Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design
Abstract
:1. Introduction
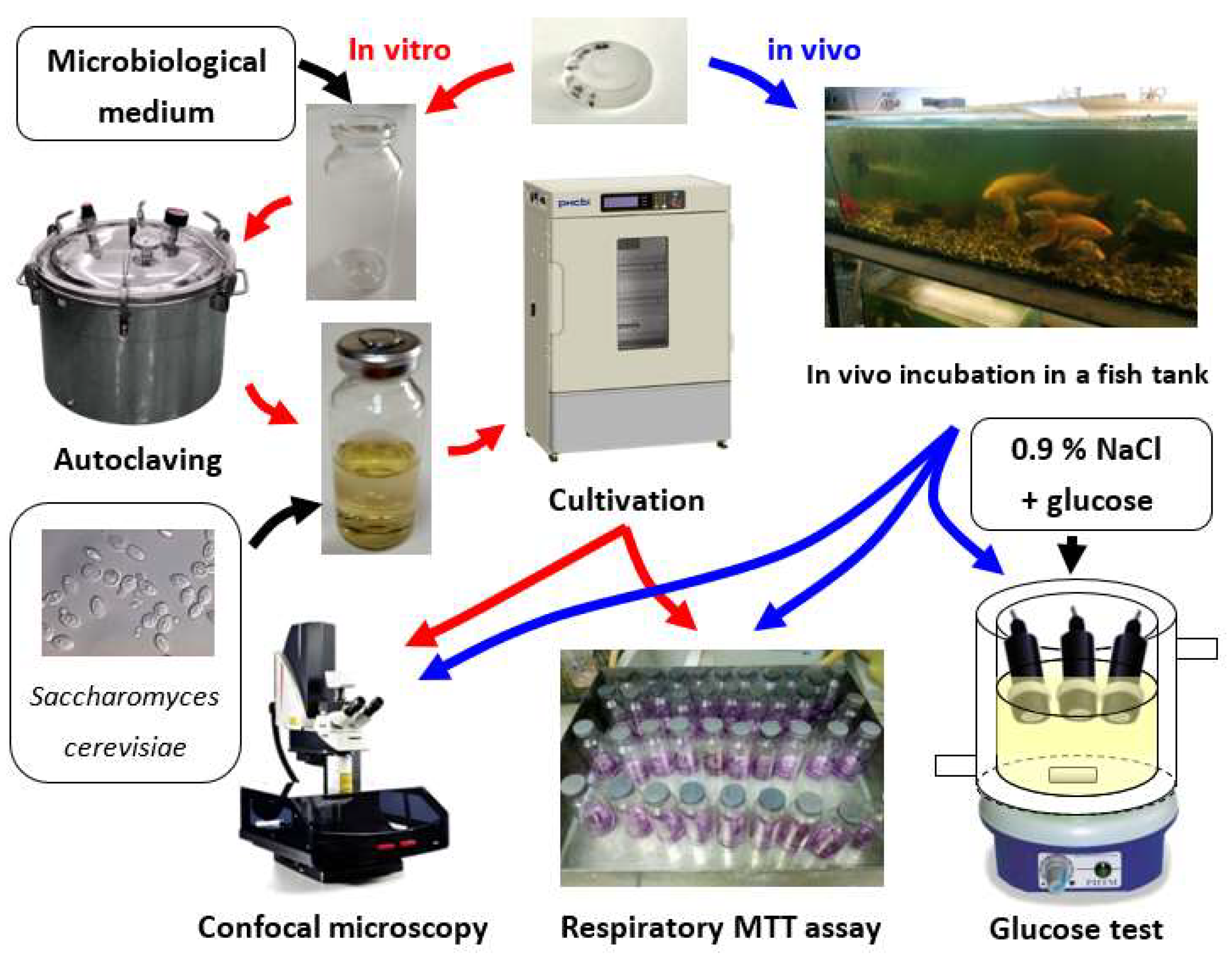
2. Materials and Methods
3. Results
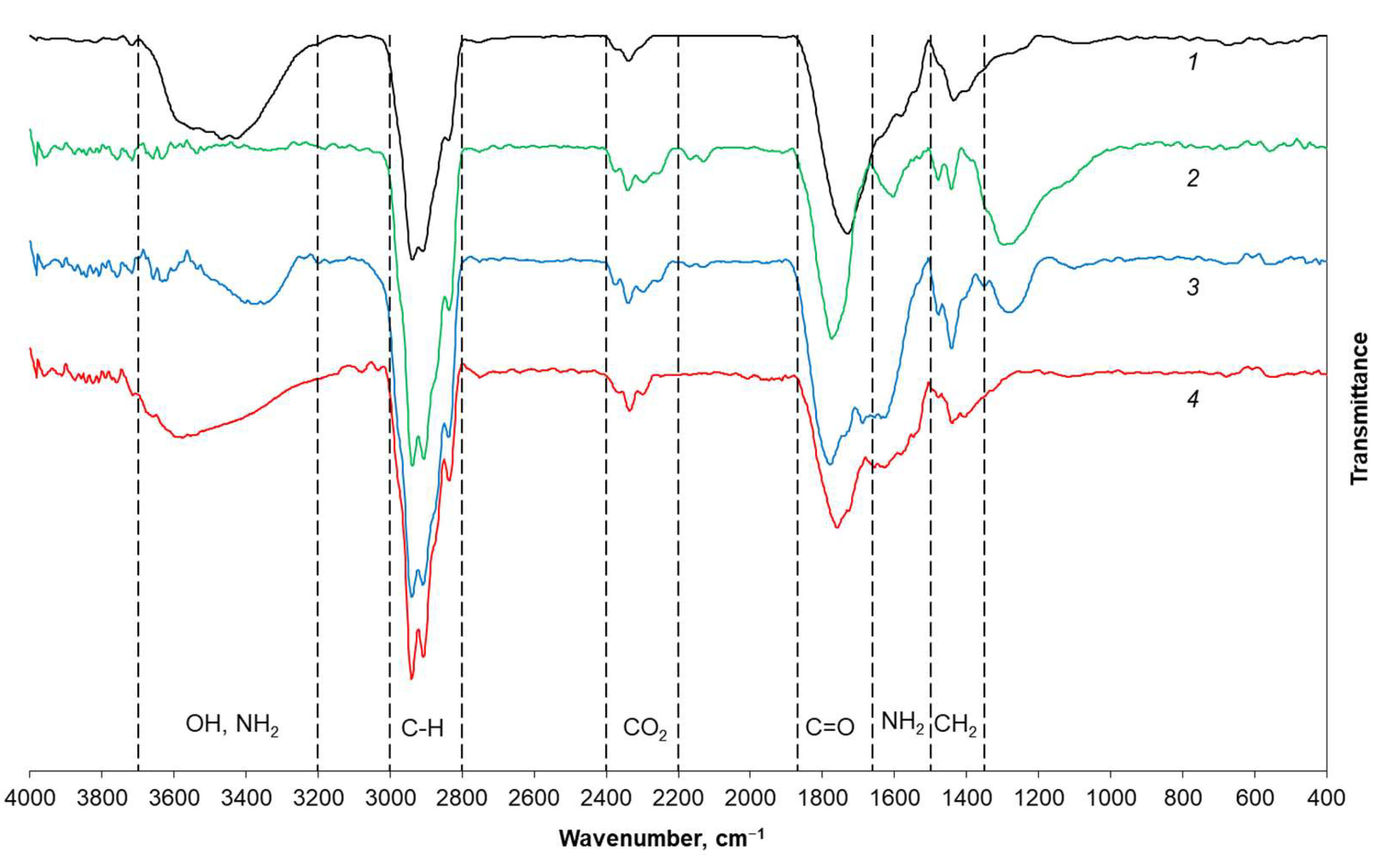
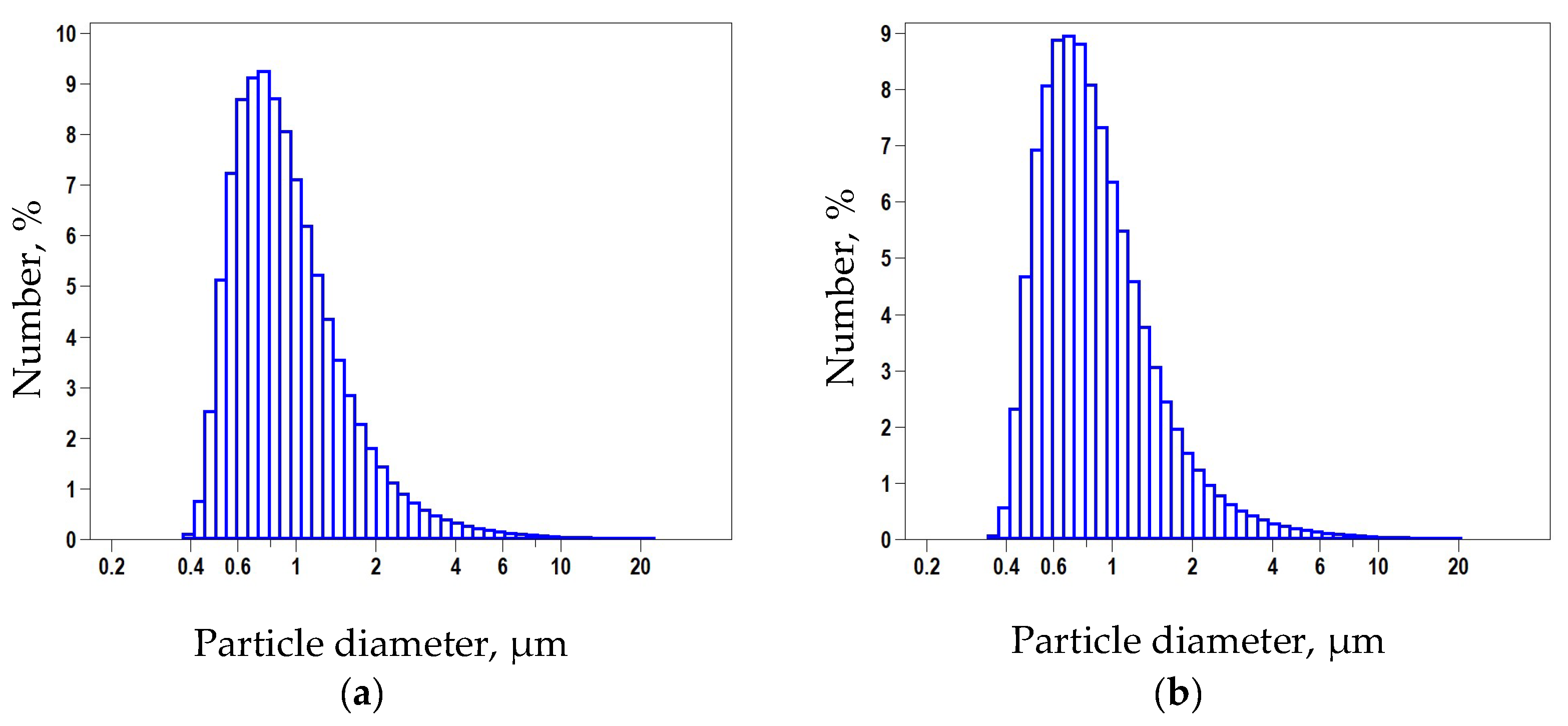
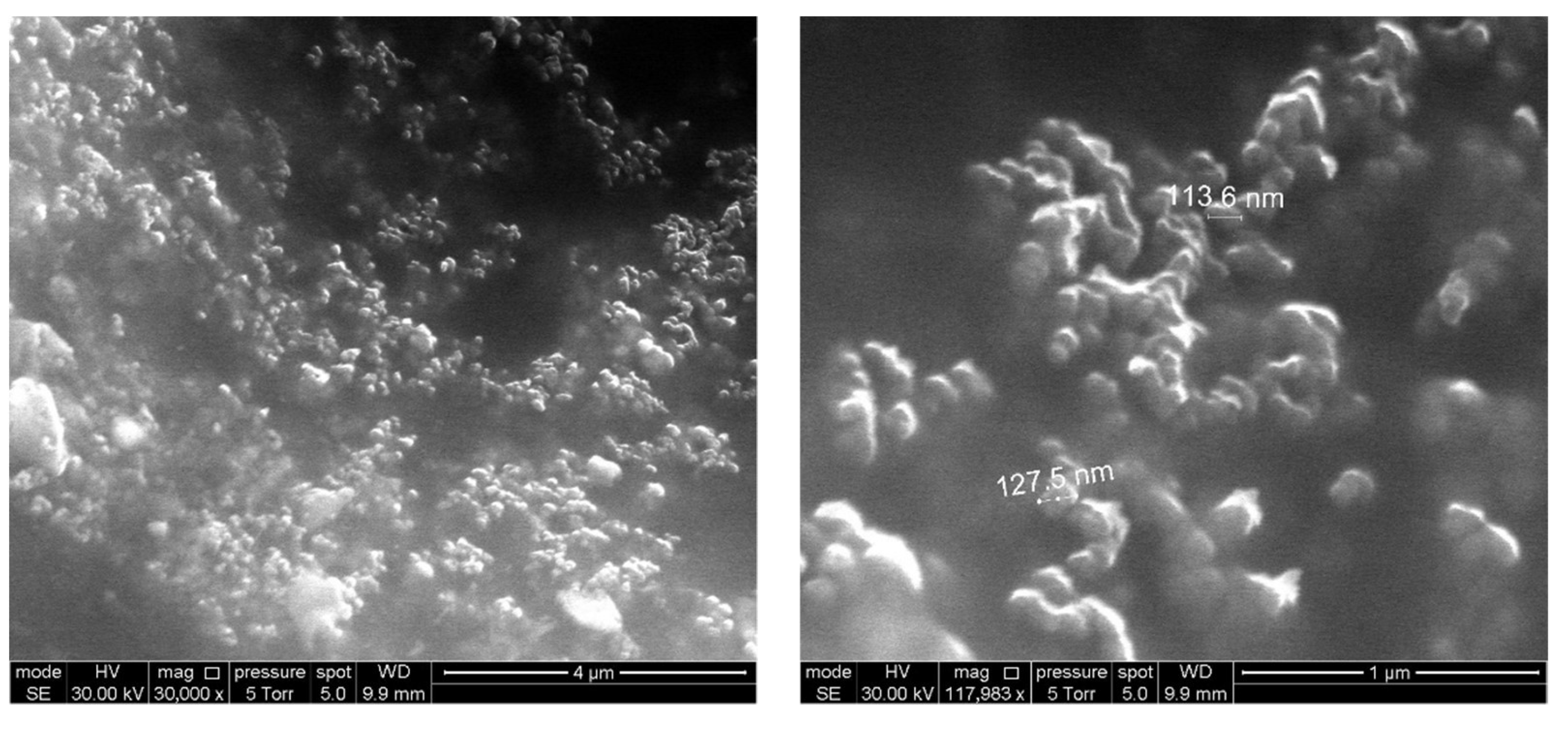
3.1. Material Properties
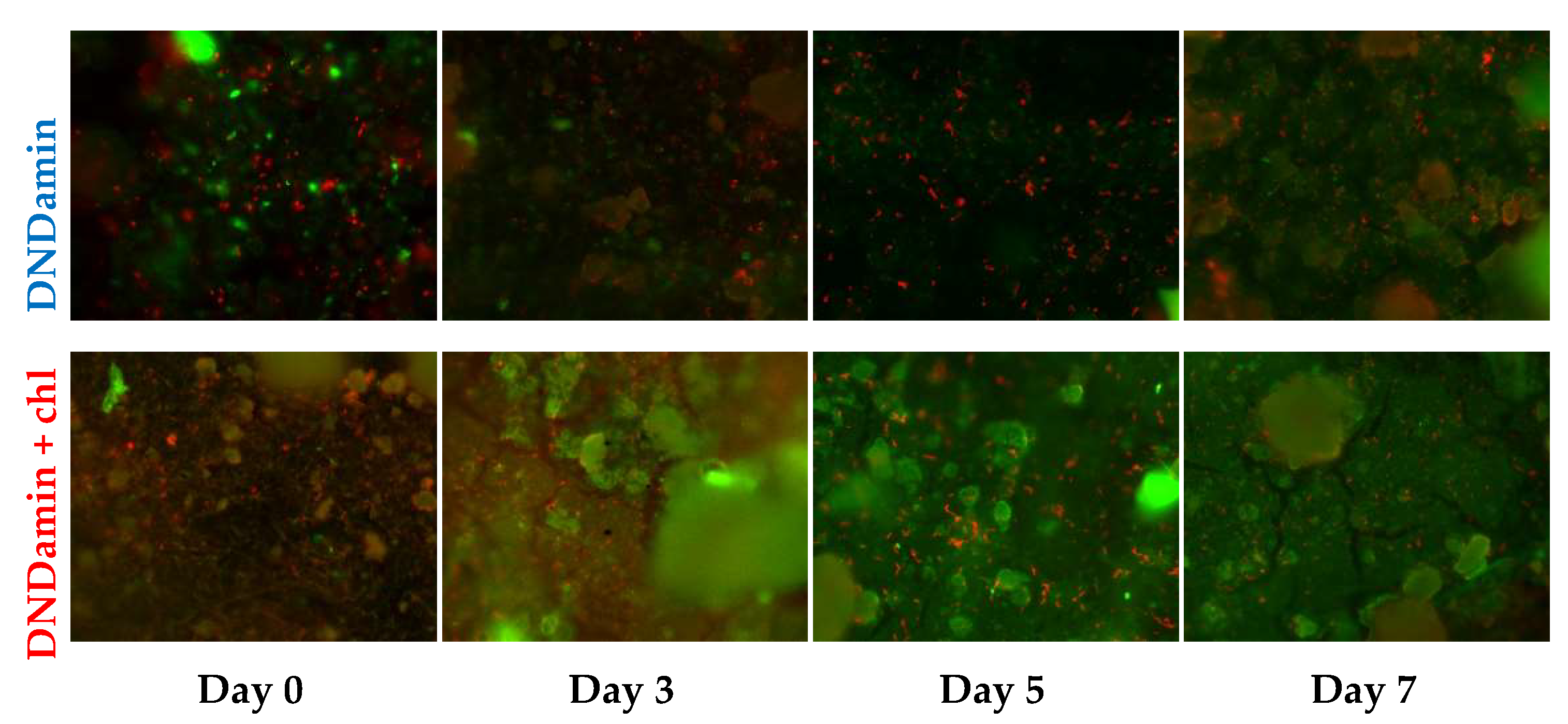
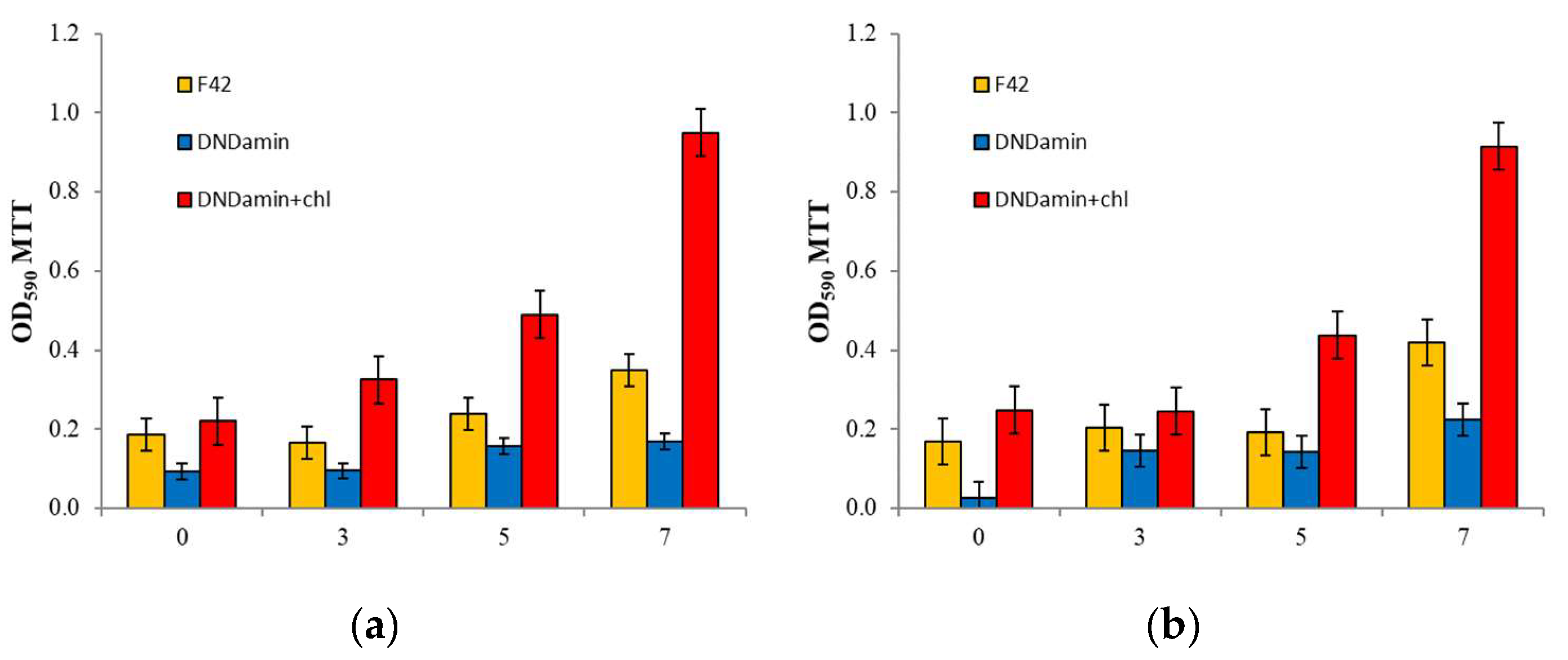
3.2. In Vitro Experiment
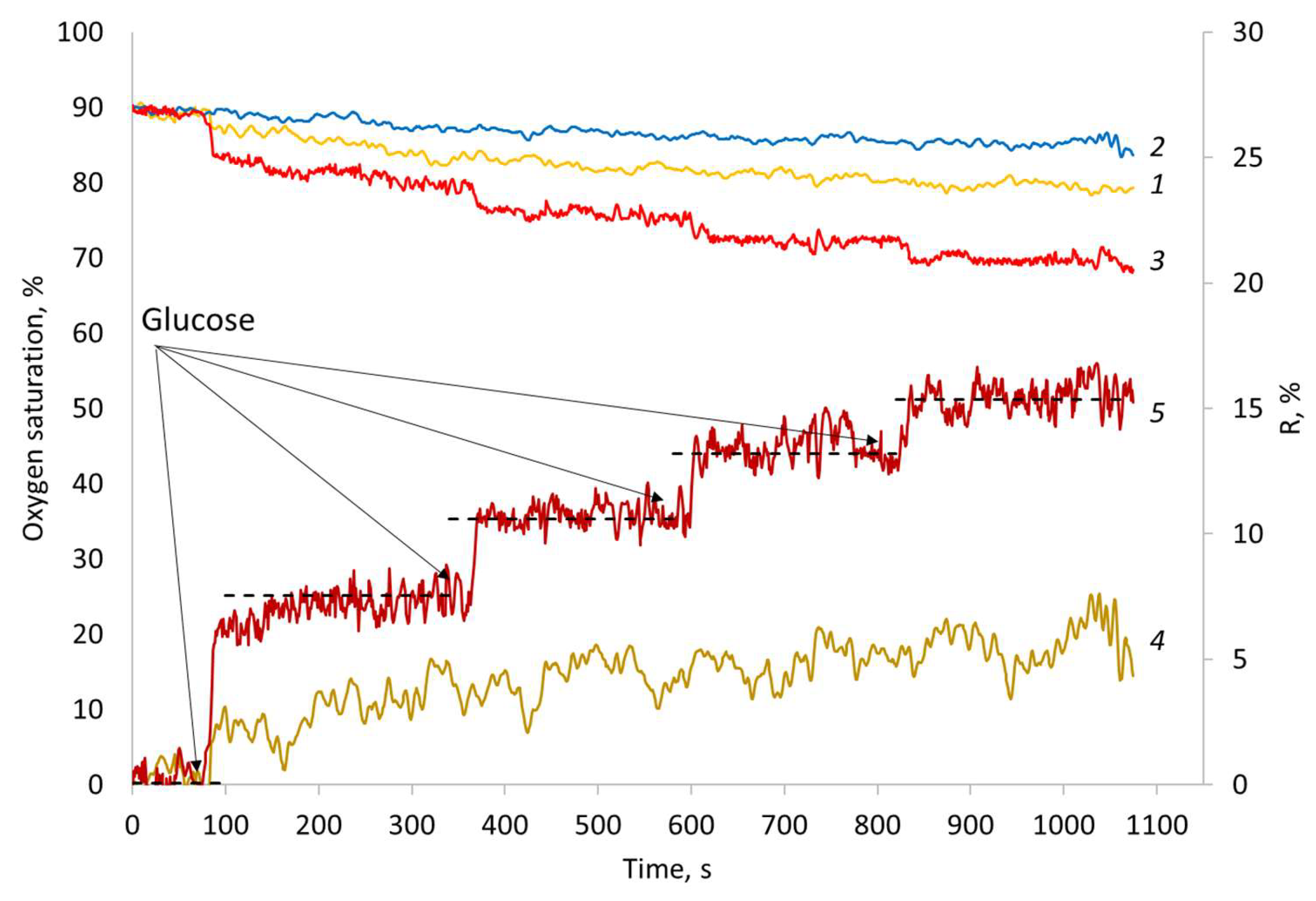
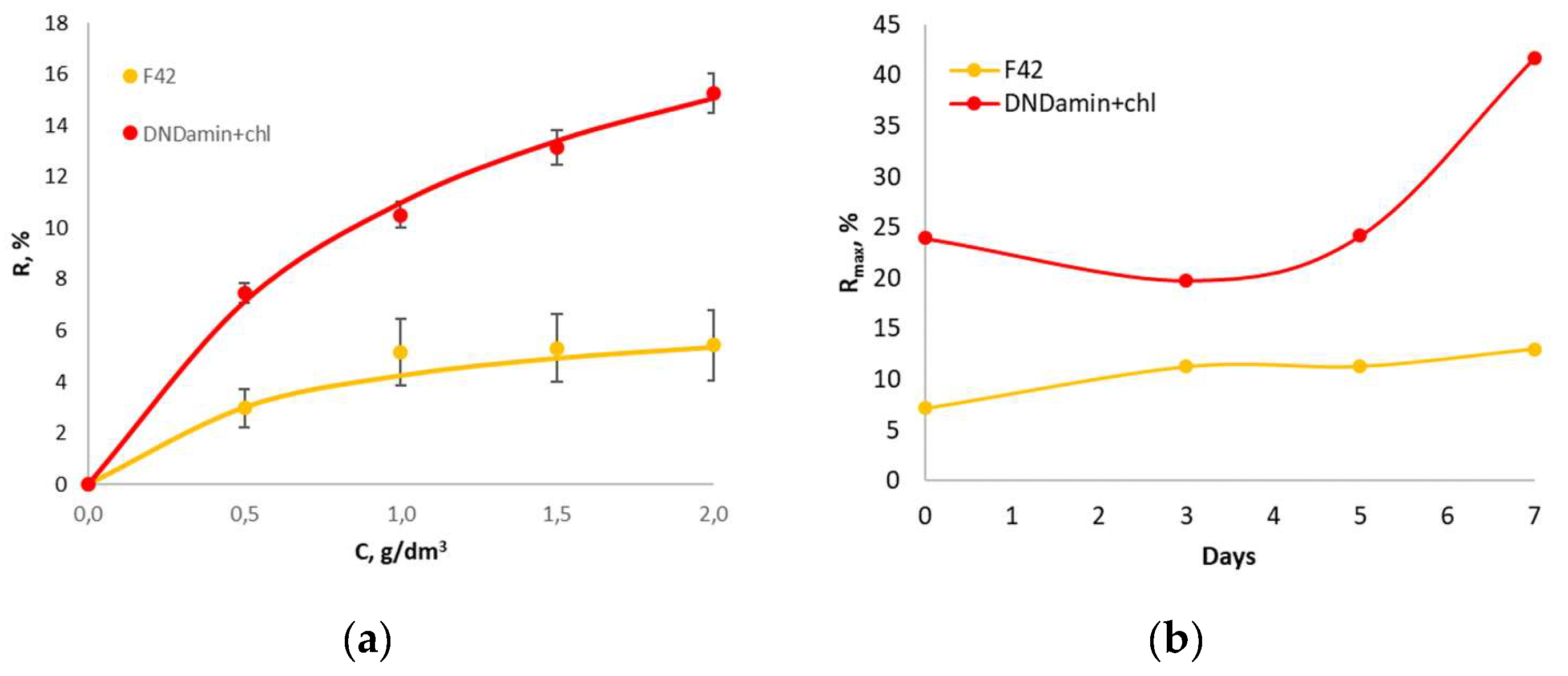
3.3. In Vivo Experiment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [Green Version]
- Piskarev, M.S.; Gilman, A.B.; Shchegolikhin, A.N.; Shmakova, N.A.; Yablokov, M.Y.U.; Kuznetsov, A.A. Alteration in the surface properties of direct-current discharge-treated tetrafluoroethylene-vinylidene fluoride copolymer films. High Energy Chem. 2013, 47, 251–257. [Google Scholar] [CrossRef]
- Fabbri, P.; Messori, M. 5—Surface modification of polymers: Chemical, physical, and biological routes. In Modification of Polymer Properties; Jasso-Gastinel, C.F., Kenny, J.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 109–130. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novák, I.; Lehocký, M.; Sedlarík, V.; Vesel, A.; Junkar, I.; Sáha, P.; Chodák, I. A physicochemical approach to render antibacterial surfaces on plasma-treated medical-grade PVC: Irgasan coating. Plasma Process. Polym. 2010, 7, 504–514. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Kang, C.; Li, J.; Zhu, Y.; Li, W.; Huang, Q.; Zhu, Z. Biodistribution and toxicity of nanodiamonds in mice after intratracheal instillation. Toxicol. Lett. 2010, 198, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Irfan Ul Haq, M.; Anand, A.; Mohan, S.; Kumar, R.; Jayalakshmi, S.; Arvind Singh, R. Nanodiamond Particles as Secondary Additive for Polyalphaolefin Oil Lubrication of Steel–Aluminium Contact. Nanomaterials 2021, 11, 1438. [Google Scholar] [CrossRef]
- Mangal, U.; Seo, J.-Y.; Yu, J.; Kwon, J.-S.; Choi, S.-H. Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances. Nanomaterials 2020, 10, 827. [Google Scholar] [CrossRef]
- Fox, K.; Ratwatte, R.; Booth, M.A.; Tran, H.M.; Tran, P.A. High Nanodiamond Content-PCL Composite for Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xu, K. FTIR study of ultradispersed diamond powder synthesized by explosive detonation. Carbon 1995, 33, 1663–1671. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, K.; Ji, S. FTIR studies on the spectral changes of the surface functional groups of ultradispersed diamond powder synthesized by explosive detonation after treatment in hydrogen, nitrogen, methane and air at different temperatures. J. Chem. Soc. Faraday Trans. 1996, 92, 3401–3406. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Chuvilin, A.L.; Butenko, Y.V.; Mal’kov, I.Y.; Titov, V.M. Onion-like carbon from ultra-disperse diamond. Chem. Phys. Lett. 1994, 222, 343–348. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Z.; Margrave, J.L.; Khabashesku, V.N. Functionalization of Nanoscale Diamond Powder: Fluoro-, Alkyl-, Amino-, and Amino Acid-Nanodiamond Derivatives. Chem. Mater. 2004, 16, 3924–3930. [Google Scholar] [CrossRef]
- Krüger, A.; Liang, Y.; Jarre, G.; Stegk, J. Surface functionalisation of detonation diamond suitable for biological applications. J. Mater. Chem. 2006, 16, 2322–2328. [Google Scholar] [CrossRef]
- Jung, H.-S.; Neuman, K.C. Surface Modification of Fluorescent Nanodiamonds for Biological Applications. Nanomaterials 2021, 11, 153. [Google Scholar] [CrossRef]
- Miller, B.S.; Bezinge, L.; Gliddon, H.D.; Huang, D.; Dold, G.; Gray, E.R.; Heaney, J.; Dobson, P.J.; Nastouli, E.; Morton, J.J.L.; et al. Spin-enhanced nanodiamond biosensing for ultrasensitive diagnostics. Nature 2020, 587, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Neburkova, J.; Vavra, J.; Cigler, P. Coating nanodiamonds with biocompatible shells for applications in biology and medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 43–53. [Google Scholar] [CrossRef]
- Raja, I.S.; Song, S.-J.; Kang, M.S.; Lee, Y.B.; Kim, B.; Hong, S.W.; Jeong, S.J.; Lee, J.-C.; Han, D.-W. Toxicity of Zero- and One-Dimensional Carbon Nanomaterials. Nanomaterials 2019, 9, 1214. [Google Scholar] [CrossRef] [Green Version]
- Marcon, L.; Riquet, F.; Vicogne, D.; Szunerits, S.; Bodart, J.-F.; Boukherroub, R. Cellular and in vivo toxicity of functionalized nanodiamond in Xenopus embryos. J. Mater. Chem. 2010, 20, 8064–8069. [Google Scholar] [CrossRef]
- Isakova, A.A.; Safonov, A.V.; Alexandrovskaya AYu Galushko, T.B.; Indenbom, A.V.; Spitsyn, B.V. The effect of nanodiamond surface modification on interaction with Pseudomonas putida K12. Prot. Met. Phys. Chem. Surf. 2017, 53, 220–223. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Kamanin, S.S.; Kharkova, A.S.; Arlyapov, V.A. Glucose biosensor based on screen-printed electrode modified with silicone sol–gel conducting matrix containing carbon nanotubes. 3 Biotech 2019, 9, 290. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Avtukh, A.N.; Starodumova, I.P.; Reshetilov, A.N. Mediator BOD Biosensor Based on Cells of Microorganisms Isolated from Activated Sludge. Appl. Biochem. Microbiol. 2019, 55, 189–197. [Google Scholar] [CrossRef]
- Kamanin, S.S.; Arlyapov, V.A.; Rogova, T.V.; Reshetilov, A.N. Screen-printed electrodes modified with glucose oxidase immobilized in hybrid organosilicon sol-gel matrix. Appl. Biochem. Microbiol. 2014, 50, 835–841. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Aleksandrovskaya, A.Y.; Safonov, A.V.; Popova, N.M.; Spitsin, B.V.; Naumova, A.O.; Zaitsev, N.K. Tuning the wetting angle of fluorinated polymer with modified nanodiamonds: Towards new type of biosensors. Mendeleev Commun. 2020, 30, 453–455. [Google Scholar] [CrossRef]
- Spitsyn, B.V.; Davidson, J.L.; Gradoboev, M.N.; Galushko, T.B.; Serebryakova, N.V.; Karpukhina, T.A.; Kulakova, I.I.; Melnik, N.N. Inroad to modification of detonation nanodiamond. Diam. Relat. Mater. 2006, 15, 296–299. [Google Scholar] [CrossRef]
- Antropov, A.P.; Ragutkin, A.V.; Melnikov, P.V.; Luchnikov, P.A.; Zaitsev, N.K. Composite material for optical oxygen sensor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 289, 012031. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Naumova, A.O.; Alexandrovskaya, A.Y.; Zaitsev, N.K. Optimizing Production Conditions for a Composite Optical Oxygen Sensor Using Mesoporous SiO2. Nanotechnol. Russ. 2018, 13, 602–608. [Google Scholar] [CrossRef]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnikov, P.; Kozhukhova, A.; Naumova, A.; Yashtulov, N.; Zaitsev, N. Optical analyzer for continuous monitoring of dissolved oxygen in aviation fuel and other non-aqueous media. Int. J. Eng. Trans. B Appl. 2019, 32, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Alexandrovskaya, A.Y.; Melnikov, P.V.; Safonov, A.V.; Naumova, A.O.; Zaytsev, N.K. A comprehensive study of the resistance to biofouling of different polymers for optical oxygen sensors. The advantage of the novel fluorinated composite based on core-dye-shell structure. Mater. Today Commun. 2020, 23C, 100916. [Google Scholar] [CrossRef]
- Naumova, A.O.; Melnikov, P.V.; Dolganova, E.V.; Yashtulov, N.A.; Zaitsev, N.K. Shifts in the pKa value of acid–base indicators caused by immobilization on solid substrates via water-soluble polycationic polymers: A case study of Congo Red. Fine Chem. Technol. 2020, 15, 59–70. [Google Scholar] [CrossRef]
- Aleksandrovskaya, A.Y.; Melnikov, P.V.; Safonov, A.V.; Abaturova, N.A.; Spitsyn, B.V.; Naumova, A.O.; Zaitsev, N.K. The Effect of Modified Nanodiamonds on the Wettability of the Surface of an Optical Oxygen Sensor and Biological Fouling During Long-Term in Situ Measurements. Nanotechnol. Russ. 2019, 14, 389–396. [Google Scholar] [CrossRef]
- Gimeno, C.J.; Fink, G.R. Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol. Cell. Biol. 1994, 14, 2100–2112. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Buttke, T.M.; McCubrey, J.A.; Owen, T.C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J. Immun. Methods 1993, 157, 233–240. [Google Scholar] [CrossRef]
- Burton, E.; Yakandawala, N.; LoVetri, K.; Madhyastha, M.S. A microplate spectrofluorometric assay for bacterial biofilms. J. Ind. Microbiol. Biotechnol. 2007, 34, 1–4. [Google Scholar] [CrossRef]
- Zaitsev, N.K.; Dvorkin, V.I.; Melnikov, P.V.; Kozhukhova, A.E. A Dissolved Oxygen Analyzer with an Optical Sensor. J. Anal. Chem. 2018, 73, 102–108. [Google Scholar] [CrossRef]
- Burleson, T.; Yusuf, N.; Stanishevsky, A. Surface modification of nanodiamonds for biomedical application and analysis by infrared spectroscopy. J. Achievem. Mater. Manuf. Eng. 2009, 37, 258–263. [Google Scholar]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.M. Covalent Incorporation of Aminated Nanodiamond into an Epoxy Polymer Network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, A.S.; Arlyapov, V.A.; Yudina, N.Y.; Alferov, S.V.; Reshetilov, A.N. Use of one- and two-mediator systems for developing a BOD biosensor based on the yeast Debaryomyces hansenii. Enzyme Microb. Technol. 2017, 98, 43–51. [Google Scholar] [CrossRef]
- Kamanin, S.S.; Arlyapov, V.A.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Biosensors based on modified screen-printed enzyme electrodes for monitoring of fermentation processes. Russ. J. Appl. Chem. 2015, 88, 463–472. [Google Scholar] [CrossRef]
- Ivanova, M.V.; Burtseva, E.I.; Ivanova, V.T.; Trushakova, S.V.; Isaeva, E.I.; Shevchenko, E.S.; Isakova, A.A.; Manykin, A.A.; Spitsyn, B.V. Adsorption of influenza A and B viruses on detonation nanodiamonds materials. MRS Online Proc. Libr. 2012, 1452, 14–19. [Google Scholar] [CrossRef]
- Qian, L.; Guan, Y.; He, B.; Xiao, H. Modified guanidine polymers: Synthesis and antimicrobial mechanism revealed by AFM. Polymer 2008, 49, 2471–2475. [Google Scholar] [CrossRef]
- Escamilla-García, E.; Alcázar-Pizaña, A.G.; Segoviano-Ramírez, J.C.; Del Angel-Mosqueda, C.; López-Lozano, A.P.; Cárdenas-Estrada, E.; De La Garza-Ramos, M.A.; Medina-De La Garza, C.E.; Márquez, M. Antimicrobial Activity of a Cationic Guanidine Compound against Two Pathogenic Oral Bacteria. Int. J. Microbiol. 2017, 5924717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.S.; Shatalov, D.O.; Kedik, S.A.; Sedishev, I.P.; Beliakov, S.V.; Trachuk, K.N.; Komarova, V.V. An effective method for preparation of high purity oligohexamethylene guanidine salts. Fine Chem. Technol. 2020, 15, 31–38. [Google Scholar] [CrossRef]
- Svadlakova, T.; Hubatka, F.; Turanek Knotigova, P.; Kulich, P.; Masek, J.; Kotoucek, J.; Macak, J.; Motola, M.; Kalbac, M.; Kolackova, M.; et al. Proinflammatory Effect of Carbon-Based Nanomaterials: In Vitro Study on Stimulation of Inflammasome NLRP3 via Destabilisation of Lysosomes. Nanomaterials 2020, 10, 418. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knötigová, P.T.; Mašek, J.; Hubatka, F.; Kotouˇcek, J.; Kulich, P.; Šiměcková, P.; Bartheldyová, E.; Machala, M.; Švadláková, T.; Krejsek, J.; et al. Application of Advanced Microscopic Methods to Study the Interaction of Carboxylated Fluorescent Nanodiamonds with Membrane Structures in THP-1 Cells: Activation of Inflammasome NLRP3 as the Result of Lysosome Destabilization. Mol. Pharm. 2019, 16, 3441–3451. [Google Scholar] [CrossRef]
- Zolti, A.; Green, S.J.; Sela, N.; Hadar, Y.; Minz, D. The microbiome as a biosensor: Functional profiles elucidate hidden stress in hosts. Microbiome 2020, 8, 71. [Google Scholar] [CrossRef]
- Sosnowski, K.; Akarapipad, P.; Yoon, J.-Y. The future of microbiome analysis: Biosensor methods for big data collection and clinical diagnostics. Med. Devices Sens. 2020, 3, e10085. [Google Scholar] [CrossRef]
- Woo, S.-G.; Moon, S.-J.; Kim, S.K.; Kim, T.H.; Lim, H.S.; Yeon, G.-H.; Sung, B.H.; Lee, C.-H.; Lee, S.-G.; Hwang, J.H.; et al. A designed whole-cell biosensor for live diagnosis of gut inflammation through nitrate sensing. Biosens. Bioelectron. 2020, 168, 112523. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Popova, N.M.; Spitsyn, B.V.; Zaitsev, N.K.; Yashtulov, N.A. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials 2021, 11, 2980. https://doi.org/10.3390/nano11112980
Melnikov PV, Alexandrovskaya AY, Naumova AO, Popova NM, Spitsyn BV, Zaitsev NK, Yashtulov NA. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials. 2021; 11(11):2980. https://doi.org/10.3390/nano11112980
Chicago/Turabian StyleMelnikov, Pavel V., Anastasia Yu. Alexandrovskaya, Alina O. Naumova, Nadezhda M. Popova, Boris V. Spitsyn, Nikolay K. Zaitsev, and Nikolay A. Yashtulov. 2021. "Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design" Nanomaterials 11, no. 11: 2980. https://doi.org/10.3390/nano11112980
APA StyleMelnikov, P. V., Alexandrovskaya, A. Y., Naumova, A. O., Popova, N. M., Spitsyn, B. V., Zaitsev, N. K., & Yashtulov, N. A. (2021). Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials, 11(11), 2980. https://doi.org/10.3390/nano11112980









