, M.E.; Inyang, U.G.; Asuquo, D.E.; Eyoh, I.J.; Adeoye, O.S. A critical review of drilling mud rheological models. J. Pet. Sci. Eng. 2021, 203, 108659. [Google Scholar] [CrossRef]
- Paredes, X.; Comuñas, M.J.; Pensado, A.S.; Bazile, J.-P.; Boned, C.; Fernández, J. High pressure viscosity characterization of four vegetable and mineral hydraulic oils. Ind. Crop. Prod. 2014, 54, 281–290. [Google Scholar] [CrossRef]
- Wu, X.-J.; Wang, Y.; Yang, W.; Xie, B.-H.; Yang, M.-B.; Dan, W. A rheological study on temperature dependent microstructural changes of fumed silica gels in dodecane. Soft Matter 2012, 8, 10457–10463. [Google Scholar] [CrossRef]



| C12:0 Lauric (wt%) | C14:0 Myristic (wt%) | C16:0 Palmitic (wt%) | C18:0 Stearic (wt%) | C18:1 Oleic (wt%) | C18:2 Linoleic (wt%) | C18:3 Linolenic (wt%) | Others (wt%) |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.06 | 6.15 | 3.30 | 24.83 | 64.48 | 0.06 | 1.12 |
| BET (m2/g) | Average Primary Particle Size (nm) | C Content (wt.%) | Fumed Silica Modified with: | |
|---|---|---|---|---|
| R106 | 250 ± 30 | 7 | 1.4–3.0 | Octamethylcyclotetrasiloxane 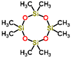 |
| R805 | 150 ± 25 | 12 | 4.5–6.5 | Trimethoxy(octyl)silane 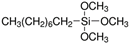 |
| R974 | 170 ± 20 | 12 | 0.7–1.5 | Dimethyldichlorosilane  |
| Degradation Temperatures (°C) | ||||
|---|---|---|---|---|
| Samples | T1 (°C) (T at 1% Weight Loss) | T5 (°C) (T at 5% Weight Loss) | Tonset (°C) | Tpeak (°C) |
| S | 330.8 | 372.4 | 391.0 | 426.0 |
| R106S | 334.0 | 381.8 | 402.1 | 434.2 |
| R805S | 337.0 | 383.8 | 402.3 | 437.3 |
| R974S | 330.9 | 391.9 | 401.7 | 437.6 |
| Sample | Crystallization (°C) | Melting (°C) | ||||
|---|---|---|---|---|---|---|
| Onset (°C) | Peak 1 (°C) | Peak 2 (°C) | Peak 1 (°C) | Peak 2 (°C) | ΔHm (J/g) | |
| S-1st | −9.1 | −17.5 | −41.2 | −37.5 | −27.9 | 74.6 |
| S-2nd | −9.8 | −18.5 | −41.7 | −37.8 | −27.4 | |
| R106S-1st | −8.7 | −18.9 | −40.9 | −30.1 | 54.8 | |
| R805S-2nd | −9.0 | −18.3 | −41.2 | - | −31.2 | |
| R805S-1st | −9.4 | −18.1 | −41.3 | - | −29.6 | 46.6 |
| R106S-2nd | −8.9 | −18.7 | −41.0 | - | −29.5 | |
| R974S-1st | −9.2 | −18.8 | −40.9 | - | −29.7 | 56.6 |
| R974S-2nd | −9.7 | −17.7 | −41.2 | - | −28.3 | |
| Limiting Viscosity (ηꝏ), Pa·s | Consistency Index (k0), Pa·sn | Flow Index (n0) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | 60 °C | 80 °C | 100 °C | 120 °C | 140 °C | 60 °C | 80 °C | 100 °C | 120 °C | 140 °C | 60 °C | 80 °C | 100 °C | 120 °C | 140 °C |
| S | 17.08 × 10−3 | 10.01 × 10−3 | 7.09 × 10−3 | 5.16 × 10−3 | 3.83 × 10−3 | ||||||||||
| R106S | 26.18 × 10−3 | 15.33 × 10−3 | 9.79 × 10−3 | 7.75 × 10−3 | 5.95 × 10−3 | 0.028 | 0.022 | 0.021 | 0.019 | 0.018 | 0.638 | 0.671 | 0.672 | 0.614 | 0.599 |
| R805S | 35.91 × 10−3 | 25.12 × 10−3 | 16.76 × 10−3 | 12.72 × 10−3 | 9.18 × 10−3 | 0.269 | 0.153 | 0.121 | 0.085 | 0.068 | 0.208 | 0.168 | 0.181 | 0.167 | 0.223 |
| R974S | 23.43 × 10−3 | 15.45 × 10−3 | 9.65 × 10−3 | 7.59 × 10−3 | 5.82 × 10−3 | 0.047 | 0.04 | 0.033 | 0.028 | 0.026 | 0.577 | 0.517 | 0.557 | 0.509 | 0.492 |
| Aged Suspensions | Limiting Viscosity (ηꝏ), Pa·s | Consistency Index (k0), Pa·sn | Flow Index (n0) |
|---|---|---|---|
| Aged_S | 17.99 × 10−3 | - | - |
| Aged_R106S | 32.53 × 10−3 | 0.032 | 0.515 |
| Aged_R805S | 35.85 × 10−3 | 0.322 | 0.197 |
| Aged_R974S | 30.54 × 10−3 | 0.020 | 0.456 |
| S | R106S | R805S | R974S | |
|---|---|---|---|---|
| A/Pa·s | 2.13 × 10−4 | 3.60 × 10−4 | 6.75 × 10−4 | 3.35 × 10−4 |
| B/°C | 698.1 | 733.3 | 565.5 | 720.6 |
| C/°C | −102.3 | −104.3 | −89.37 | −107.2 |
| a1/bar−1 | 2.23 × 10−4 | 8.72 × 10−5 | 2.24 × 10−4 | 2.32 × 10−4 |
| a2/bar−2 | 0 | 0 | 0 | 0 |
| b1/°C·bar−1 | 0.1740 | 0.1778 | 0.1424 | 0.1726 |
| b2/°C·bar−2 | −1.90 × 10−5 | −1.35 × 10−5 | −1.26 × 10−5 | −1.67 × 10−5 |
| b3/°C·bar−3 | 0 | 0 | 0 | 0 |
| Pr/bar | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Alfonso, M.J.; Pozo, J.; Delgado-Sánchez, C.; Martínez-Boza, F.J. Thermal and Rheological Properties of Hydrophobic Nanosilica in Sunflower Oil Suspensions at High Pressures. Nanomaterials 2021, 11, 3037. https://doi.org/10.3390/nano11113037
Martín-Alfonso MJ, Pozo J, Delgado-Sánchez C, Martínez-Boza FJ. Thermal and Rheological Properties of Hydrophobic Nanosilica in Sunflower Oil Suspensions at High Pressures. Nanomaterials. 2021; 11(11):3037. https://doi.org/10.3390/nano11113037
Chicago/Turabian StyleMartín-Alfonso, María J., Javier Pozo, Clara Delgado-Sánchez, and Francisco José Martínez-Boza. 2021. "Thermal and Rheological Properties of Hydrophobic Nanosilica in Sunflower Oil Suspensions at High Pressures" Nanomaterials 11, no. 11: 3037. https://doi.org/10.3390/nano11113037
APA StyleMartín-Alfonso, M. J., Pozo, J., Delgado-Sánchez, C., & Martínez-Boza, F. J. (2021). Thermal and Rheological Properties of Hydrophobic Nanosilica in Sunflower Oil Suspensions at High Pressures. Nanomaterials, 11(11), 3037. https://doi.org/10.3390/nano11113037








