Nonporous, Strong, Stretchable, and Transparent Electrospun Aromatic Polyurea Nanocomposites as Potential Anticorrosion Coating Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation and Experimental Setup
2.3. Characterization
2.3.1. Tensile Testing
2.3.2. Fourier-Transform Infrared (FTIR)
2.3.3. Water Contact Angle
2.3.4. Contact Transparency
2.3.5. Field Emission Scanning Electron Microscope (FE-SEM)
2.3.6. Modulated Thermogravimetry (MTGA)
2.3.7. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
3.1. Thickness and Transparency of Polyurea Membranes
3.2. Mechanical Properties of Polyurea Nanocomposite Membranes
3.3. Chemical Interaction
3.4. Degree of Hydrophobicity
3.5. Surface Morphology of Aromatic Polyurea Membranes
3.6. Modulated Thermogravimetry (MTGA)
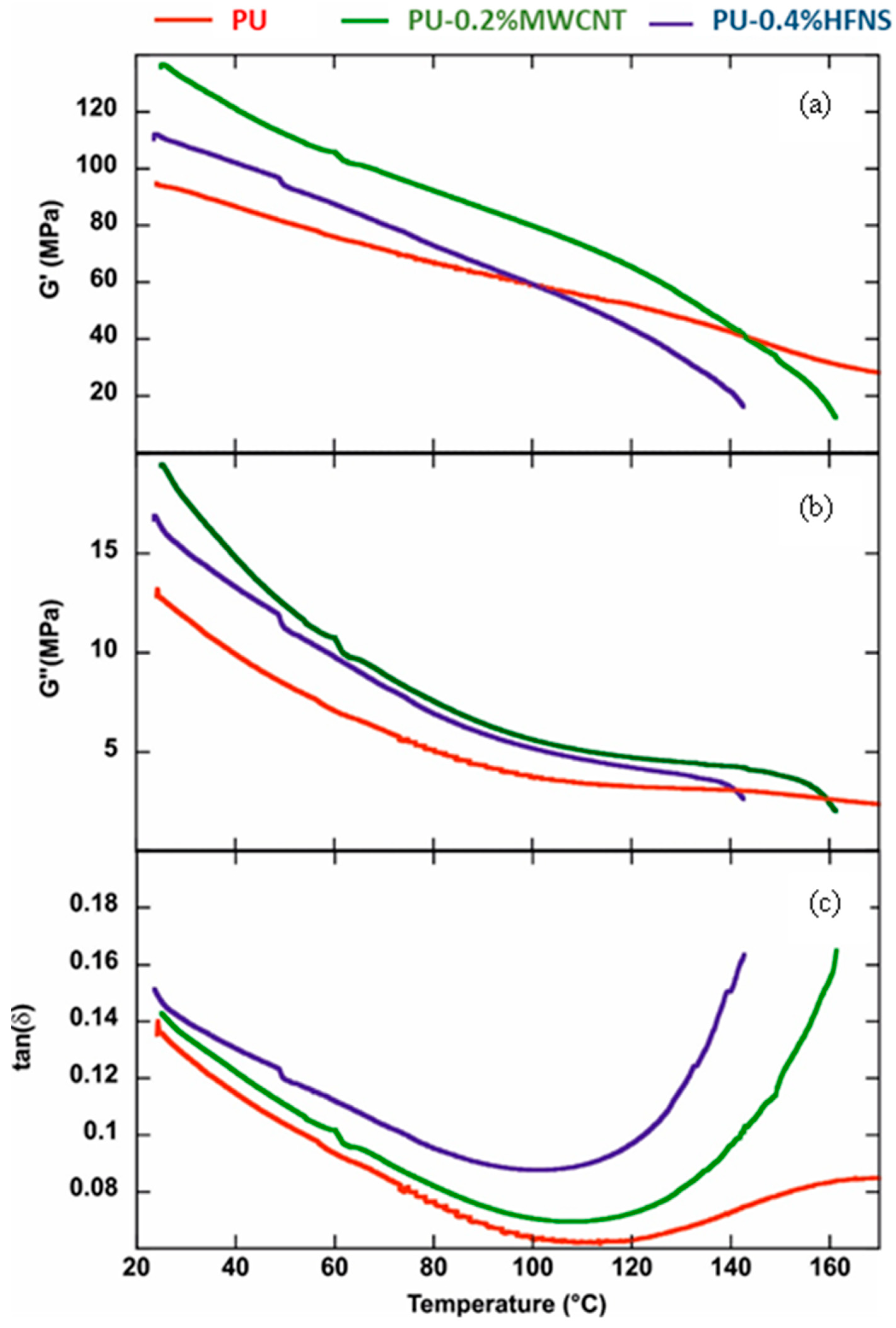
3.7. Viscoelastic Properties Polyurea Nanocomposites Membranes
4. Prospects of Electrospun Polyurea Films as Anti-Corrosive Coatings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhang, W.; Wei, L.; Pu, L.; Liu, J.; Liu, H.; Li, Y.; Fan, J.; Ding, T.; Guo, Z. Alternating Multilayer Structural Epoxy Composite Coating for Corrosion Protection of Steel. Macromol. Mater. Eng. 2019, 304, 1900374. [Google Scholar] [CrossRef]
- Barnoush, A.; Hosemann, P.; Molina-Aldareguia, J.; Wheeler, J.M. In situ small-scale mechanical testing under extreme environments. MRS Bull. 2019, 44, 471–477. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Nie, T.; Bai, J.; Zhao, F.; Ma, S. Enhancement of barrier and anti-corrosive performance of zinc-rich epoxy coatings using nano-silica/graphene oxide hybrid. Corros. Rev. 2020, 38, 497–513. [Google Scholar] [CrossRef]
- Habibpour, S.; Um, J.G.; Jun, Y.; Bhargava, P.; Park, C.B.; Yu, A. Structural Impact of Graphene Nanoribbon on Mechanical Properties and Anti-corrosion Performance of Polyurethane Nanocomposites. Chem. Eng. J. 2021, 405, 126858. [Google Scholar] [CrossRef]
- Rajitha, K.; Mohana, K.N.S.; Hegde, M.B.; Nayak, S.R.; Swamy, N.K. Fabrication of ZnO/rGO and ZnO/MWCNT nanohybrids to reinforce the anticorrosion performance of polyurethane coating. FlatChem 2020, 24, 100208. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, G.; Yu, H.; Hu, C.; Yan, X.; Guo, T.; Li, J. Comparison of anti-corrosion properties of polyurethane based composite coatings with low infrared emissivity. Appl. Surf. Sci. 2011, 257, 4743–4748. [Google Scholar] [CrossRef]
- Nayak, S.R.; Mohana, K.N.S.; Hegde, M.B.; Rajitha, K.; Madhusudhana, A.M.; Naik, S.R. Functionalized multi-walled carbon nanotube/polyindole incorporated epoxy: An effective anti-corrosion coating material for mild steel. J. Alloys Compd. 2021, 856, 158057. [Google Scholar] [CrossRef]
- Lorwanishpaisarn, N.; Srikhao, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Theerakulpisut, S.; Okhawilai, M.; Kasemsiri, P. Self-healing Ability of Epoxy Vitrimer Nanocomposites Containing Bio-Based Curing Agents and Carbon Nanotubes for Corrosion Protection. J. Polym. Environ. 2021, 1–11. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, C.; Cao, Y. Corrosion behavior of epoxy composite coatings reinforced with reduced graphene oxide nanosheets in the high salinity environments. Appl. Surf. Sci. 2019, 493, 889–896. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Ou, J.; Li, W. Effect of PDMS on the waterproofing performance and corrosion resistance of cement mortar. Appl. Surf. Sci. 2020, 507, 145016. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Z. Robust, hydrophobic anti-corrosion coating prepared by PDMS modified epoxy composite with graphite nanoplatelets/nano-silica hybrid nanofillers. Surf. Coat. Technol. 2021, 421, 127440. [Google Scholar] [CrossRef]
- Badi, N.; Khasim, S.; Pasha, A.; Alatawi, A.S.; Lakshmi, M. Silver Nanoparticles Intercalated Polyaniline Composites for High Electrochemical Anti-Corrosion Performance in 6061 Aluminum Alloy-Based Solar Energy Frameworks. J. Bio Tribo Corros. 2020, 6, 123. [Google Scholar] [CrossRef]
- Xu, H.; Fan, S.; Lu, Y.; Feng, H.; Qiu, J. Proposal and Verification of a Novel Superhydrophobic-Conductive Anti-Corrosion Polyaniline-Silica Coating. Bull. Chem. Soc. Jpn. 2020, 93, 1114–1120. [Google Scholar] [CrossRef]
- Yadav, P.K.; Prakash, O.; Ray, B.; Maiti, P. Functionalized polythiophene for corrosion inhibition and photovoltaic application. J. Appl. Polym. Sci. 2021, 138, 51306. [Google Scholar] [CrossRef]
- Xue, S.; Ma, Y.; Miao, Y.; Li, W. Anti-Corrosion Performance of Conductive Copolymers of Polyaniline/Polythiophene on a Stainless Steel Surface in Acidic Media. Int. J. Nanosci. 2020, 19, 1950023. [Google Scholar] [CrossRef]
- Lu, F.; Liu, C.; Chen, Z.; Veerabagu, U.; Chen, Z.; Liu, M.; Hu, L.; Xia, H.; Cha, L.; Zhang, W. Polypyrrole-functionalized boron nitride nanosheets for high-performance anti-corrosion composite coating. Surf. Coat. Technol. 2021, 420, 127273. [Google Scholar] [CrossRef]
- Rajkumar, R.; Vedhi, C. Study of the corrosion protection efficiency of polypyrrole/metal oxide nanocomposites as additives in anticorrosion coating. Anti Corros. Methods Mater. 2020, 67, 305–312. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wen, S.; Li, S.; Chen, Y.; Wang, J.; Wang, Y.; Wang, C.; Yu, X.; Mao, Y. Waterborne Polyurea Coatings Filled with Sulfonated Graphene Improved Anti-Corrosion Performance. Coatings 2021, 11, 251. [Google Scholar] [CrossRef]
- Mo, Q.; Qin, G.; Ling, K.; Lv, X.; Wang, N.; Li, W. Layer-by-layer self-assembled polyurea layers onto MAO surface for enhancing corrosion protection to aluminum alloy 6063. Surf. Coat. Technol. 2021, 405, 126653. [Google Scholar] [CrossRef]
- Fellet, M.; Nyborg, R. Understanding corrosion of flexible pipes at subsea oil and gas wells. MRS Bull. 2018, 43, 654–655. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, D.S.; Quraishi, M.A.; Ansari, K.R.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coat. 2020, 147, 105741. [Google Scholar] [CrossRef]
- Tong, Y.; Bohm, S.; Song, M. The capability of graphene on improving the electrical conductivity and anti-corrosion properties of Polyurethane coatings. Appl. Surf. Sci. 2017, 424, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Poniatowska, A.; Trzaskowska, P.A.; Trzaskowski, M.; Ciach, T. Physicochemical and Biological Properties of Graphene-Oxide-Coated Metallic Materials. Materials 2021, 14, 5752. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, L.; Li, G. Properties of Waterborne Polyurethane Conductive Coating with Low MWCNTs Content by Electrostatic Spraying. Polymers 2018, 10, 1406. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Suleiman, R.; Kim, H. Do Effect of functionalized multiwalled carbon nanotubes on weather degradation and corrosion of waterborne polyurethane coatings. Korean J. Chem. Eng. 2017, 34, 2480–2487. [Google Scholar] [CrossRef]
- Liu, J. Super Slipery Coating By ZnO Based on Aluminum and Its Anti-Corrosion Performance. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- Sanmugam, A.; Vikraman, D.; Karuppasamy, K.; Lee, J.Y.; Kim, H.-S. Evaluation of the Corrosion Resistance Properties of Electroplated Chitosan-Zn1−xCuxO Composite Thin Films. Nanomaterials 2017, 7, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipour, K.; Nasirpouri, F. Effect of Morphology and Surface Modification of Silica Nanoparticles on the Electrodeposition and Corrosion Behavior of Zinc-Based Nanocomposite Coatings. J. Electrochem. Soc. 2019, 166, D1–D9. [Google Scholar] [CrossRef]
- Esteban-Arranz, A.; de la Osa, A.R.; García-Lorefice, W.E.; Sacristan, J.; Sánchez-Silva, L. Long-Term Performance of Nanomodified Coated Concrete Structures under Hostile Marine Climate Conditions. Nanomaterials 2021, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ruiz, J.; Bautista-Ruiz, W.A.; Aperador, W. Anti-corrosive characterization of silicon, titanium, and zirconium oxide coatings deposited on aeronautical aluminum substrates via sol-gel. J. Phys. Conf. Ser. 2020, 1708, 12003. [Google Scholar] [CrossRef]
- Ning, C.; Mingyan, L.; Weidong, Z. Fouling and Corrosion Properties of SiO2 Coatings on Copper in Geothermal Water. Ind. Eng. Chem. Res. 2012, 51, 6001–6017. [Google Scholar] [CrossRef]
- Asmatulu, R.; Mahmud, G.A.; Hille, C.; Misak, H.E. Effects of UV degradation on surface hydrophobicity, crack, and thickness of MWCNT-based nanocomposite coatings. Prog. Org. Coat. 2011, 72, 553–561. [Google Scholar] [CrossRef]
- Zachariah, S.; Liu, Y.-L. Nanocomposites of polybenzoxazine-functionalized multiwalled carbon nanotubes and polybenzoxazine for anticorrosion application. Compos. Sci. Technol. 2020, 194, 108169. [Google Scholar] [CrossRef]
- Jeon, H.; Park, J.; Shon, M. Corrosion protection by epoxy coating containing multi-walled carbon nanotubes. J. Ind. Eng. Chem. 2013, 19, 849–853. [Google Scholar] [CrossRef]
- Bakhtiary-Noodeh, M.; Moradian, S.; Ranjbar, Z. Edge protection improvement of automotive electrocoatings in the presence of silica nanoparticles. Surf. Coat. Technol. 2017, 317, 134–147. [Google Scholar] [CrossRef]
- Charitidis, P.J. The Effect of Nanoparticles in Single Lap Joints Studied by Numerical Analyses. Eur. J. Eng. Technol. Res. 2020, 5. [Google Scholar] [CrossRef]
- Danglad-Flores, J.; Eickelmann, S.; Riegler, H. Deposition of polymer films by spin casting: A quantitative analysis. Chem. Eng. Sci. 2018, 179, 257–264. [Google Scholar] [CrossRef]
- Fowler, P.D.; Ruscher, C.; McGraw, J.D.; Forrest, J.A.; Dalnoki-Veress, K. Controlling Marangoni-induced instabilities in spin-cast polymer films: How to prepare uniform films. Eur. Phys. J. E 2016, 39, 90. [Google Scholar] [CrossRef] [PubMed]
- Grohens, Y.; Brogly, M.; Labbe, C.; Schultz, J. Chain flattening of spin-cast PMMMA on aluminum mirrors: Influence of polymer tacticity. Eur. Polym. J. 1997, 33, 691–697. [Google Scholar] [CrossRef]
- Shui, X.; Li, J.; Zhang, M.; Fang, C.; Zhu, L. Tailoring ultrathin microporous polyamide films with rapid solvent transport by molecular layer-by-layer deposition. J. Memb. Sci. 2021, 628, 119249. [Google Scholar] [CrossRef]
- Mulhearn, W.D.; Oleshko, V.P.; Stafford, C.M. Thickness-dependent permeance of molecular layer-by-layer polyamide membranes. J. Memb. Sci. 2021, 618, 118637. [Google Scholar] [CrossRef] [PubMed]
- Hoat, P.D.; Ha, H.-H.; Hung, P.T.; Hien, V.X.; Lee, S.; Lee, J.-H.; Heo, Y.-W. Synthesis of Cs2SnI6 perovskite thin film by low-pressure chemical vapor deposition method. Thin Solid Films 2021, 732, 138799. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Sevgili, E.; Karaman, M.; Lau, W.J.; Ismail, A.F. Hydroxypropyl methacrylate thin film coating on polyvinylidene fluoride hollow fiber membranes via initiated chemical vapor deposition. Eur. Polym. J. 2020, 122, 109360. [Google Scholar] [CrossRef]
- Sadeghi, E.; Markocsan, N.; Joshi, S. Advances in Corrosion-Resistant Thermal Spray Coatings for Renewable Energy Power Plants. Part I: Effect of Composition and Microstructure. J. Therm. Spray Technol. 2019, 28, 1749–1788. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, K.; Zhang, D.; Guo, Z. Stable and Durable Conductive Superhydrophobic Coatings Prepared by Double-Layer Spray Coating Method. Nanomaterials 2021, 11, 1506. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, D.-G.; Ha, T.; Chan Won, J.; Jang, K.-S.; Kim, Y.H. Solution-Processable, Thin, and High-κ Dielectric Polyurea Gate Insulator with Strong Hydrogen Bonding for Low-Voltage Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2018, 10, 32462–32470. [Google Scholar] [CrossRef]
- Park, Y.-S.; Choi, S.-E.; Kim, H.; Lee, J.S. Fine-Tunable Absorption of Uniformly Aligned Polyurea Thin Films for Optical Filters Using Sequentially Self-Limited Molecular Layer Deposition. ACS Appl. Mater. Interfaces 2016, 8, 11788–11795. [Google Scholar] [CrossRef]
- Nye, R.A.; Kelliher, A.P.; Gaskins, J.T.; Hopkins, P.E.; Parsons, G.N. Understanding Molecular Layer Deposition Growth Mechanisms in Polyurea via Picosecond Acoustics Analysis. Chem. Mater. 2020, 32, 1553–1563. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsuji, A.; Shimoyama, A.; Hayakawa, M.; Matsubara, R.; Kubono, A. Electrical properties of crosslinked aliphatic polyurea thin films prepared by vapor deposition polymerization. Jpn. J. Appl. Phys. 2020, 59, 36502. [Google Scholar] [CrossRef]
- Zhou, H.; Bent, S.F. Fabrication of organic interfacial layers by molecular layer deposition: Present status and future opportunities. J. Vac. Sci. Technol. A 2013, 31, 40801. [Google Scholar] [CrossRef]
- Meng, X. An overview of molecular layer deposition for organic and organic-inorganic hybrid materials: Mechanisms, growth characteristics, and promising applications. J. Mater. Chem. A 2017, 5, 18326–18378. [Google Scholar] [CrossRef]
- Song, G.-L.; Feng, Z. Modification, Degradation and Evaluation of a Few Organic Coatings for Some Marine Applications. Corros. Mater. Degrad. 2020, 1, 19. [Google Scholar] [CrossRef]
- Rivero, P.J.; Redin, D.M.; Rodríguez, R.J. Electrospinning: A Powerful Tool to Improve the Corrosion Resistance of Metallic Surfaces Using Nanofibrous Coatings. Metals 2020, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Cocchi, D.; Musiari, F.; Brugo, T.M.; Pirondi, A.; Zucchelli, A.; Campanini, F.; Leoni, E.; Mazzocchetti, L. Characterization of aluminum alloy-epoxy bonded joints with nanofibers obtained by electrospinning. J. Adhes. 2020, 96, 384–401. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Barakat, N.A.M.; Lim, J.K. Influence of electrospinning and dip-coating techniques on the degradation and cytocompatibility of Mg-based alloy. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 420, 37–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Yu, L.; Jiang, T. Hydrophobic polystyrene/electro-spun polyaniline coatings for corrosion protection. Synth. Met. 2017, 234, 166–174. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef] [Green Version]
- Pires, R.F.; Bonifácio, V.D.B. Polyureas. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Yi, J.; Boyce, M.C.; Lee, G.F.; Balizer, E. Large deformation rate-dependent stress-strain behavior of polyurea and polyurethanes. Polymer 2006, 47, 319–329. [Google Scholar] [CrossRef]
- Roland, C.M.; Twigg, J.N.; Vu, Y.; Mott, P.H. High strain rate mechanical behavior of polyurea. Polymer 2007, 48, 574–578. [Google Scholar] [CrossRef]
- James, E.; Mark Myer, K. 6—Thermoset Elastomer. In Applied Plastics Engineering Handbook—Processing, Materials, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-323-39040-8. Available online: https://app.knovel.com/hotlink/toc/id:kpAPEHPMA5/applied-plastics-engineering/applied-plastics-engineering (accessed on 3 November 2021).
- Malik, M.; Kaur, R. Influence of aliphatic and aromatic isocyanates on the properties of poly(ether ester) polyol based PU adhesive system. Polym. Eng. Sci. 2018, 58, 112–117. [Google Scholar] [CrossRef]
- He, Y.; Xie, D.; Zhang, X. The structure, microphase-separated morphology, and property of polyurethanes and polyureas. J. Mater. Sci. 2014, 49, 7339–7352. [Google Scholar] [CrossRef]
- Tripathi, M.; Parthasarathy, S.; Roy, P.K. Mechanically robust polyurea nanofibers processed through electrospinning technique. Mater. Today Commun. 2020, 22, 100771. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhong, Q.; Cui, C.; Ma, L.; Liu, S.; Zhang, Q.; Wu, Y.; An, L.; Cheng, Y.; Ye, S.; et al. Extremely Tough, Puncture-Resistant, Transparent, and Photoluminescent Polyurethane Elastomers for Crack Self-Diagnose and Healing Tracking. ACS Appl. Mater. Interfaces 2020, 12, 30847–30855. [Google Scholar] [CrossRef] [PubMed]
- Wildner, W.; Drummer, D. Nanofiller materials for transparent polymer composites: Influences on the properties and on the transparency—A review. J. Thermoplast. Compos. Mater. 2019, 32, 1547–1565. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Bai, H.; Ming, W. ZnO Nanoparticles Coated with Amphiphilic Polyurethane for Transparent Polyurethane Nanocomposites with Enhanced Mechanical and UV-Shielding Performance. ACS Appl. Nano Mater. 2020, 3, 59–67. [Google Scholar] [CrossRef]
- Casalini, R.; Bogoslovova, R.; Qadrib, S.B.; Roland, C.M. Nanofiller reinforcement of elastomeric polyurea. Polymer 2012, 53, 1282–1287. [Google Scholar] [CrossRef]
- Gao, C.; Jin, Y.Z.; Kong, H.; Whitby, R.L.D.; Acquah, S.F.A.; Chen, G.Y.; Qian, H.; Hartschuh, A.; Silva, S.R.P.; Henley, S.; et al. Polyurea-Functionalized Multiwalled Carbon Nanotubes: Synthesis, Morphology, and Raman Spectroscopy. J. Phys. Chem. B 2005, 109, 11925–11932. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.; Parthasarathy, S.; Kumar, D.; Chandel, P.; Sharma, P.; Roy, P.K. Strain rate sensitivity of polyurea coatings: Viscous and elastic contributions. Polym. Test. 2020, 86, 106488. [Google Scholar] [CrossRef]
- Riehle, N.; Athanasopulu, K.; Kutuzova, L.; Götz, T.; Kandelbauer, A.; Tovar, G.E.M.; Lorenz, G. Influence of Hard Segment Content and Diisocyanate Structure on the Transparency and Mechanical Properties of Poly(dimethylsiloxane)-Based Urea Elastomers for Biomedical Applications. Polymers 2021, 13, 212. [Google Scholar] [CrossRef]
- Castagna, A.M.; Pangon, A.; Choi, T.; Dillon, G.P.; Runt, J. The role of soft segment molecular weight on microphase separation and dynamics of bulk polymerized polyureas. Macromolecules 2012, 45, 8438–8444. [Google Scholar] [CrossRef]
- Iqbal, N.; Tripathi, M.; Parthasarathy, S.; Kumar, D.; Roy, P.K. Tuning the properties of segmented polyurea by regulating soft-segment length. J. Appl. Polym. Sci. 2018, 135, 46284. [Google Scholar] [CrossRef]
- Blagojević, S.L.; Buhin, Z.; Pustak, A.; Lukić Kovačić, R. Influence of nanosilica on the morphology, thermal and mechanical properties of polyurethane elastomer. J. Appl. Polym. Sci. 2012, 125, E181–E190. [Google Scholar] [CrossRef]
- Kakade, B.A.; Pillai, V.K. Tuning the wetting properties of multiwalled carbon nanotubes by surface functionalization. J. Phys. Chem. C 2008, 112, 3183–3186. [Google Scholar] [CrossRef]
- Wu, M.; Ge, S.; Jiao, C.; Yan, Z.; Jiang, H.; Zhu, Y.; Dong, B.; Dong, M.; Guo, Z. Improving electrical, mechanical, thermal and hydrophobic properties of waterborne acrylic resin-glycidyl methacrylate (GMA) by adding multi-walled carbon nanotubes. Polymer 2020, 200, 122547. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Westerberg, L.G.; Li, J.; Höglund, E.; Lugt, P.M.; Baart, P. Free-surface grease flow on a rotating plate. Tribol. Lett. 2014, 56, 317–325. [Google Scholar] [CrossRef]
- Kim, D.S.; Kwon, T.H. Modeling, analysis and design of centrifugal force driven transient filling flow into rectangular microchannel. Microsyst. Technol. 2006, 12, 822–838. [Google Scholar] [CrossRef]
- Greenhalgh, E.S. (Ed.) 4—Delamination-dominated failures in polymer composites. In Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2009; pp. 164–237. ISBN 978-1-84569-217-9. [Google Scholar]
- Santmarti, A.; Teh, J.W.; Lee, K.-Y. Transparent Poly(methyl methacrylate) Composites Based on Bacterial Cellulose Nanofiber Networks with Improved Fracture Resistance and Impact Strength. ACS Omega 2019, 4, 9896–9903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Qiu, J.; Sakai, E.; Wei, X. Effects of multiwalled carbon nanotube mass fraction on microstructures and electrical resistivity of polycarbonate-based conductive composites. Sci. Eng. Compos. Mater. 2017, 24, 163–175. [Google Scholar] [CrossRef]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling surface morphology of electrospun polystyrene fibers: Effect of humidity and molecular weight in the electrospinning process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Lin, L.; Wang, H.; Huang, X.; Xue, H.; Gao, J. Highly stretchable, anti-corrosive and wearable strain sensors based on the PDMS/CNTs decorated elastomer nanofiber composite. Chem. Eng. J. 2019, 362, 89–98. [Google Scholar] [CrossRef]
- Pakuła, D.; Dobrosielska, M.; Jarkowski, M.; Popiół, M.; Klonowski, B.; Brząkalski, D.; Marciniec, B.; Przekop, R. A New Microsilica Filler for Polyurea Systems. 2019. Available online: https://www.semanticscholar.org/paper/MICROSILICA-FILLER-FOR-POLYUREA-SYSTEMS-Paku%C5%82a-Dobrosielska/d3454beb65d9e55207bbf3bee75ad597986945df (accessed on 3 November 2021).
- Awad, W.H.; Wilkie, C.A. Investigation of the thermal degradation of polyurea: The effect of ammonium polyphosphate and expandable graphite. Polymer 2010, 51, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Yilgor, I.; Yilgor, E.; Wilkes, G.L. Probing the urea hard domain connectivity in segmented, non-chain extended polyureas using hydrogen-bond screening agents. Polymer 2008, 49, 174–179. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef]
- Maria, M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S. Non-isothermal thermogravimetry as an accelerated tool for the shelf-life prediction of paracetamol formulations. Thermochim. Acta 2021, 700, 178940. [Google Scholar] [CrossRef]
- Blaine, R.L.; Hahn, B.K. Obtaining kinetic parameters by modulated thermogravimetry. J. Therm. Anal. Calorim. 1998, 54, 695–704. [Google Scholar] [CrossRef]
- Matějka, L.; Špírková, M.; Dybal, J.; Kredatusová, J.; Hodan, J.; Zhigunov, A.; Šlouf, M. Structure evolution during order–disorder transitions in aliphatic polycarbonate based polyurethanes. Self-healing polymer. Chem. Eng. J. 2019, 357, 611–624. [Google Scholar] [CrossRef]
- Miljkovic, N.; Wang, E.N. Condensation heat transfer on superhydrophobic surfaces. MRS Bull. 2013, 38, 397–406. [Google Scholar] [CrossRef]
- Faes, W.; Lecompte, S.; Ahmed, Z.Y.; Van Bael, J.; Salenbien, R.; Verbeken, K.; De Paepe, M. Corrosion and corrosion prevention in heat exchangers. Corros. Rev. 2019, 37, 131–155. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Finn, M.; Tan, S.T.M.; Dickey, M.D.; Lipomi, D.J. Stretchable bioelectronics—Current and future. MRS Bull. 2017, 42, 960–967. [Google Scholar] [CrossRef]
- Sung, J.; Li, Y.; Sun, X.S. Plasticization effects of dihydroxyl soybean oil improve flexibilities of epoxy-based films for coating applications. J. Appl. Polym. Sci. 2015, 132, 41773. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, P.K.; Yadav, K.L.; Kumar, K. Thermo-mechanical and anti-corrosive properties of MWCNT/epoxy nanocomposite fabricated by innovative dispersion technique. Compos. Part B Eng. 2017, 113, 291–299. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, P.; Zhang, H.; Yan, C.; Zheng, Z.; Wu, B.; Yu, Y. A Transparent, Highly Stretchable, Autonomous Self-Healing Poly(dimethyl siloxane) Elastomer. Macromol. Rapid Commun. 2017, 38, 1700110. [Google Scholar] [CrossRef]
- Fang, G.; Ren, J.; Shi, J.; Gao, X.; Song, Y. Thermal Stress Analysis of Environmental Barrier Coatings Considering Interfacial Roughness. Coatings 2020, 10, 947. [Google Scholar] [CrossRef]
- Yanhai, C.; Lu, R.; Xianliang, M.; Jinyong, Y.; Xianhua, T. The effect of PTFE addition on mechanical and anti-corrosion properties of coating of heat exchangers. Mater. Res. Express 2019, 6, 85207. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, W.; Zhao, S.; Zhang, X.; Pei, L.; Zhao, G.; Wang, Z. Flexible and thermally stable superhydrophobic surface with excellent anti-corrosion behavior. J. Mater. Sci. 2020, 55, 2215–2225. [Google Scholar] [CrossRef]















| Film | Td1 (°C) | Td2 (°C) | Ea (kJ mol−1) |
|---|---|---|---|
| PU | 302.1 | 380.2 | 160 ± 9 |
| PU-0.2%MWCNT | 307.7 | 387.4 | 182 ± 8 |
| PU-0.4%HFNS | 307.7 | 386.1 | 187 ± 9 |
| Film | Storage Modulus (MPa) | Loss Modulus (MPa) | tan(δ) |
|---|---|---|---|
| PU | 93.9 | 12.75 | 0.135 |
| PU-0.2%MWCNT | 135.9 | 19.43 | 0.143 |
| PU-0.4%HFNS | 111.2 | 16.35 | 0.147 |
| Reference | Sung et al. [97] | Kumar et al. [98] | Yanhai et al. [101] | Zhu et al. [102] | Zhang et al. [99] | Our Study |
|---|---|---|---|---|---|---|
| Metal | - | Mild steel | Mild steel (1015) | Tin | Stainless steel | - |
| Polymer blend | 3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexanecarboxylate (ECHM) and Dihydroxyl Soybean Oil blend (DSO) | MWCNT/Epoxy resin (Cam coat 2071) | Polytetrafluoroethylene in Ni-Cu-P coating | Bisphenol A-based benzoxazine (BA-a), polyurethane, and mesoporous SiO2 (SBA-15) | bis(amine)-terminated poly(dimethylsiloxane) (H2N-PDMS-NH2) and 1,3,5-triformylbenzene (TFB) | Aromatic polyurea |
| Deposition method | Casting | Spray coating | Electroless plating | Spray coating | Casting | Electrospinning |
| Tensile Strength | 34.5 MPa (After 10 days) | ~75 MPa (with 0.75 wt% MWCNT) | - | - | 0.035 MPa | 14.1 MPa(Neat) 20.8 MPa (0.2 wt% MWCNT) |
| Elongation | 3.7% | 1.1% | - | - | 150% (20 mm/min) | 360% (Neat) 402% (0.2 wt% MWCNT) (500 mm/min) |
| Water contact angle | - | - | - | 150° | 123.2° | 90° |
| Degradation temperature | 428 °C (50% mass loss) | 342 °C (10% mass loss) | - | - | Stable until 525 °C | 15% mass loss Neat: 302.1 °C 0.2% MWCNT: 307.7 °C |
| Activation energy (kJ/mol) | - | - | 290 | 149.3 | - | Neat: 160 0.2% MWCNT: 182 0.4% HFNS: 187 |
| Transparency | 90% | - | - | - | 80% | Neat PU: Optically transparent |
| Corrosion resistance before coating | - | - | 1.75 mg/cm2 mass loss (7 days in 3.5% NaCl solution) | 2.14 × 10−4 A cm−2 corrosion current density (10 days in 3.5% NaCl solution) | - | - |
| Corrosion resistance after coating | - | 99.99% protection efficiency (0.75 wt% MWCNT) in 3.5% NaCl solution. | 0.1 mg/cm2 mass loss (7 days in 3.5% NaCl solution) | 8.9 × 10−5 A cm−2 corrosion current density (10 days in 3.5% NaCl solution) | Contact angle showed no significant change 8 days in 4% NaCl | - |
| Targeted application | Coating applications | General anti-corrosion coating | Heat exchanger | Superhydrophobic surfaces | Anti-corrosion coating and flexible electronics | General coating for anti-corrosion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auckloo, S.A.B.; Palaniandy, K.; Hung, Y.M.; Lazzara, G.; Chai, S.-P.; Pasbakhsh, P. Nonporous, Strong, Stretchable, and Transparent Electrospun Aromatic Polyurea Nanocomposites as Potential Anticorrosion Coating Films. Nanomaterials 2021, 11, 2998. https://doi.org/10.3390/nano11112998
Auckloo SAB, Palaniandy K, Hung YM, Lazzara G, Chai S-P, Pasbakhsh P. Nonporous, Strong, Stretchable, and Transparent Electrospun Aromatic Polyurea Nanocomposites as Potential Anticorrosion Coating Films. Nanomaterials. 2021; 11(11):2998. https://doi.org/10.3390/nano11112998
Chicago/Turabian StyleAuckloo, Sheik Ambarine Banon, Khanisya Palaniandy, Yew Mun Hung, Giuseppe Lazzara, Siang-Piao Chai, and Pooria Pasbakhsh. 2021. "Nonporous, Strong, Stretchable, and Transparent Electrospun Aromatic Polyurea Nanocomposites as Potential Anticorrosion Coating Films" Nanomaterials 11, no. 11: 2998. https://doi.org/10.3390/nano11112998
APA StyleAuckloo, S. A. B., Palaniandy, K., Hung, Y. M., Lazzara, G., Chai, S.-P., & Pasbakhsh, P. (2021). Nonporous, Strong, Stretchable, and Transparent Electrospun Aromatic Polyurea Nanocomposites as Potential Anticorrosion Coating Films. Nanomaterials, 11(11), 2998. https://doi.org/10.3390/nano11112998












