Temperature-Dependent Growth of 36 Inner Nanotubes inside Nickelocene, Cobaltocene and Ferrocene-Filled Single-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. SEM and TEM Studies
3.2. Multifrequency Raman Spectroscopy Studies on Inner Tube Growth
3.3. Evaluation of Growth Temperatures of Inner Tubes
3.4. Dependence of Growth Temperatures of Inner Tubes on Their Diameter and Metal Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Guan, L.; Shi, Z.; Li, M.; Gu, Z. Ferrocene-filled single-walled carbon nanotubes. Carbon 2005, 43, 2780–2785. [Google Scholar] [CrossRef]
- Li, L.J.; Khlobystov, A.N.; Wiltshire, J.G.; Briggs, G.A.D.; Nicholas, R.J. Diameter-selective encapsulation of metallocenes in single-walled carbon nanotubes. Nat. Mater. 2005, 4, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, H.; Pichler, T.; Gruneis, A.; Pfeiffer, R.; Kuzmany, H.; Liu, Z. A catalytic reaction inside a single-walled carbon nanotube. Adv. Mater. 2008, 20, 1443–1449. [Google Scholar] [CrossRef]
- Shiozawa, H.; Pichler, T.; Kramberger, C.; Gruneis, A.; Knupfer, M.; Buchner, B. Fine tuning the charge transfer in carbon nanotubes via the interconversion of encapsulated molecules. Phys. Rev. B 2008, 77, 153402. [Google Scholar] [CrossRef] [Green Version]
- Plank, W.; Pfeiffer, R.; Schaman, C.; Kuzmany, H.; Calvaresi, M.; Zerbetto, F.; Meyer, J. Electronic structure of carbon nanotubes with ultrahigh curvature. ACS Nano 2010, 4, 4515–4522. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Sauer, M.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Doping of single-walled carbon nanotubes controlled via chemical transformation of encapsulated nickelocene. Nanoscale 2015, 7, 1383–1391. [Google Scholar] [CrossRef] [Green Version]
- Fukumaru, T.; Fujigaya, T.; Nakashima, N. Development of n-type cobaltocene-encapsulated carbon nanotubes with remarkable thermoelectric property. Sci. Rep. 2015, 5, 7951. [Google Scholar] [CrossRef] [Green Version]
- Shiozawa, H.; Pichler, T.; Kramberger, C.; Rummeli, M.; Batchelor, D.; Liu, Z.; Suenaga, K.; Kataura, H.; Silva, S.R.P. Screening the missing electron: Nanochemistry in action. Phys. Rev. Lett. 2009, 102, 046804. [Google Scholar] [CrossRef] [Green Version]
- Gueorguiev, G.K.; Czigány, Z.; Furlan, A.; Stafström, S.; Hultman, L. Intercalation of P atoms in Fullerene-like CPx. Chem. Phys. Lett. 2011, 501, 400–403. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Greczynski, G.; Goyenola, C.; Gueorguiev, G.K.; Czigány, Z.; Jensen, J.; Ivanov, I.G.; Hultman, L. CFx thin solid films deposited by high power impulse magnetron sputtering: Synthesis and characterization. Surface and Coatings. Technology 2011, 206, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Gueorguiev, G.K.; Goyenola, C.; Schmidt, S.; Hultman, L. CFx: A first-principles study of structural patterns arising during synthetic growth. Chem. Phys. Lett. 2011, 516, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Xie, K.; Jia, Q.; Zhang, X.; Fu, L.; Zhao, G. Electronic and Magnetic Properties of Stone–Wales Defected Graphene Decorated with the Half-Metallocene of M (M = Fe, Co, Ni): A First Principle Study. Nanomaterials 2018, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Xie, K.; An, N.; Zhang, Y.; Liu, G.; Zhang, F.; Zhang, Y.; Jiao, F. Two-dimensional porphyrin sheet as an electric and optical sensor material for pH detection: A DFT study. Comput. Mater. Sci. 2020, 174, 109485. [Google Scholar] [CrossRef]
- Enriquez, J.; Villagracia, A. Hydrogen adsorption on pristine, defected, and 3d-block transition metal-doped penta-graphene. Int. J. Hydrog. Energy 2016, 41, 12157–12166. [Google Scholar] [CrossRef]
- Liu, X.J.; Kuzmany, H.; Saito, T.; Pichler, T. Temperature dependence of inner tube growth from ferrocene-filled single-walled carbon nanotubes. Phys. Status Solidi B 2011, 248, 2492–2495. [Google Scholar] [CrossRef]
- Briones, A.; Liu, X.J.; Kramberger, C.; Saito, T.; Pichler, T. Nanochemical reactions by laser annealing of ferrocene filled single-walled carbon nanotubes. Phys. Status Solidi B 2011, 248, 2488–2491. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Sauer, M.; Saito, T.; Krause, S.; Liu, X.; Yanagi, K.; Pichler, T.; Shiozawa, H. Inner tube growth properties and electronic structure of ferrocene-filled large diameter single-walled carbon nanotubes. Phys. Status Solidi B 2013, 250, 2575–2580. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Sauer, M.; Egorov, A.; Kramberger, C.; Saito, T.; Pichler, T.; Shiozawa, H. Temperature-dependent inner tube growth and electronic structure of nickelocene-filled single-walled carbon nanotubes. Phys. Status Solidi B 2015, 252, 2485–2490. [Google Scholar] [CrossRef]
- Fabian, G.; Kramberger, C.; Friedrich, A.; Simon, F.; Pichler, T. A broadband and high throughput single-monochromator Raman spectrometer: Application for single-wall carbon nanotubes. Rev. Sci. Instrum. 2011, 82, 023905. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V.; Kramberger, C. Metal Cluster Size-Dependent Activation Energies of Growth of Single-Chirality Single-Walled Carbon Nanotubes inside Metallocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Shiozawa, H.; Pichler, T. Growth dynamics of inner tubes inside cobaltocene-filled single-walled carbon nanotubes. Appl. Phys. A 2016, 122, 749. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Araujo, P.T.; Maciel, I.O.; Pesce, P.B.C.; Pimenta, M.A.; Doorn, S.K.; Qian, H.; Hartschuh, A.; Steiner, M.; Grigorian, L.; Hata, K.; et al. Nature of the constant factor in the relation between radial breathing mode frequency and tube diameter for single-wall carbon nanotubes. Phys. Rev. B 2008, 77, 241403. [Google Scholar] [CrossRef] [Green Version]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Pichler, T. Diameter and metal-dependent growth properties of inner tubes inside metallocene-filled single-walled carbon nanotubes. Fullerenes, Nanotubes and Carbon Nanostructures 2020, 28, 20–26. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Souza, A.G.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Brown, S.D.M.; Corio, P.; Marucci, A.; Dresselhaus, M.S.; Pimenta, M.A.; Kneipp, K. Anti-Stokes Raman spectra of single-walled carbon nanotubes. Phys. Rev. B 2000, 61, R5137–R5140. [Google Scholar] [CrossRef]
- Jorio, A.; Souza, A.G.; Dresselhaus, G.; Dresselhaus, M.S.; Swan, A.K.; Unlu, M.S.; Goldberg, B.B.; Pimenta, M.A.; Hafner, J.H.; Lieber, C.M.; et al. G-band resonant Raman study of 62 isolated single-wall carbon nanotubes. Phys. Rev. B 2002, 65, 155412. [Google Scholar] [CrossRef] [Green Version]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Chirality-dependent growth of single-wall carbon nanotubes as revealed inside nano-test tubes. Nanoscale 2017, 9, 7998–8006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharlamova, M.V.; Kramberger, C.; Sato, Y.; Saito, T.; Suenaga, K.; Pichler, T.; Shiozawa, H. Chiral vector and metal catalyst-dependent growth kinetics of single-wall carbon nanotubes. Carbon 2018, 133, 283–292. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef] [Green Version]
- Somorjai, G.A. Introduction to Surface Chemistry and Catalysis; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Shiozawa, H.; Kramberger, C.; Pfeiffer, R.; Kuzmany, H.; Pichler, T.; Liu, Z.; Suenaga, K.; Kataura, H.; Silva, S.R.P. Catalyst and chirality dependent growth of carbon nanotubes determined through nano-test tube chemistry. Adv. Mater. 2010, 22, 3685–3689. [Google Scholar] [CrossRef]
- Shiozawa, H.; Silva, S.R.P.; Liu, Z.; Suenaga, K.; Kataura, H.; Kramberger, C.; Pfeiffer, R.; Kuzmany, H.; Pichler, T. Low-temperature growth of single-wall carbon nanotubes inside nano test tubes. Phys. Status Solidi B 2010, 247, 2730–2733. [Google Scholar] [CrossRef]
- Baker, R.T.K. Catalytic growth of carbon filaments. Carbon 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Bower, C.; Zhou, O.; Zhu, W.; Werder, D.J.; Jin, S.H. Nucleation and growth of carbon nanotubes by microwave plasma chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 2767–2769. [Google Scholar] [CrossRef]
- Choi, Y.C.; Shin, Y.M.; Lee, Y.H.; Lee, B.S.; Park, G.S.; Choi, W.B.; Lee, N.S.; Kim, J.M. Controlling the diameter, growth rate, and density of vertically aligned carbon nanotubes synthesized by microwave plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2000, 76, 2367–2369. [Google Scholar] [CrossRef] [Green Version]
- Chiang, W.H.; Sankaran, R.M. Relating carbon nanotube growth parameters to the size and composition of nanocatalysts. Diam. Relat. Mater. 2009, 18, 946–952. [Google Scholar] [CrossRef]
- Patole, S.P.; Kim, H.; Choi, J.; Kim, Y.; Baik, S.; Yoo, J.B. Kinetics of catalyst size dependent carbon nanotube growth by growth interruption studies. Appl. Phys. Lett. 2010, 96, 094101. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Sodi, F.; McNicholas, T.P.; Simmons, J.G.; Liu, J.; Csanyi, G.; Ferrari, A.C.; Curtarolo, S. Viscous state effect on the activity of Fe nanocatalysts. ACS Nano 2010, 4, 6950–6956. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.S.; Lee, Y.T.; Park, J.H.; Ryu, H.; Lee, H.J.; Choi, S.Y.; Choo, J.B. Dependence of the vertically aligned growth of carbon nanotubes on the catalysts. J. Phys. Chem. B 2002, 106, 9286–9290. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C.; Sauer, M.; Yanagi, K.; Saito, T.; Pichler, T. Inner tube growth and electronic properties of metallicity-sorted nickelocene-filled semiconducting single-walled carbon nanotubes. Appl. Phys. A 2018, 124, 247. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Nickelocene-Filled Purely Metallic Single-Walled Carbon Nanotubes: Sorting and Tuning the Electronic Properties. Nanomaterials 2021, 11, 2500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Min, X.; Ding, Z.; Chen, S.; Ai, C.; Liu, Z.; Yang, T.; Wu, X.; Liu, Y.; Lin, S.; et al. Metal-Based Nanocatalysts via a Universal Design on Cellular Structure. Adv. Sci. 2020, 7, 1902051. [Google Scholar] [CrossRef] [Green Version]
- Min, X.; Sun, B.; Chen, S.; Fang, M.; Wu, X.; Liu, Y.; Abdelkader, A.; Huang, Z.; Liu, T.; Xi, K.; et al. A textile-based SnO2 ultra-flexible electrode for lithium-ion batteries. Energy Storage Mater. 2019, 16, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Min, X.; Xiao, J.; Fang, M.; Wang, W.; Zhao, Y.; Liu, Y.; Abdelkader, A.; Xi, K.; Kumar, R.; Huang, Z. Potassium-ion batteries: Outlook on present and future technologies. Energy Environ. Sci. 2021, 14, 2186–2243. [Google Scholar] [CrossRef]
- Gao, C.; Min, X.; Fang, M.; Tao, T.; Zheng, X.; Liu, Y.; Wu, X.; Huang, Z. Innovative Materials Science via Machine Learning. Adv. Funct. Mater. 2021, 2108044. [Google Scholar] [CrossRef]



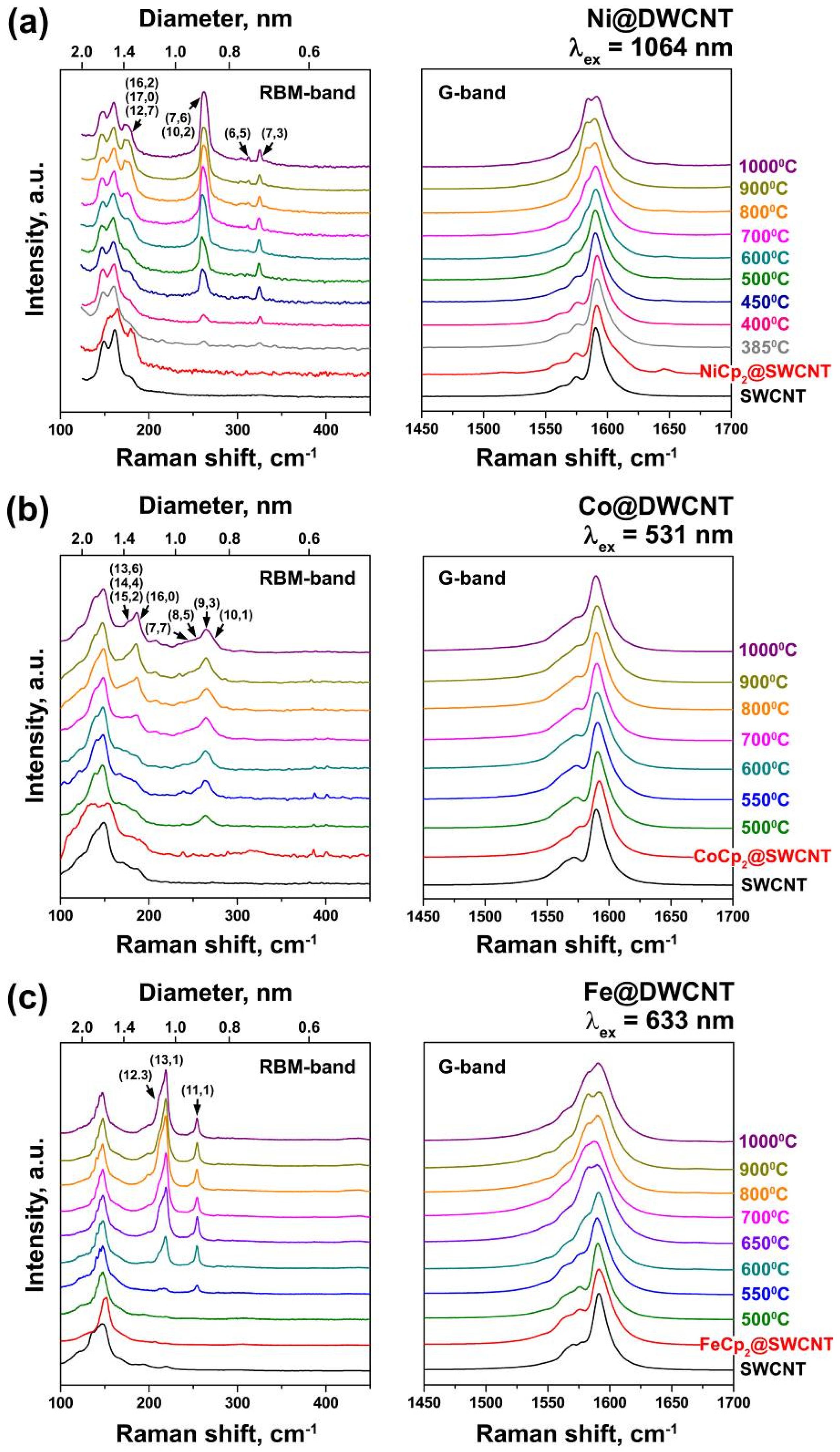
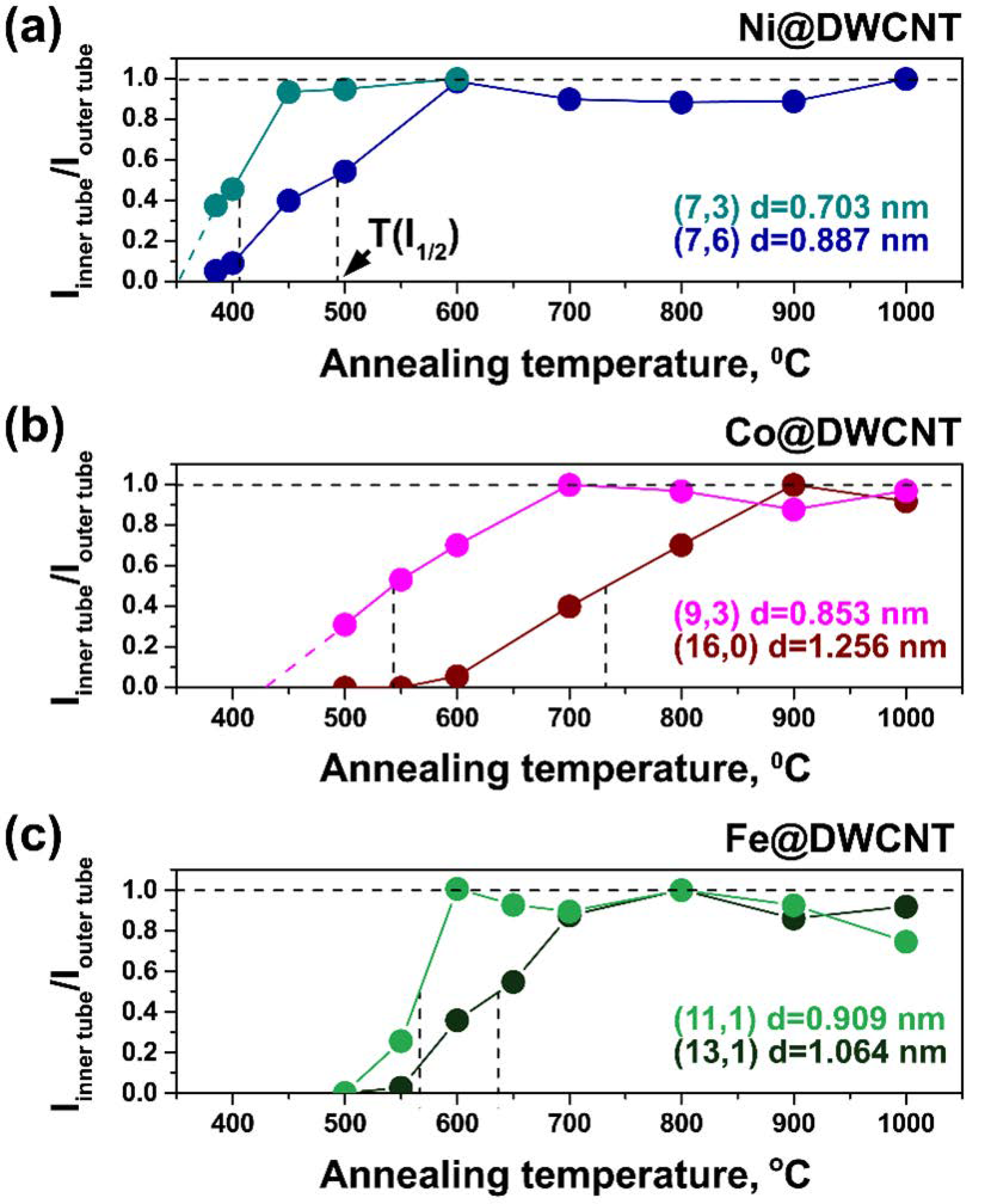

| dt, nm | (n,m) | RBM, cm−1 | λex, nm | T(I1/2), °C | ||
|---|---|---|---|---|---|---|
| Precursor | ||||||
| NiCp2 | CoCp2 | FeCp2 | ||||
| 0.703 | (7,3) | 325 | 1064 | 405 | 500 | 500 |
| 0.778 | (8,3) | 295 | 647 | 515 | 500 | 555 |
| 0.823 | (7,5) | 279 | 647 | 510 | 515 | 550 |
| 0.834 | (8,4) | 279 | 458 | 440 | 500 | 565 |
| 0.853 | (9,3) | 267 | 514 | 480 | 530 | 567 |
| 0.853 | (9,3) | 265 | 531 | 473 | 540 | 577 |
| 0.853 | (9,3) | 269 | 568 | 520 | 560 | 550 |
| 0.878 | (10,2) | 264 | 1064 | 530 | 520 | 560 |
| 0.887 | (7,6) | 260 | 1064 | 490 | 520 | 554 |
| 0.895 | (8,5) | 258 | 514 | 500 | 564 | 570 |
| 0.895 | (8,5) | 255 | 531 | 490 | 530 | 570 |
| 0.909 | (11,1) | 253 | 458 | 530 | 548 | 600 |
| 0.909 | (11,1) | 254 | 633 | 520 | 535 | 565 |
| 0.909 | (11,1) | 256 | 647 | 550 | 570 | 565 |
| 0.928 | (10,3) | 251 | 647 | 540 | 530 | 565 |
| 0.954 | (7,7) | 245 | 514 | 515 | 590 | 590 |
| 0.954 | (7,7) | 247 | 531 | 538 | 576 | 580 |
| 0.983 | (10,4) | 235 | 568 | 540 | 580 | 600 |
| 1.023 | (13,0) | 230 | 488 | 594 | 650 | 650 |
| 1.028 | (9,6) | 226 | 568 | 550 | 580 | 640 |
| 1.031 | (12,2) | 225 | 488 | 564 | 660 | 660 |
| 1.058 | (11,4) | 221 | 458 | 560 | 572 | 625 |
| 1.064 | (13,1) | 219 | 633 | 600 | 595 | 635 |
| 1.064 | (13,1) | 219 | 647 | 605 | 620 | 640 |
| 1.081 | (12,3) | 214 | 633 | 540 | 600 | 640 |
| 1.081 | (12,3) | 216 | 647 | 560 | 600 | 645 |
| 1.089 | (8,8) | 215 | 568 | 610 | 600 | 650 |
| 1.100 | (10,6) | 212 | 458 | 595 | 605 | 640 |
| 1.142 | (14,1) | 207 | 514 | 620 | 667 | 675 |
| 1.157 | (9,8) | 202 | 458 | 675 | 677 | 650 |
| 1.157 | (13,3) | 202 | 514 | 630 | 644 | 685 |
| 1.187 | (14,2) | 196 | 647 | 605 | 620 | 670 |
| 1.188 | (12,5) | 200 | 488 | 616 | 697 | 700 |
| 1.247 | (12,6) | 190 | 647 | 600 | 670 | 690 |
| 1.256 | (16,0) | 186 | 531 | 673 | 730 | 770 |
| 1.264 | (15,2) | 184 | 531 | 693 | 720 | 760 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharlamova, M.V.; Kramberger, C. Temperature-Dependent Growth of 36 Inner Nanotubes inside Nickelocene, Cobaltocene and Ferrocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 2984. https://doi.org/10.3390/nano11112984
Kharlamova MV, Kramberger C. Temperature-Dependent Growth of 36 Inner Nanotubes inside Nickelocene, Cobaltocene and Ferrocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials. 2021; 11(11):2984. https://doi.org/10.3390/nano11112984
Chicago/Turabian StyleKharlamova, Marianna V., and Christian Kramberger. 2021. "Temperature-Dependent Growth of 36 Inner Nanotubes inside Nickelocene, Cobaltocene and Ferrocene-Filled Single-Walled Carbon Nanotubes" Nanomaterials 11, no. 11: 2984. https://doi.org/10.3390/nano11112984
APA StyleKharlamova, M. V., & Kramberger, C. (2021). Temperature-Dependent Growth of 36 Inner Nanotubes inside Nickelocene, Cobaltocene and Ferrocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials, 11(11), 2984. https://doi.org/10.3390/nano11112984







