Recent Progress in Technetium-99m-Labeled Nanoparticles for Molecular Imaging and Cancer Therapy
Abstract
:1. Introduction
2. Radiolabeling of Nanomaterials
3. Radiolabeling of Nanomaterial Using 99mTc
Technetium-99m (99mTc) Properties and Production
4. Inorganic Nanoparticles
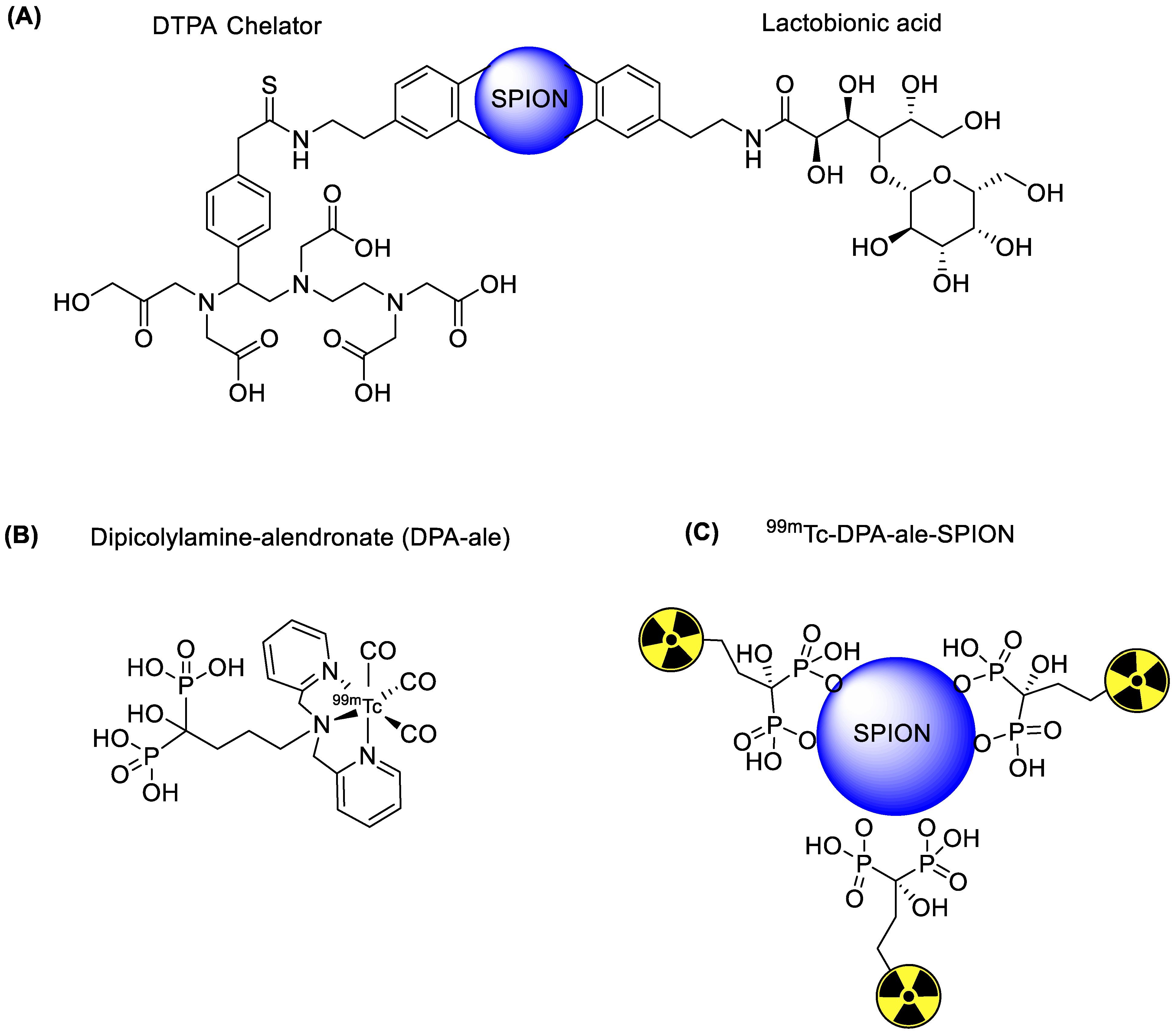
4.1. Iron Oxide Nanoparticles
4.2. Gold Nanoparticles
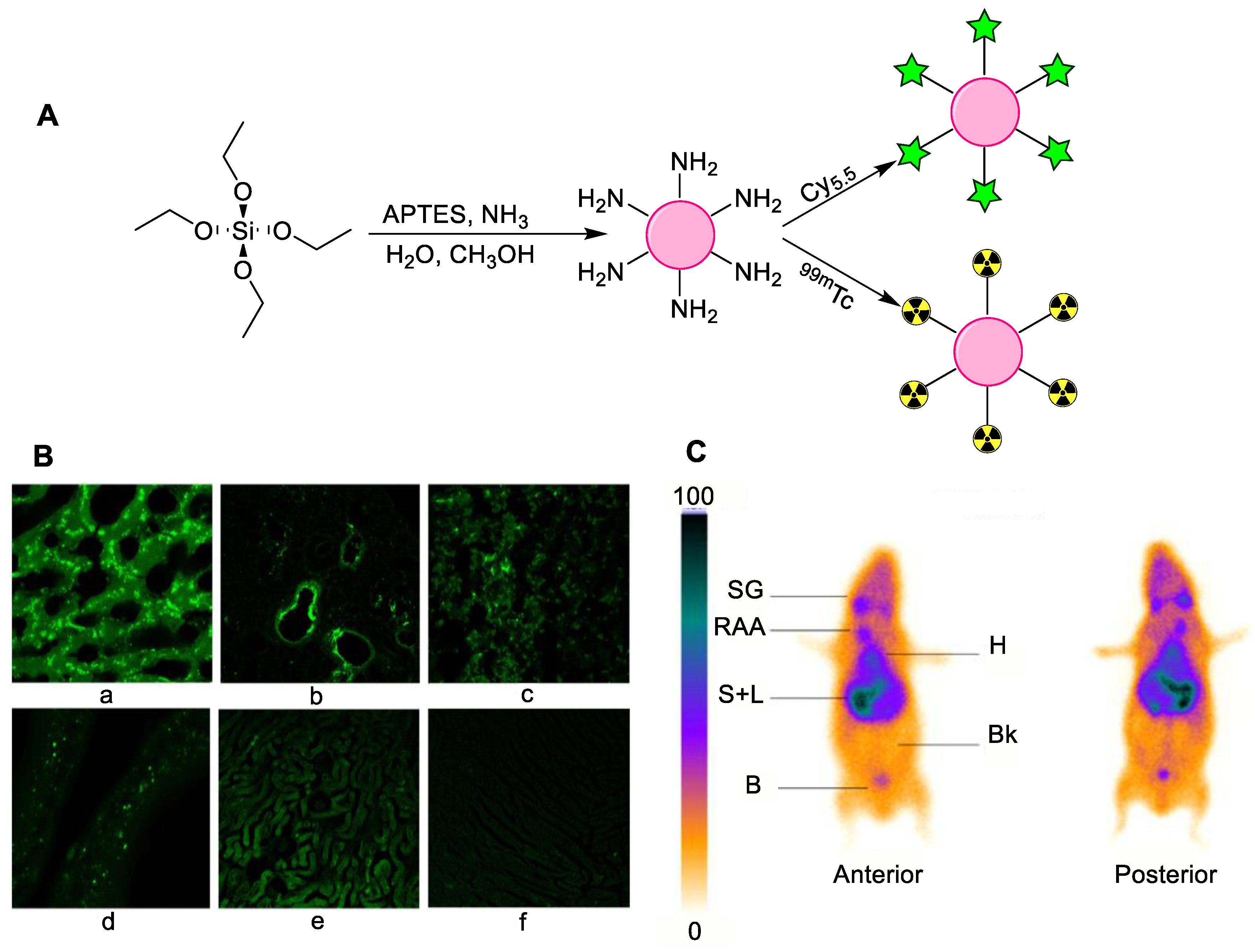
4.3. Silica Nanoparticles
4.4. Titanium Nanoparticles
| Nanoparticle | Application | Drug Loaded on NPs | Radiolabeling Method | Ref. |
|---|---|---|---|---|
| Iron oxide Nanoparticles | Hyperthermia procedure | Without drug | Direct radiolabeling using tetrahydroborate exchange resin (reducing agent) | [27] |
| SPECT and MRI based imaging of hepatocytes | Lactobionic acid | Chelator-based radiolabeling using DTPA and stannous chloride (reducing agent) | [28] | |
| The liver and spleen imaging | Without drug | Chelator-based radiolabeling using bisphosphonate chelator | [29] | |
| The liver and spleen imaging | Dimerccaptosuccinic acid (DMSA) | Chelator-based radiolabeling using DMSA | [30] | |
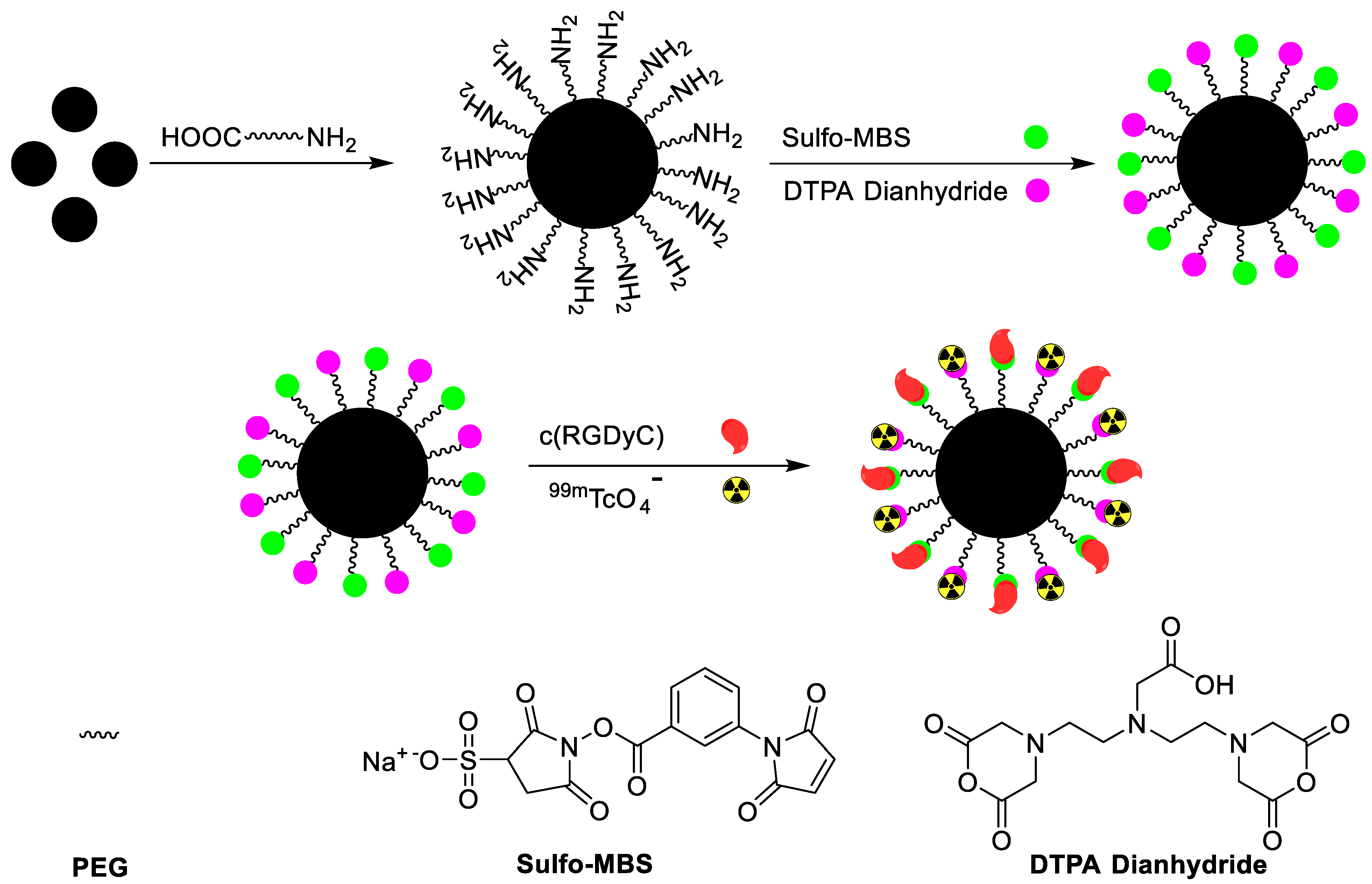
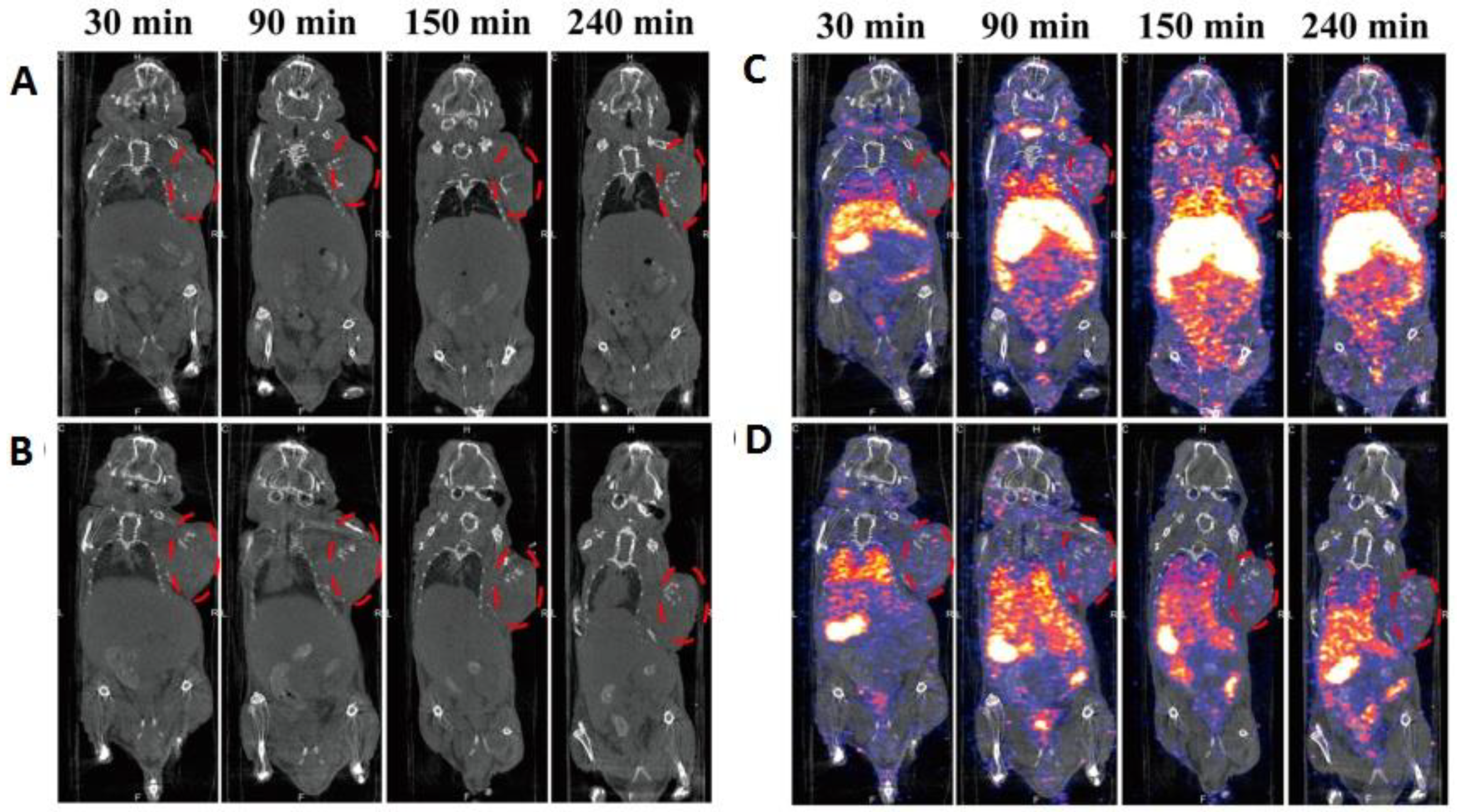
| SPECT and MRI imaging of H1299 αvβ3-positive cells | c(RGDyC) peptide | Chelator-based radiolabeling using DTPA and stannous chloride (reducing agent) | [31] | |
| αvβ3-positive tumor imaging | c(RGDyC) and glutathione (GSH) | Direct radiolabeling using stannous chloride (reducing agent) | [32] | |
| Fe3O4 and CoFe2O4 nanoparticles for liver and spleen imaging | Without drug | Direct radiolabeling using stannous chloride (reducing agent) | [33] | |
| Gold Nanoparticles | GRP-r receptor-based therapy of prostate cancer | Lys3-bombesin and HYNIC-Gly-Gly-Cys-NH2 [HYNIC (hydrazinonicotinamide), GGC(Gly-Gly-Cys) peptide | Chelator-based radiolabeling using HYNIC and stannous chloride (reducing agent) | [35] |
| Lymph node (SLN) imaging | Without drug | Chelator-based radiolabeling using HYNIC and stannous chloride (reducing agent) | [36] | |
| αvβ3-positive tumor imaging | HYNIC-GGC and cyclic[Arg-Gly-Asp-Phe-Lys(Cys)] {c[RGDfK(C)]}. | Chelator-based radiolabeling using HYNIC and stannous chloride (reducing agent) | [37] | |
| GRP-r-positive tumors, SLN detection and αvβ3 positive tumors | Lys3-bombesin, thiol-mannose or cyclo[Arg–Gly–Asp–D–Phe–Lys–(Cys)] c[RDGfK(C)] | Chelator-based radiolabeling using HYNIC and stannous chloride (reducing agent) | [38] | |
| Plasmonic photothermal therapy | HIV Tat (49–57) peptide and bombesin | Chelator-based radiolabeling using HYNIC and stannous chloride (reducing agent) | [39] | |
| SPECT/CT imaging of tumor xenografted model | Poly(amidoamine) (PAMAM) dendrimers | Chelator-based radiolabeling using DTPA and stannous chloride (reducing agent) | [40] | |
| HT29 cells | Resveratrol (Res) | Direct radiolabeling using stannous chloride (reducing agent) | [41] | |
| αvβ3-positive tumor imaging | c(RGDyC) peptide | Chelator-based radiolabeling using DTPA and stannous chloride (reducing agent) | [43] | |
| Monitoring of tumor apoptosis | Duramycin | Chelator-based radiolabeling using DOTA and stannous chloride (reducing agent) | [44] | |
| Tumor targeting | Alkoxyphenylacylsulfonamide (APAS) | Chelator-based radiolabeling using DTPA and stannous chloride (reducing agent) | [45] | |
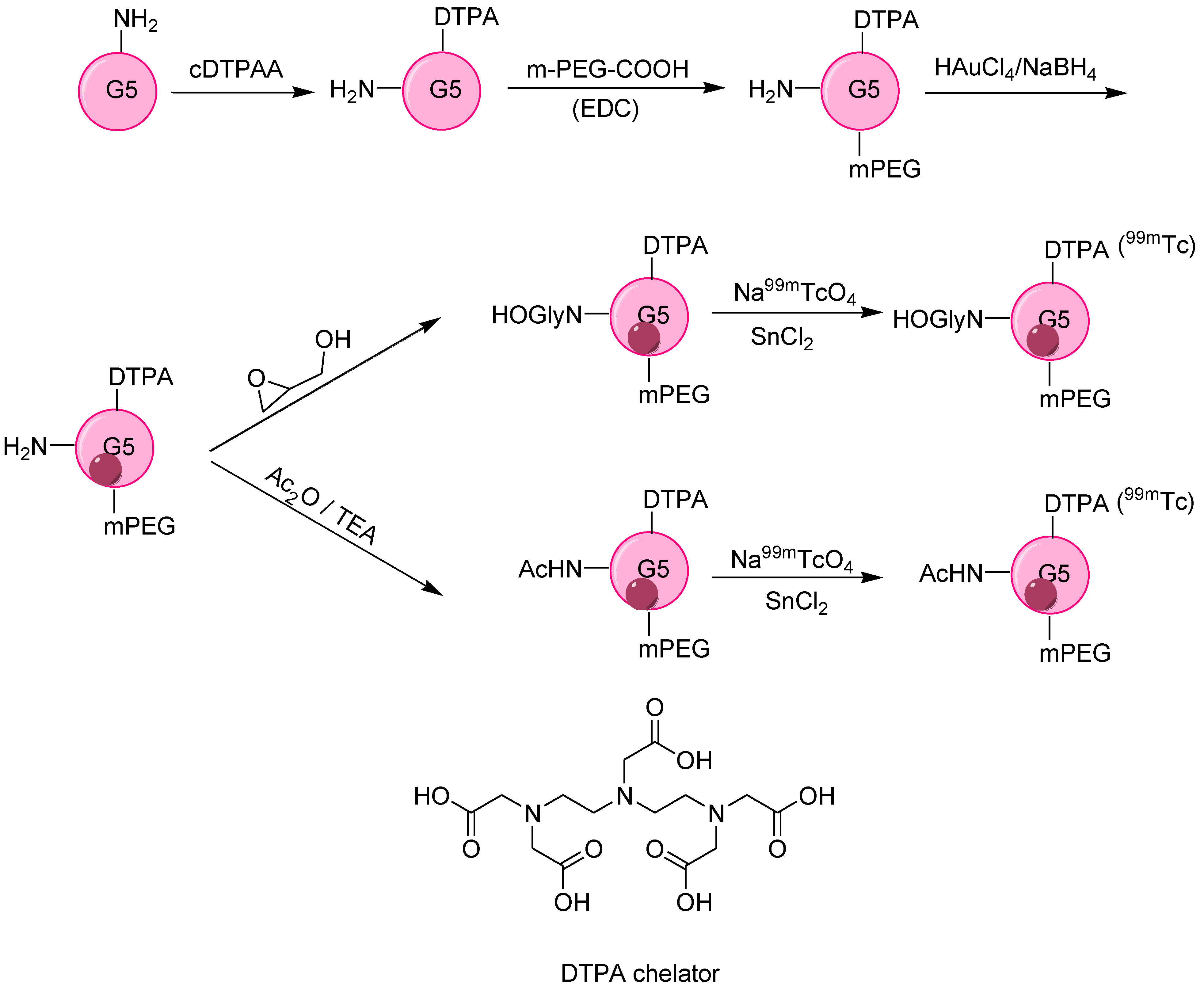
| Tumor targeting | Generation 5 dendrimer, G5-NH2 | Chelator-based radiolabeling using DTPA and stannous chloride (reducing agent) | [46] | |
| Silica Nanoparticles | General biodistribution study | Without drug | Direct radiolabeling using stannous chloride (reducing agent) | [48] |
| General biodistribution study | Without drug and with Cy5.5 fluorescent agent | Direct radiolabeling using stannous chloride (reducing agent) | [49] | |
| General biodistribution study | APTES modified nanoparticles | Chelator-based radiolabeling using DTPA and stannous chloride (reducing agent) | [50] | |
| HER2 receptors targeting in tumors | Anti-HER2 antibody | Direct radiolabeling using stannous chloride (reducing agent),Chelator-based radiolabeling using DTPA and MAG3 | [51] | |
| To target HER2 positive breast cancer | Trastuzumab (TZ) | Chelator-based radiolabeling using His-Tag | [52] | |
| To target HER2 positive breast cancer | Trastuzumab (TZ) and DOX | Direct radiolabeling | [53] | |
| Melanoma treatment | Dacarbazine | Direct radiolabeling using stannous chloride (reducing agent) | [54] | |
| General biodistribution study | Without the drug, radiotherapy using 186/188Re and optical imaging using eosin isothiocyanate (EOITC) | Direct radiolabeling using a tricarbonyl kit | [55] | |
| General biodistribution study and dual SPECT and MRI imaging agent | DOX drug for chemotherapy and MnO for MRI imaging | Direct radiolabeling using stannous chloride (reducing agent) | [56] | |
| Titanium Nanoparticles | General biodistribution study | Without drug | Direct radiolabeling on the surface of nanoparticles | [57] |
5. Organic Nanoparticles
5.1. Dendrimers
5.2. Polymeric Nanoparticles
5.3. Lipid Nanoparticles
5.4. Liposomes
5.5. Oligomers
5.6. Protein Nanoparticles
| Nanoparticle | Application | Drug Loaded on NPs | Radiolabeling Method | Ref. |
|---|---|---|---|---|
| Dendrimer | Human breast cancer (MCF-7) imaging | Without drug | Chelator-based radiolabeling using HYNIC and stannous chloride (reducing agent) | [58] |
| A549 tumor imaging | Without drug | Direct radiolabeling using stannous chloride (reducing agent) | [59] | |
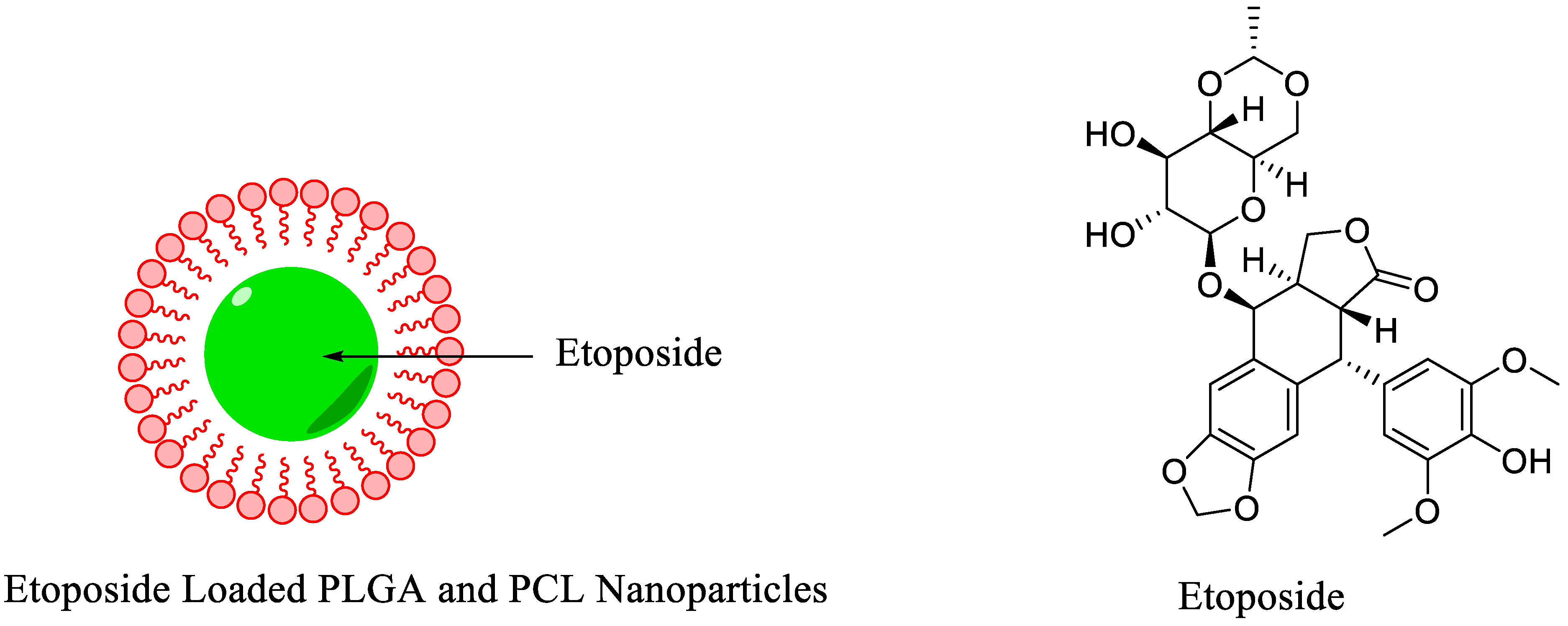
| Polymeric Nanoparticles | General biodistribution study | Etoposide | Direct radiolabeling using stannous chloride (reducing agent) | [60] |
| Sentinel lymph node | Without drug | Direct radiolabeling using stannous chloride (reducing agent),chelator-based radiolabeling using DTPA | [61] | |
| Comparative study using various reducing agents for 99mTc radiolabeling | Without drug | Direct radiolabeling using stannous chloride, sodium dithionite or sodium borohydride (reducing agent) | [62] | |
| Imaging of folate receptor overexpressed tumors | Folic acid | Direct radiolabeling using stannous chloride (reducing agent) | [63] | |
| Imaging of folate receptor or vascular endothelial growth factor receptor-2 (VEGFR-2) overexpressed tumors | Folic acid (FA) to target folate receptor and K237 (HTMYYHHYQHHL) peptide | Direct radiolabeling using stannous chloride (reducing agent) | [64] | |
| General biodistribution study | Gemcitabine | Direct radiolabeling using tricarbonyl kit | [65] | |
| Lipid nanoparticles | Imaging of folate receptor overexpressed tumors | Cisplatin and folic acid | Direct radiolabeling using stannous chloride (reducing agent) | [68] |
| Bone imaging | Methylene diphosphonate (MDP) | Direct radiolabeling using stannous chloride (reducing agent) | [69] | |
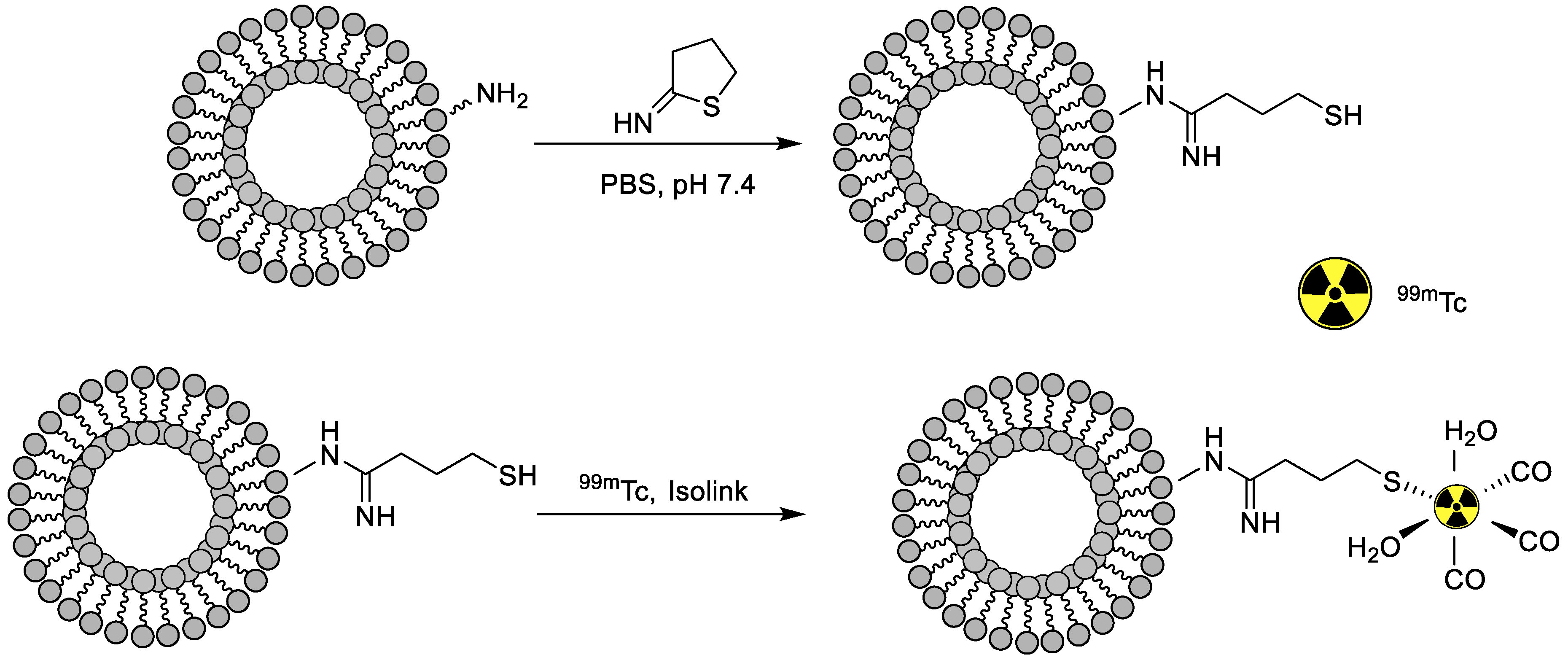
| Liposomes nanoparticles | General biodistribution study | Without drug | Direct radiolabeling on the surface of nanoparticles | [71] |
| General biodistribution study | Without drug | Chelator-based radiolabeling using 2-Iminothiolane and tricrbonyl kit | [72] | |
| Oligomers | Human HepG2 cells targeting | Folic acid | Direct radiolabeling using stannous chloride (reducing agent) | [74] |
| Protein nanoparticles (Human Serum Albumin) | General biodistribution study | Bevacizumab | Direct radiolabeling using tricarbonyl kit | [77] |
| General biodistribution study | HPMC, GMN2, GPM2 and GTM2 polymer coating | Direct radiolabeling using stannous chloride (reducing agent) | [78] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.R.; Hasan, A.; Ertsey, R.; et al. Intraoperative pancreatic cancer detection using tumor-specific multimodality molecular imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shu, J. Multimodal molecular imaging: Current status and future directions. Contrast Media Mol. Imaging. 2018, 2018, 1382183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez Vargas, S.H.; Ghosh, S.C.; Azhdarinia, A. New developments in dual-labeled molecular imaging agents. J. Nucl. Med. 2019, 60, 459–465. [Google Scholar] [CrossRef] [Green Version]
- De La Pena, H.; Sharma, A.; Glicksman, C.; Joseph, J.; Subesinghe, M.; Traill, Z.; Verrill, C.; Sullivan, M.; Redgwell, J.; Bataillard, E.; et al. No longer any role for routine follow-up chest x-rays in men with stage I germ cell cancer. Eur. J. Cancer 2017, 84, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabyani, W.; Gomaa, M.; Khaled, H.; Fahmy, A. Dataset of breast ultrasound images. Data Brief. 2020, 28, 104863. [Google Scholar] [CrossRef]
- Duan, F.; Hao, D.; Xu, W.; Zhong, X.; Luo, T. Correlations of twist expression with pathological and computed tomography (CT) characteristics and prognosis of Non-Small Cell Lung Cancer (NSCLC). Med. Sci. Monit. 2019, 25, 977–983. [Google Scholar] [CrossRef]
- Qi, J.; Chen, C.; Zhang, X.; Hu, X.; Ji, S.; Kwok, R.T.K.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Light-driven transformable optical agent with adaptive functions for boosting cancer surgery outcomes. Nat. Commun. 2018, 9, 1848. [Google Scholar] [CrossRef] [PubMed]
- Stavrinides, V.; Giganti, F.; Trock, B.; Punwani, S.; Allen, C.; Kirkham, A.; Freeman, A.; Haider, A.; Ball, R.; McCartan, N.; et al. Five-year outcomes of magnetic resonance imaging–based active surveillance for prostate cancer: A large cohort study. Eur. Urol. 2020, 78, 443–451. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, H.J. Single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging for radiotherapy planning in patients with lung cancer: A meta-analysis. Sci. Rep. 2020, 10, 14864. [Google Scholar] [CrossRef]
- Corfield, J.; Perera, M.; Bolton, D.; Lawrentschuk, N. 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: A systematic review. World J. Urol. 2018, 36, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, S.; Liu, H.; Meng, B.; Zhou, F.; He, F.; Shi, X.; Yang, H. Detection of vertebral metastases: A meta-analysis comparing MRI, CT, PET, BS and BS with SPECT. J. Cancer Res. Clin. Oncol. 2017, 143, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Martí-Bonmatí, L.; Sopena, R.; Bartumeus, P.; Sopena, P. Multimodality imaging techniques. Contrast Media Mol. Imaging 2010, 5, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Holland, J.P. Advanced methods for radiolabeling multimodality nanomedicines for SPECT/MRI and PET/MRI. J. Nucl. Med. 2018, 59, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Fan, W.; Zhang, Z.; Wen, Y.; Xiong, L.; Chen, X. Advanced nanotechnology leading the way to multimodal imaging-guided precision surgical therapy. Adv. Mater. 2019, 31, e1904329. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Salvanou, E.A.; Bouziotis, P.; Tsoukalas, C. Radiolabeled nanoparticles in nuclear oncology. Adv. Drug Deliv. Rev. 2018, 1, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, R.; Bahadur, J.; Lohar, S.; Sarma, H.D.; Sen, D.; Mishra, R.; Chakraborty, S.; Dash, A. Solid state synthesis of mesoporous alumina: A viable strategy for preparation of an advanced nanosorbent for 99Mo/99mTc generator technology. Microporous Mesoporous Mater. 2019, 287, 271–279. [Google Scholar] [CrossRef]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.A.; Xu, L.C.; Parker, N.; Vetzel, M.; Leamon, C.P. Preclinical evaluation of 99mTc-EC20 for imaging folate receptor–positive tumors. J. Nucl. Med. 2004, 45, 857–866. [Google Scholar] [PubMed]
- Carpenet, H.; Cuvillier, A.; Monteil, J.; Quelven, I. Anti-CD20 immunoglobulin G radiolabeling with a 99mTc-tricarbonyl core: In vitro and in vivo evaluations. PLoS ONE 2015, 10, e0139835. [Google Scholar]
- Felber, M.; Alberto, R. 99mTc radiolabelling of Fe3O4-Au core-shell and Au–Fe3O4 dumbbell-like nanoparticles. Nanoscale 2015, 7, 6653–6660. [Google Scholar] [CrossRef] [Green Version]
- Putra, A.R.; Lestary, E.; Maskur, M.; Tahyan, Y. Validation of [99mTc] Tc-DTPA radiochemical testing method using one-system paper chromatography. J. Phys. Conf. Ser. 2020, 1436, 012001. [Google Scholar] [CrossRef]
- Ling, Y.; Wei, K.; Luo, Y.; Gao, X.; Zhong, S. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 7139–7150. [Google Scholar] [CrossRef]
- Park, S.H.; Gwon, H.J.; Choi, S.M. Preparation of 99mTc-Labeled iron oxide nanoparticles for in vivo imaging in hyperthermia. Chem. Lett. 2007, 36, 1282–1283. [Google Scholar] [CrossRef]
- Lee, C.M.; Jeong, H.J.; Kim, E.M.; Kim, D.W.; Lim, S.T.; Kim, H.T.; Park, I.K.; Jeong, Y.Y.; Kim, J.W.; Sohn, M.H. Superparamagnetic iron oxide nanoparticles as a dual imaging probe for targeting hepatocytes in vivo. Magn. Reson. Med. 2009, 62, 1440–1446. [Google Scholar] [CrossRef]
- Torres Martin de Rosales, R.; Tavaré, R.; Glaria, A.; Varma, G.; Protti, A.; Blower, P.J. 99mTc-bisphosphonate-iron oxide nanoparticle conjugates for dual-modality biomedical imaging. Bioconjug. Chem. 2011, 22, 455–465. [Google Scholar] [CrossRef]
- Fatahian, S.; Shahbazi-Gahrouei, D.; Pouladian, M.; Yousefi, M.H.; Amiri, G.R.; Noori, A. Biodistribution and toxicity assessment of radiolabeled and DMSA coated ferrite nanoparticles in mice. J. Radioanal. Nucl. Chem. 2012, 293, 915–921. [Google Scholar] [CrossRef]
- Xue, S.; Zhang, C.; Yang, Y.; Zhang, L.; Cheng, D.; Zhang, J.; Shi, H.; Zhang, Y. 99mTc-labeled iron oxide nanoparticles for dual-contrast (T1/T2) magnetic resonance and dual-modality imaging of tumor angiogenesis. J. Biomed. Nanotechnol. 2015, 11, 1027–1037. [Google Scholar] [CrossRef]
- Gao, Z.; Hou, Y.; Zeng, J.; Chen, L.; Liu, C.; Yang, W.; Gao, M. Tumor microenvironment-triggered aggregation of antiphagocytosis 99mTc-Labeled Fe3O4 nanoprobes for enhanced tumor imaging in vivo. Adv Mater. 2017, 29, 1701095. [Google Scholar] [CrossRef]
- Psimadas, D.; Baldi, G.; Ravagli, C.; Franchini, M.C.; Locatelli, E.; Innocenti, C.; San Gregorio, C.; Loudos, G. Comparison of the magnetic, radiolabeling, hyperthermic and biodistribution properties of hybrid nanoparticles bearing CoFe2O4 and Fe3O4 metal cores. Nanotechnology 2013, 25, 025101. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 9, 309–315. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, A.N.; Ferro-Flores, G.; Ocampo-García, B.E.; Morales-Avila, E.; de M. Ramírez, F.D.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; Medina, L.A.; Rojas-Calderón, E.L.; Camacho-López, M.A. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging. J. Biomed. Nanotechnol. 2010, 6, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-García, B.E.; Ramírez, F.D.; Ferro-Flores, G.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; Morales-Avila, E.; de Murphy, C.A.; Pedraza-López, M.; Medina, L.A.; Camacho-López, M.A. 99mTc-labelled gold nanoparticles capped with HYNIC-peptide/mannose for sentinel lymph node detection. Nucl. Med. Biol. 2011, 38, 1–11. [Google Scholar] [CrossRef]
- Morales-Avila, E.; Ferro-Flores, G.; Ocampo-García, B.E.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; García-Becerra, R.; Medina, L.A.; Gómez-Oliván, L. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c [RGDfK(C)] for molecular imaging of tumor α(v)β(3) expression. Bioconjug. Chem. 2011, 22, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-García, B.; Ferro-Flores, G.; Morales-Avila, E.; de MaríaRamírez, F. Kit for preparation of multimeric receptor-specific 99mTc-radiopharmaceuticals based on gold nanoparticles. Nucl. Med. Commun. 2011, 32, 1095–1104. [Google Scholar] [CrossRef]
- Jiménez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-García, B.; Luna-Gutiérrez, M.; Azorín-Vega, E.; Isaac-Olivé, K.; Camacho-López, M.; Torres-García, E. Multifunctional targeted therapy system based on 99mTc/177Lu-labeled gold nanoparticles-Tat (49-57)-Lys3-bombesin internalized in nuclei of prostate cancer cells. J. Label. Comp. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Z.; Xu, X.; Luo, Y.; Peng, C.; Shen, M.; Shi, X. 99mTc-labeled multifunctional low-generation dendrimer-entrapped gold nanoparticles for targeted SPECT/CT dual-mode imaging of tumors. ACS Appl. Mater. Interfaces 2016, 8, 19883–19891. [Google Scholar] [CrossRef]
- Kamal, R.; Chadha, V.D.; Dhawan, D.K. Physiological uptake and retention of radiolabeled resveratrol loaded gold nanoparticles (99mTc-Res-AuNP) in colon cancer tissue. Nanomedicine 2018, 14, 1059–1071. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, R.; Chen, F.; Zhao, L.; Wang, P.; Li, X.; Bányai, I.; Ouyang, Q.; Shi, X.; Shen, M. 99mTc-labeled RGD–polyethylenimine conjugates with entrapped gold nanoparticles in the cavities for dual-mode SPECT/CT imaging of hepatic carcinoma. ACS Appl. Mater. Interfaces 2018, 10, 6146–6154. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhu, J.; Zhao, L.; Xiong, Z.; Li, Y.; Wu, S.; Chand, G.; Shi, X.; Zhao, J. SPECT/CT imaging of chemotherapy-induced tumor apoptosis using 99mTc-labeled dendrimer-entrapped gold nanoparticles. Drug Deliv. 2018, 25, 1384–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhao, L.; Yang, J.; Chen, L.; Shi, J.; Zhao, J.; Shi, X. 99mTc-Labeled polyethylenimine-entrapped gold nanoparticles with pH-responsive charge conversion property for enhanced dual mode SPECT/CT imaging of cancer cells. Langmuir 2019, 35, 13405–13412. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, L.; Zhao, Q.; Li, D.; Liu, C.; Yu, Z.; Shen, M.; Majoral, J.P.; Mignani, S.; Zhao, J.; et al. A promising dual mode SPECT/CT imaging platform based on 99mTc-labeled multifunctional dendrimer-entrapped gold nanoparticles. J. Mater. Chem. B 2017, 5, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Tarn, D.; Ashley, C.E.; Xue, M.I.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Sá, L.T.; Pessoa, C.; Meira, A.S.; Da Silva, M.I.; Missailidis, S.; Santos-Oliveira, R. Development of nanoaptamers using a mesoporous silica model labeled with 99mTc for cancer targeting. Oncology 2012, 82, 213–217. [Google Scholar] [CrossRef]
- Tamba, B.I.; Dondas, A.; Leon, M.; Neagu, A.N.; Dodi, G.; Stefanescu, C.; Tijani, A. Silica nanoparticles: Preparation, characterization and in vitro/in vivo biodistribution studies. Eur. J. Pharm. Sci. 2015, 71, 46–55. [Google Scholar] [CrossRef]
- De Barros, A.L.B.; de Oliveira Ferraz, K.S.; Dantas, T.C.S.; Andrade, G.F.; Cardoso, V.N.; De Sousa, E.M. Synthesis, characterization, and biodistribution studies of 99mTc-labeled SBA-16 mesoporous silica nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 181–188. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Tsuchimochi, M.; Hayama, K.; Kawase, T.; Tsubokawa, N. Dual-labeled near-infrared/99mTc imaging probes using PAMAM-coated silica nanoparticles for the imaging of HER2-expressing cancer cells. Int. J. Mol. Sci. 2016, 17, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainone, P.; Riva, B.; Belloli, S.; Sudati, F.; Ripamonti, M.; Verderio, P.; Colombo, M.; Colzani, B.; Gilardi, M.C.; Moresco, R.M.; et al. Development of 99mTc-radiolabeled nanosilica for targeted detection of HER2-positive breast cancer. Int. J. Nanomed. 2017, 12, 3447–3461. [Google Scholar] [CrossRef] [Green Version]
- Rainone, P.; De Palma, A.; Sudati, F.; Roffia, V.; Rigamonti, V.; Salvioni, L.; Colombo, M.; Ripamonti, M.; Spinelli, A.E.; Mazza, D.; et al. 99mTc-radiolabeled silica nanocarriers for targeted detection and treatment of HER2-positive breast cancer. Int. J. Nanomed. 2021, 16, 1943–1960. [Google Scholar] [CrossRef] [PubMed]
- Portilho, F.L.; Helal-Neto, E.; Cabezas, S.S.; Pinto, S.R.; Dos Santos, S.N.; Pozzo, L.; Sancenón, F.; Martínez-Máñez, R.; Santos-Oliveira, R. Magnetic core mesoporous silica nanoparticles doped with dacarbazine and labelled with 99mTc for early and differential detection of metastatic melanoma by single photon emission computed tomography. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Wuillemin, M.A.; Reber, M.J.; Fox, T.; Spingler, B.; Brühwiler, D.; Alberto, R.; Braband, H. Towards 99mTc- and Re-based multifunctional silica platforms for theranostic applications. Inorganics 2019, 7, 134. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Liu, X.; Tang, W.; Niu, D.; Zhou, B.; Zhang, H.; Liu, W.; Gu, B.; Zhou, X.; Zheng, Y.; et al. 99mTc-conjugated manganese-based mesoporous silica nanoparticles for SPECT, pH-responsive MRI and anti-cancer drug delivery. Nanoscale 2016, 8, 19573–19580. [Google Scholar] [CrossRef] [PubMed]
- Suchánková, P.; Kukleva, E.; Nykl, E.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Hydroxyapatite and titanium dioxide nanoparticles: Radiolabelling and in vitro stability of prospective theranostic nanocarriers for 223Ra and 99mTc. Nanomaterials 2020, 10, 1632. [Google Scholar] [CrossRef]
- Narmani, A.; Yavari, K.; Mohammadnejad, J. Imaging, biodistribution and in vitro study of smart 99mTc-PAMAM G4 dendrimer as novel nano-complex. Colloids Surf. B Biointerfaces 2017, 159, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Khalaj, A.; Sabzevari, O.; Badrzadeh, L.; Mohammadzadeh, P.; Mousavi Motlagh, S.S.; Bitarafan-Rajabi, A.; Shafiee Ardestani, M. Technetium-99m chelator-free radiolabeling of specific glutamine tumor imaging nanoprobe: In vitro and in vivo evaluations. Int. J. Nanomed. 2018, 13, 4671–4683. [Google Scholar] [CrossRef] [Green Version]
- Snehalatha, M.; Venugopal, K.; Saha, R.N.; Babbar, A.K.; Sharma, R.K. Etoposide loaded PLGA and PCL nanoparticles II: Biodistribution and pharmacokinetics after radiolabeling with Tc-99m. Drug Deliv. 2008, 15, 277–287. [Google Scholar] [CrossRef]
- Subramanian, S.; Dandekar, P.; Jain, R.; Pandey, U.; Samuel, G.; Hassan, P.A.; Patravale, V.; Venkatesh, M. Technetium-99m–labeled poly (dl-Lactide-co-Glycolide) nanoparticles as an alternative for sentinel lymph node imaging. Cancer Biother. Radiopharm. 2010, 25, 637–644. [Google Scholar] [CrossRef]
- Geskovski, N.; Kuzmanovska, S.; Simonoska Crcarevska, M.; Calis, S.; Dimchevska, S.; Petrusevska, M.; Zdravkovski, P.; Goracinova, K. Comparative biodistribution studies of technetium-99m radiolabeled amphiphilic nanoparticles using three different reducing agents during the labeling procedure. J. Label. Comp. Radiopharm. 2013, 56, 689–695. [Google Scholar] [CrossRef]
- Polyák, A.; Hajdu, I.; Bodnár, M.; Trencsényi, G.; Pöstényi, Z.; Haász, V.; Jánoki, G.; Jánoki, G.A.; Balogh, L.; Borbély, J. 99mTc-labelled nanosystem as tumour imaging agent for SPECT and SPECT/CT modalities. Int. J. Pharm. 2013, 449, 10–17. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, X.; Huang, J.; Wu, Y.; Huang, X.; Chen, J.; Xia, J.; Jiang, H.; Ma, J.; Wu, J. Immune activity and biodistribution of polypeptide K237 and folic acid conjugated amphiphilic PEG-PLGA copolymer nanoparticles radiolabeled with 99mTc. Oncotarget 2016, 7, 76635–76646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- İçhedef, Ç.; Teksöz, S.; Çetin, O.; Aydın, B.; Sarıkavak, İ.; Parlak, Y.; Bilgin, B.E. Design of 99mTc radiolabeled gemcitabine polymeric nanoparticles as drug delivery system and in vivo evaluation. Mater. Chem. Phys. 2021, 263, 124380. [Google Scholar] [CrossRef]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oumzil, K.; Khiati, S.; Camplo, M.; Koquely, M.; Chuttani, K.; Chaturvedi, S.; Mishra, A.K.; Barthélémy, P. Nucleolipids as building blocks for the synthesis of 99mTc-labeled nanoparticles functionalized with folic acid. New J. Chem. 2014, 38, 5240–5246. [Google Scholar] [CrossRef]
- Mandiwana, V.; Kalombo, L.; Grobler, A.; Zeevaart, J.R. 99mTc-MDP as an imaging tool to evaluate the in vivo biodistribution of solid lipid nanoparticles. Appl. Radiat. Isot. 2018, 141, 51–56. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Choi, Y.; Huh, E.J.; Lee, K.Y.; Song, H.C.; Sun, M.J.; Jeong, H.J.; Cho, C.S.; Bom, H.S. Polyethylene glycol (PEG) modified 99mTc-HMPAOliposome for improving blood circulation and biodistribution: The effect of the extent of pegylation. Cancer Biother. Radiopharm. 2005, 20, 620–628. [Google Scholar] [CrossRef]
- Varga, Z.; Szigyártó, I.C.; Gyurkó, I.; Dóczi, R.; Horváth, I.; Máthé, D.; Szigeti, K. Radiolabeling and quantitative in vivo SPECT/CT imaging study of liposomes using the novel iminothiolane-99mTc-tricarbonyl complex. Contrast Media Mol. Imaging 2017, 2017, 4693417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galzitskaya, O.V. Oligomers are promising targets for drug development in the treatment of proteinopathies. Front. Mol. Neurosci. 2019, 12, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Zhang, X.; Su, X.; Li, J.; Tian, Y.; Xue, H.; Xu, H. 99mTc-labeled oligomeric nanoparticles as potential agents for folate receptor-positive tumor targeting. J. Label. Comp. Radiopharm. 2018, 61, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed. Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Ramos-Membrive, R.; Erhard, Á.; de Redín, I.L.; Quincoces, G.; Collantes, M.; Ecay, M.; Irache, J.M.; Peñuelas, I. In vivo SPECT-CT imaging and characterization of technetium-99m-labeled bevacizumab-loaded human serum albumin pegylated nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 64, 101809. [Google Scholar] [CrossRef]
- de Arcocha-Torres, M.; Quincoces, G.; Martínez-López, A.L.; Erhard, A.; Collantes, M.; Martínez-Rodríguez, I.; Ecay, M.; Banzo, I.; Irache, J.M.; Peñuelas, I. Preparation, radiolabeling with 99mTc and 67Ga and biodistribution studies of albumin nanoparticles coated with polymers. Rev. Esp. Med. Nucl. Imagen Mol. 2020, 39, 225–232. [Google Scholar] [CrossRef]





| Modality | Contrast Agent (Examples) | Spatial Resolution (mm) | Advantages | Limitations | Clinical Application |
|---|---|---|---|---|---|
| CT | Iodine; nanoparticles; barium; krypton | 0.5–0.625 mm | Whole-body imaging available; high spatial resolution; short imaging time; unlimited depth penetration, inexpensive | Use of ionizing radiation; limited soft tissue contrast; molecular imaging not available; no real-time imaging | Yes |
| US | Microbubbles | 0.04–0.1 (micro), 0.1–2 (clinical) | High sensitivity; non-ionizing radiation; real-time imaging; inexpensive, short acquisition time | Whole-body imaging not possible; limited depth penetration; limited contrast agents | Yes |
| Optical | Fluorescent dye; Nanoparticles | 1–5 mm | High sensitivity; non-ionizing radiation; real time imaging; inexpensive, short acquisition time | Whole body imaging not possible; limited depth penetration; limited contrast agents | Yes |
| MRI | Gadolinium; iron oxide nanoparticles; manganese nanoparticles | 0.01–0.1 (micro); 0.5–1.5 (clinical) | Non ionizing radiation; high spatial resolution; high soft tissue contrast; whole-body imaging possible; unlimited depth penetration | Expensive imaging; long acquisition time; low sensitivity | Yes |
| PET | Radioisotopes | 1–2 (micro); 5–10 (clinical) | High sensitivity; whole-body imaging possible; unlimited depth penetration; quantitative imaging; can combine with other imaging technologies and therapy | Expensive imaging; low spatial resolution; long acquisition time; ionizing radiation exposure; need cyclotron or nuclear reactor | Yes |
| SPECT | Radioisotopes | 0.5–2 (micro); 6–15 (clinical) | High sensitivity; whole-body imaging possible; unlimited depth penetration; quantitative imaging; can combine with other imaging technologies and therapy | Expensive imaging; low spatial resolution; long acquisition time; ionizing radiation exposure; need cyclotron or nuclear reactor | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushtaq, S.; Bibi, A.; Park, J.E.; Jeon, J. Recent Progress in Technetium-99m-Labeled Nanoparticles for Molecular Imaging and Cancer Therapy. Nanomaterials 2021, 11, 3022. https://doi.org/10.3390/nano11113022
Mushtaq S, Bibi A, Park JE, Jeon J. Recent Progress in Technetium-99m-Labeled Nanoparticles for Molecular Imaging and Cancer Therapy. Nanomaterials. 2021; 11(11):3022. https://doi.org/10.3390/nano11113022
Chicago/Turabian StyleMushtaq, Sajid, Asia Bibi, Jung Eun Park, and Jongho Jeon. 2021. "Recent Progress in Technetium-99m-Labeled Nanoparticles for Molecular Imaging and Cancer Therapy" Nanomaterials 11, no. 11: 3022. https://doi.org/10.3390/nano11113022
APA StyleMushtaq, S., Bibi, A., Park, J. E., & Jeon, J. (2021). Recent Progress in Technetium-99m-Labeled Nanoparticles for Molecular Imaging and Cancer Therapy. Nanomaterials, 11(11), 3022. https://doi.org/10.3390/nano11113022







