Carbon Nanomaterials Modified Biomimetic Dental Implants for Diabetic Patients
Abstract
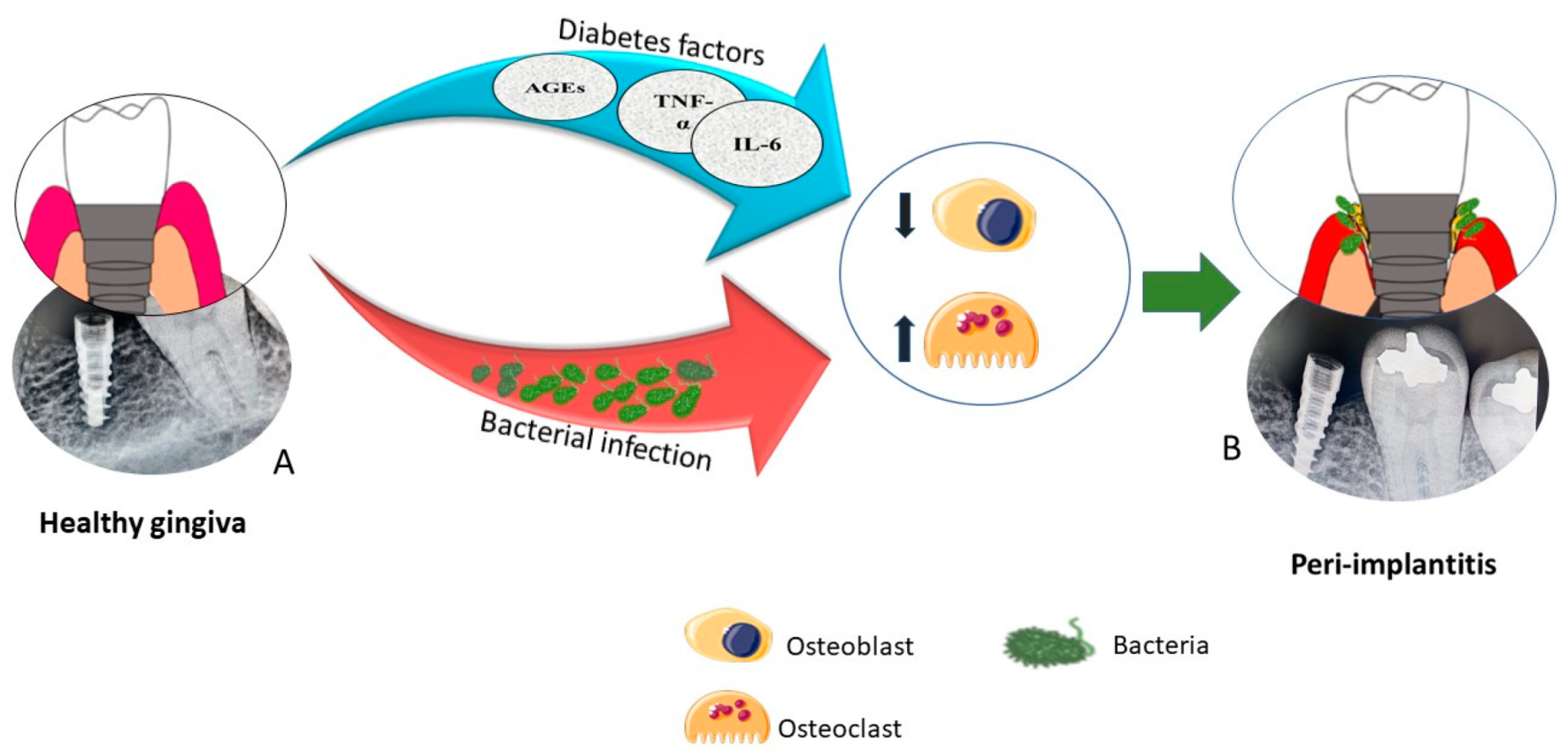
:1. Introduction
2. Biomimetic Dental Implants
2.1. Bio-Inspired Patterns Used to Increase Antimicrobial and Antifouling Properties
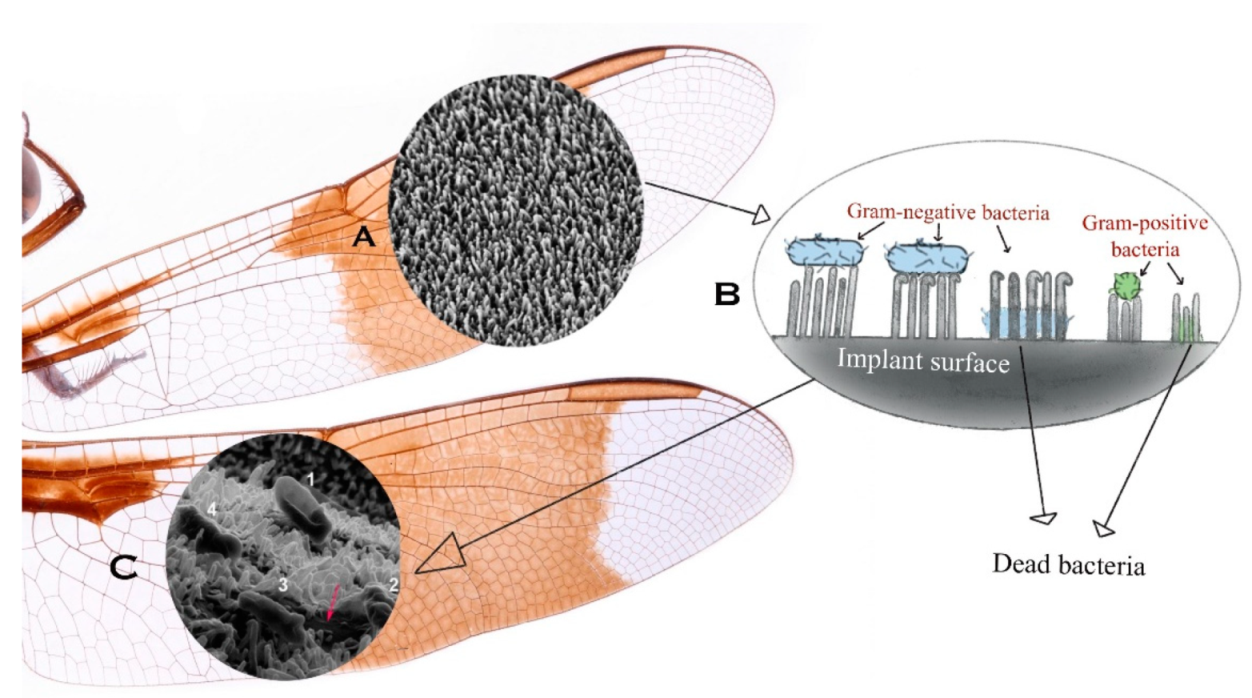
2.1.1. Insect-Inspired Patterns
2.1.2. Animal-Inspired Patterns
2.1.3. Plant-Inspired Patterns
2.2. Bio-Inspired Patterns Used to Increase Osseointegration
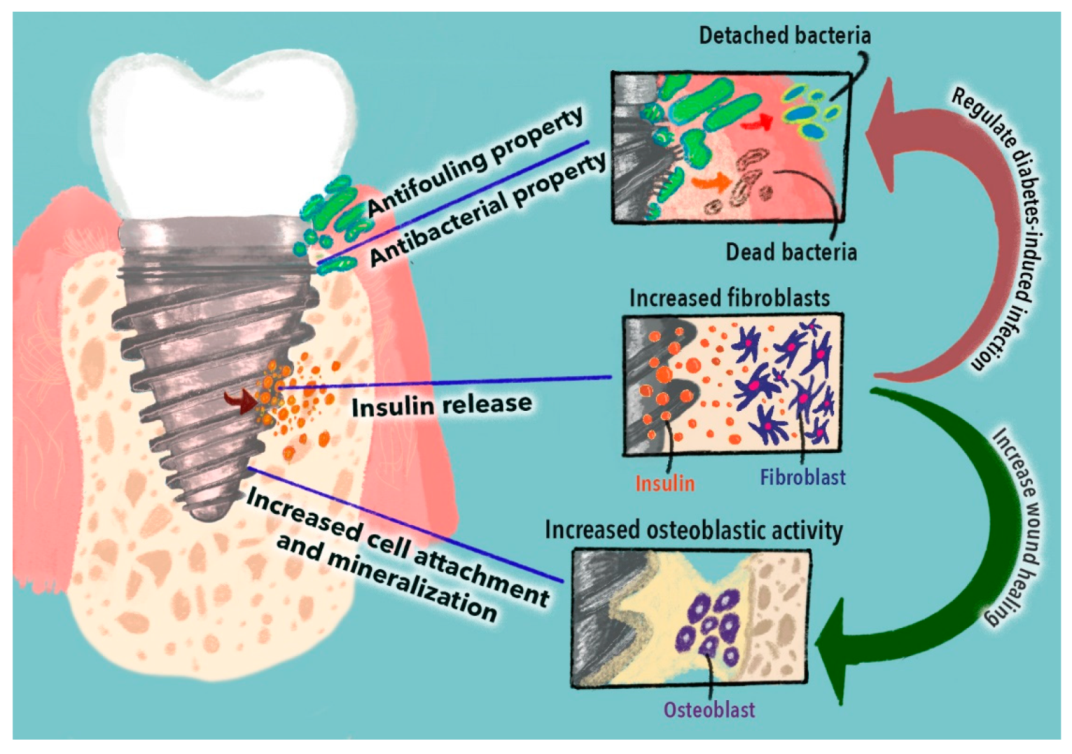
3. The Delivery of Insulin to Improve Dental Implants for Diabetic Patients
4. Capabilities of Carbon Nanomaterials to Functionalize Biomimetic Implants
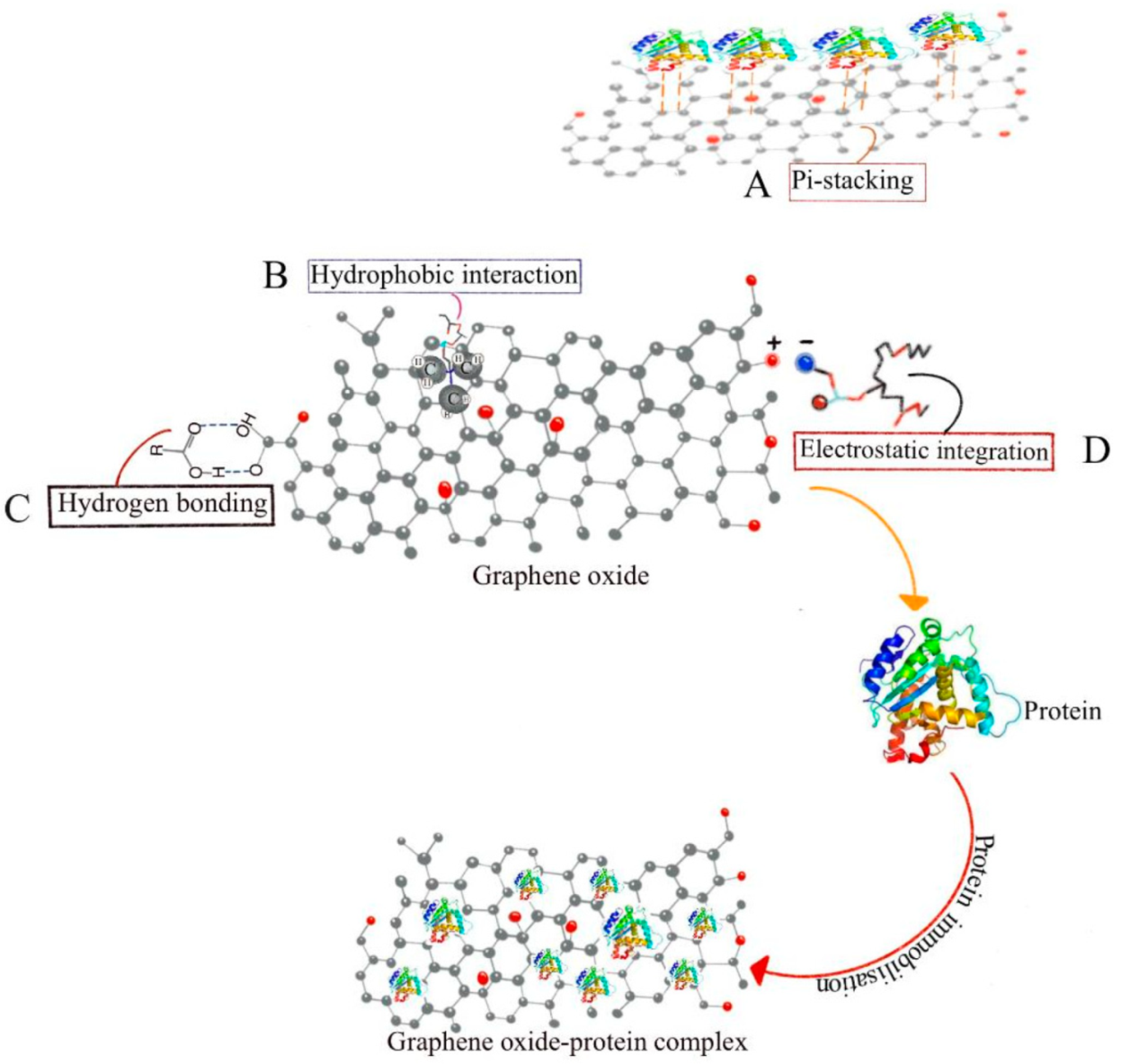
4.1. Drug Delivery Property
4.2. Antibacterial Property
4.3. Anti-Inflammatory Property
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Misch, C.E. Dental Implant Prosthetics-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental implants and diabetes mellitus—A systematic review. Int. J. Implant Dent. 2016, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar]
- Souza, J.C.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontol. 2000 2019, 79, 178–189. [Google Scholar] [PubMed]
- Alberti, A.; Morandi, P.; Zotti, B.; Tironi, F.; Francetti, L.; Taschieri, S.; Corbella, S. Influence of diabetes on implant failure and peri-implant diseases: A retrospective study. Dent. J. 2020, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Bazli, L.; Chahardehi, A.M.; Arsad, H.; Malekpouri, B.; Jazi, M.A.; Azizabadi, N. Factors influencing the failure of dental implants: A Systematic Review. J. Compos. Compd. 2020, 2, 18–25. [Google Scholar]
- Lorusso, F.; Postiglione, F.; Delvecchio, M.; Rapone, B.; Scarano, A. The impact of diabetes in implant oral rehabilitations: A bibliometric study and literature review. Acta Med. 2020, 36, 3333. [Google Scholar]
- Javed, F.; Romanos, G.E. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: A systematic literature review. J. Periodontol. 2009, 80, 1719–1730. [Google Scholar]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, P.G.F.P.; Bonfante, E.A.; Bergamo, E.T.; de Souza, S.L.S.; Riella, L.; Torroni, A.; Jalkh, E.B.B.; Witek, L.; Lopez, C.D.; Zambuzzi, W.F. Obesity/Metabolic Syndrome and Diabetes Mellitus on Peri-implantitis. Trends Endocrinol. Metab. 2020, 31, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and its effect on bone and fracture healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Suresh Kumar, N.; Padma Suvarna, R.; Chandra Babu Naidu, K.; Banerjee, P.; Ratnamala, A.; Manjunatha, H. A review on biological and biomimetic materials and their applications. Appl. Phys. A 2020, 126, 445. [Google Scholar] [CrossRef]
- Upadhyay, A.; Pillai, S.; Khayambashi, P.; Sabri, H.; Lee, K.T.; Tarar, M.; Zhou, S.; Harb, I.; Tran, S.D. Biomimetic Aspects of Oral and Dentofacial Regeneration. Biomimetics 2020, 5, 51. [Google Scholar]
- Goswami, S. Biomimetic dentistry. J. Oral Res. Rev. 2018, 10, 28. [Google Scholar] [CrossRef]
- Sheeparamatti, B.; Sheeparamatti, R.; Kadadevaramath, J. Nanotechnology: Inspiration from nature. IETE Tech. Rev. 2007, 24, 5–8. [Google Scholar]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Li, X. Bactericidal mechanism of nanopatterned surfaces. Phys. Chem. Chem. Phys. 2016, 18, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, W.; Dong, C.; Sreeprasad, T.S.; Xia, Z. Biomimetic self-cleaning surfaces: Synthesis, mechanism and applications. J. R. Soc. Interface 2016, 13, 20160300. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz-Zochniak, A.; Jarzynka, S.; Iwańska, A.; Strom, K.; Iwańczyk, B.; Bartel, M.; Mazur, M.; Pietruczuk-Padzik, A.; Konieczna, M.; Augustynowicz-Kopeć, E. Biofilm Formation on Dental Implant Biomaterials by Staphylococcus aureus Strains Isolated from Patients with Cystic Fibrosis. Materials 2021, 14, 2030. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. Iscience 2020, 24, 102001. [Google Scholar] [CrossRef]
- Da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395. [Google Scholar] [CrossRef] [Green Version]
- Meyyappan, M.; Delzeit, L.; Cassell, A.; Hash, D. Carbon nanotube growth by PECVD: A review. Plasma Sources Sci. Technol. 2003, 12, 205. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.; Junizah, A. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Puskar, L.; Hasan, J.; Webb, H.K.; Hirschmugl, C.J.; Nasse, M.J.; Gervinskas, G.; Juodkazis, S.; Watson, G.S.; Watson, J.A. High-spatial-resolution mapping of superhydrophobic cicada wing surface chemistry using infrared microspectroscopy and infrared imaging at two synchrotron beamlines. J. Synchrotron Radiat. 2013, 20, 482–489. [Google Scholar] [PubMed] [Green Version]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-based nanomaterials for biomedical applications: A recent study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-based nanomaterials for tissue engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, J. Effects of Topical Insulin on Wound Healing: A Review of Animal and Human Evidences. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Bhatlu, M.L.D.; Sukanya, K.; Jayan, N. Nanovaccination: Evolution and Review. J. Crit. Rev. 2020, 7, 971–975. [Google Scholar]
- Lee, D.-K.; Kim, S.V.; Limansubroto, A.N.; Yen, A.; Soundia, A.; Wang, C.-Y.; Shi, W.; Hong, C.; Tetradis, S.; Kim, Y. Nanodiamond–gutta percha composite biomaterials for root canal therapy. ACS Nano 2015, 9, 11490–11501. [Google Scholar] [CrossRef]
- Krok, E.; Balakin, S.; Jung, J.; Gross, F.; Opitz, J.; Cuniberti, G. Modification of titanium implants using biofunctional nanodiamonds for enhanced antimicrobial properties. Nanotechnology 2020, 31, 205603. [Google Scholar] [CrossRef]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Yen, A.; Zhang, K.; Daneshgaran, G.; Kim, H.-J.; Ho, D. A Chemopreventive Nanodiamond Platform for Oral Cancer Treatment. J. Calif. Dent. Assoc. 2016, 44, 121–127. [Google Scholar] [PubMed]
- Najeeb, S.; Khurshid, Z.; Agwan, A.S.; Zafar, M.S.; Alrahabi, M.; Qasim, S.B.; Sefat, F. Dental applications of nanodiamonds. Sci. Adv. Mater. 2016, 8, 2064–2070. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Park, S.; Lee, D.; Choi, K.S.; Lim, H.-P.; Kim, J. Graphene as an enabling strategy for dental implant and tissue regeneration. Tissue Eng. Regen. Med. 2017, 14, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wan, Q.; Pei, X. Graphene family materials in bone tissue regeneration: Perspectives and challenges. Nanoscale Res. Lett. 2018, 13, 289. [Google Scholar] [PubMed] [Green Version]
- Guo, S.; Lu, Y.; Wan, X.; Wu, F.; Zhao, T.; Shen, C. Preparation, characterization of highly dispersed reduced graphene oxide/epoxy resin and its application in alkali-activated slag composites. Cem. Concr. Compos. 2020, 105, 103424. [Google Scholar]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials 2018, 8, 349. [Google Scholar]
- Nizami, M.Z.I.; Takashiba, S.; Nishina, Y. Graphene oxide: A new direction in dentistry. Appl. Mater. Today 2020, 19, 100576. [Google Scholar]
- Abdallah, B.; Elhissi, A.M.; Ahmed, W.; Najlah, M. Carbon nanotubes drug delivery system for cancer treatment. In Advances in Medical and Surgical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 313–332. [Google Scholar]
- Szymański, T.; Mieloch, A.A.; Richter, M.; Trzeciak, T.; Florek, E.; Rybka, J.D.; Giersig, M. Utilization of Carbon Nanotubes in Manufacturing of 3D Cartilage and Bone Scaffolds. Materials 2020, 13, 4039. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Pandruvada, S.; Puleo, D.; Hartsfield, J.; Huja, S. In vitro evaluation of osteoblast responses to carbon nanotube-coated titanium surfaces. Prog. Orthod. 2016, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [Green Version]
- Martins-Júnior, P.; Alcântara, C.; Resende, R.; Ferreira, A. Carbon nanotubes: Directions and perspectives in oral regenerative medicine. J. Dent. Res. 2013, 92, 575–583. [Google Scholar] [CrossRef]
- Mozetič, M. Surface modification to improve properties of materials. Materials 2019, 12, 441. [Google Scholar]
- Matos, G.R.M. Surface roughness of dental implant and osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar]
- Chen, Y.-C.; Huang, Z.-S.; Yang, H. Cicada-wing-inspired self-cleaning antireflection coatings on polymer substrates. ACS Appl. Mater. Interfaces 2015, 7, 25495–25505. [Google Scholar] [CrossRef]
- Oh, J.; Yin, S.; Dana, C.E.; Hong, S.; Roman, J.K.; Jo, K.D.; Chavan, S.; Cropek, D.; Alleyne, M.; Miljkovic, N. Cicada-inspired self-cleaning superhydrophobic surfaces. J. Heat Transf. 2019, 141, 100905. [Google Scholar] [CrossRef]
- Diu, T.; Faruqui, N.; Sjöström, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-inspired cell-instructive nanopatterned arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, J.; Raj, S.; Yadav, L.; Chatterjee, K. Engineering a nanostructured “super surface” with superhydrophobic and superkilling properties. RSC Adv. 2015, 5, 44953–44959. [Google Scholar] [CrossRef] [Green Version]
- Chien, H.-W.; Chen, X.-Y.; Tsai, W.-P.; Lee, M. Inhibition of biofilm formation by rough shark skin-patterned surfaces. Colloids Surf. B Biointerfaces 2020, 186, 110738. [Google Scholar] [PubMed]
- Wang, L.; Xia, M.; Wang, D.; Yan, J.; Huang, X.; Luo, J.; Xue, H.-G.; Gao, J.-F. Bioinspired Superhydrophobic and Durable Octadecanoic Acid/Ag Nanoparticle-Decorated Rubber Composites for High-Performance Strain Sensors. ACS Sustain. Chem. Eng. 2021, 9, 7245–7254. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhou, Y.; Xu, X.; Shi, W.; Hu, J.; Li, G.; Qu, X.; Guo, Y.; Tian, X.; Zaman, A. Progress in construction of bio-inspired physico-antimicrobial surfaces. Nanotechnol. Rev. 2020, 9, 1562–1575. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Xin, J.H. Super-hydrophobic surfaces from a simple coating method: A bionic nanoengineering approach. Nanotechnology 2006, 17, 3259. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Wang, N. Chemical fabrication strategies for achieving bioinspired superhydrophobic surfaces with micro and nanostructures: A review. Adv. Eng. Mater. 2021, 23, 2001083. [Google Scholar] [CrossRef]
- Al-Zubaidi, S.M.; Madfa, A.A.; Mufadhal, A.A.; Aldawla, M.A.; Hameed, O.S.; Yue, X.-G. Improvements in Clinical Durability From Functional Biomimetic Metallic Dental Implants. Front. Mater. 2020, 7, 106. [Google Scholar] [CrossRef]
- Mendhi, J.; Asgari, M.; Ratheesh, G.; Prasadam, I.; Yang, Y.; Xiao, Y. Dose controlled nitric oxide-based strategies for antibacterial property in biomedical devices. Applied Materials Today 2020, 19, 100562. [Google Scholar] [CrossRef]
- Mendhi, J.; Ramachandra, S.S.; Prasadam, I.; Ivanovski, S.; Yang, Y.; Xiao, Y. Endogenous nitric oxide-generating surfaces via polydopamine-copper coatings for preventing biofilm dispersal and promoting microbial killing. Mater. Sci. Eng. C 2021, 128, 112297. [Google Scholar]
- Ahmad, R.; Haque, M. Oral Health Messiers: Diabetes Mellitus Relevance. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3001–3015. [Google Scholar]
- Li, S.; Yi, G.; Peng, H.; Li, Z.; Chen, S.; Zhong, H.; Chen, Y.; Wang, Z.; Deng, Q.; Fu, M. How ocular surface microbiota debuts in type 2 diabetes mellitus. Front. Cell. Infect. Microbiol. 2019, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Barik, S.; Barik, N.; Mahapatra, A.; Lenka, S.; Rathore, K. Impact of Glycemic Level in Type 2 Diabeties. Indian J. Public Health Res. Dev. 2019, 10, 1203–1207. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Eskow, C.C.; Oates, T.W. Dental implant survival and complication rate over 2 years for individuals with poorly controlled type 2 diabetes mellitus. Clin. Implant Dent. Relat. Res. 2017, 19, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Pulluri, P.; Mallappa, J.; Karibasappa, S.N.; Mehta, D.S. Management of peri-implantitis: Remedy for the malady. Int. J. Oral Health Sci. 2017, 7, 56. [Google Scholar] [CrossRef]
- Abd-Ul-Salam, H. Peri-implantitis. In Innovative Perspectives in Oral and Maxillofacial Surgery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 47–59. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacing missing teeth: Antibiotics at dental implant placement to prevent complications. Cochrane Database Syst. Rev. 2013, CD004152. [Google Scholar] [CrossRef]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [PubMed] [Green Version]
- Wychowański, P.; Starzyńska, A.; Adamska, P.; Słupecka-Ziemilska, M.; Sobocki, B.K.; Chmielewska, A.; Wysocki, B.; Alterio, D.; Marvaso, G.; Jereczek-Fossa, B.A.; et al. Methods of topical administration of drugs and biological active substances for dental implants—A narrative review. Antibiotics 2021, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, M.T.; Vallecillo-Rivas, M.; Toledano-Osorio, M.; Rodríguez-Archilla, A.; Toledano, R.; Osorio, R. Efficacy of local antibiotic therapy in the treatment of peri-implantitis: A systematic review and meta-analysis. J. Dent. 2021, 113, 103790. [Google Scholar]
- Prathapachandran, J.; Suresh, N. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516–521. [Google Scholar] [CrossRef]
- Bhatavadekar, N.B.; Gharpure, A.S. The Graft Infusion Technique (GIT) for Treatment of Peri-Implantitis Defects: Case Series. J. Dent. 2021. [Google Scholar] [CrossRef]
- Thomsen, T.; Klok, H.-A. Chemical Cell Surface Modification and Analysis of Nanoparticle-Modified Living Cells. ACS Appl. Biomater. 2021, 4, 2293–2306. [Google Scholar] [CrossRef]
- Barik, A.; Chakravorty, N. Targeted drug delivery from titanium implants: A review of challenges and approaches. In Trends in Biomedical Research; Springer: Cham, Switzerland, 2019; pp. 1–17. [Google Scholar]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A. Drug delivery (nano) platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- Ge, X.; Zhao, J.; Esmeryan, K.D.; Lu, X.; Li, Z.; Wang, K.; Ren, F.; Wang, Q.; Wang, M.; Qian, B. Cicada-inspired fluoridated hydroxyapatite nanostructured surfaces synthesized by electrochemical additive manufacturing. Mater. Des. 2020, 193, 108790. [Google Scholar] [CrossRef]
- Narayana, P.; Srihari, P. Biofilm resistant surfaces and coatings on implants: A review. Mater. Today Proc. 2019, 18, 4847–4853. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. Apmis 2017, 125, 392–417. [Google Scholar] [PubMed] [Green Version]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on superhydrophobic surfaces. Chem. Commun. 2014, 50, 3900–3913. [Google Scholar]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrcanovic, B.; Albrektsson, T.; Wennerberg, A. Diabetes and oral implant failure: A systematic review. J. Dent. Res. 2014, 93, 859–867. [Google Scholar] [CrossRef]
- Ishak, M.I.; Liu, X.; Jenkins, J.; Nobbs, A.H.; Su, B. Protruding nanostructured surfaces for antimicrobial and osteogenic titanium implants. Coatings 2020, 10, 756. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Recent advances of bioinspired functional materials with specific wettability: From nature and beyond nature. Nanoscale Horiz. 2019, 4, 52–76. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure—A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, Y.; Ren, B.; Wang, H.; Yu, M.; Liu, A.; Liu, Z. Preparation of superhydrophobic surface on titanium alloy via micro-milling, anodic oxidation and fluorination. Micromachines 2020, 11, 316. [Google Scholar]
- Zhao, N.; Li, H.; Tian, C.; Xie, Y.; Feng, Z.; Wang, Z.; Yan, X.; Wang, W.; Yu, H. Bioscaffold arrays decorated with Ag nanoparticles as a SERS substrate for direct detection of melamine in infant formula. RSC Adv. 2019, 9, 21771–21776. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, J.; Xie, G.; Liu, Z.; Shao, H. Cicada wings: A stamp from nature for nanoimprint lithography. Small 2006, 2, 1440–1443. [Google Scholar]
- Ganjian, M.; Modaresifar, K.; Zhang, H.; Hagedoorn, P.-L.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Reactive ion etching for fabrication of biofunctional titanium nanostructures. Sci. Rep. 2019, 9, 18815. [Google Scholar] [PubMed] [Green Version]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar]
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Zarzycki, A.; Kac, S.; Perzanowski, M.; Marszalek, M. Mechanical properties of different nanopatterned TiO2 substrates and their effect on hydrothermally synthesized bioactive hydroxyapatite coatings. Materials 2020, 13, 5290. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhou, Y.; Cai, M.; Lin, K.; Fang, B.; Xia, L. The synergistic promotion of osseointegration by nanostructure design and silicon substitution of hydroxyapatite coatings in a diabetic model. J. Mater. Chem. B 2020, 8, 2754–2767. [Google Scholar] [CrossRef]
- Song, H.N.; Webb, H.; Mahon, P.; Crawford, R.; Ivanova, E. Natural insect and plant micro-/nanostructured surfaces: An excellent selection of valuable templates with superhydrophobic and self-cleaning properties. Molecules 2014, 19, 13614–13630. [Google Scholar]
- Chien, C.-Y.; Chen, Y.-H.; Chen, R.-Y.; Yang, H. Dragonfly-Wing-Inspired Inclined Irregular Conical Structures for Broadband Omnidirectional Antireflection Coatings. ACS Appl. Nano Mater. 2019, 3, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Selvakumar, R.; Karuppanan, K.K.; Pezhinkattil, R. Analysis on surface nanostructures present in hindwing of dragon fly (Sympetrum vulgatum) using atomic force microscopy. Micron 2012, 43, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Aburto-Medina, A.; Le, P.; Baulin, V.A.; Nguyen, H.K.D.; Curtain, R.; Hanssen, E.; Gervinskas, G.; Ng, S.H. Antifungal versus antibacterial defence of insect wings. J. Colloid Interface Sci. 2021, 603, 886–897. [Google Scholar] [CrossRef]
- Afhea, J.A. A Study of Nanotextured Surface Production for Bactericidal Surfaces on Orthopaedic Implants Using the Hydrothermal Method. Ph.D. Thesis, Queensland University of Technology, Brisbane, QLD, Australia, 2019. [Google Scholar]
- Guillem-Marti, J.; Delgado, L.; Godoy-Gallardo, M.; Pegueroles, M.; Herrero, M.; Gil, F. Fibroblast adhesion and activation onto micro-machined titanium surfaces. Clin. Oral Implants Res. 2013, 24, 770–780. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, H.; Jia, Y.; Liu, J.; Zhang, H.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of biomimetic polydimethylsiloxane (PDMS) films with superhydrophobic, self-healing and drag reduction properties via replication of shark skin and SI-ATRP. Chem. Eng. J. 2019, 356, 318–328. [Google Scholar]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bixler, G.D.; Bhushan, B. Bioinspired rice leaf and butterfly wing surface structures combining shark skin and lotus effects. Soft Matter 2012, 8, 11271–11284. [Google Scholar] [CrossRef]
- Peng, Y.L.; Lin, C.G.; Wang, L. The preliminary study on antifouling mechanism of shark skin. Adv. Mater. Res. 2009, 79–82, 977–980. [Google Scholar]
- Yarlagadda, T.; Sharma, S.; Yarlagadda, P.K.; Sharma, J. Recent developments in the Field of nanotechnology for development of medical implants. Procedia Manuf. 2019, 30, 544–551. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 82. [Google Scholar] [CrossRef]
- Wu, S.; Altenried, S.; Zogg, A.; Zuber, F.; Maniura-Weber, K.; Ren, Q. Role of the surface nanoscale roughness of stainless steel on bacterial adhesion and microcolony formation. ACS Omega 2018, 3, 6456–6464. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Mescola, A.; Rosace, G.; Stocchi, R.; Rossi, G.; Alessandrini, A.; Preziuso, S.; Scarano, A.; Rea, S.; Loschi, A.R. Antibacterial Effect of Stainless Steel Surfaces Treated with a Nanotechnological Coating Approved for Food Contact. Microorganisms 2021, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Mitropoulos, A.C. Advanced Low-Cost Separation Techniques in Interface Science; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Khanmohammadi Chenab, K.; Sohrabi, B.; Rahmanzadeh, A. Superhydrophobicity: Advanced biological and biomedical applications. Biomater. Sci. 2019, 7, 3110–3137. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, B.; Wang, J.; Fan, X.; Zhang, J. Hydrothermally structured superhydrophobic surface with superior anti-corrosion, anti-bacterial and anti-icing behaviors. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126820. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.; Costa, R.C.; Cordeiro, J.M.; Nagay, B.E.; De Almeida, A.B.; Retamal-Valdes, B.; Nociti, F.H.; Feres, M.; Rangel, E.C.; et al. Targeting Pathogenic Biofilms: Newly Developed Superhydrophobic Coating Favors a Host-Compatible Microbial Profile on the Titanium Surface. ACS Appl. Mater. Interfaces 2020, 12, 10118–10129. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar]
- Santos, A.; Sinn Aw, M.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef]
- Ho, C.-C.; Ding, S.-J. Structure, properties and applications of mussel-inspired polydopamine. J. Biomed. Nanotechnol. 2014, 10, 3063–3084. [Google Scholar] [CrossRef] [PubMed]
- Owji, N.; Mandakhbayar, N.; Gregory, D.A.; Marcello, E.; Kim, H.W.; Roy, I.; Knowles, J.C. Mussel Inspired Chemistry and Bacteria Derived Polymers for Oral Mucosal Adhesion and Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 663764. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, I.S.; Shin, G.S.; Lyu, S.K.; Ahn, S.G.; Bae, T.S.; Lee, M.H. Effects of polydopamine coating on the bioactivity of titanium for dental implants. Int. J. Precis. Eng. Manuf. 2014, 15, 1647–1655. [Google Scholar] [CrossRef]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene oxide coated titanium surfaces with osteoimmunomodulatory role to enhance osteogenesis. Mater. Sci. Eng. C 2020, 113, 110983. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar]
- Wu, J.; Liu, Y.; Cao, Q.; Yu, T.; Zhang, J.; Liu, Q.; Yang, X. Growth factors enhanced angiogenesis and osteogenesis on polydopamine coated titanium surface for bone regeneration. Mater. Des. 2020, 196, 109162. [Google Scholar]
- Yang, J.; Zhang, H.; Chan, S.M.; Li, R.; Wu, Y.; Cai, M.; Wang, A.; Wang, Y. TiO2 nanotubes alleviate diabetes-induced osteogenetic inhibition. Int. J. Nanomed. 2020, 15, 3523. [Google Scholar]
- Liu, X.; Tan, N.; Zhou, Y.; Wei, H.; Ren, S.; Yu, F.; Chen, H.; Jia, C.; Yang, G.; Song, Y. Delivery of antagomiR204-conjugated gold nanoparticles from PLGA sheets and its implication in promoting osseointegration of titanium implant in type 2 diabetes mellitus. Int. J. Nanomed. 2017, 12, 7089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, G.E.; Bosshardt, D.D.; Lang, N.P.; Abrahamsson, I.; Berglundh, T.; Lindhe, J.; Ivanovski, S.; Donos, N. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontol. 2000 2015, 68, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef] [PubMed]
- Dasmah, A.; Kashani, H.; Thor, A.; Rasmusson, L. Integration of fluoridated implants in onlay autogenous bone grafts—An experimental study in the rabbit tibia. J. Cranio-Maxillofac. Surg. 2014, 42, 796–800. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018, 27, 1055–1059. [Google Scholar] [CrossRef]
- Takanche, J.S.; Kim, J.-E.; Kim, J.-S.; Lee, M.-H.; Jeon, J.-G.; Park, I.-S.; Yi, H.-K. Chitosan-gold nanoparticles mediated gene delivery of c-myb facilitates osseointegration of dental implants in ovariectomized rat. Artif. Cells Nanomed. Biotechnol. 2018, 46, S807–S817. [Google Scholar] [CrossRef] [Green Version]
- Azzawi, Z.G.; Hamad, T.I.; Kadhim, S.A.; Naji, G.A.-H. Osseointegration evaluation of laser-deposited titanium dioxide nanoparticles on commercially pure titanium dental implants. J. Mater. Sci. Mater. Med. 2018, 29, 1–11. [Google Scholar] [CrossRef]
- Turky, R.N.; Jassim, R.K. The Electrophoretic Deposition of Nano Al2O3 and AgNO3 on CpTi Dental Implant (An in vitro and in vivo study). J. Baghdad Coll. Dent. 2016, 28, 41–47. [Google Scholar]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. J. Cell. Physiol. 2019, 234, 298–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Hong, Y.; Zhang, X. Applications of nanostructured calcium phosphate in tissue engineering. Biomater. Sci. 2013, 1, 1012–1028. [Google Scholar] [PubMed]
- Khabadze, Z.; Sheroziia, M.; Generalova, J.; Nedashkovsky, A.; Hrytsenko, K.; Omarova, K.; Balashova, M.; Gracheva, A.; Mordanov, O. Features of Dental Implantation in Patients with Type II Diabetes. J. Int. Dent. Med. Res. 2021, 14, 376–383. [Google Scholar]
- Benni, J.M.; Patil, P.A. Non-diabetic clinical applications of insulin. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 445–456. [Google Scholar]
- Liu, H.; Wang, J.; Deng, Y.; Zou, G.; Xu, J. Effects of topical insulin on wound healing: A meta-analysis of animal and clinical studies. Endocr. J. 2021, 68, 969–979. [Google Scholar] [CrossRef]
- Martínez-Jiménez, M.A.; Valadez-Castillo, F.J.; Aguilar-García, J.; Ramírez-GarciaLuna, J.L.; Gaitán-Gaona, F.I.; Pierdant-Perez, M.; Valdes-Rodríguez, R.; Sánchez-Aguilar, J.M. Effects of local use of insulin on wound healing in non-diabetic patients. Plast. Surg. 2018, 26, 75–79. [Google Scholar] [CrossRef]
- Goel, V.; Kaur, P.; Singla, L.D.; Choudhury, D. Biomedical Evaluation of Lansium parasiticum Extract-Protected Silver Nanoparticles Against Haemonchus contortus, a Parasitic Worm. Front. Mol. Biosci. 2020, 7, 396. [Google Scholar] [CrossRef]
- Malekzadeh, B.; Ransjo, M.; Tengvall, P.; Mladenovic, Z.; Westerlund, A. Insulin released from titanium discs with insulin coatings—Kinetics and biological activity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1847–1854. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivaramakrishnan, G. Efficacy of topical insulin in wound healing: A preliminary systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2017, 25, 279–287. [Google Scholar] [CrossRef]
- Ganazzoli, F.; Raffaini, G. Classical atomistic simulations of protein adsorption on carbon nanomaterials. Curr. Opin. Colloid Interface Sci. 2019, 41, 11–26. [Google Scholar]
- Patila, M.; Chalmpes, N.; Dounousi, E.; Stamatis, H.; Gournis, D. Use of functionalized carbon nanotubes for the development of robust nanobiocatalysts. Methods Enzymol. 2020, 630, 263–301. [Google Scholar] [PubMed]
- Contreras, M.L.; Torres, C.; Villarroel, I.; Rozas, R. Molecular dynamics assessment of doxorubicin–carbon nanotubes molecular interactions for the design of drug delivery systems. Struct. Chem. 2019, 30, 369–384. [Google Scholar] [CrossRef]
- Lather, V. Interaction and Surface Modification of Biomaterials with Cells Based on Carbon Nanomaterials. Appl. Chem. 2019. [Google Scholar] [CrossRef]
- Dhasaiyan, P.; Prasad, B.L.V. Self-Assembly of Bolaamphiphilic Molecules. Chem. Rec. 2017, 17, 597–610. [Google Scholar] [CrossRef]
- Yoo, J.W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Zhuang, W.R.; Wang, Y.; Cui, P.F.; Xing, L.; Lee, J.; Kim, D.; Jiang, H.L.; Oh, Y.K. Applications of π-π stacking interactions in the design of drug-delivery systems. J. Control. Release 2019, 294, 311–326. [Google Scholar] [CrossRef]
- Majumder, M.; Stinchcomb, A.; Hinds, B.J. Towards mimicking natural protein channels with aligned carbon nanotube membranes for active drug delivery. Life Sci. 2010, 86, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—A minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Maas, M. Carbon nanomaterials as antibacterial colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Bandosz, T.J. Carbon quantum dot surface-chemistry-dependent Ag release governs the high antibacterial activity of Ag-metal–organic framework composites. ACS Appl. Biomater. 2018, 1, 693–707. [Google Scholar] [CrossRef]
- El-Shabasy, R.M.; Elsadek, M.F.; Ahmed, B.M.; Farahat, M.F.; Mosleh, K.M.; Taher, M.M. Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes 2021, 9, 388. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar]
- Amjadi, M.; Hallaj, T.; Asadollahi, H.; Song, Z.; de Frutos, M.; Hildebrandt, N. Facile synthesis of carbon quantum dot/silver nanocomposite and its application for colorimetric detection of methimazole. Sens. Actuators B Chem. 2017, 244, 425–432. [Google Scholar]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Gupta, D.K.; Singh, A.K. Dental implant survival in diabetic patients; review and recommendations. Natl. J. Maxillofac. Surg. 2013, 4, 142. [Google Scholar] [CrossRef] [Green Version]
- Soltani, R.; Guo, S.; Bianco, A.; Ménard-Moyon, C. Carbon Nanomaterials Applied for the Treatment of Inflammatory Diseases: Preclinical Evidence. Adv. Ther. 2020, 3, 2000051. [Google Scholar] [CrossRef]
| Carbon Nanoparticles | Dental Applications |
|---|---|
| Carbon nanodots |
|
| Nano diamonds | |
| Graphene | |
| Carbon nanotubes |
| Type of Materials | Advantage as Dental Implant Coating on Osseointegration |
|---|---|
| Titanium oxide nanotubes coated surface |
|
| Hydroxyapatite-coated surface |
|
| Hydroxyapatite and silicon-based coating |
|
| Chitosan gold nanoparticle coating |
|
| Laser-deposited titanium dioxide nanoparticles |
|
| Aluminium oxide nanoparticles |
|
| Polydopamine coated dental implants | |
| Gold nanoparticles coupled with miR204 |
|
| Calcium phosphate |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijay, R.; Mendhi, J.; Prasad, K.; Xiao, Y.; MacLeod, J.; Ostrikov, K.; Zhou, Y. Carbon Nanomaterials Modified Biomimetic Dental Implants for Diabetic Patients. Nanomaterials 2021, 11, 2977. https://doi.org/10.3390/nano11112977
Vijay R, Mendhi J, Prasad K, Xiao Y, MacLeod J, Ostrikov K, Zhou Y. Carbon Nanomaterials Modified Biomimetic Dental Implants for Diabetic Patients. Nanomaterials. 2021; 11(11):2977. https://doi.org/10.3390/nano11112977
Chicago/Turabian StyleVijay, Renjini, Jayanti Mendhi, Karthika Prasad, Yin Xiao, Jennifer MacLeod, Kostya (Ken) Ostrikov, and Yinghong Zhou. 2021. "Carbon Nanomaterials Modified Biomimetic Dental Implants for Diabetic Patients" Nanomaterials 11, no. 11: 2977. https://doi.org/10.3390/nano11112977
APA StyleVijay, R., Mendhi, J., Prasad, K., Xiao, Y., MacLeod, J., Ostrikov, K., & Zhou, Y. (2021). Carbon Nanomaterials Modified Biomimetic Dental Implants for Diabetic Patients. Nanomaterials, 11(11), 2977. https://doi.org/10.3390/nano11112977






_Ostrikov.png)

