Graphene and Carbon Nanotubes Fibrous Composite Decorated with PdMg Alloy Nanoparticles with Enhanced Absorption–Desorption Kinetics for Hydrogen Storage Application
Abstract
:1. Introduction
2. Materials and Methods
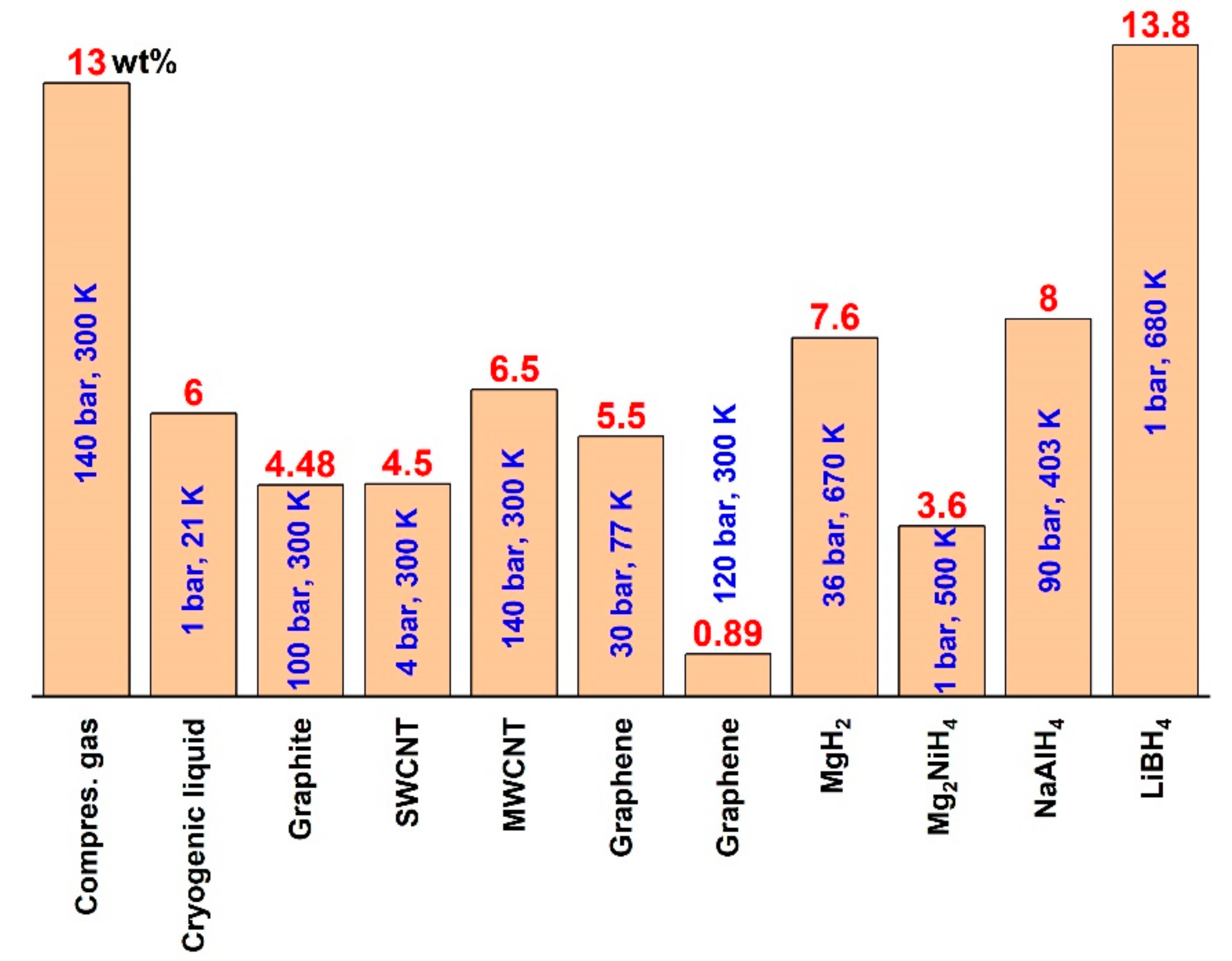
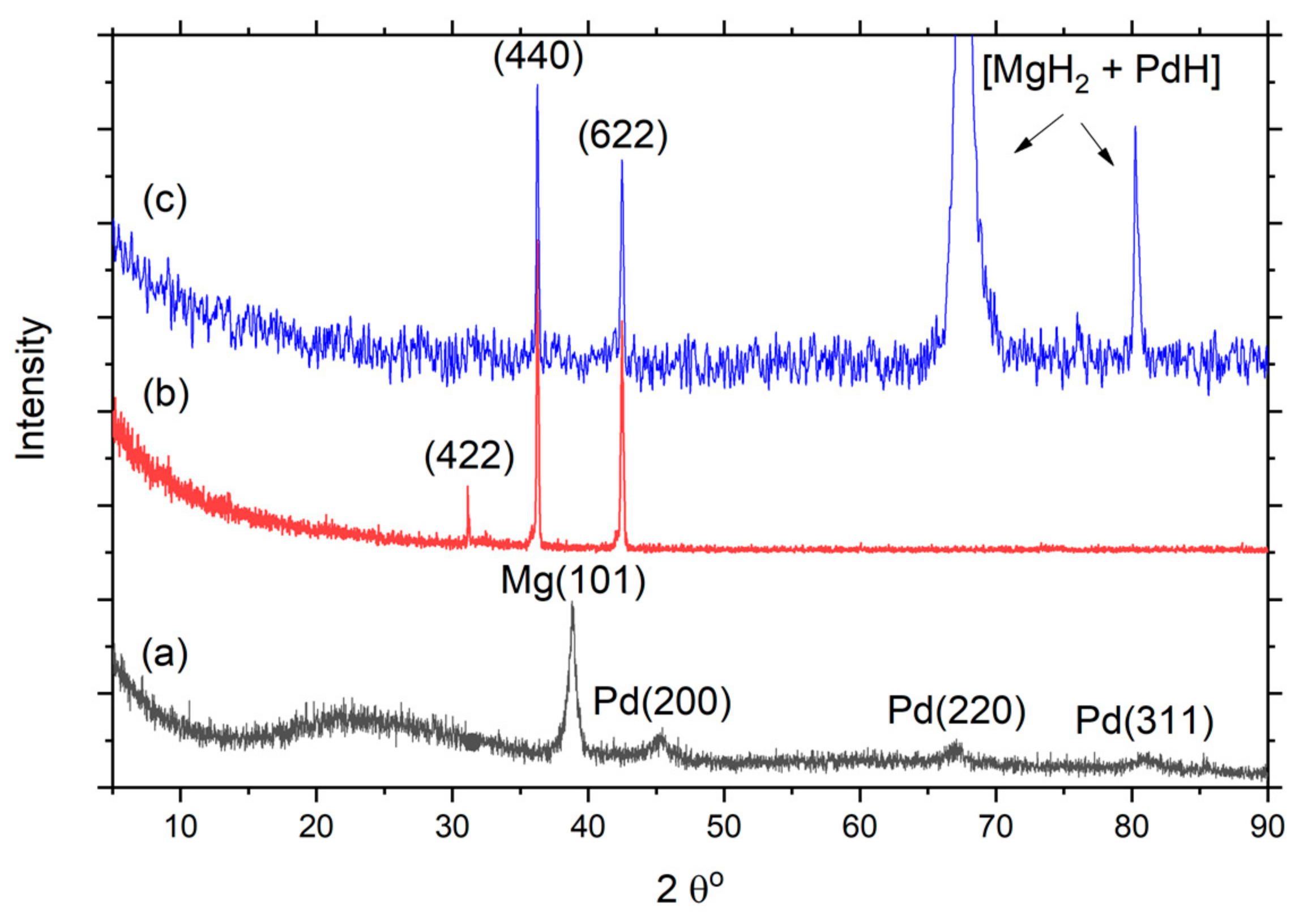
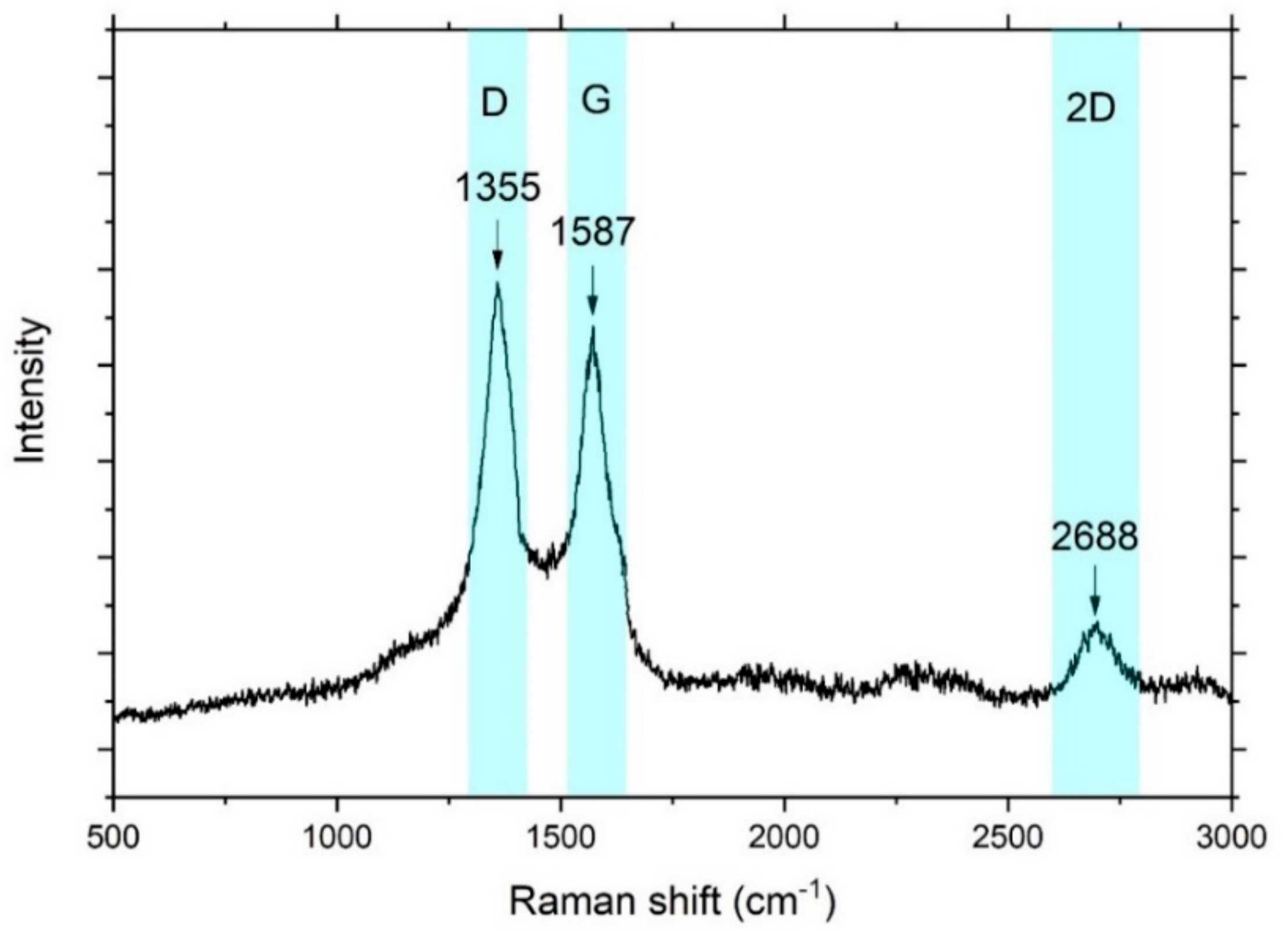
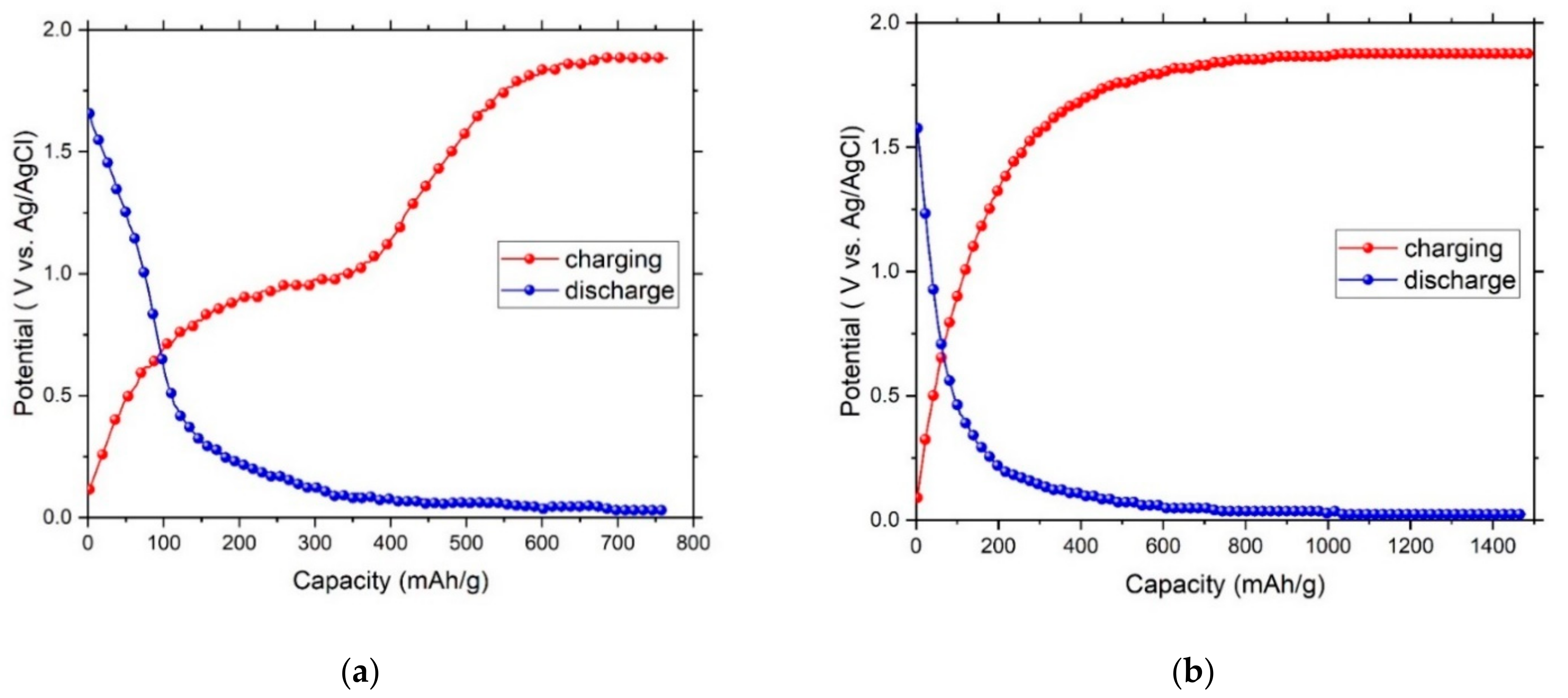
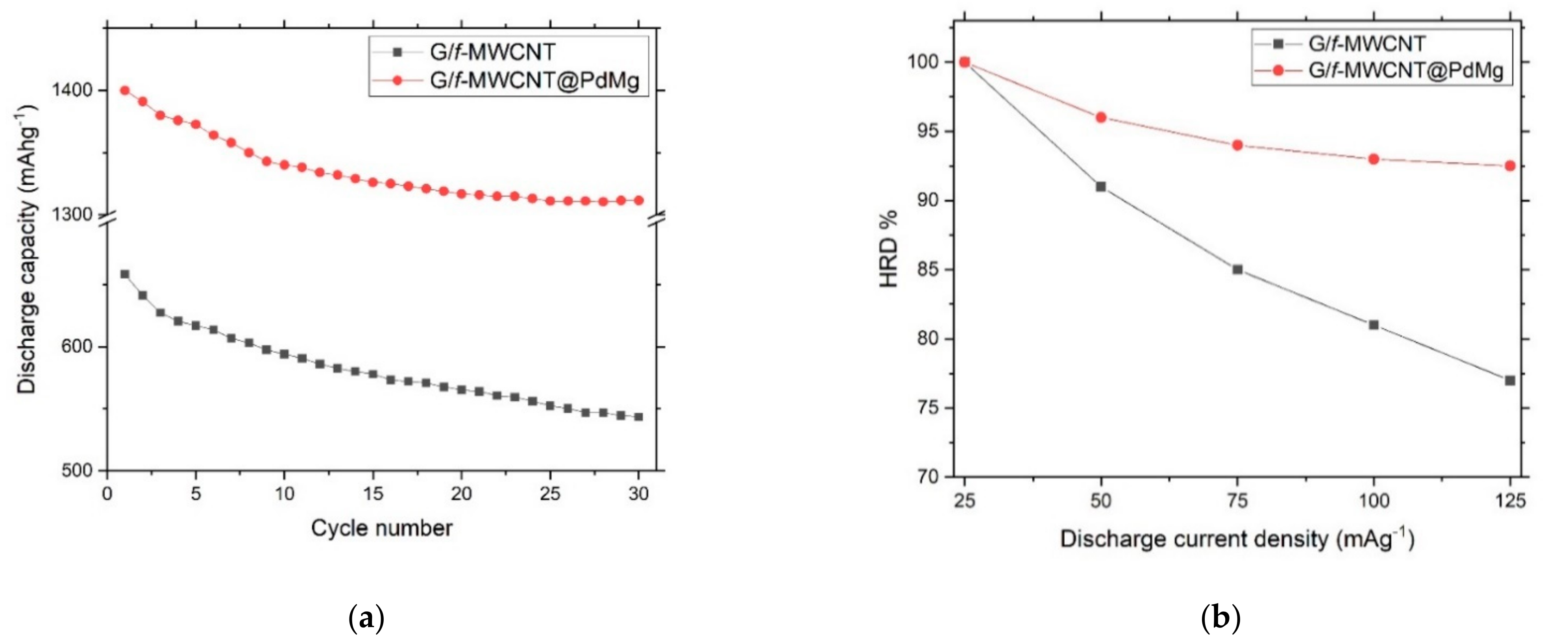
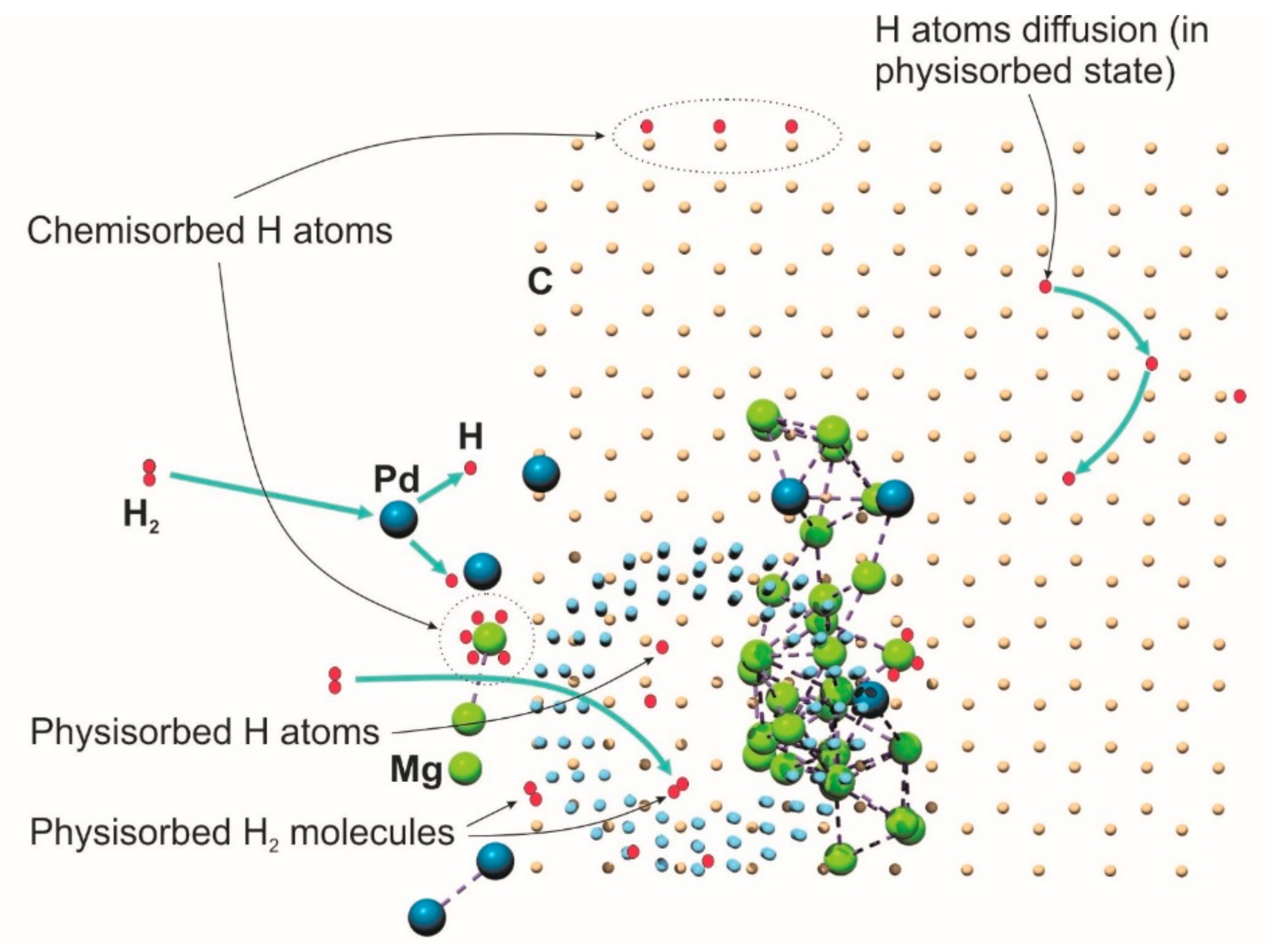
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Van Renssen, S. The hydrogen solution? Nat. Clim. Chang. 2020, 10, 799–801. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Salem, S.M.; Ali, A.N.; Banyan, M.; Al-Ajmi, F.; Al-Duweesh, A. From gangue to the fuel-cells application. Sci. Rep. 2020, 10, 20022. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.M.; Borrás-Morell, J.F.; García-Baños, B.; Balaguer, M.; Plaza-González, P.; Santos-Blasco, J.; Catalán-Martínez, D.; Navarrete, L.; Catalá-Civera, J.M. Hydrogen production via microwave-induced water splitting at low temperature. Nat. Energy 2020, 5, 910–919. [Google Scholar] [CrossRef]
- Chu, S.; Majumdasr, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- He, T.; Cao, H.; Chen, P. Complex Hydrides for Energy Storage, Conversion, and Utilization. Adv. Mater. 2019, 31, 1902757. [Google Scholar] [CrossRef] [PubMed]
- Firlej, L.; Pfeifer, P.; Kuchta, B. Understanding Universal Adsorption Limits for Hydrogen Storage in Nano Porous Systems. Adv. Mater. 2013, 25, 5971–5974. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zhang, L. Hydrogen Storage in Metal–Organic Frameworks. Adv. Mater. 2010, 22, E117–E130. [Google Scholar] [CrossRef]
- Kato, R.; Nishide, H. Polymers for carrying and storing hydrogen. Polym. J. 2018, 50, 77–82. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Jin, J.; Fu, L.; Yang, H.; Ouyang, J. Carbon hybridized halloysite nanotubes for high-performance hydrogen storage capacities. Sci. Rep. 2015, 5, 12429. [Google Scholar] [CrossRef]
- Mohtadi, R.; Orimo, S.-i. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2016, 2, 16091. [Google Scholar] [CrossRef]
- Adametz, P.; Müller, K.; Arlt, W. Energetic evaluation of hydrogen storage in metal hydrides. Int. J. Energy Res. 2016, 40, 1820–1831. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrog. Energy 2020, 46, 13647–13657. [Google Scholar] [CrossRef]
- Kumar, K.; Alam, M.; Verma, S.; Dutta, V. Analysis of metal hydride storage on the basis of thermophysical properties and its application in microgrid. Energy Convers. Manag. 2020, 222, 113217. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Investigation on hydrogen dissociation pressure, heat of formation and strain energy of metal hydrides. J. Alloys Compd. 2020, 840, 155686. [Google Scholar] [CrossRef]
- Lim, K.L.; Kazemian, H.; Yaakob, Z.; Daud, W.R.W. Solid-state Materials and Methods for Hydrogen Storage: A Critical Review. Chem. Eng. Technol. 2010, 33, 213–226. [Google Scholar] [CrossRef]
- Lin, I.H.; Tong, Y.-J.; Hsieh, H.-J.; Huang, H.-W.; Chen, H.-T. Hydrogen adsorption and storage in boron-substituted and nitrogen-substituted nano-carbon materials decorated with alkaline earth metals. Int. J. Energy Res. 2016, 40, 230–240. [Google Scholar] [CrossRef]
- Salehabadi, A.; Umar, M.F.; Ahmad, A.; Ahmad, M.I.; Ismail, N.; Rafatullah, M. Carbon-based nanocomposites in solid-state hydrogen storage technology: An overview. Int. J. Energy Res. 2020, 44, 11044–11058. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Wang, C.; Tang, C.; Fu, L. An effective method to screen carbon (boron, nitrogen) based two-dimensional hydrogen storage materials. Int. J. Hydrog. Energy 2020, 45, 25054–25064. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Dong, H.; Zheng, M.; Liu, Y. Polyacrylonitrile-based highly porous carbon materials for exceptional hydrogen storage. Int. J. Hydrog. Energy 2019, 44, 23210–23215. [Google Scholar] [CrossRef]
- Salehabadi, A.; Salavati-Niasari, M.; Ghiyasiyan-Arani, M. Self-assembly of hydrogen storage materials based multi-walled carbon nanotubes (MWCNTs) and Dy3Fe5O12 (DFO) nanoparticles. J. Alloys Compd. 2018, 745, 789–797. [Google Scholar] [CrossRef]
- Yang, S.J.; Jung, H.; Kim, T.; Park, C.R. Recent advances in hydrogen storage technologies based on nanoporous carbon materials. Prog. Nat. Sci. Mater. Int. 2012, 22, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Papp, G.; Csorba, J.; Laurenczy, G.; Joó, F. A Charge/Discharge Device for Chemical Hydrogen Storage and Generation. Angew. Chem. Int. Ed. 2011, 50, 10433–10435. [Google Scholar] [CrossRef]
- Jiang , H.-L.; Singh , S.K.; Yan , J.-M.; Zhang, X.-B.; Xu, Q. Liquid-Phase Chemical Hydrogen Storage: Catalytic Hydrogen Generation under Ambient Conditions. ChemSusChem 2010, 3, 541–549. [Google Scholar] [CrossRef]
- Moury, R.; Demirci, U.B.; Ichikawa, T.; Filinchuk, Y.; Chiriac, R.; van der Lee, A.; Miele, P. Sodium Hydrazinidoborane: A Chemical Hydrogen-Storage Material. ChemSusChem 2013, 6, 667–673. [Google Scholar] [CrossRef]
- Moussa, G.; Moury, R.; Demirci, U.B.; Şener, T.; Miele, P. Boron-based hydrides for chemical hydrogen storage. Int. J. Energy Res. 2013, 37, 825–842. [Google Scholar] [CrossRef]
- Lang, C.; Jia, Y.; Yao, X. Recent advances in liquid-phase chemical hydrogen storage. Energy Storage Mater. 2020, 26, 290–312. [Google Scholar] [CrossRef]
- Brooks, K.P.; Sprik, S.J.; Tamburello, D.A.; Thornton, M.J. Design tool for estimating chemical hydrogen storage system characteristics for light-duty fuel cell vehicles. Int. J. Hydrog. Energy 2018, 43, 8846–8858. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrog. Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Brooks, K.P. Chemical hydrogen storage material property guidelines for automotive applications. J. Power Sources 2015, 279, 593–609. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yao, Y.; He, L.; Zhou, X.J.; Yu, L.P.; Lu, X.Z.; Peng, P. Hydrogen storage properties and mechanisms of as-cast, homogenized and ECAP processed Mg98.5Y1Zn0.5 alloys containing LPSO phase. Energy 2020, 217, 119315. [Google Scholar] [CrossRef]
- Baroutaji, A.; Arjunan, A.; Ramadan, M.; Alaswad, A.; Achour, H.; Abdelkareem, M.A.; Olabi, A.-G. Nanocrystalline Mg2Ni for Hydrogen Storage. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Pandey, A.P.; Bhatnagar, A.; Shukla, V.; Soni, P.K.; Singh, S.; Verma, S.K.; Shaneeth, M.; Sekkar, V.; Srivastava, O.N. Hydrogen storage properties of carbon aerogel synthesized by ambient pressure drying using new catalyst triethylamine. Int. J. Hydrog. Energy 2020, 45, 30818–30827. [Google Scholar] [CrossRef]
- Tan, K.C.; Yu, Y.; Chen, R.; He, T.; Jing, Z.; Pei, Q.; Wang, J.; Chua, Y.S.; Wu, A.; Zhou, W.; et al. Metallo-N-Heterocycles—A new family of hydrogen storage material. Energy Storage Mater. 2020, 26, 198–202. [Google Scholar] [CrossRef]
- Jain, V.; Kandasubramanian, B. Functionalized graphene materials for hydrogen storage. J. Mater. Sci. 2020, 55, 1865–1903. [Google Scholar] [CrossRef]
- Song, L.-F.; Jiang, C.-H.; Liu, S.-S.; Jiao, C.-L.; Si, X.-L.; Wang, S.; Li, F.; Zhang, J.; Sun, L.-X.; Xu, F.; et al. Progress in improving thermodynamics and kinetics of new hydrogen storage materials. Front. Phys. 2011, 6, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Coppola, C.M.; Tolbatov, I.; Tranca, I.C.; Coletti, C.; Marrone, A.; Storchi, L.; Profio, P.D.; Re, N.; Kazandjian, M.V.; Pellecchia, A.; et al. A database approach for materials selection for hydrogen storage in aerospace technology. Rend. Lincei. Sci. Fis. E Nat. 2019, 30, 287–296. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.-W.; Chen, P. Amides and borohydrides for high-capacity solid-state hydrogen storage—materials design and kinetic improvements. MRS Bull. 2013, 38, 480–487. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, P.; Shaw, L. Solid-state hydrogen desorption of 2 MgH2 + LiBH4 nano-mixture: A kinetics mechanism study. J. Alloys Compd. 2019, 806, 350–360. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, K.; Jiang, J.; Yang, X.-S.; Zhu, M. Hydrogen storage in light-metal based systems: A review. J. Alloys Compd. 2020, 829, 154597. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic Tuning of Mg-Based Hydrogen Storage Alloys: A Review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef] [Green Version]
- Oliva, D.G.; Fuentes, M.; Borzone, E.M.; Meyer, G.O.; Aguirre, P.A. Hydrogen storage on LaNi5−xSnx. Experimental and phenomenological Model-based analysis. Energy Convers. Manag. 2018, 173, 113–122. [Google Scholar] [CrossRef]
- Konik, P.; Berdonosova, E.; Savvotin, I.; Zadorozhnyy, V.; Zadorozhnyy, M.; Semenov, D.; Korol, A.; Kvaratskheliya, A.; Klyamkin, S. Structure and hydrogenation features of mechanically activated LaNi5-type alloys. Int. J. Hydrog. Energy 2020, 46, 13638–13646. [Google Scholar] [CrossRef]
- Orgaz, E.; Gupta, M. Electronic structure of BaReH9. J. Alloys Compd. 1999, 293–295, 217–221. [Google Scholar] [CrossRef]
- Ianni, E.; Sofianos, M.V.; Rowles, M.R.; Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Synthesis of NaAlH4/Al composites and their applications in hydrogen storage. Int. J. Hydrog. Energy 2018, 43, 17309–17317. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; Zhou, Y.; Chen, Z.; Yang, W.; Ma, W.; Shaw, L. LiBH4 for hydrogen storage—New perspectives. Nano Mater. Sci. 2020, 2, 109–119. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Shaw, L. New insights into the solid-state hydrogen storage of nanostructured LiBH4-MgH2 system. Chem. Eng. J. 2020, 385, 123856. [Google Scholar] [CrossRef]
- Ding, Z.; Shaw, L. Enhancement of Hydrogen Desorption from Nanocomposite Prepared by Ball Milling MgH2 with In Situ Aerosol Spraying LiBH4. ACS Sustain. Chem. Eng. 2019, 7, 15064–15072. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High reversible capacity hydrogen storage through Nano-LiBH4 + Nano-MgH2 system. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Ding, Z.; Zhao, X.; Shaw, L.L. Reaction between LiBH4 and MgH2 induced by high-energy ball milling. J. Power Sources 2015, 293, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Petrushenko, I.K.; Petrushenko, K.B. Hydrogen adsorption on graphene, hexagonal boron nitride, and graphene-like boron nitride-carbon heterostructures: A comparative theoretical study. Int. J. Hydrog. Energy 2018, 43, 801–808. [Google Scholar] [CrossRef]
- Shalaan, E.; Inoue, A.; Al-Marzouki, F.; Al-Heniti, S.; Obaid, A.Y.; Al-Hashimi, S. Formation of nano-porous Pd(Ni) structure produced by dealloying Zr-Al-Ni-Pd base glassy alloys and their electrochemical properties. J. Non-Cryst. Solids 2019, 518, 123–127. [Google Scholar] [CrossRef]
- Wu, X.; He, G.; Ding, Y. Dealloyed nanoporous materials for rechargeable lithium batteries. Electrochem. Energy Rev. 2020, 3, 541–580. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Fu, Z.; Qu, J.; Zhong, M.; Yang, X.; Yi, Y.; Wang, C. Nanoporous Ni with High Surface Area for Potential Hydrogen Storage Application. Nanomaterials 2018, 8, 394. [Google Scholar] [CrossRef] [Green Version]
- Baburin, I.A.; Klechikov, A.; Mercier, G.; Talyzin, A.; Seifert, G. Hydrogen adsorption by perforated graphene. Int. J. Hydrog. Energy 2015, 40, 6594–6599. [Google Scholar] [CrossRef] [Green Version]
- Nagar, R.; Vinayan, B.P.; Samantaray, S.S.; Ramaprabhu, S. Recent advances in hydrogen storage using catalytically and chemically modified graphene nanocomposites. J. Mater. Chem. A 2017, 5, 22897–22912. [Google Scholar] [CrossRef]
- Rangel, E.; Sansores, E. Theoretical study of hydrogen adsorption on nitrogen doped graphene decorated with palladium clusters. Int. J. Hydrog. Energy 2014, 39, 6558–6566. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Tanimura, M.; Tachibana, M. Temperature dependence of the Raman spectra of multilayer graphene nanoribbons fabricated by unzipping method. Diam. Relat. Mater. 2020, 109, 108047. [Google Scholar] [CrossRef]
- Cao, A.; Zhu, H.; Zhang, X.; Li, X.; Ruan, D.; Xu, C.; Wei, B.; Liang, J.; Wu, D. Hydrogen storage of dense-aligned carbon nanotubes. Chem. Phys. Lett. 2001, 342, 510–514. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Castiglia, A.; Ganter, M.J.; Rogers, R.E.; Cress, C.D.; Raffaelle, R.P.; Landi, B.J. Enhanced Capacity and Rate Capability of Carbon Nanotube Based Anodes with Titanium Contacts for Lithium Ion Batteries. ACS Nano 2010, 4, 6121–6131. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.; Oakes, L.; Cohn, A.P.; Holzgrafe, J.; Zarick, H.F.; Chatterjee, S.; Bardhan, R.; Pint, C.L. Solution Assembled Single-Walled Carbon Nanotube Foams: Superior Performance in Supercapacitors, Lithium-Ion, and Lithium–Air Batteries. J. Phys. Chem. C 2014, 118, 20137–20151. [Google Scholar] [CrossRef]
- Kumara, L.S.R.; Sakata, O.; Kobayashi, H.; Song, C.; Kohara, S.; Ina, T.; Yoshimoto, T.; Yoshioka, S.; Matsumura, S.; Kitagawa, H. Hydrogen storage and stability properties of Pd–Pt solid-solution nanoparticles revealed via atomic and electronic structure. Sci. Rep. 2017, 7, 14606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Szpunar, J.A. Hydrogen Storage Performance in Pd/Graphene Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 25933–25940. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamauchi, M.; Ikeda, R.; Yamamoto, T.; Matsumura, S.; Kitagawa, H. Double enhancement of hydrogen storage capacity of Pd nanoparticles by 20 at% replacement with Ir; systematic control of hydrogen storage in Pd–M nanoparticles (M = Ir, Pt, Au). Chem. Sci. 2018, 9, 5536–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]

| Phase | 2θ° | d Spacing, Å | ∆θ° | ∆d, Å | Lattice Parameters, Å | Cell Volume, Å3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Film | Bulk | Film | Bulk | Film | Bulk | Film | Bulk | |||
| Mg (101) | 38.81 | 36.62 | 2.318 | 2.450 | 2.19 | −0.132 | - | - | ||
| Pd (200) | 45.32 | 46.66 | 1.998 | 1.945 | −1.32 | 0.053 | 3.99 | 3.89 | 63.52 | 58.87 |
| Pd (220) | 66.98 | 68.12 | 1.396 | 1.375 | −1.14 | 0.021 | 3.95 | 61.63 | ||
| Pd (311) | 80.96 | 82.09 | 1.186 | 1.173 | −1.14 | 0.014 | 3.94 | 61.16 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkook, B.; Alshahrie, A.; Salah, N.; Aslam, M.; Aissan, S.; Al-Ojeery, A.; Al-Ghamdi, A.; Inoue, A.; Shalaan, E.-S. Graphene and Carbon Nanotubes Fibrous Composite Decorated with PdMg Alloy Nanoparticles with Enhanced Absorption–Desorption Kinetics for Hydrogen Storage Application. Nanomaterials 2021, 11, 2957. https://doi.org/10.3390/nano11112957
Arkook B, Alshahrie A, Salah N, Aslam M, Aissan S, Al-Ojeery A, Al-Ghamdi A, Inoue A, Shalaan E-S. Graphene and Carbon Nanotubes Fibrous Composite Decorated with PdMg Alloy Nanoparticles with Enhanced Absorption–Desorption Kinetics for Hydrogen Storage Application. Nanomaterials. 2021; 11(11):2957. https://doi.org/10.3390/nano11112957
Chicago/Turabian StyleArkook, Bassim, Ahmed Alshahrie, Numan Salah, Mohammad Aslam, Saeed Aissan, Ashwaq Al-Ojeery, Ahmed Al-Ghamdi, Akihisa Inoue, and El-Sayed Shalaan. 2021. "Graphene and Carbon Nanotubes Fibrous Composite Decorated with PdMg Alloy Nanoparticles with Enhanced Absorption–Desorption Kinetics for Hydrogen Storage Application" Nanomaterials 11, no. 11: 2957. https://doi.org/10.3390/nano11112957
APA StyleArkook, B., Alshahrie, A., Salah, N., Aslam, M., Aissan, S., Al-Ojeery, A., Al-Ghamdi, A., Inoue, A., & Shalaan, E.-S. (2021). Graphene and Carbon Nanotubes Fibrous Composite Decorated with PdMg Alloy Nanoparticles with Enhanced Absorption–Desorption Kinetics for Hydrogen Storage Application. Nanomaterials, 11(11), 2957. https://doi.org/10.3390/nano11112957







