Visualization of Spatial Charge in Thermally Poled Glasses via Nanoparticles Formation
Abstract
1. Introduction
2. Materials and Methods
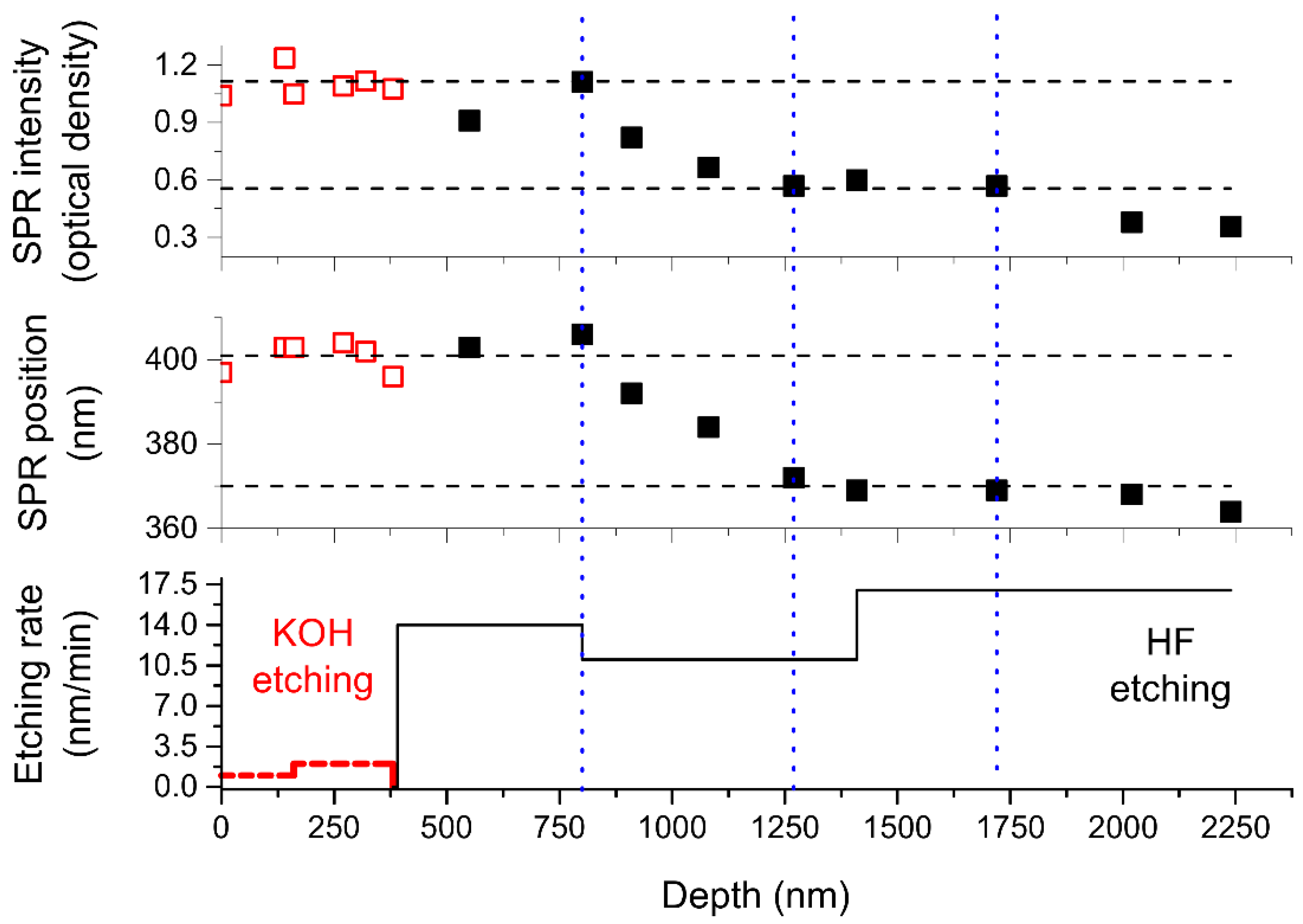
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lind, F.; Palles, D.; Möncke, D.; Kamitsos, E.I.; Wondraczek, L. Modifying the surface wetting behavior of soda-lime silicate glass substrates through thermal poling. J. Non-Cryst. Solids 2017, 462, 47–50. [Google Scholar] [CrossRef]
- Lepicard, A.; Cardinal, T.; Fargin, E.; Adamietz, F.; Rodriguez, V.; Richardson, K.; Dussauze, M. Surface Reactivity Control of a Borosilicate Glass Using Thermal Poling. J. Phys. Chem. C 2015, 119, 22999–23007. [Google Scholar] [CrossRef]
- Lepicard, A.; Cardinal, T.; Fargin, E.; Adamietz, F.; Rodriguez, V.; Richardson, K.; Dussauze, M. Micro-structuring the surface reactivity of a borosilicate glass via thermal poling. Chem. Phys. Lett. 2016, 664, 10–15. [Google Scholar] [CrossRef]
- Kamenskii, A.N.; Reduto, I.V.; Petrikov, V.D.; Lipovskii, A.A. Effective diffraction gratings via acidic etching of thermally poled glass. Opt. Mater. 2016, 62, 250–254. [Google Scholar] [CrossRef]
- Ikutame, N.; Kawaguchi, K.; Ikeda, H.; Sakai, D.; Harada, K.; Funatsu, S.; Nishii, J. Low-temperature fabrication of fine structures on glass using electrical nanoimprint and chemical etching. J. Appl. Phys. 2013, 114, 083514. [Google Scholar] [CrossRef]
- Ziemath, E.C.; Araújo, V.D.; Escanhoela, C.A. Compositional and structural changes at the anodic surface of thermally poled soda-lime float glass. J. Appl. Phys. 2008, 104, 054912. [Google Scholar] [CrossRef]
- Dussauze, M.; Kamitsos, E.I.; Fargin, E.; Rodriguez, V. Refractive index distribution in the non-linear optical layer of thermally poled oxide glasses. Chem. Phys. Lett. 2009, 470, 63–66. [Google Scholar] [CrossRef]
- Luo, J.; Bae, S.; Yuan, M.; Schneider, E.; Lanagan, M.T.; Pantano, C.G.; Kim, S.H. Chemical structure and mechanical properties of soda lime silica glass surfaces treated by thermal poling in inert and reactive ambient gases. J. Am. Ceram. Soc. 2018, 101, 2951–2964. [Google Scholar] [CrossRef]
- Paraillous, M.; Dussauze, M.; Fargin, E.; Poulon-Quintin, A.; Cardinal, T. Hardness reinforcement by surface engineering of soda lime silicate glass under thermal poling. In Proceedings of the Photonics and Fiber Technology 2016 (ACOFT, BGPP, NP); OSA: Washington, DC, USA, 2016; p. BT5B.1. [Google Scholar]
- Redkov, A.V.; Melehin, V.G.; Statcenko, V.V.; Lipovskii, A.A. Nanoprofiling of alkali-silicate glasses by thermal poling. J. Non-Cryst. Solids 2015, 409, 166–169. [Google Scholar] [CrossRef]
- An, H.; Fleming, S. Second-order optical nonlinearity and accompanying near-surface structural modifications in thermally poled soda-lime silicate glasses. J. Opt. Soc. Am. B 2006, 23, 2303. [Google Scholar] [CrossRef]
- Dergachev, A.; Kaasik, V.; Lipovskii, A.; Melehin, V.; Redkov, A.; Reshetov, I.; Tagantsev, D. Control of soda-lime glass surface crystallization with thermal poling. J. Non-Cryst. Solids 2020, 533, 119899. [Google Scholar] [CrossRef]
- Karam, L.; Adamietz, F.; Michau, D.; Gonçalves, C.; Kang, M.; Sharma, R.; Murugan, G.S.; Cardinal, T.; Fargin, E.; Rodriguez, V.; et al. Electrically Micro-Polarized Amorphous Sodo-Niobate Film Competing with Crystalline Lithium Niobate Second-Order Optical Response. Adv. Opt. Mater. 2020, 8, 2000202. [Google Scholar] [CrossRef]
- Dussauze, M.; Rodriguez, V.; Lipovskii, A.; Petrov, M.; Smith, C.; Richardson, K.; Cardinal, T.; Fargin, E.; Kamitsos, E.I. How does thermal poling affect the structure of soda-lime glass? J. Phys. Chem. C 2010, 114, 12754–12759. [Google Scholar] [CrossRef]
- Lepienski, C.M.; Giacometti, J.A.; Leal Ferreira, G.F.; Freire, F.L.; Achete, C.A. Electric field distribution and near-surface modifications in soda-lime glass submitted to a dc potential. J. Non-Cryst. Solids 1993, 159, 204–212. [Google Scholar] [CrossRef]
- Dussauze, M.; Fargin, E.; Lahaye, M.; Rodriguez, V.; Adamietz, F. Large second-harmonic generation of thermally poled sodium borophosphate glasses. Opt. Express 2005, 13, 4064. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.L.; de Araujo, M.T.; Vermelho, M.V.D.; Aitchison, J.S. Improved stability of the induced second-order nonlinearity in soft glass by thermal poling. J. Appl. Phys. 2006, 100, 033509. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, H.Y. Thermal poling induced second-order optical nonlinearity in phosphosilicate glass thin films. J. Mod. Opt. 2019, 66, 2053–2062. [Google Scholar] [CrossRef]
- Yudistira, D.; Faccio, D.; Corbari, C.; Kazansky, P.G.; Benchabane, S.; Pruneri, V. Electric surface potential and frozen-in field direct measurements in thermally poled silica. Appl. Phys. Lett. 2008, 92, 3–5. [Google Scholar] [CrossRef]
- Alvarado, R.; Karam, L.; Dahmani, R.; Lepicard, A.; Calzavara, F.; Piarristeguy, A.; Pradel, A.; Cardinal, T.; Adamietz, F.; Fargin, E.; et al. Patterning of the Surface Electrical Potential on Chalcogenide Glasses by a Thermoelectrical Imprinting Process. J. Phys. Chem. C 2020, 124, 23150–23157. [Google Scholar] [CrossRef]
- Scherbak, S.A.; Kaasik, V.P.; Zhurikhina, V.V.; Lipovskii, A.A. SEM-visualization of a spatial charge and a giant potassium peak in a corona-poled glass. J. Phys. Condens. Matter 2021, 33, 235702. [Google Scholar] [CrossRef]
- Doremus, R.H. Mechanism of electrical polarization of silica glass. Appl. Phys. Lett. 2005, 87, 1–2. [Google Scholar] [CrossRef]
- Redkov, A.V.; Melehin, V.G.; Lipovskii, A.A. How Does Thermal Poling Produce Interstitial Molecular Oxygen in Silicate Glasses? J. Phys. Chem. C 2015, 119, 17298–17307. [Google Scholar] [CrossRef]
- Von Hippel, A.; Gross, E.P.; Jelatis, J.G.; Geller, M. Photocurrent, Space-Charge Buildup, and Field Emission in Alkali Halide Crystals. Phys. Rev. 1953, 91, 568–579. [Google Scholar] [CrossRef]
- Microscope Slides. Available online: www.agarscientific.com/microscope-slides.html (accessed on 12 October 2021).
- An, H.; Fleming, S.C. Near-anode phase separation in thermally poled soda lime glass. Appl. Phys. Lett. 2006, 88, 181106. [Google Scholar] [CrossRef]
- Liñares, J.; Sotelo, D.; Lipovskii, A.A.; Zhurihina, V.V.; Tagantsev, D.K.; Turunen, J. New glasses for graded-index optics: Influence of non-linear diffusion in the formation of optical microstructures. Opt. Mater. 2000, 14, 145–153. [Google Scholar] [CrossRef]
- Spierings, G.A.C.M. Optical absorption of Ag+ ions in 11(Na,Ag)2O · 11B2O3 · 78SiO2 glass. J. Non-Cryst. Solids 1987, 94, 407–411. [Google Scholar] [CrossRef]
- Babich, E.; Reduto, I.; Redkov, A.; Reshetov, I.; Zhurikhina, V.; Lipovskii, A. Thermal poling of glasses to fabricate masks for ion exchange. J. Phys. Conf. Ser. 2020, 1695, 012107. [Google Scholar] [CrossRef]
- Babich, E.; Raskhodchikov, D.; Lubyankina, E.; Lipovskii, A. Depth of glass poling—Via optical transmission spectra. Optik 2021, 244, 167600. [Google Scholar] [CrossRef]
- Fabijanić, I.; Pervan, P.; Okorn, B.; Sancho-Parramon, J.; Janicki, V. Ellipsometry-based study of glass refractive index depth profiles obtained by applying different poling conditions. Appl. Opt. 2020, 59, A69. [Google Scholar] [CrossRef] [PubMed]
- Zhurikhina, V.V.; Brunkov, P.N.; Melehin, V.G.; Kaplas, T.; Svirko, Y.; Rutckaia, V.V.; Lipovskii, A.A. Self-assembled silver nanoislands formed on glass surface via out-diffusion for multiple usages in SERS applications. Nanoscale Res. Lett. 2012, 7, 1–5. [Google Scholar] [CrossRef]
- Smith, N.J.; Pantano, C.G. Structural and compositional modification of a barium boroaluminosilicate glass surface by thermal poling. Appl. Phys. A Mater. Sci. Process. 2014, 116, 529–543. [Google Scholar] [CrossRef]
- Majérus, O.; Lehuédé, P.; Biron, I.; Alloteau, F.; Narayanasamy, S.; Caurant, D. Glass alteration in atmospheric conditions: Crossing perspectives from cultural heritage, glass industry, and nuclear waste management. NPJ Mater. Degrad. 2020, 4, 27. [Google Scholar] [CrossRef]
- Balavandy, S.K.; Shameli, K.; Biak, D.R.B.A.; Abidin, Z.Z. Stirring time effect of silver nanoparticles prepared in glutathione mediated by green method. Chem. Cent. J. 2014, 8, 11. [Google Scholar] [CrossRef]
- Chen, Y.; Karvonen, L.; Säynätjoki, A.; Ye, C.; Tervonen, A.; Honkanen, S. Ag nanoparticles embedded in glass by two-step ion exchange and their SERS application. Opt. Mater. Express 2011, 1, 164. [Google Scholar] [CrossRef]
- Sancho-Parramon, J.; Janicki, V.; Dubček, P.; Karlušić, M.; Gracin, D.; Jakšić, M.; Bernstorff, S.; Meljanac, D.; Juraić, K. Optical and structural properties of silver nanoparticles in glass matrix formed by thermal annealing of field assisted film dissolution. Opt. Mater. 2010, 32, 510–514. [Google Scholar] [CrossRef][Green Version]
- Kaasik, V.P.; Lipovskii, A.A.; Raskhodchikov, D.V.; Reshetov, I.V.; Tagantsev, D.K. How to reveal the correct elemental concentration profiles in poled multicomponent silicate glasses from the data of secondary ion mass spectrometry (SIMS). J. Non-Cryst. Solids 2019, 503–504, 397–399. [Google Scholar] [CrossRef]
- Petrov, M.I.; Lepen’kin, Y.A.; Lipovskii, A.A. Polarization of glass containing fast and slow ions. J. Appl. Phys. 2012, 112, 043101. [Google Scholar] [CrossRef]
- Xu, W.; Arentoft, J.; Wong, D.; Fleming, S. Evidence of space-charge effects in thermal poling. IEEE Photonics Technol. Lett. 1999, 11, 1265–1267. [Google Scholar] [CrossRef]


| SiO2 | Na2O | CaO | MgO | K2O | Al2O | Others |
|---|---|---|---|---|---|---|
| 72.2 | 14.3 | 6.4 | 4.3 | 1.2 | 1.2 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babich, E.; Lubyankina, E.; Kaasik, V.; Mozharov, A.; Mukhin, I.; Zhurikhina, V.; Lipovskii, A. Visualization of Spatial Charge in Thermally Poled Glasses via Nanoparticles Formation. Nanomaterials 2021, 11, 2973. https://doi.org/10.3390/nano11112973
Babich E, Lubyankina E, Kaasik V, Mozharov A, Mukhin I, Zhurikhina V, Lipovskii A. Visualization of Spatial Charge in Thermally Poled Glasses via Nanoparticles Formation. Nanomaterials. 2021; 11(11):2973. https://doi.org/10.3390/nano11112973
Chicago/Turabian StyleBabich, Ekaterina, Ekaterina Lubyankina, Vladimir Kaasik, Alexey Mozharov, Ivan Mukhin, Valentina Zhurikhina, and Andrey Lipovskii. 2021. "Visualization of Spatial Charge in Thermally Poled Glasses via Nanoparticles Formation" Nanomaterials 11, no. 11: 2973. https://doi.org/10.3390/nano11112973
APA StyleBabich, E., Lubyankina, E., Kaasik, V., Mozharov, A., Mukhin, I., Zhurikhina, V., & Lipovskii, A. (2021). Visualization of Spatial Charge in Thermally Poled Glasses via Nanoparticles Formation. Nanomaterials, 11(11), 2973. https://doi.org/10.3390/nano11112973








