Tailoring the Structural and Optical Properties of Germanium Telluride Phase-Change Materials by Indium Incorporation
Abstract
:1. Introduction
2. Computational Details
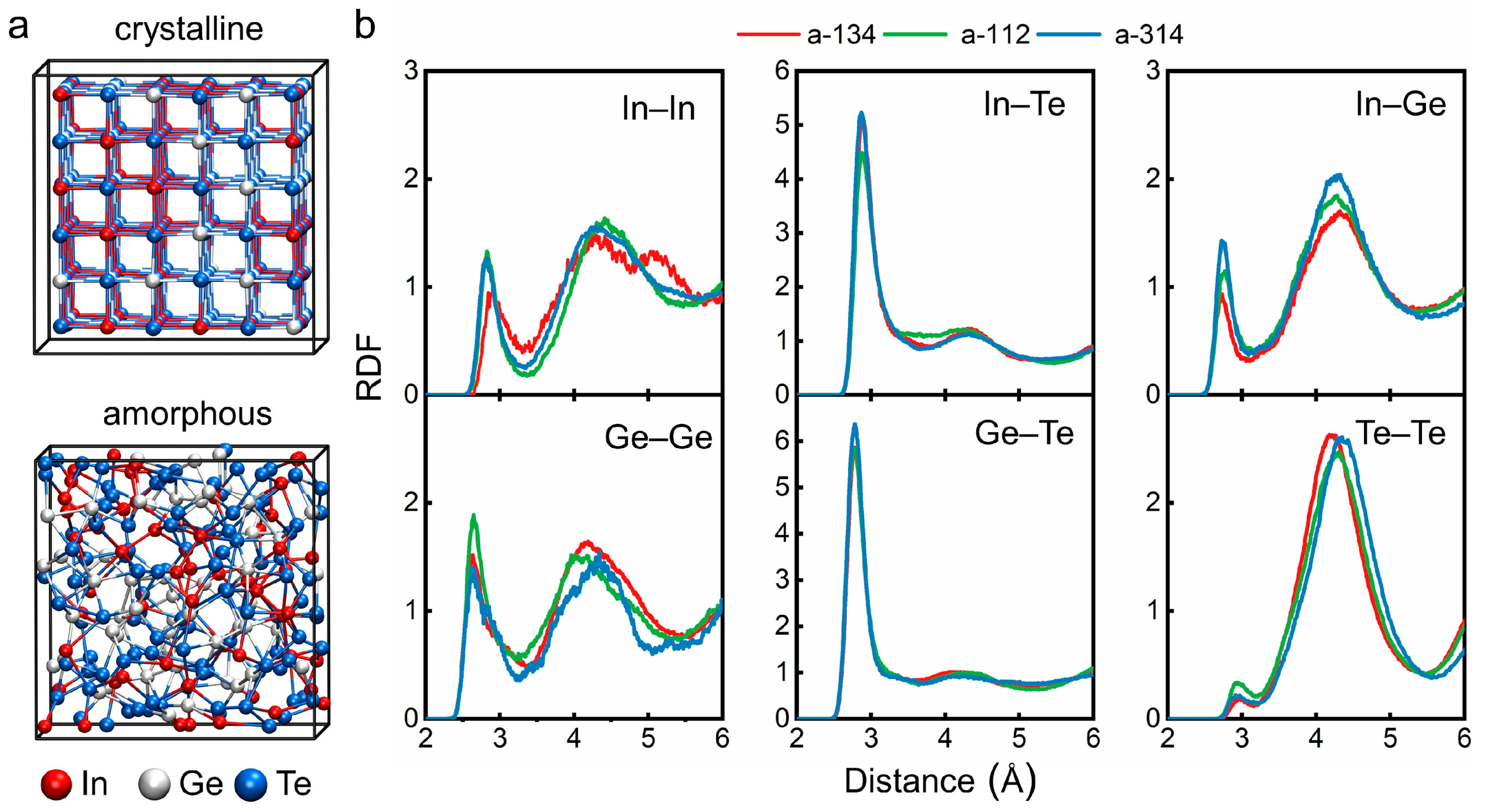
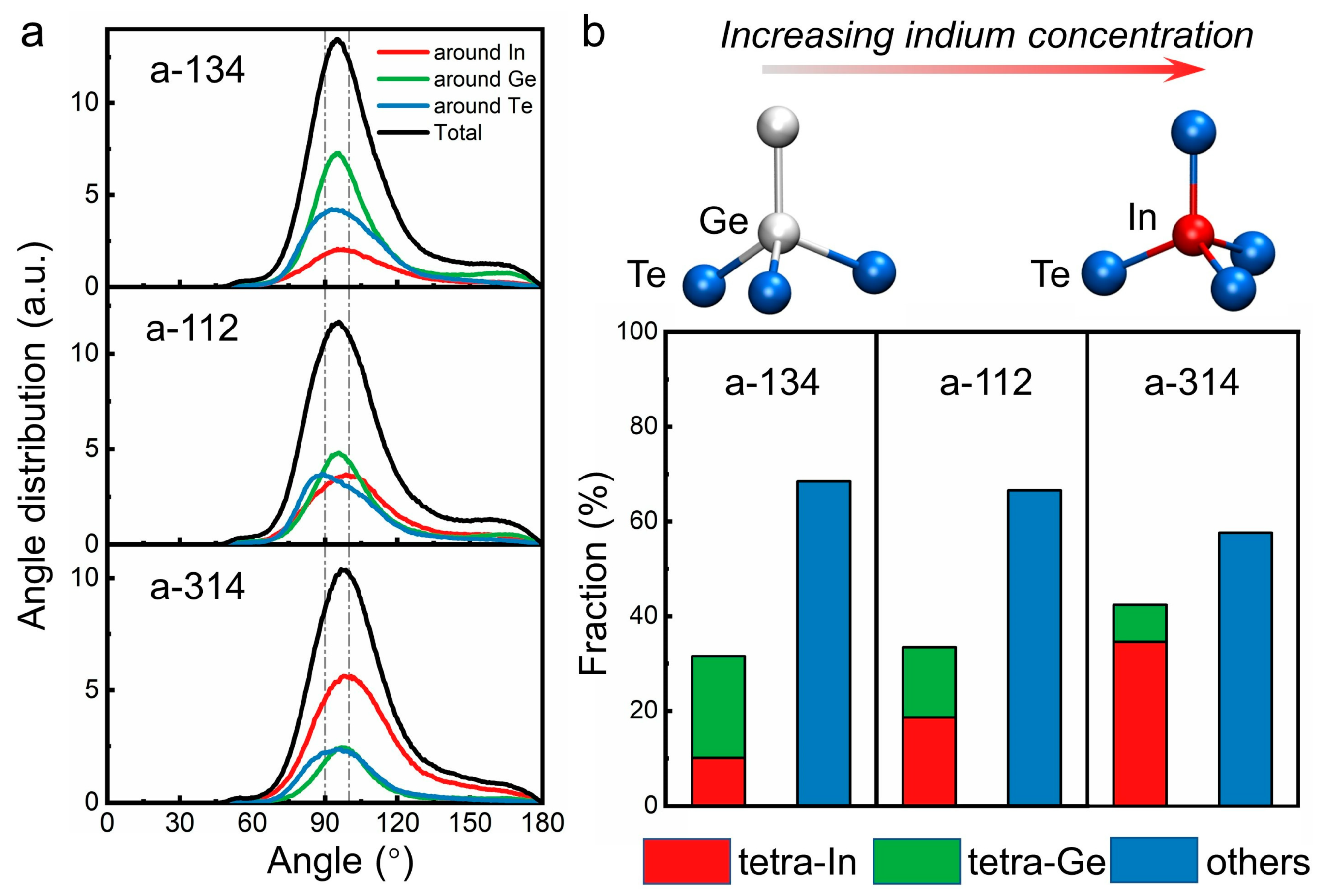
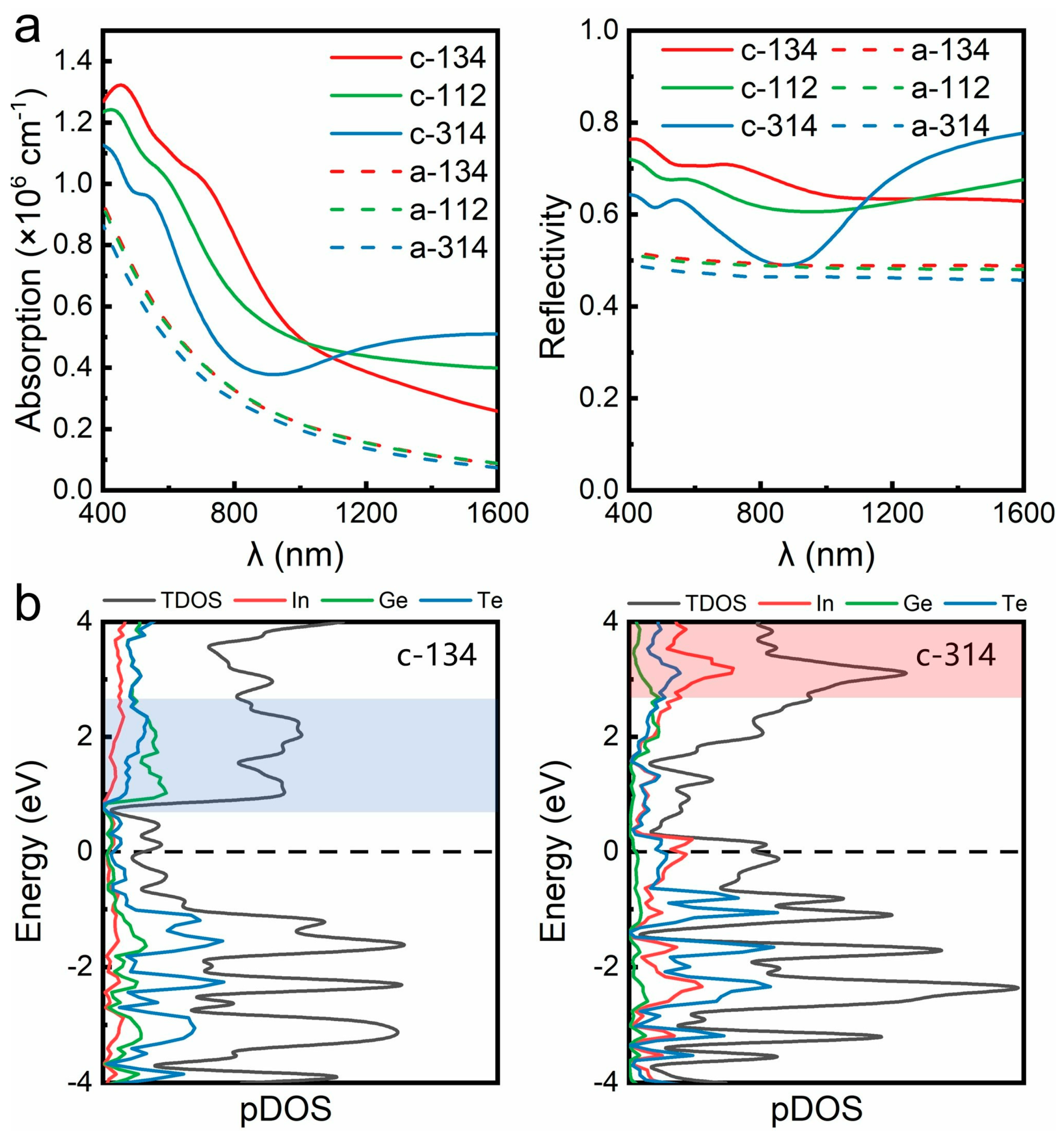
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef]
- Wong, H.-S.P.; Raoux, S.; Kim, S.B.; Liang, J.; Reifenberg, J.P.; Rajendran, B.; Asheghi, M.; Goodson, K.E. Phase Change Memory. Proc. IEEE 2010, 98, 2201. [Google Scholar] [CrossRef]
- Raoux, S.; Welnic, W.; Ielmini, D. Phase change materials and their application to nonvolatile memories. Chem. Rev. 2010, 110, 240–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, E. Unveiling the structural origin to control resistance drift in phase-change memory materials. Mater. Today 2020, 41, 156–176. [Google Scholar] [CrossRef]
- Zhang, W.; Wuttig, M. Phase Change Materials and Superlattices for Non-Volatile Memories. Phys. Status Solidi RRL 2019, 13, 1900130. [Google Scholar] [CrossRef]
- Li, X.-B.; Chen, N.-K.; Wang, X.-P.; Sun, H.-B. Phase-Change Superlattice Materials toward Low Power Consumption and High Density Data Storage: Microscopic Picture, Working Principles, and Optimization. Adv. Funct. Mater. 2018, 28, 1803380. [Google Scholar] [CrossRef]
- Cappelletti, P.; Annunziata, R.; Arnaud, F.; Disegni, F.; Maurelli, A.; Zuliani, P. Phase change memory for automotive grade embedded NVM applications. J. Phys. D Appl. Phys. 2020, 53, 193002. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Xie, C.; Cai, D.; Song, S.; Chen, Y.; Lei, Y.; Zhu, M.; Song, Z. Enhancing the Performance of Phase Change Memory for Embedded Applications. Phys. Status Solidi RRL 2019, 13, 1800558. [Google Scholar] [CrossRef]
- Sebastian, A.; Le Gallo, M.; Burr, G.W.; Kim, S.; BrightSky, M.; Eleftheriou, E. Tutorial: Brain-inspired computing using phase-change memory devices. J. Appl. Phys. 2018, 124, 111101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Mazzarello, R.; Wuttig, M.; Ma, E. Designing crystallization in phase-change materials for universal memory and neuro-inspired computing. Nat. Rev. Mater. 2019, 4, 150–168. [Google Scholar] [CrossRef]
- Ambrogio, S.; Narayanan, P.; Tsai, H.; Shelby, R.M.; Boybat, I.; Burr, G.W. Equivalent-accuracy accelerated neural network training using analogue memory. Nature 2018, 558, 60–67. [Google Scholar] [CrossRef]
- Sebastian, A.; Le Gallo, M.; Khaddam-Aljameh, R.; Eleftheriou, E. Memory devices and applications for in-memory computing. Nat. Nanotechnol. 2020, 15, 529–544. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, T.; Yang, Y.; Huang, R. A comprehensive review on emerging artificial neuromorphic devices. Appl. Phys. Rev. 2020, 7, 011312. [Google Scholar] [CrossRef]
- Xu, M.; Mai, X.; Lin, J.; Zhang, W.; Li, Y.; He, Y.; Tong, H.; Hou, X.; Zhou, P.; Miao, X. Recent Advances on Neuromorphic Devices Based on Chalcogenide Phase-Change Materials. Adv. Funct. Mater. 2020, 30, 2003419. [Google Scholar] [CrossRef]
- Ding, K.; Wang, J.; Zhou, Y.; Tian, H.; Lu, L.; Mazzarello, R.; Jia, C.; Zhang, W.; Rao, F.; Ma, E. Phase-change heterostructure enables ultralow noise and drift for memory operation. Science 2019, 366, 210–215. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Yan, L.; Zhang, T.; Yang, Y.; Song, Z.; Huang, R. Accelerated Local Training of CNNs by Optimized Direct Feedback Alignment Based on Stochasticity of 4 Mb C-doped Ge2Sb2Te5 PCM Chip in 40 nm Node. In Proceedings of the 2020 IEEE International Electron Devices Meeting, San Francisco, CA, USA, 12–18 December 2020. [Google Scholar]
- Lim, D.H.; Wu, S.; Zhao, R.; Lee, J.H.; Jeong, H.; Shi, L. Spontaneous sparse learning for PCM-based memristor neural networks. Nat. Commun. 2021, 12, 319. [Google Scholar] [CrossRef]
- Khan, A.I.; Daus, A.; Islam, R.; Neilson, K.M.; Lee, H.R.; Wong, H.-S.P.; Pop, E. Ultralow switching current density multilevel phase-change memory on a flexible substrate. Science 2021, 373, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, P.; Wright, C.D.; Bhaskaran, H. An optoelectronic framework enabled by low-dimensional phase-change films. Nature 2014, 511, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Milne, T.; Salter, P.; Kim, J.S.; Humphrey, S.; Booth, M.; Bhaskaran, H. Antimony thin films demonstrate programmable optical nonlinearity. Sci. Adv. 2021, 7, eabd7097. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, W.; Wang, H.; Lu, L.; Ruan, Q.; Tan, Y.S.; Simpson, R.E.; Yang, J.K.W. Rewritable color nanoprints in antimony trisulfide films. Sci. Adv. 2020, 6, eabb7171. [Google Scholar] [CrossRef]
- Ovshinsky, S.R. Optical Cognitive Information Processing—A New Field. Jpn. J. Appl. Phys. 2004, 43, 4695–4699. [Google Scholar] [CrossRef]
- Ríos, C.; Stegmaier, M.; Hosseini, P.; Wang, D.; Scherer, T.; Wright, C.D.; Bhaskaran, H.; Pernice, W.H.P. Integrated all-photonic non-volatile multi-level memory. Nat. Photon. 2015, 9, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Ríos, C.; Youngblood, N.; Wright, C.D.; Pernice, W.H.P.; Bhaskaran, H. Device-Level Photonic Memories and Logic Applications Using Phase-Change Materials. Adv. Mater. 2018, 30, 1802435. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, J.; Youngblood, N.; Karpov, M.; Gehring, H.; Li, X.; Stappers, M.; Le Gallo, M.; Fu, X.; Lukashchuk, A.; Raja, A.S.; et al. Parallel convolutional processing using an integrated photonic tensor core. Nature 2021, 589, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, L.; Lu, L.; Xu, J.; Wang, N.; Hu, H.; Rahman, B.M.A.; Zhou, Z.; Chen, J. Miniature Multilevel Optical Memristive Switch Using Phase Change Material. ACS Photon. 2019, 6, 2205–2212. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chou, J.B.; Li, J.; Li, H.; Du, Q.; Yadav, A.; Zhou, S.; Shalaginov, M.Y.; Fang, Z.; Zhong, H.; et al. Broadband transparent optical phase change materials for high-performance nonvolatile photonics. Nat. Commun. 2019, 10, 4279. [Google Scholar] [CrossRef]
- Zhang, Y.; Fowler, C.; Liang, J.; Azhar, B.; Shalaginov, M.Y.; Deckoff-Jones, S.; An, S.; Chou, J.B.; Roberts, C.M.; Liberman, V.; et al. Electrically reconfigurable non-volatile metasurface using low-loss optical phase-change material. Nat. Nanotechnol. 2021, 16, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Qiu, Y.; Zhou, X.; Banas, A.; Banas, K.; Breese, M.B.H.; Cao, T.; Simpson, R.E. Tunable Mid-Infrared Phase-Change Metasurface. Adv. Opt. Mater. 2018, 6, 1701346. [Google Scholar] [CrossRef]
- Leitis, A.; Heßler, A.; Wahl, S.; Wuttig, M.; Taubner, T.; Tittl, A.; Altug, H. All-Dielectric Programmable Huygens’ Metasurfaces. Adv. Funct. Mater. 2020, 30, 1910259. [Google Scholar] [CrossRef]
- Wang, Y.; Landreman, P.; Schoen, D.; Okabe, K.; Marshall, A.; Celano, U.; Wong, H.S.P.; Park, J.; Brongersma, M.L. Electrical tuning of phase-change antennas and metasurfaces. Nat. Nanotechnol. 2021, 16, 667–672. [Google Scholar] [CrossRef]
- Tian, J.; Luo, H.; Yang, Y.; Ding, F.; Qu, Y.; Zhao, D.; Qiu, M.; Bozhevolnyi, S.I. Active control of anapole states by structuring the phase-change alloy Ge2Sb2Te5. Nat. Commun. 2019, 10, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholipour, B. The promise of phase-change materials. Science 2019, 366, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Gerislioglu, B.; Bakan, G.; Ahuja, R.; Adam, J.; Mishra, Y.K.; Ahmadivand, A. The role of Ge2Sb2Te5 in enhancing the performance of functional plasmonic devices. Mater. Today Phys. 2020, 12, 100178. [Google Scholar] [CrossRef]
- Bakan, G.; Gerislioglu, B.; Dirisaglik, F.; Jurado, Z.; Sullivan, L.; Dana, A.; Lam, C.; Gokirmak, A.; Silva, H. Extracting the temperature distribution on a phase-change memory cell during crystallization. J. Appl. Phys. 2016, 120, 164504. [Google Scholar] [CrossRef] [Green Version]
- Yamada, N.; Ohno, E.; Nishiuchi, K.; Akahira, N.; Takao, M. Rapid-phase transitions of GeTe-Sb2Te3 pseudobinary amorphous thin films for an optical disk memory. J. Appl. Phys. 1991, 69, 2849–2856. [Google Scholar] [CrossRef]
- van Pieterson, L.; Lankhorst, M.H.R.; van Schijndel, M.; Kuiper, A.E.T.; Roosen, J.H.J. Phase-change recording materials with a growth-dominated crystallization mechanism: A materials overview. J. Appl. Phys. 2005, 97, 083520. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Hsu, T.H.; Raoux, S.; Wu, J.Y.; Du, P.Y.; Breitwisch, M.; Zhu, Y.; Lai, E.K.; Joseph, E.; Mittal, S.; et al. A High Performance Phase Change Memory with Fast Switching Speed and High Temperature Retention by Engineering the GexSbyTez Phase Change Material. In Proceedings of the 2011 International Electron Devices Meeting, Washington, DC, USA, 5–7 December 2011. [Google Scholar]
- Rao, F.; Ding, K.; Zhou, Y.; Zheng, Y.; Xia, M.; Lv, S.; Song, Z.; Feng, S.; Ronneberger, I.; Mazzarello, R.; et al. Reducing the stochasticity of crystal nucleation to enable subnanosecond memory writing. Science 2017, 358, 1423–1427. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, K.; Liu, W.; Zhou, J.; Wu, L.; Song, Z.; Elliott, S.R.; Sun, Z. Multi-level phase-change memory with ultralow power consumption and resistance drift. Sci. Bull. 2021, 66, 2217. [Google Scholar] [CrossRef]
- Zewdie, G.M.; Zhou, Y.-X.; Sun, L.; Rao, F.; Deringer, V.L.; Mazzarello, R.; Zhang, W. Chemical design principles for cache-type Sc-Sb-Te phase-change memory materials. Chem. Mater. 2019, 31, 4008–4015. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, X.; Zhang, W.; Schafer, L.; Reindl, J.; Vom Bruch, F.; Zhou, Y.; Evang, V.; Wang, J.J.; Deringer, V.L.; et al. Materials Screening for Disorder-Controlled Chalcogenide Crystals for Phase-Change Memory Applications. Adv. Mater. 2021, 33, 2006221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, L.; Zewdie, G.M.; Mazzarello, R.; Deringer, V.L.; Ma, E.; Zhang, W. Bonding similarities and differences between Y–Sb–Te and Sc–Sb–Te phase-change memory materials. J. Mater. Chem. C 2020, 8, 3646–3654. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, Y.; Wang, X.; Chen, Y.; Deringer, V.L.; Mazzarello, R.; Zhang, W. Ab initio molecular dynamics and materials design for embedded phase-change memory. NPJ Comput. Mater. 2021, 7, 29. [Google Scholar] [CrossRef]
- Iwasaki, H.; Harigaya, M.; Nonoyama, O.; Kageyama, Y.; Takahashi, M.; Yamada, K.; Deguchi, H.; Ide, Y. Completely Erasable Phase Change Optical Disc II: Application of Ag-In-Sb-Te Mixed-Phase System for Rewritable Compact Disc Compatible with CD-Velocity and Double CD-Velocity. Jpn. J. Appl. Phys. 1993, 32, 5241–5247. [Google Scholar] [CrossRef]
- Matsunaga, T.; Akola, J.; Kohara, S.; Honma, T.; Kobayashi, K.; Ikenaga, E.; Jones, R.O.; Yamada, N.; Takata, M.; Kojima, R. From local structure to nanosecond recrystallization dynamics in AgInSbTe phase-change materials. Nat. Mater. 2011, 10, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Salinga, M.; Carria, E.; Kaldenbach, A.; Bornhöfft, M.; Benke, J.; Mayer, J.; Wuttig, M. Measurement of crystal growth velocity in a melt-quenched phase-change material. Nat. Commun. 2013, 4, 2371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ronneberger, I.; Zalden, P.; Xu, M.; Salinga, M.; Wuttig, M.; Mazzarello, R. How fragility makes phase-change data storage robust: Insights from ab initio simulations. Sci. Rep. 2014, 4, 6529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morikawa, T.; Kurotsuchi, K.; Kinoshita, M.; Matsuzaki, N.; Matsui, Y.; Fujisaki, Y.; Hanzawa, S.; Kotabe, A.; Terao, M.; Moriya, H.; et al. Doped In-Ge-Te Phase Change Memory Featuring Stable Operation and Good Data Retention. In Proceedings of the 2007 IEEE International Electron Devices Meeting, Washington, DC, USA, 10–12 December 2007. [Google Scholar]
- Spreafico, E.; Caravati, S.; Bernasconi, M. First-principles study of liquid and amorphous InGeTe2. Phys. Rev. B 2011, 83, 144205. [Google Scholar] [CrossRef]
- Cecchini, R.; Martella, C.; Lamperti, A.; Brivio, S.; Rossi, F.; Lazzarini, L.; Varesi, E.; Longo, M. Fabrication of ordered Sb–Te and In–Ge–Te nanostructures by selective MOCVD. J. Phys. D Appl. Phys. 2020, 53, 144002. [Google Scholar] [CrossRef]
- Song, K.-H.; Beak, S.-C.; Lee, H.-Y. Amorphous-to-crystalline phase transition of (InTe)x(GeTe) thin films. J. Appl. Phys. 2010, 108, 024506. [Google Scholar] [CrossRef]
- Song, Z.; Wang, R.; Xue, Y.; Song, S. The “gene” of reversible phase transformation of phase change materials: Octahedral motif. Nano Res. 2021. [Google Scholar] [CrossRef]
- Wang, R.; Song, Z.; Song, W.; Xin, T.; Lv, S.; Song, S.; Liu, J. Phase-change memory based on matched Ge-Te, Sb-Te, and In-Te octahedrons: Improved electrical performances and robust thermal stability. InfoMat 2021, 3, 1008–1015. [Google Scholar] [CrossRef]
- Maeda, Y.; Andoh, H.; Ikuta, I.; Minemura, H. Reversible phase-change optical data storage in InSbTe alloy films. J. Appl. Phys. 1988, 64, 1715. [Google Scholar] [CrossRef]
- Miao, N.; Sa, B.; Zhou, J.; Sun, Z.; Blomqvist, A.; Ahuja, R. First-principles investigation on the phase stability and chemical bonding of mInSb·nInTe phase-change random alloys. Solid State Commun. 2010, 150, 1375–1377. [Google Scholar] [CrossRef]
- Los, J.H.; Kühne, T.D.; Gabardi, S.; Bernasconi, M. First-principles study of the amorphous In3SbTe2 phase change compound. Phys. Rev. B 2013, 88, 174203. [Google Scholar] [CrossRef]
- Deringer, V.L.; Zhang, W.; Rausch, P.; Mazzarello, R.; Dronskowski, R.; Wuttig, M. A chemical link between Ge–Sb–Te and In–Sb–Te phase-change materials. J. Mater. Chem. C 2015, 3, 9519–9523. [Google Scholar] [CrossRef] [Green Version]
- Heßler, A.; Wahl, S.; Leuteritz, T.; Antonopoulos, A.; Stergianou, C.; Schön, C.-F.; Naumann, L.; Eicker, N.; Lewin, M.; Maß, T.W.W.; et al. In3SbTe2 as a programmable nanophotonics material platform for the infrared. Nat. Commun. 2021, 12, 924. [Google Scholar] [CrossRef]
- Park, S.J.; Park, S.-J.; Park, D.; An, M.; Cho, M.-H.; Kim, J.; Na, H.; Park, S.h.; Sohn, H. High-mobility property of crystallized In-Te chalcogenide materials. Electron. Mater. Lett. 2012, 8, 175–178. [Google Scholar] [CrossRef]
- Zhang, W.; Deringer, V.L.; Dronskowski, R.; Mazzarello, R.; Ma, E.; Wuttig, M. Density functional theory guided advances in phase-change materials and memories. MRS Bull. 2015, 40, 856–865. [Google Scholar] [CrossRef] [Green Version]
- Kühne, T.; Krack, M.; Mohamed, F.; Parrinello, M. Efficient and Accurate Car-Parrinello-like Approach to Born-Oppenheimer Molecular Dynamics. Phys. Rev. Lett. 2007, 98, 066401. [Google Scholar] [CrossRef] [Green Version]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. cp2k:atomistic simulations of condensed matter systems. WIREs Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, R.; Ertural, C.; George, J.; Deringer, V.L.; Hautier, G.; Dronskowski, R. LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory. J. Comput. Chem. 2020, 41, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Caravati, S.; Bernasconi, M.; Parrinello, M. First principles study of the optical contrast in phase change materials. J. Phys. Condens. Matter 2010, 22, 315801. [Google Scholar] [CrossRef]
- Wełnic, W.; Wuttig, M.; Botti, S.; Reining, L. Local atomic order and optical properties in amorphous and laser-crystallized GeTe. Comptes Rendus Phys. 2009, 10, 514–527. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, X.; Zhou, Y.; Sun, L.; Mazzarello, R.; Zhang, W. Unraveling the optical contrast in Sb2Te and AgInSbTe phase-change materials. J. Phys. Photon. 2021, 3, 03401. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Woolley, J.C. Solid Solution in the GeTe-InTe System. J. Electrochem. Soc. 1965, 112, 906. [Google Scholar] [CrossRef]
- Deringer, V.L.; Zhang, W.; Lumeij, M.; Maintz, S.; Wuttig, M.; Mazzarello, R.; Dronskowski, R. Bonding nature of local structural motifs in amorphous GeTe. Angew. Chem. Int. Ed. 2014, 53, 10817–10820. [Google Scholar] [CrossRef] [PubMed]
- Errington, J.R.; Debenedetti, P.G. Relationship between structural order and the anomalies of liquid water. Nature 2001, 409, 318–321. [Google Scholar] [CrossRef]
- Caravati, S.; Bernasconi, M.; Kühne, T.D.; Krack, M.; Parrinello, M. Coexistence of tetrahedral- and octahedral-like sites in amorphous phase change materials. Appl. Phys. Lett. 2007, 91, 171906. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Zhou, Y.; Zewdie, G.M.; Deringer, V.L.; Mazzarello, R.; Zhang, W. Chemical understanding of resistance drift suppression in Ge-Sn-Te phase-change memory materials. J. Mater. Chem. C 2020, 8, 71–77. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Zhang, B.; Wang, X.-D.; Zhang, W. Density dependent local structures in InTe phase-change materials. arXiv 2021, arXiv:2109.14253. [Google Scholar]
- Mazzarello, R.; Caravati, S.; Angioletti-Uberti, S.; Bernasconi, M.; Parrinello, M. Signature of Tetrahedral Ge in the Raman Spectrum of Amorphous Phase-Change Materials. Phys. Rev. Lett. 2010, 104, 085503. [Google Scholar] [CrossRef] [Green Version]
- Akola, J.; Jones, R.O. Structural phase transitions on the nanoscale: The crucial pattern in the phase-change materials Ge2Sb2Te5 and GeTe. Phys. Rev. B 2007, 76, 235201. [Google Scholar] [CrossRef] [Green Version]
- Raty, J.-Y.; Zhang, W.; Luckas, J.; Chen, C.; Bichara, C.; Mazzarello, R.; Wuttig, M. Aging mechanisms of amorphous phase-change materials. Nat. Commun. 2015, 6, 7467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegrist, T.; Jost, P.; Volker, H.; Woda, M.; Merkelbach, P.; Schlockermann, C.; Wuttig, M. Disorder-induced localization in crystalline phase-change materials. Nat. Mater. 2011, 10, 202–208. [Google Scholar] [CrossRef]
- Cobelli, M.; Galante, M.; Gabardi, S.; Sanvito, S.; Bernasconi, M. First-Principles Study of Electromigration in the Metallic Liquid State of GeTe and Sb2Te3 Phase-Change Compounds. J. Phys. Chem. C 2020, 124, 9599–9603. [Google Scholar] [CrossRef]
- Xie, Y.; Kim, W.; Kim, Y.; Kim, S.; Gonsalves, J.; BrightSky, M.; Lam, C.; Zhu, Y.; Cha, J.J. Self-Healing of a Confined Phase Change Memory Device with a Metallic Surfactant Layer. Adv. Mater. 2018, 30, 1705587. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Baek, K.; Son, S.K.; Song, K.; Oh, J.W.; Jeon, S.-J.; Kim, W.; Yoo, J.H.; Lee, K.J. In situ TEM observation of void formation and migration in phase change memory devices with confined nanoscale Ge2Sb2Te5. Nanoscale Adv. 2020, 2, 3841–3848. [Google Scholar] [CrossRef]
- Wuttig, M.; Bhaskaran, H.; Taubner, T. Phase-change materials for non-volatile photonic applications. Nat. Photon. 2017, 11, 465–476. [Google Scholar] [CrossRef]
- Shportko, K.; Kremers, S.; Woda, M.; Lencer, D.; Robertson, J.; Wuttig, M. Resonant bonding in crystalline phase-change materials. Nat. Mater. 2008, 7, 653–658. [Google Scholar] [CrossRef]
- Lencer, D.; Salinga, M.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Wuttig, M. A map for phase-change materials. Nat. Mater. 2008, 7, 972–977. [Google Scholar] [CrossRef]
- Huang, B.; Robertson, J. Bonding origin of optical contrast in phase-change memory materials. Phys. Rev. B 2010, 81, 081204. [Google Scholar] [CrossRef]
- Wuttig, M.; Deringer, V.L.; Gonze, X.; Bichara, C.; Raty, J.Y. Incipient Metals: Functional Materials with a Unique Bonding Mechanism. Adv. Mater. 2018, 30, 1803777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Cojocaru-Mirédin, O.; Mio, A.M.; Keutgen, J.; Küpers, M.; Yu, Y.; Cho, J.-Y.; Dronskowski, R.; Wuttig, M. Unique Bond Breaking in Crystalline Phase Change Materials and the Quest for Metavalent Bonding. Adv. Mater. 2018, 30, 1706735. [Google Scholar] [CrossRef]
- Raty, J.Y.; Schumacher, M.; Golub, P.; Deringer, V.L.; Gatti, C.; Wuttig, M. A Quantum-Mechanical Map for Bonding and Properties in Solids. Adv. Mater. 2019, 31, 1806280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cojocaru-Mirédin, O.; Keutgen, J.; Yu, Y.; Küpers, M.; Schumacher, M.; Golub, P.; Raty, J.-Y.; Dronskowski, R.; Wuttig, M. Understanding the Structure and Properties of Sesqui-Chalcogenides (i.e., V2VI3 or Pn2Ch3 (Pn = Pnictogen, Ch = Chalcogen) Compounds) from a Bonding Perspective. Adv. Mater. 2019, 31, 1904316. [Google Scholar] [CrossRef] [Green Version]
- Kooi, B.J.; Wuttig, M. Chalcogenides by Design: Functionality through Metavalent Bonding and Confinement. Adv. Mater. 2020, 32, 1908302. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shen, X.; Sun, S.; Zhang, W. Tailoring the Structural and Optical Properties of Germanium Telluride Phase-Change Materials by Indium Incorporation. Nanomaterials 2021, 11, 3029. https://doi.org/10.3390/nano11113029
Wang X, Shen X, Sun S, Zhang W. Tailoring the Structural and Optical Properties of Germanium Telluride Phase-Change Materials by Indium Incorporation. Nanomaterials. 2021; 11(11):3029. https://doi.org/10.3390/nano11113029
Chicago/Turabian StyleWang, Xudong, Xueyang Shen, Suyang Sun, and Wei Zhang. 2021. "Tailoring the Structural and Optical Properties of Germanium Telluride Phase-Change Materials by Indium Incorporation" Nanomaterials 11, no. 11: 3029. https://doi.org/10.3390/nano11113029
APA StyleWang, X., Shen, X., Sun, S., & Zhang, W. (2021). Tailoring the Structural and Optical Properties of Germanium Telluride Phase-Change Materials by Indium Incorporation. Nanomaterials, 11(11), 3029. https://doi.org/10.3390/nano11113029






