Challenges in Coopted Hydrophilic and Lipophilic Herbal Bioactives in the Same Nanostructured Carriers for Effective Bioavailability and Anti-Inflammatory Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Nanostructured Lipid Carriers Loaded with DSG and/or Wild Yam
2.2.2. Particle Size Analysis
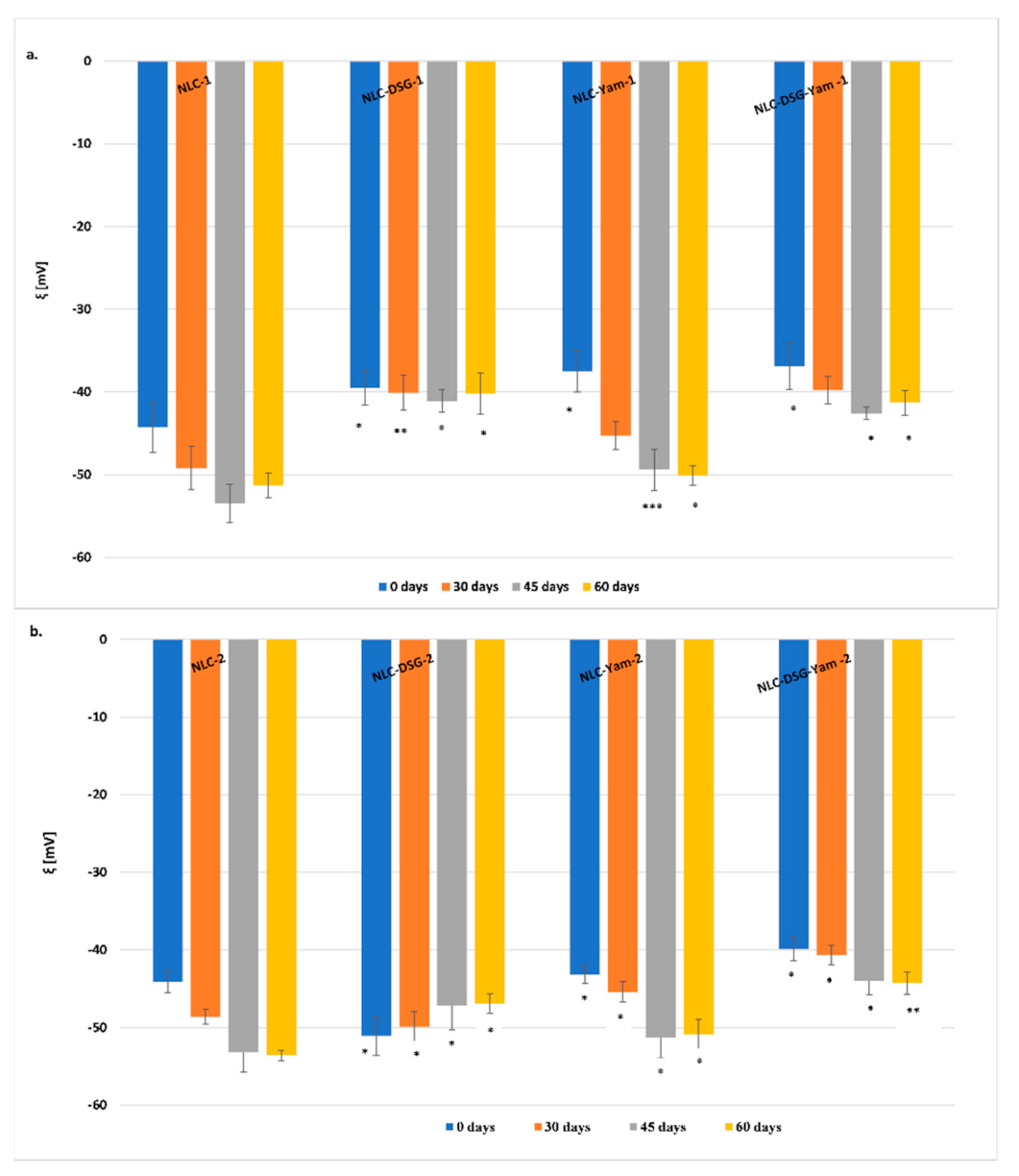
2.2.3. Electrokinetic Potential Analysis
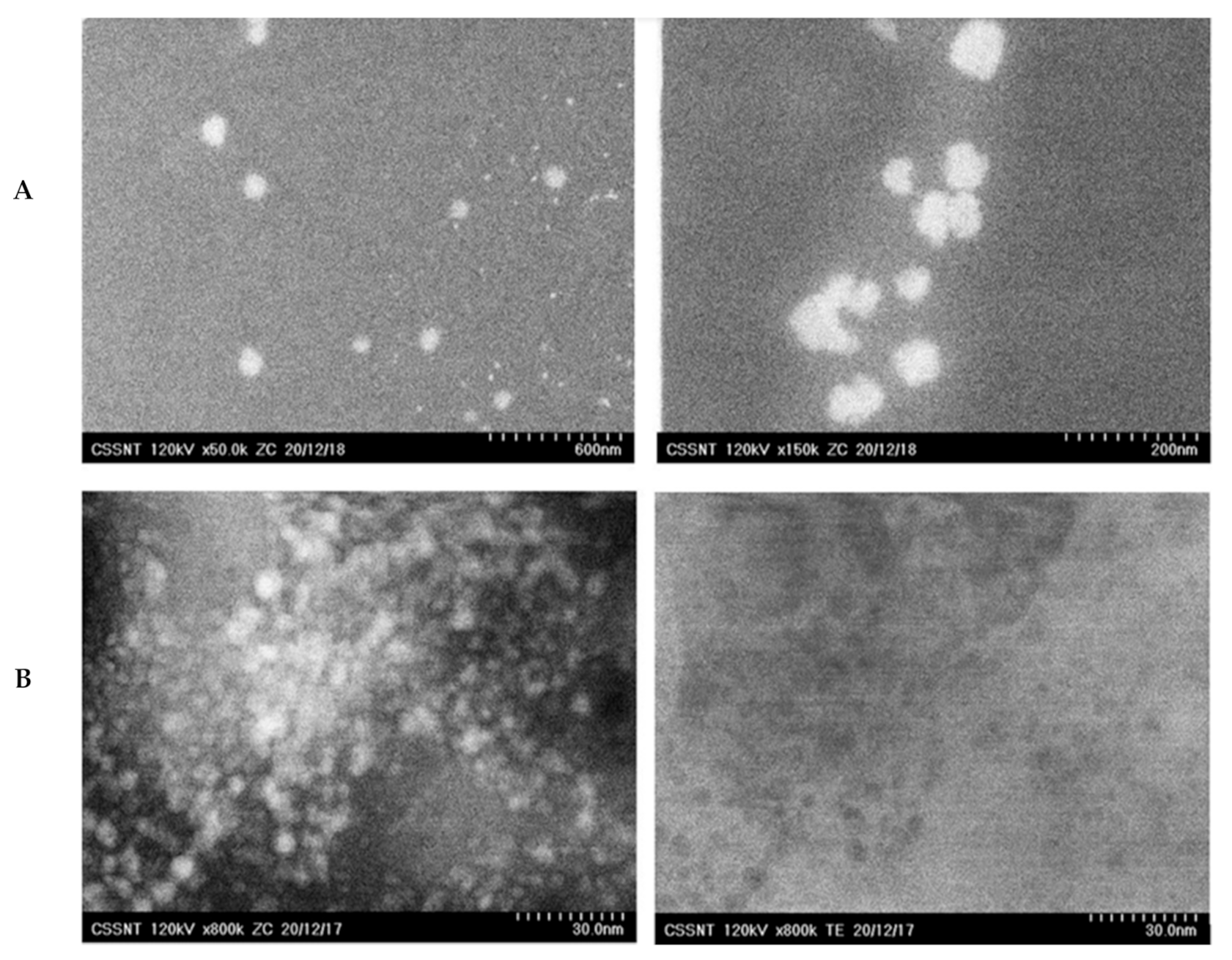
2.2.4. Morphological Characterization
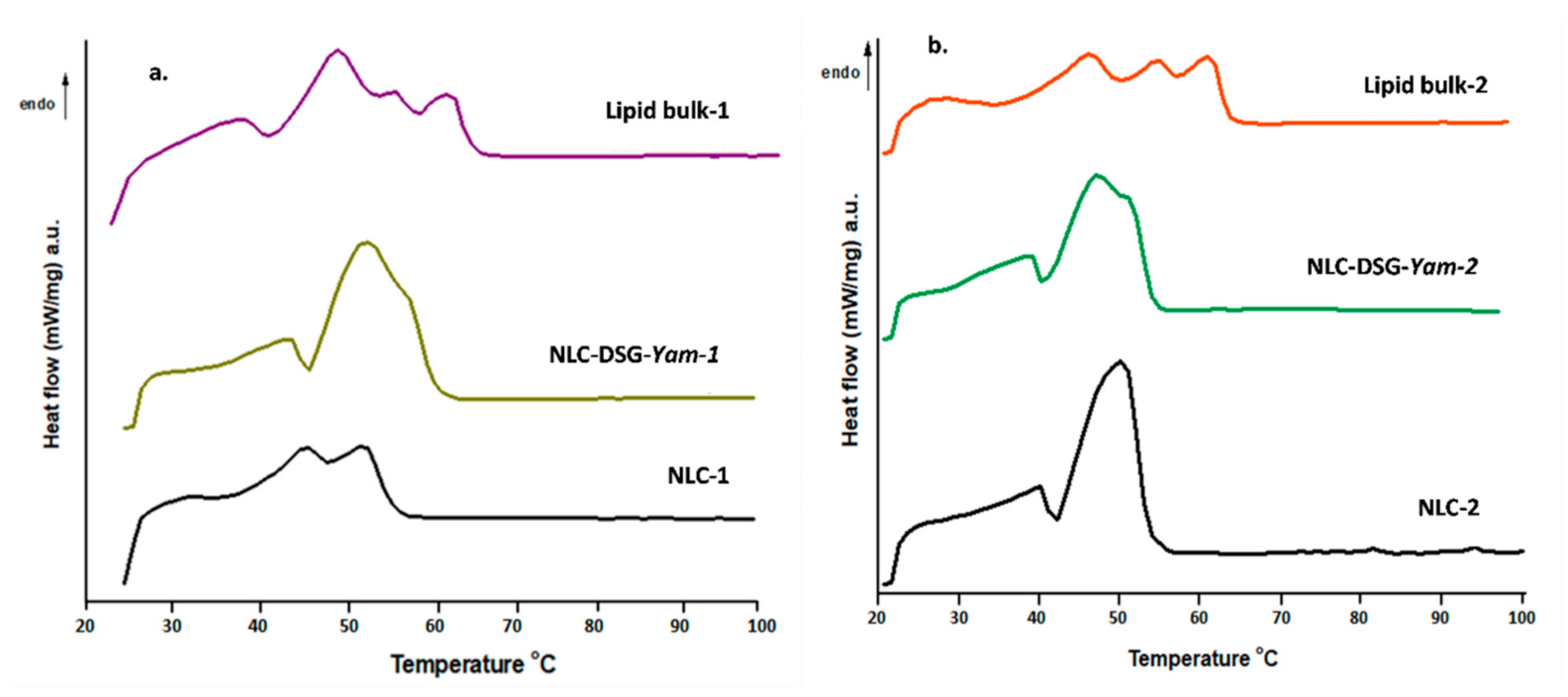
2.2.5. Differential Scanning Calorimetry Analysis
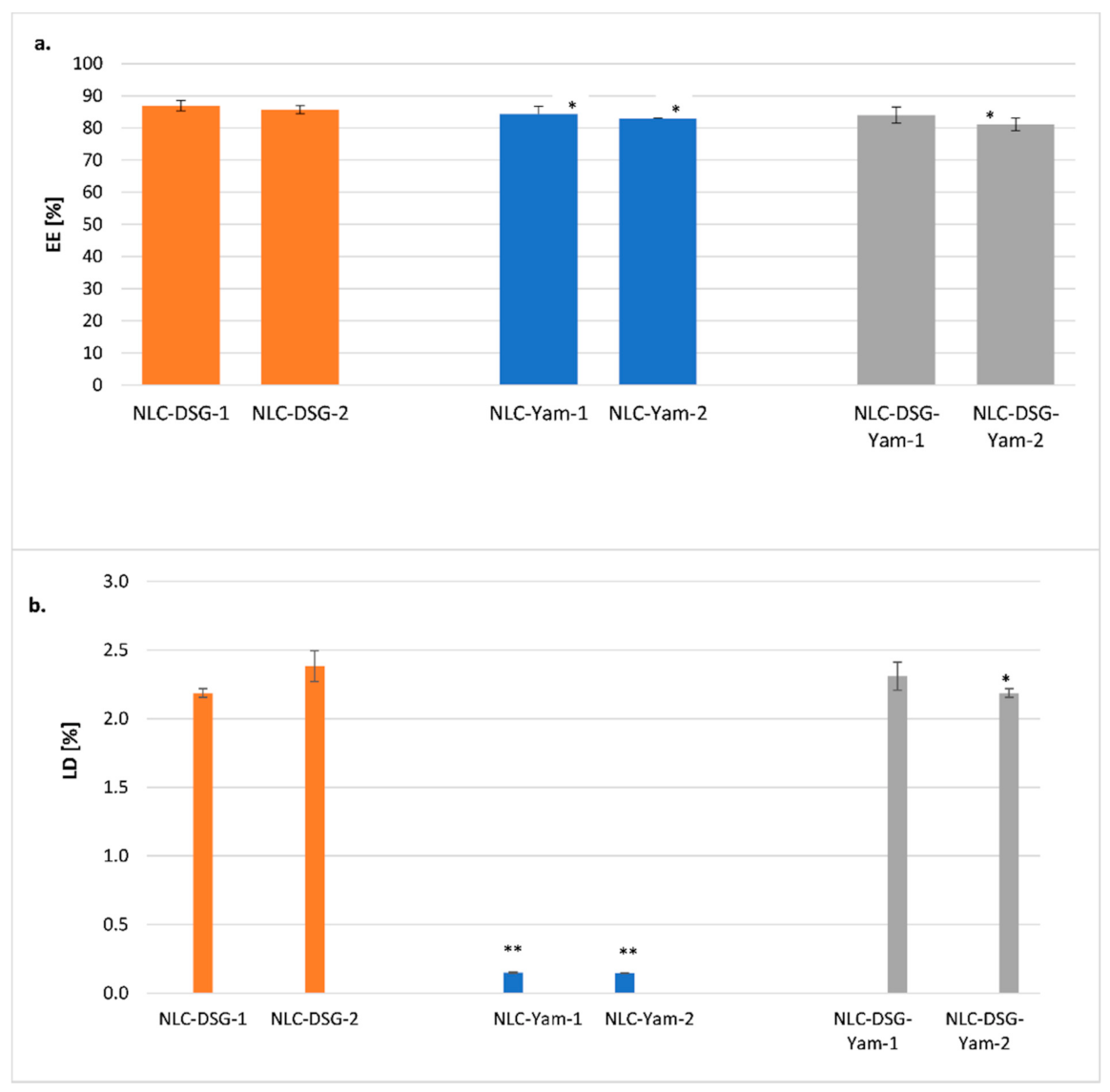
2.2.6. Encapsulation Efficiency (EE%) and Drug Loading (DL%)
2.2.7. In Vitro Antioxidant Activity of NLC against Long/Short-Life Free Radicals
Chemiluminescence Assay
ABTS Assay
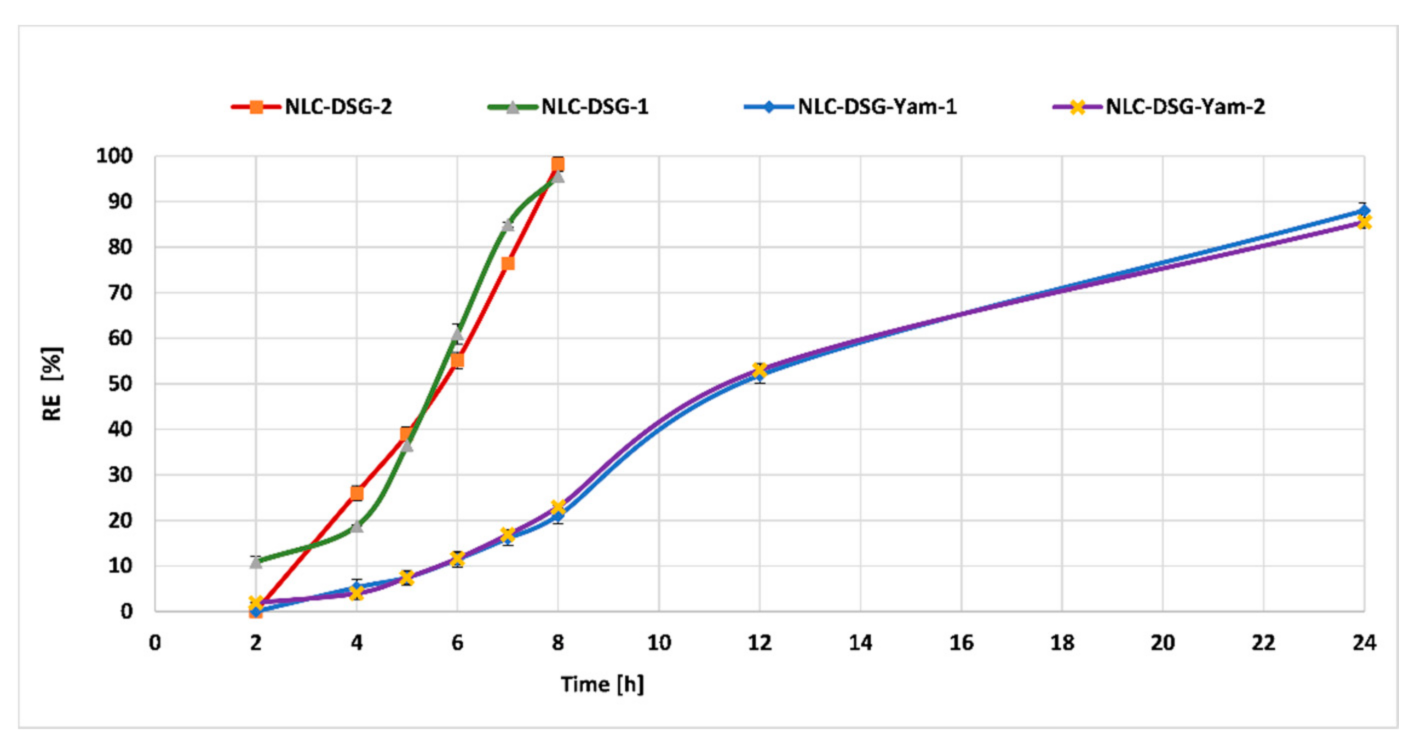
2.2.8. In Vitro Controlled Release
2.2.9. In Vitro Assessment of Cell Viability
Cytotoxicity by MTS Assay
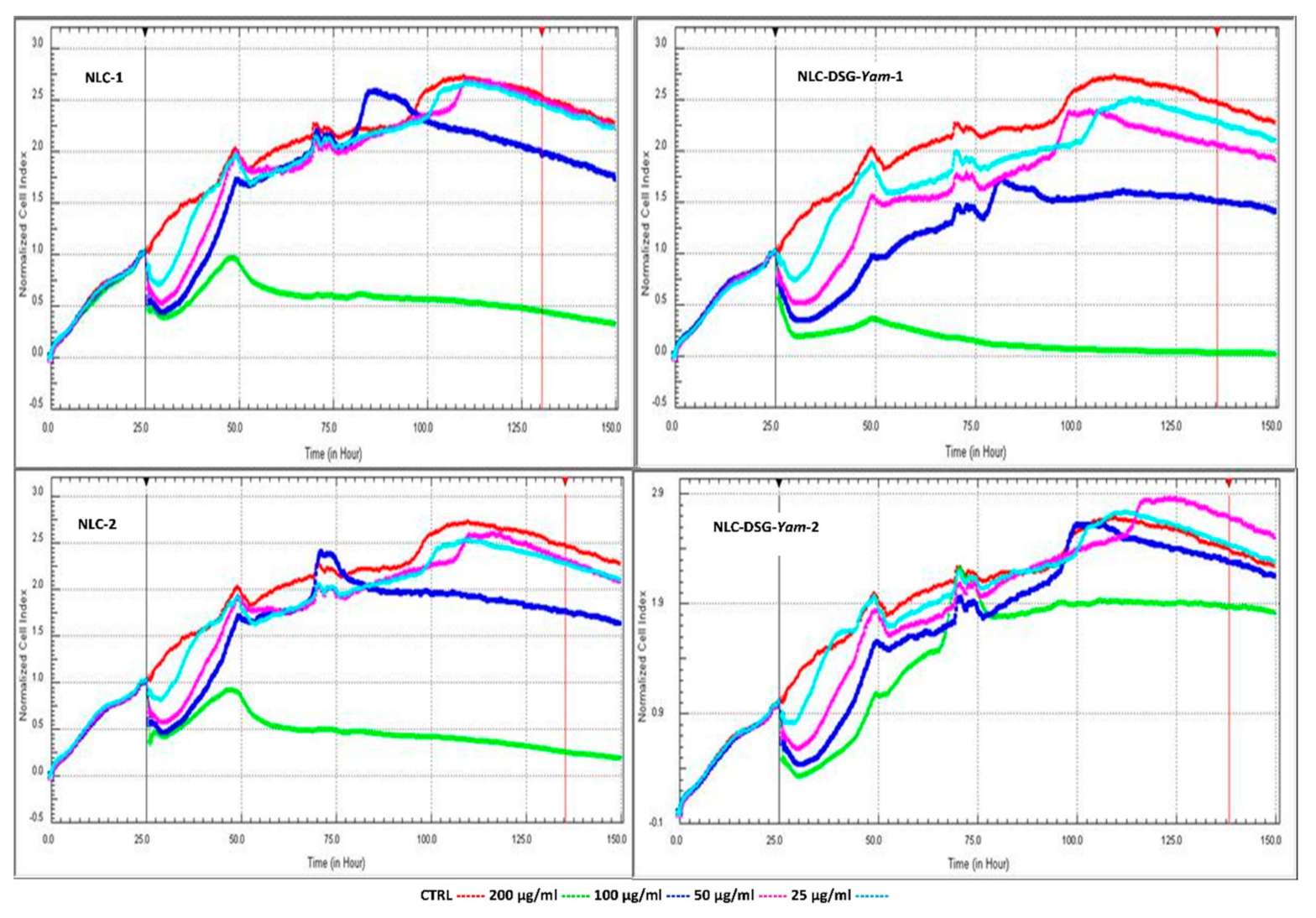
Cytotoxicity by Real-Time Cell Analysis (RTCA)
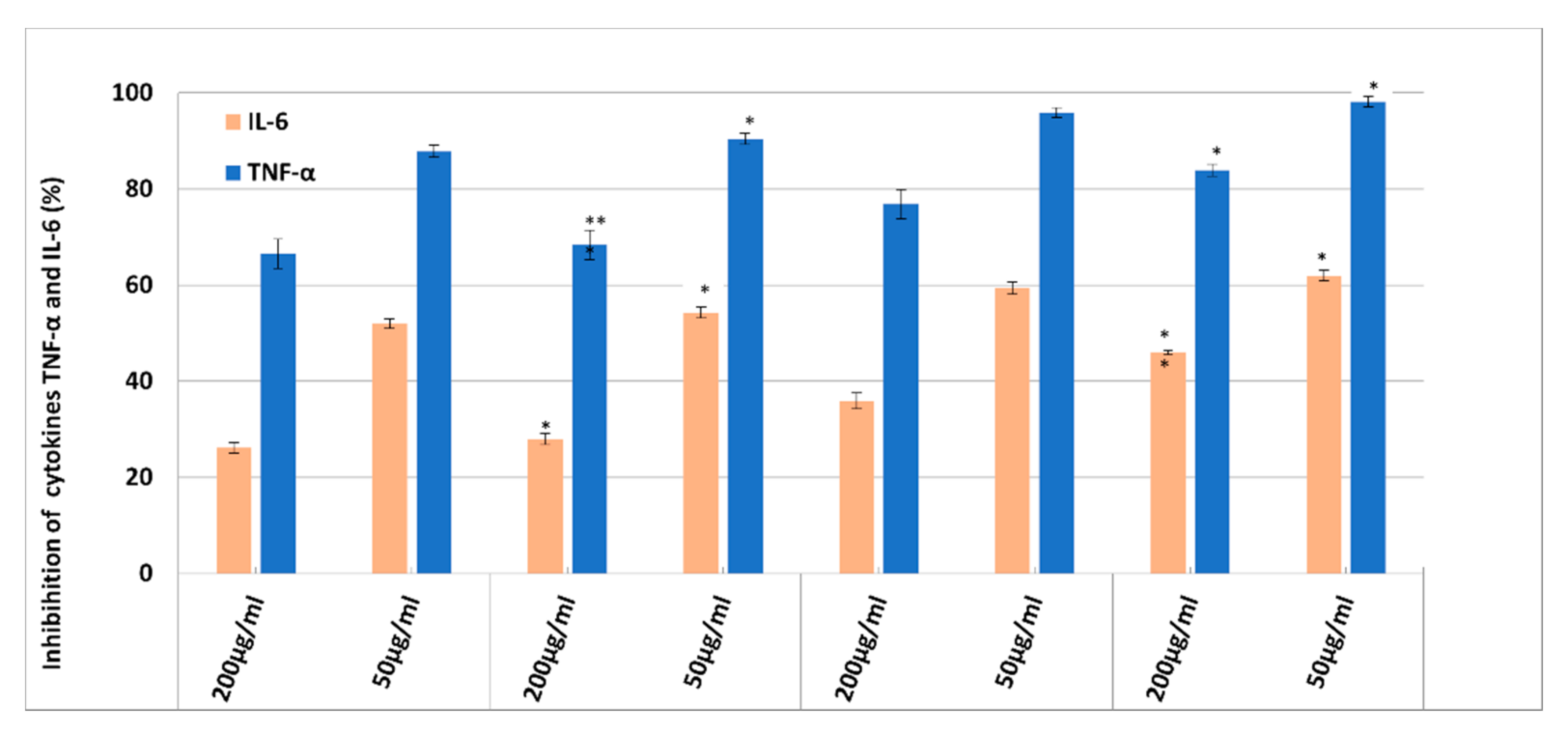
2.2.10. The Assessment of the Anti-Inflammatory Activity (ELISA Assay)
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Size and Stability Features of the NLC Loaded with Hydrophilic and Lipophilic Herbal Bioactive
3.2. The Thermal Behaviour and Entrapment Characteristics of Lipid Nanocarriers Loaded with Herbal Actives
3.3. Assigning In Vitro Cytotoxicity to NLC Systems
3.4. In Vitro Antioxidant Activity through TEAC and Chemiluminescence Method
3.5. In Vitro Controlled Release
3.6. In Vitro Anti-Inflammatory Effect of NLC-DSG-Yam
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [Green Version]
- Segneanu, A.E.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive Molecules Profile from Natural Compounds. In Amino Acid—New Insights and Roles in Plant and Animal; Asao, T., Asaduzzaman, M., Eds.; IntechOpen Book Series: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed 2014, 4, S1–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.R. Natural products chemistry: The emerging trends, and prospective goals. Saudi Pharm. J. 2018, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, Y.; Wang, R.; Yu, F.; Yu, F. Self-assembled nanomaterials for enhanced phototherapy of cancer. ACS Appl. Bio Mater. 2020, 3, 86–106. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, H.; Xing, Y.; Wang, R.; Cheng, Z.; Lv, C.; Lv, Z.; Yu, F. Tumor microenvironment-specific functional nanomaterials for biomedical applications. J. Biomed. Nanotechnol. 2020, 16, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Food Sci. Nutr. 2018, 59, 3129–3151. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Culita, D.C.; Patron, L.; Calderon-Moreno, J.M.; Preda, S.; Oprea, O.; Osiceanu, P.; Pineda, E.M. Investigation of nanocrystalline zinc chromite obtained by two soft chemical routes. Mater. Res. Bull. 2014, 49, 151–159. [Google Scholar] [CrossRef]
- Ganesana, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.A.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Badea, G.; Brasoveanu, L.; Stan, R.; Ott, C.; Oprea, O.; Meghea, A. Ivy leaves extract based—lipid nanocarriers and their bioefficacy on antioxidant and antitumor activities. RSC Adv. 2016, 6, 77243–77255. [Google Scholar] [CrossRef]
- Istrati, D.; Lacatusu, I.; Bordei, N.; Badea, G.; Oprea, O.; Stefan, L.M.; Stan, R.; Badea, N.; Meghea, A. Phyto-mediated nanostructured carriers based on dual vegetable actives involved in the prevention of cellular damage. Mater. Sci. Eng. C 2016, 64, 249–259. [Google Scholar] [CrossRef]
- Lacatusu, I.; Istrati, D.; Bordei, N.; Popescu, M.; Seciu, A.M.; Panteli, L.M.; Badea, N. Synergism of plant extract and vegetable oils-based lipid nanocarriers: Emerging trends in development of advanced cosmetic prototype products. Mater. Sci. Eng. C 2020, 108, 110412. [Google Scholar] [CrossRef]
- Arsenie, L.V.; Lacatusu, I.; Oprea, O.; Borde, N.; Bacalumc, M.; Badea, N. Azelaic acid-willow bark extract-panthenol—Loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crops Prod. 2020, 154, 112658. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, G.; Popescu, M.; Bordei, N.; Istrati, D.; Moldovan, L.; Seciu, A.M.; Pandeli, M.I.; Rasit, I.; Badea, N. Marigold extract, azelaic acid and black caraway oil into lipid nanocarriers provides a strong anti-inflammatory effect in vivo. Ind. Crops Prod. 2017, 109, 141–150. [Google Scholar] [CrossRef]
- Badea, G.; Badea, N.; Brasoveanu, L.I.; Mihaila, M.; Stan, R.; Istrati, D.; Balaci, T.; Lacatusu, I. Naringenin improves the sunscreen performance of hydrogel formulations based on vegetable nanocarriers. New Chem. J. 2017, 41, 480–492. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Udeanu, D.; Coc, L.; Pop, A.; Cioates Negut, C.; Tanase, C.; Stan, R.; Meghea, A. Improved anti-obesity effect of herbal active and endogenous lipids co-loaded lipid nanocarriers: Preparation, in vitro and in vivo evaluation. Mater. Sci. Eng. C 2019, 99, 12–24. [Google Scholar] [CrossRef]
- Padhan, B.; Panda, D. Potential of neglected and underutilized yams (Dioscorea spp.) for improving nutritional security and health benefits. Front. Pharmacol. 2020, 11, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramaa, D.; Boruahb, M.; Yachnaa, K.; Ranaa, V.; Banika, K.; Harshaa, C.; Thakura, K.K.; Duttab, U.; Aryac, A.; Maod, X.; et al. Diosgenin, a steroidal saponin, and its analogs: Effective therapies against different chronic diseases. Life Sci. 2020, 280, 118–182. [Google Scholar] [CrossRef] [PubMed]
- Padhan, B.; Nayak, J.K.; Panda, D. Natural antioxidant potential of selected underutilized wild yams (Dioscorea spp.) for health benefit. J. Food Sci. Technol. 2020, 57, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Satija, S.; Gupta, P.R.; Mahajan, S.; Sharma, N.; Khurana, N.; Kalsi, V.; Duggal, N.; Singh, A.; Mehta, M. Chromatographic fingerprinting, antioxidant, and anti-inflammatory potential of Dioscorea villosa (wild yam) leaves. Int. J. Green Pharm. 2018, 12, 102–106. [Google Scholar] [CrossRef]
- Yan, C.; You-Mei, T.; Su-Lan, Y.; Yu-Wei, H.; Jun-Ping, H.; Bao-Lin, L.; Bo-Yang, Y. Advances in the pharmacological activities and mechanisms of diosgenin. Chin. J. Nat. Med. 2015, 13, 578–587. [Google Scholar] [CrossRef]
- Raju, J.; Rao, C.V. 7—Diosgenin, a Steroid Saponin Constituent of Yams and Fenugreek: Emerging Evidence for Applications in Medicine. In Bioactive Compounds in Phytomedicine; Rasooli, I., Ed.; IntechOpen Book Series: London, UK, 2012. [Google Scholar]
- Hajizadeh, M.R.; Parvaz, N.; Barani, M.; Khoshdel, A.; Fahmidehkar, M.A.; Mahmoodi, M.; Torkzadeh-Mahani, M. Diosgenin-loaded niosome as an effective phytochemical nanocarrier: Physicochemical characterization, loading efficiency, and cytotoxicity assay. J. Pharm. Sci. 2019, 27, 329–339. [Google Scholar] [CrossRef]
- Liu, C.-Z.; Chang, J.-H.; Zhang, L.; Xue, H.F.; Liu, X.G.; Liu, P.; Fu, Q. Preparation and evaluation of diosgenin nanocrystals to improve oral bioavailability. AAPS Pharm. Sci. Tech. 2017, 18, 2067–2076. [Google Scholar] [CrossRef]
- Okawara, M.; Hashimoto, F.; Todo, H.; Sugibayashi, K.; Tokudome, Y. Effect of liquid crystals with cyclodextrin on the bioavailability of a poorly water-soluble compound, diosgenin, after its oral administration to rats. Int. J. Pharm. 2014, 472, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, A.-M.; Zhang, Y.; Hou, Y.; Huang, J.-H.; Hui, M.; Lee, K.-K.; Youl Lee, K.; Ju Chun, C. Preparation, characterization, and cytotoxicity evaluation of self-assembled nanoparticles of diosgenin-cytarabine conjugate. Food Chem. Toxicol. 2021, 151, 202114. [Google Scholar] [CrossRef]
- Pathak, S.R.; Kumar, R.; Mehndiratta, P.; Mishra, N.K.; Thukral, A.; Singh, R. Bioavailability enhancement of poorly water-soluble nano diosgenin by encapsulation using chitosan/bovine serum albumin bilayers. Asian J. Pharm. 2018, 12, 115–119. [Google Scholar] [CrossRef]
- Kumar, R.; Siril, P.F. Controlling the size and morphology of griseofulvin nanoparticles using polymeric stabilizers by evaporation-assisted solvent–antisolvent, interaction method. J. Nanopart. Res. 2015, 17, 256. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.; Sharma, K.; Dhasmana, D.; Garg, N.; Siril, P.F. Preparation, characterization, and in vitro cytotoxicity of Fenofibrate and Nabumetone loaded solid lipid nanoparticles. Mater. Sci. Eng. C 2020, 106, 110184. [Google Scholar] [CrossRef]



| Type of NLC * | Lipid Phase (10%), g | Surfactant Mixture (2.5%) g | Herbal Bioactives ***, g | |||||
|---|---|---|---|---|---|---|---|---|
| GMS | CP | EPO/SOY | Polox 188 | Tw 20 | SC | DSG | Yam | |
| NLC-1/2 ** | 3.5 | 3.5 | 3.0 | 0.375 | 0.875 | 1.25 | - | - |
| NLC-DSG-1/2 | 0.5 | - | ||||||
| NLC-Yam-1/2 | - | 0.5 | ||||||
| NLC-DSG-Yam-1/2 | 0.5 | 0.5 | ||||||
| Sample | Tm (°C) | ΔHm (J/g) | IC | Tc (°C) | ΔHc (J/g) |
|---|---|---|---|---|---|
| NLC-1 | 46.2 | 6.65 | - | 35.8 | 28.7 |
| 52.2 | 51.08 | 27.67 | |||
| NLC-DSG-Yam-1 | 44.2 | 9.46 | - | 37.4 | 21.58 |
| 53.1 | 64.17 | 34.76 | 42 | 2.935 | |
| Lipids bulk 1 (MSG + PC + EPO) | 37.4 | 7.61 | 100 | 37.9 | 16.26 |
| 48.6 | 18.46 | 41.5 | 1.98 | ||
| 55.9 | 1.84 | 47.3 | 8.08 | ||
| 61.7 | 9.01 | - | - | ||
| NLC-2 | 39.9 | 8.59 | - | 38.5 | 25.02 |
| 50 | 58.66 | 44.81 | 43.2 | 4.736 | |
| NLC-DSG-Yam-2 | 38.8 | 7.232 | - | 38 | 15.75 |
| 48.5 | 51.84 | 39.60 | 42.7 | 3.288 | |
| Lipids bulk 2 (MSG + PC + SOY) | 47.1 | 13.09 | 100 | 38.8 | 15.82 |
| 55.5 | 3.8 | 42.4 | 2.8 | ||
| 62 | 9.51 | 48.1 | 8.169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordache, T.-A.; Badea, N.; Mihaila, M.; Crisan, S.; Pop, A.L.; Lacatusu, I. Challenges in Coopted Hydrophilic and Lipophilic Herbal Bioactives in the Same Nanostructured Carriers for Effective Bioavailability and Anti-Inflammatory Action. Nanomaterials 2021, 11, 3035. https://doi.org/10.3390/nano11113035
Iordache T-A, Badea N, Mihaila M, Crisan S, Pop AL, Lacatusu I. Challenges in Coopted Hydrophilic and Lipophilic Herbal Bioactives in the Same Nanostructured Carriers for Effective Bioavailability and Anti-Inflammatory Action. Nanomaterials. 2021; 11(11):3035. https://doi.org/10.3390/nano11113035
Chicago/Turabian StyleIordache, Teodora-Alexandra, Nicoleta Badea, Mirela Mihaila, Simona Crisan, Anca Lucia Pop, and Ioana Lacatusu. 2021. "Challenges in Coopted Hydrophilic and Lipophilic Herbal Bioactives in the Same Nanostructured Carriers for Effective Bioavailability and Anti-Inflammatory Action" Nanomaterials 11, no. 11: 3035. https://doi.org/10.3390/nano11113035







