Iron-Nickel Alloy with Starfish-like Shape and Its Unique Magnetic Properties: Effect of Reaction Volume and Metal Concentration on the Synthesized Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
2.3.1. X-ray Diffraction (XRD) Analysis
2.3.2. Scanning Electron Microscopy (SEM) Imaging and X-ray Spectroscopy (EDX) Analysis
2.3.3. Scanning-Transmission Electron Microscope (STEM) Imaging and X-ray Spectroscopy (EDX) Analysis
2.3.4. Vibrating Sample Magnetometer (VSM) Analysis
2.3.5. Differential Scanning Calorimeter (DSC) Analysis
3. Results and Discussion
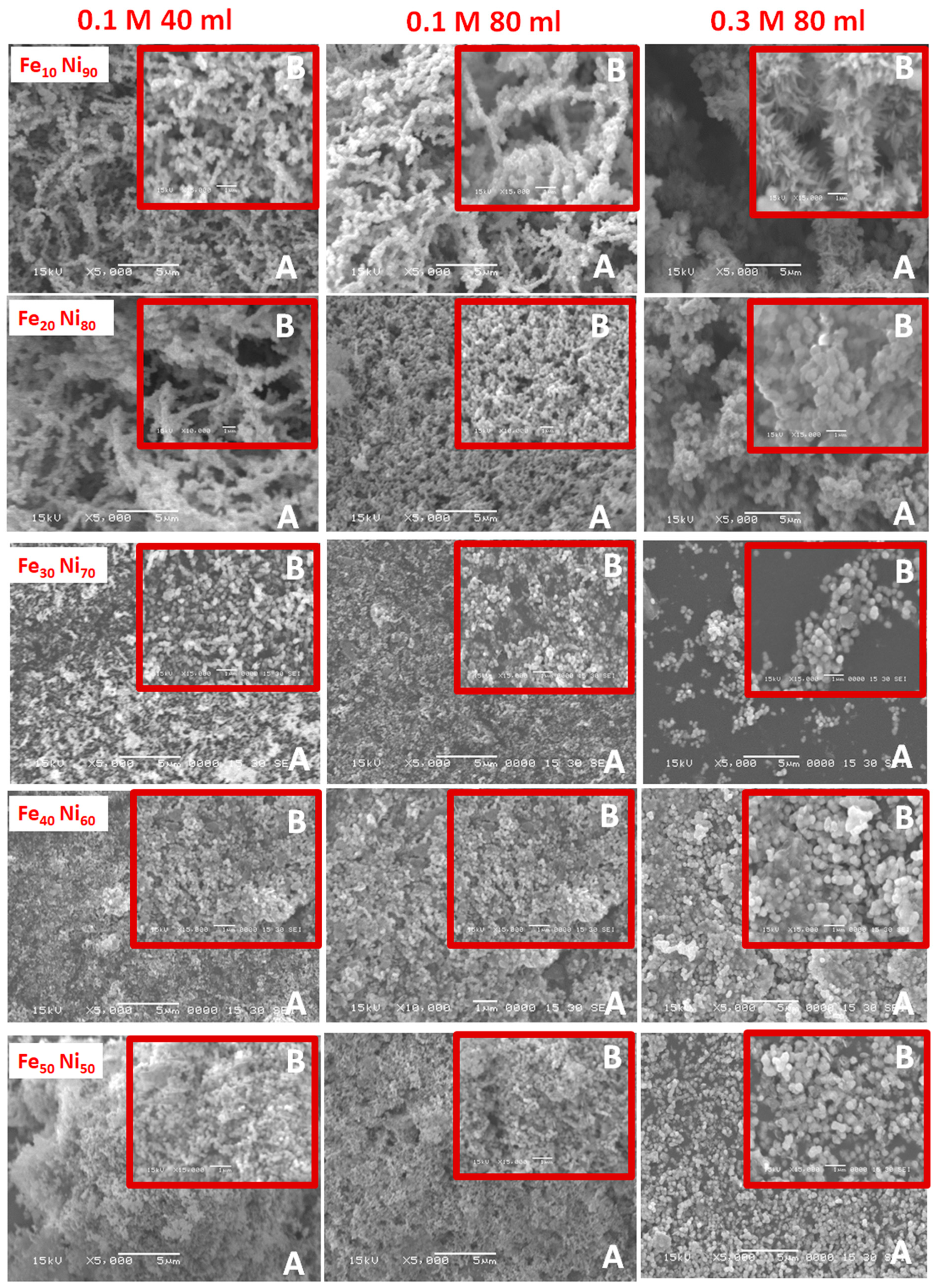
3.1. Alloy Morphology
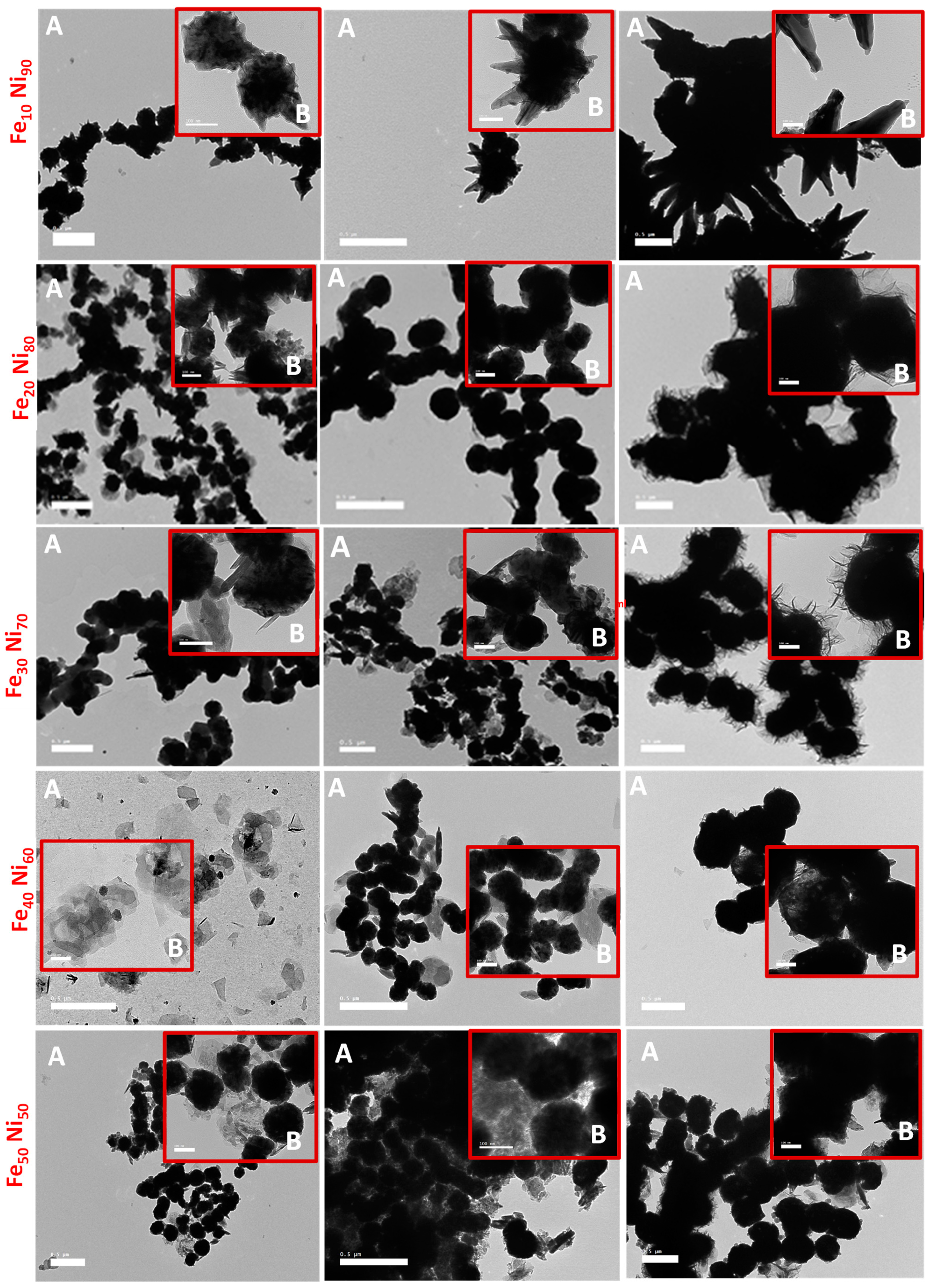
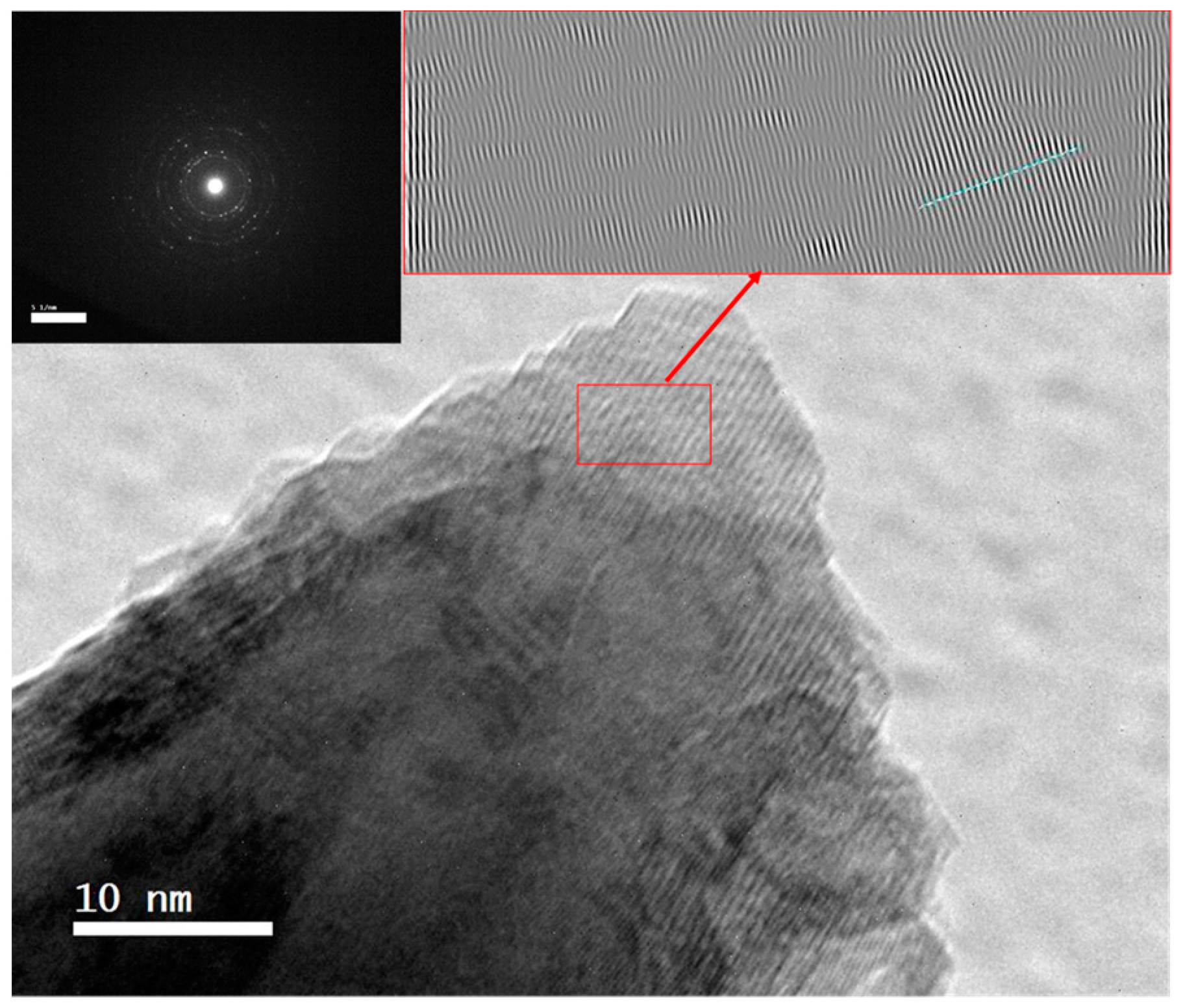
3.2. Alloy Particle Size
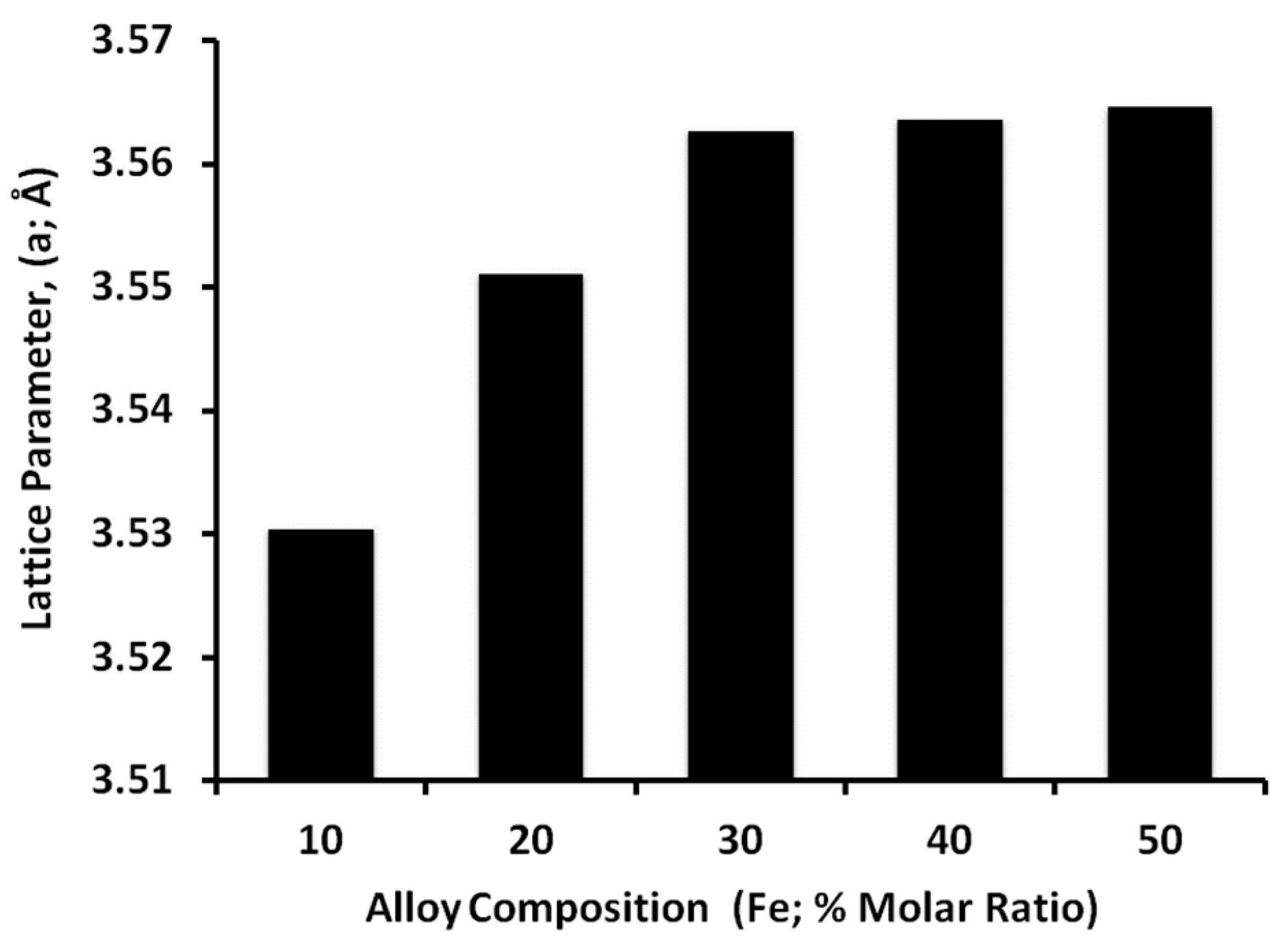
3.3. Alloy Composition
3.4. Alloy Reaction Mechanism
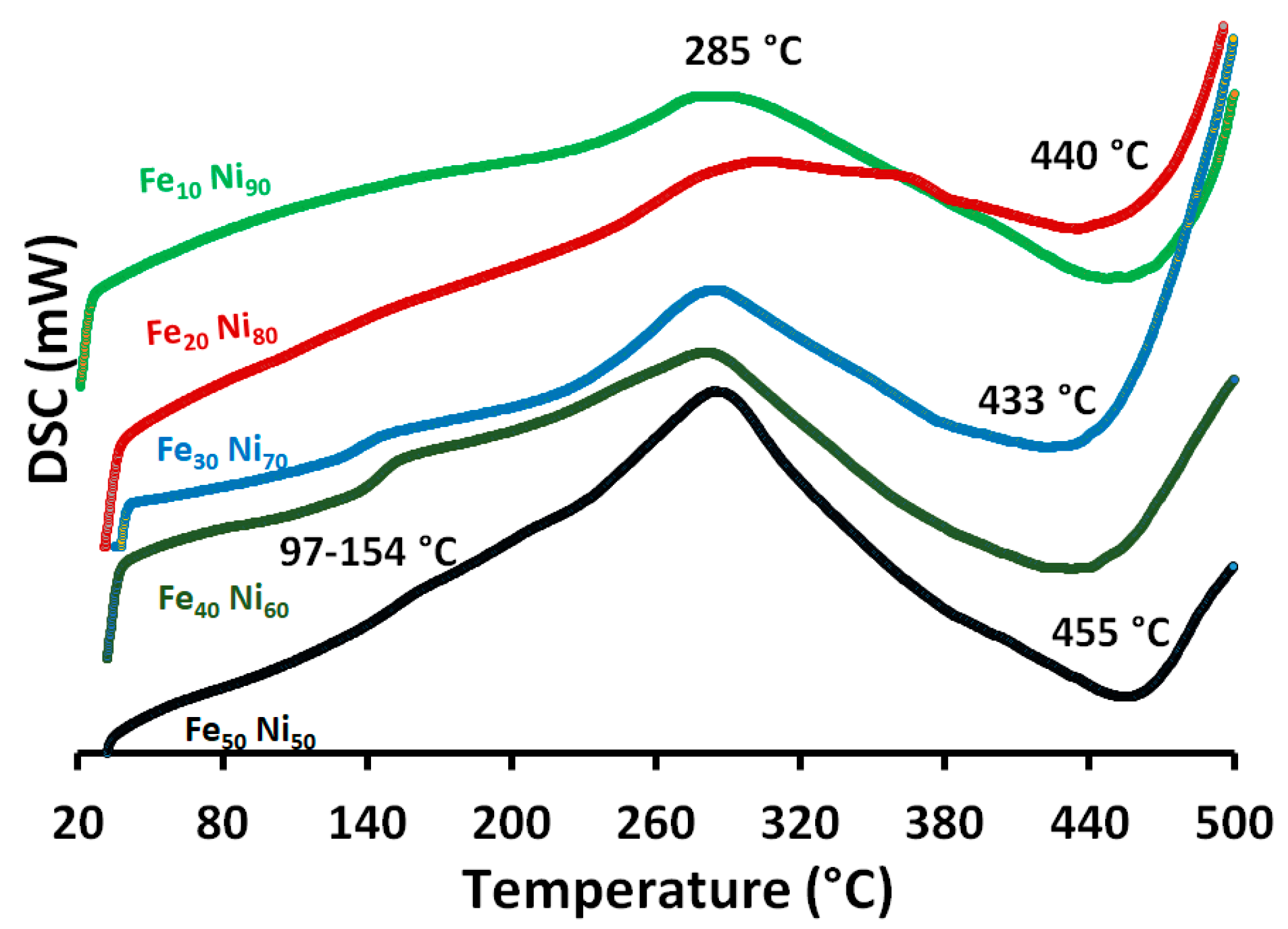
3.5. Thermal Properties
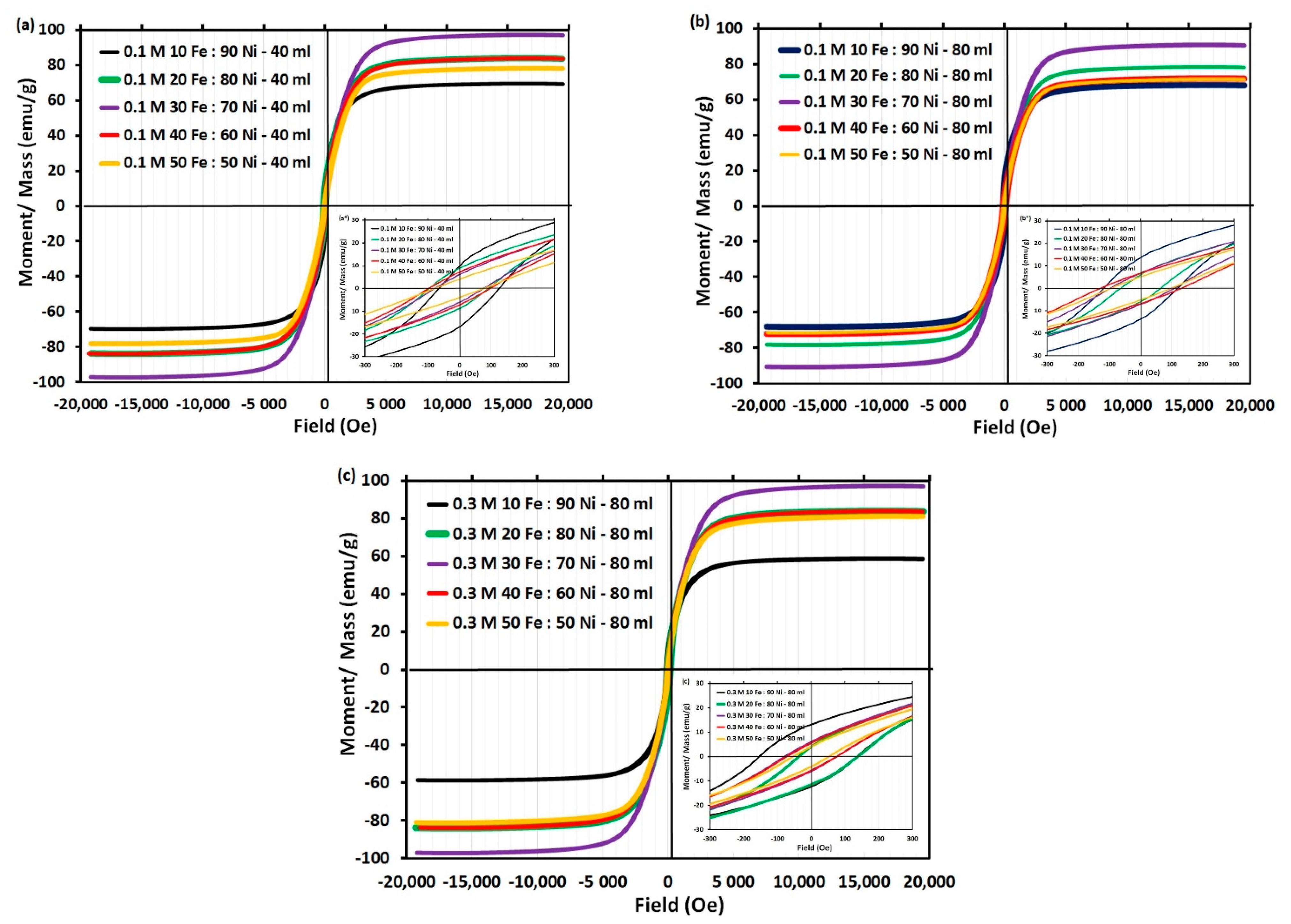
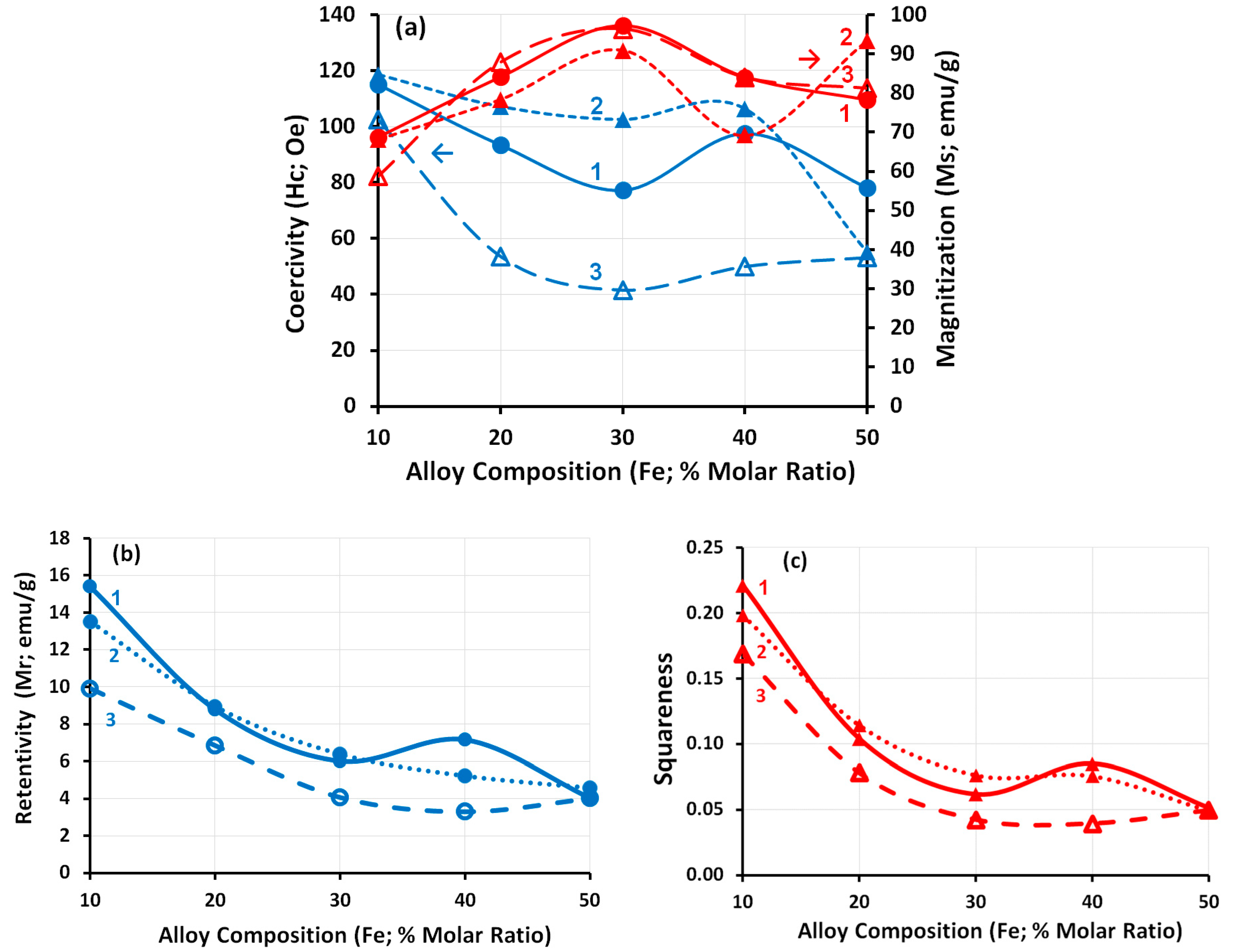
3.6. Magnetic Properties
4. General Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, R.; Hasan, A.; Iqbal, N.; Alam, S.; Saini, M.K.; Raza, S.K. Synthesis and surface engineering of magnetic nanoparticles for environmental cleanup and pesticide residue analysis: A review. J. Sep. Sci. 2014, 37, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, X.; Liu, J.; Huang, Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 2020, 188, 106943. [Google Scholar] [CrossRef]
- Jiang, B.; Lian, L.; Xing, Y.; Zhang, N.; Chen, Y.; Lu, P.; Zhang, D. Advances of magnetic nanoparticles in environmental application: Environmental remediation and (bio) sensors as case studies. Environ. Sci. Pollut. Res. 2018, 25, 30863–30879. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Kongsat, P.; Kudkaew, K.; Tangjai, J.; Edgar, A.; Pongprayoon, T. Synthesis of structure-controlled hematite nanoparticles by a surfactant-assisted hydrothermal method and property analysis. J. Phys. Chem. Solids 2021, 148, 109685. [Google Scholar] [CrossRef]
- Hudson, R.; Feng, Y.; Varma, R.S.; Moores, A. Bare magnetic nanoparticles: Sustainable synthesis and applications in catalytic organic transformations. Green Chem. 2014, 16, 4493–4505. [Google Scholar] [CrossRef]
- Fatima, H.; Kim, K.-S. Iron-based magnetic nanoparticles for magnetic resonance imaging. Adv. Powder Technol. 2018, 29, 2678–2685. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–relaxivity relationships of magnetic nanoparticles for magnetic resonance imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Kuwa, M.; Sato, R.; Teranishi, T.; Takahashi, M.; Maenosono, S. Cation Distribution in Monodispersed MFe2O4 (M = Mn, Fe, Co, Ni, and Zn) Nanoparticles Investigated by X-ray Absorption Fine Structure Spectroscopy: Implications for Magnetic Data Storage, Catalysts, Sensors, and Ferrofluids. ACS Appl. Nano Mater. 2020, 3, 8389–8402. [Google Scholar] [CrossRef]
- Döpke, C.; Grothe, T.; Steblinski, P.; Klöcker, M.; Sabantina, L.; Kosmalska, D.; Blachowicz, T.; Ehrmann, A. Magnetic nanofiber mats for data storage and transfer. Nanomaterials 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef] [PubMed]
- Matatagui, D.; Kolokoltsev, O.; Qureshi, N.; Mejía-Uriarte, E.; Ordoñez-Romero, C.; Vázquez-Olmos, A.; Saniger, J. Magnonic sensor array based on magnetic nanoparticles to detect, discriminate and classify toxic gases. Sens. Actuators B Chem. 2017, 240, 497–502. [Google Scholar] [CrossRef]
- Jia, Z.; Kou, K.; Yin, S.; Feng, A.; Zhang, C.; Liu, X.; Cao, H.; Wu, G. Magnetic Fe nanoparticle to decorate N dotted C as an exceptionally absorption-dominate electromagnetic shielding material. Compos. Part B Eng. 2020, 189, 107895. [Google Scholar] [CrossRef]
- Liang, L.; Yang, R.; Han, G.; Feng, Y.; Zhao, B.; Zhang, R.; Wang, Y.; Liu, C. Enhanced Electromagnetic Wave-Absorbing Performance of Magnetic Nanoparticles-Anchored 2D Ti3C2T x MXene. ACS Appl. Mater. Interfaces 2019, 12, 2644–2654. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, J.; Wang, L.; Li, B.; Li, L. Properties of FeSiAl-based soft magnetic composites with AlN/Al2O3 and hybrid phosphate–silane insulation coatings. J. Alloy. Compd. 2018, 735, 1603–1610. [Google Scholar] [CrossRef]
- Mohapatra, J.; Xing, M.; Elkins, J.; Beatty, J.; Liu, J.P. Extraordinary Magnetic Hardening in Nanowire Assemblies: The Geometry and Proximity Effects. Adv. Funct. Mater. 2021, 31, 2010157. [Google Scholar] [CrossRef]
- Bashir-U-Din, A. Characterisation of amorphous metal materials for high-frequency high-power-density transformer. In Proceedings of the 2018 26th International Conference on Systems Engineering (ICSEng), Sydney, Australia, 18–20 December 2018; IEEE: Washington, DC, USA, 2018. [Google Scholar]
- Wang, S.; Sun, N.; Yamaguchi, M.; Yabukami, S. Properties of a new soft magnetic material. Nature 2000, 407, 150–151. [Google Scholar] [CrossRef]
- Pardavi-Horvath, M. Microwave applications of soft ferrites. J. Magn. Magn. Mater. 2000, 215, 171–183. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lim, Y.-S.; Choi, I.-H.; Lee, D.-I.; Kim, S.-B. Effective combination of soft magnetic materials for magnetic shielding. IEEE Trans. Magn. 2012, 48, 4550–4553. [Google Scholar] [CrossRef]
- Jiles, D. Recent advances and future directions in magnetic materials. Acta Mater. 2003, 51, 5907–5939. [Google Scholar] [CrossRef]
- Krajewski, M.; Tokarczyk, M.; Stefaniuk, T.; Słomińska, H.; Małolepszy, A.; Kowalski, G.; Lewińska, S.; Ślawska-Waniewska, A. Magnetic-field-induced synthesis of amorphous iron-nickel wire-like nanostructures. Mater. Chem. Phys. 2020, 246, 122812. [Google Scholar] [CrossRef]
- Wu, K.-L.; Yu, R.; Wei, X.-W. Monodispersed FeNi2 alloy nanostructures: Solvothermal synthesis, magnetic properties and size-dependent catalytic activity. CrystEngComm 2012, 14, 7626–7632. [Google Scholar] [CrossRef]
- Malaeru, T.; Patroi, E.A.; Patroi, D.; Manta, E.; Marinescu, V.; Georgescu, G. Influence of Synthesis Parameters of FeNi3 Alloy Nanoparticles Obtained by Chemical Reduction Method in Aqueous Solution. Rev. Chim. 2019, 70, 1118–1124. [Google Scholar] [CrossRef]
- Gurmen, S.; Ebin, B.; Stopić, S.; Friedrich, B. Nanocrystalline spherical iron–nickel (Fe–Ni) alloy particles prepared by ultrasonic spray pyrolysis and hydrogen reduction (USP-HR). J. Alloy. Compd. 2009, 480, 529–533. [Google Scholar] [CrossRef]
- Mohamed, M.A.; El-Maghraby, A.H.; Abd El-Latif, M.M.; Farag, H.A. Optimum synthesis conditions of nanometric Fe50 Ni50 alloy formed by chemical reduction in aqueous solution. Bull. Mater. Sci. 2013, 36, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wu, H.; You, M.; Li, Z.; Zhang, Y. Improving wear resistance and friction stability of FeNi matrix coating by in-situ multi-carbide WC-TiC via PTA metallurgical reaction. Surf. Coat. Technol. 2019, 378, 124957. [Google Scholar] [CrossRef]
- Ban, I.; Drofenik, M.; Makovec, D. The synthesis of iron–nickel alloy nanoparticles using a reverse micelle technique. J. Magn. Magn. Mater. 2006, 307, 250–256. [Google Scholar] [CrossRef]
- Tanaka, A.; Yoon, S.-H.; Mochida, I. Formation of fine Fe–Ni particles for the non-supported catalytic synthesis of uniform carbon nanofibers. Carbon 2004, 42, 1291–1298. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Sun, Y.; Jin, C.; Lv, Y. Enhanced microwave absorption of flower-like FeNi@ C nanocomposites by dual dielectric relaxation and multiple magnetic resonance. RSC Adv. 2014, 4, 22710–22715. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, N.; Zhang, J.L. Synthesis and Characterization of Nanoscale Fe/Ni Alloy Particles by Hydrothermal Method. in Adv. Mater. Res. 2013, 658, 13–18. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Qiu, T. Synthesis, characterization, and microwave absorption properties of Fe–40 wt% Ni alloy prepared by mechanical alloying and annealing. J. Magn. Magn. Mater. 2011, 323, 3071–3076. [Google Scholar] [CrossRef]
- Liu, L.; Guan, J.; Shi, W.; Sun, Z.; Zhao, J. Facile synthesis and growth mechanism of flowerlike Ni− Fe alloy nanostructures. J. Phys. Chem. C 2010, 114, 13565–13570. [Google Scholar] [CrossRef]
- Maxwell, J.; Pegna, J.; Deangelis, D.; Messia, D. Three-dimensional laser chemical vapor deposition of nickel-iron alloys. MRS Online Proc. Libr. 1995, 397, 601–606. [Google Scholar] [CrossRef]
- Davis, J. Materials for Medical Devices; ASM Handbook Series; ASM International: Materials Park, OH, USA, 2003. [Google Scholar]
- Jin, M.; Zhou, W.; Jin, X.; Fukuda, T.; Kakeshita, T. Promoting martensitic transformation in Fe–Ni nanoparticles prepared by a Sol–gel and Reduction method. Mater. Lett. 2013, 109, 190–194. [Google Scholar] [CrossRef]
- Yermekova, Z.; Roslyakov, S.I.; Kovalev, D.Y.; Danghyan, V.; Mukasyan, A.S. One-step synthesis of pure γ-FeNi alloy by reactive sol–gel combustion route: Mechanism and properties. J. Sol-Gel Sci. Technol. 2020, 94, 310–321. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Z.; Zhao, X.; Chuang, Y.; Jin, S.; Sun, W. The preparation and characterization of ultrafine Fe–Ni particles. J. Mater. Res. 1999, 14, 398–406. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Gao, F.; Ren, B.; Huang, Y. Fabrication and electromagnetic properties of novel FeNi alloy-coated flake graphite prepared by electroless plating. J. Alloy. Compd. 2016, 656, 51–57. [Google Scholar] [CrossRef]
- Jacob, G.A.; Sellaiyan, S.; Uedono, A.; Joseyphus, R.J. Magnetic properties of metastable bcc phase in Fe64Ni36 alloy synthesized through polyol process. Appl. Phys. A 2020, 126, 120. [Google Scholar] [CrossRef]
- Beygi, H.; Babakhani, A. Microemulsion synthesis and magnetic properties of FexNi(1−x) alloy nanoparticles. J. Magn. Magn. Mater. 2017, 421, 177–183. [Google Scholar] [CrossRef]
- Yuan, M.L.; Tao, J.H.; Yu, L.; Song, C.; Qiu, G.Z.; Li, Y.; Xu, Z.H. Synthesis and magnetic properties of Fe–Ni alloy nanoparticles obtained by hydrothermal reaction. Adv. Mater. Res. 2011, 239–242, 748–753. [Google Scholar] [CrossRef]
- Tong, G.; Yuan, J.; Wu, W.; Hu, Q.; Qian, H.; Li, L.; Shen, J. Flower-like Co superstructures: Morphology and phase evolution mechanism and novel microwave electromagnetic characteristics. CrystEngComm 2012, 14, 2071–2079. [Google Scholar] [CrossRef]
- Wang, C.; Han, X.; Zhang, X.; Hu, S.; Zhang, T.; Wang, J.; Du, Y.; Wang, X.; Xu, P. Controlled synthesis and morphology-dependent electromagnetic properties of hierarchical cobalt assemblies. J. Phys. Chem. C 2010, 114, 14826–14830. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing: Reading, MA, USA, 1956. [Google Scholar]
- Amaliya, A.P.; Anand, S.; Pauline, S. Investigation on structural, electrical and magnetic properties of titanium substituted cobalt ferrite nanocrystallites. J. Magn. Magn. Mater. 2018, 467, 14–28. [Google Scholar] [CrossRef]
- Islam, M.N.; Abbas, M.; Kim, C. Synthesis of monodisperse and high moment nickel–iron (NiFe) nanoparticles using modified polyol process. Curr. Appl. Phys. 2013, 13, 2010–2013. [Google Scholar] [CrossRef]
- Guechi, N.; Bourzami, A.; Guittoum, A.; Kharmouche, A.; Colis, S.; Meni, N. Structural, magnetic and electrical properties of FexNi100−x/Si (100) films. Phys. B Condens. Matter 2014, 441, 47–53. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3166. [Google Scholar] [CrossRef]
- Wang, P.; Cui, C.; Yang, D.; Yu, X. Seed-assisted growth of cast-mono silicon for photovoltaic application: Challenges and strategies. Sol. RRL 2020, 4, 1900486. [Google Scholar] [CrossRef]
- Yuwono, V.M.; Burrows, N.D.; Soltis, J.A.; Lee Penn, R. Oriented aggregation: Formation and transformation of mesocrystal intermediates revealed. J. Am. Chem. Soc. 2010, 132, 2163–2165. [Google Scholar] [CrossRef]
- Johnson, D.; Pinski, F.; Staunton, J. The Slater–Pauling curve: First principles calculations of the moments of Fe1−c Nic and V1−c Fec. J. Appl. Phys. 1987, 61, 3715–3717. [Google Scholar] [CrossRef]
- Dijith, K.S.; Aiswarya, R.; Praveen, M.; Pillai, S.; Surendran, K.P. Polyol derived Ni and NiFe alloys for effective shielding of electromagnetic interference. Mater. Chem. Front. 2018, 2, 1829–1841. [Google Scholar] [CrossRef]
- Tripp, S.L.; Dunin-Borkowski, R.E.; Wei, A. Flux closure in self-assembled cobalt nanoparticle rings. Angew. Chem. Int. Ed. 2003, 42, 5591–5593. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S. Insights into the formation pathway of templated ordered nanostructured carbonaceous particles under hydrothermal conditions. Langmuir 2021, 37, 10866–10874. [Google Scholar] [CrossRef] [PubMed]


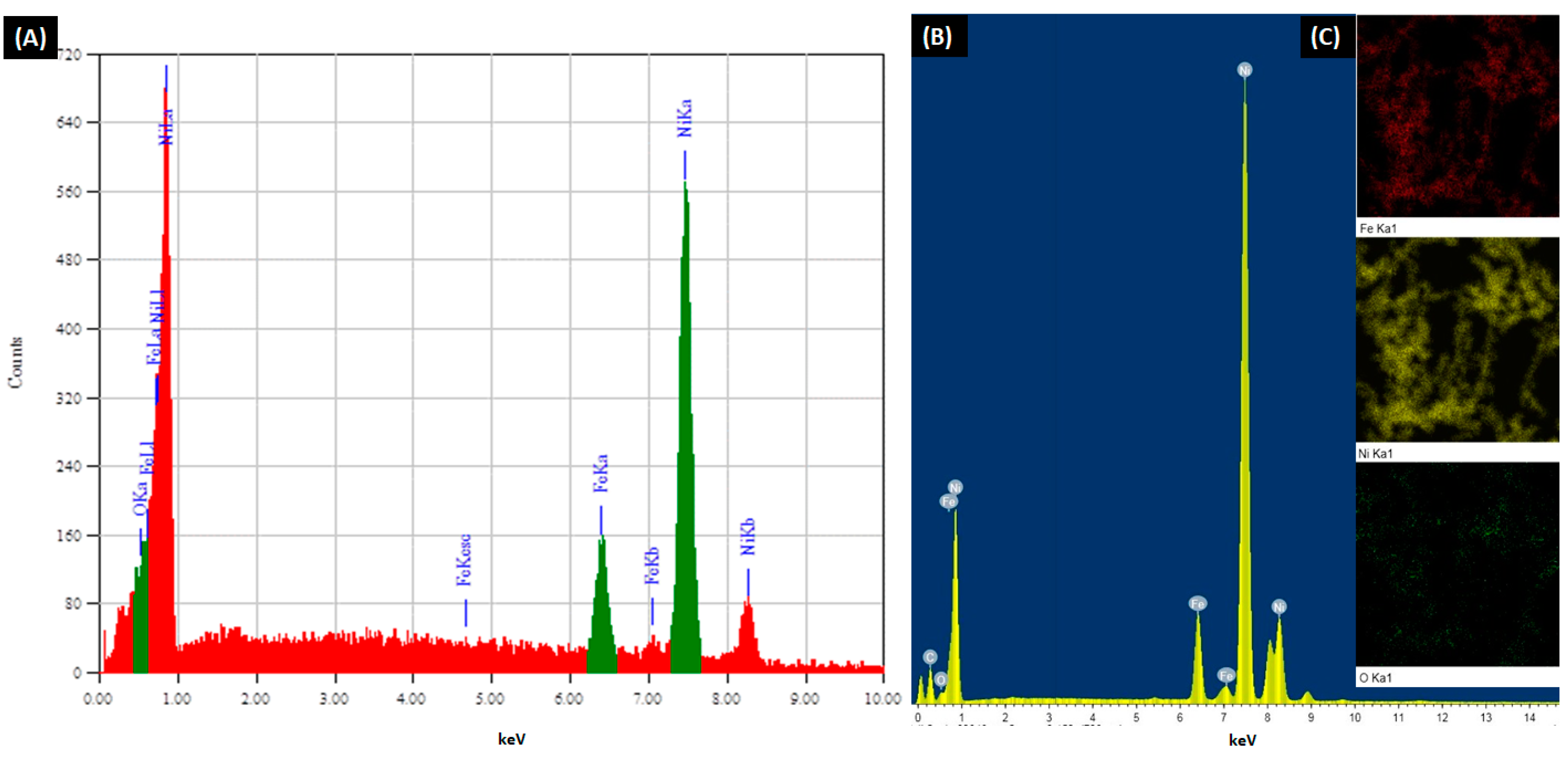

| Iron: Nickel Molar Ratio | TEM EDX Analysis | SEM EDX Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atomic % | Mass % | Atomic % | Mass % | |||||||||
| Fe | Ni | O | Fe | Ni | O | Fe | Ni | O | Fe | Ni | O | |
| 10:90 | 11.36 | 88.64 | 0.00 | 10.87 | 89.13 | 0.00 | 10.74 | 89.22 | 0.05 | 10.27 | 89.72 | 0.01 |
| 20:80 | 17.50 | 71.71 | 10.78 | 18.24 | 78.54 | 3.22 | 20.05 | 79.05 | 0.89 | 19.39 | 80.36 | 0.25 |
| 30:70 | 28.51 | 62.44 | 9.06 | 29.47 | 67.85 | 2.68 | 31.25 | 67.22 | 1.53 | 30.53 | 69.04 | 0.43 |
| 40:60 | 34.88 | 47.76 | 17.36 | 38.73 | 55.75 | 5.52 | 39.89 | 56.60 | 3.51 | 39.20 | 58.27 | 2.53 |
| 50:50 | 39.06 | 40.19 | 20.74 | 44.77 | 48.42 | 6.81 | 41.92 | 42.82 | 15.26 | 45.91 | 49.30 | 4.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nady, N.; Salem, N.; Mohamed, M.A.A.; Kandil, S.H. Iron-Nickel Alloy with Starfish-like Shape and Its Unique Magnetic Properties: Effect of Reaction Volume and Metal Concentration on the Synthesized Alloy. Nanomaterials 2021, 11, 3034. https://doi.org/10.3390/nano11113034
Nady N, Salem N, Mohamed MAA, Kandil SH. Iron-Nickel Alloy with Starfish-like Shape and Its Unique Magnetic Properties: Effect of Reaction Volume and Metal Concentration on the Synthesized Alloy. Nanomaterials. 2021; 11(11):3034. https://doi.org/10.3390/nano11113034
Chicago/Turabian StyleNady, Norhan, Noha Salem, Marwa A. A. Mohamed, and Sherif H. Kandil. 2021. "Iron-Nickel Alloy with Starfish-like Shape and Its Unique Magnetic Properties: Effect of Reaction Volume and Metal Concentration on the Synthesized Alloy" Nanomaterials 11, no. 11: 3034. https://doi.org/10.3390/nano11113034
APA StyleNady, N., Salem, N., Mohamed, M. A. A., & Kandil, S. H. (2021). Iron-Nickel Alloy with Starfish-like Shape and Its Unique Magnetic Properties: Effect of Reaction Volume and Metal Concentration on the Synthesized Alloy. Nanomaterials, 11(11), 3034. https://doi.org/10.3390/nano11113034






