Iron, Copper, and Zinc Homeostasis: Physiology, Physiopathology, and Nanomediated Applications
Abstract
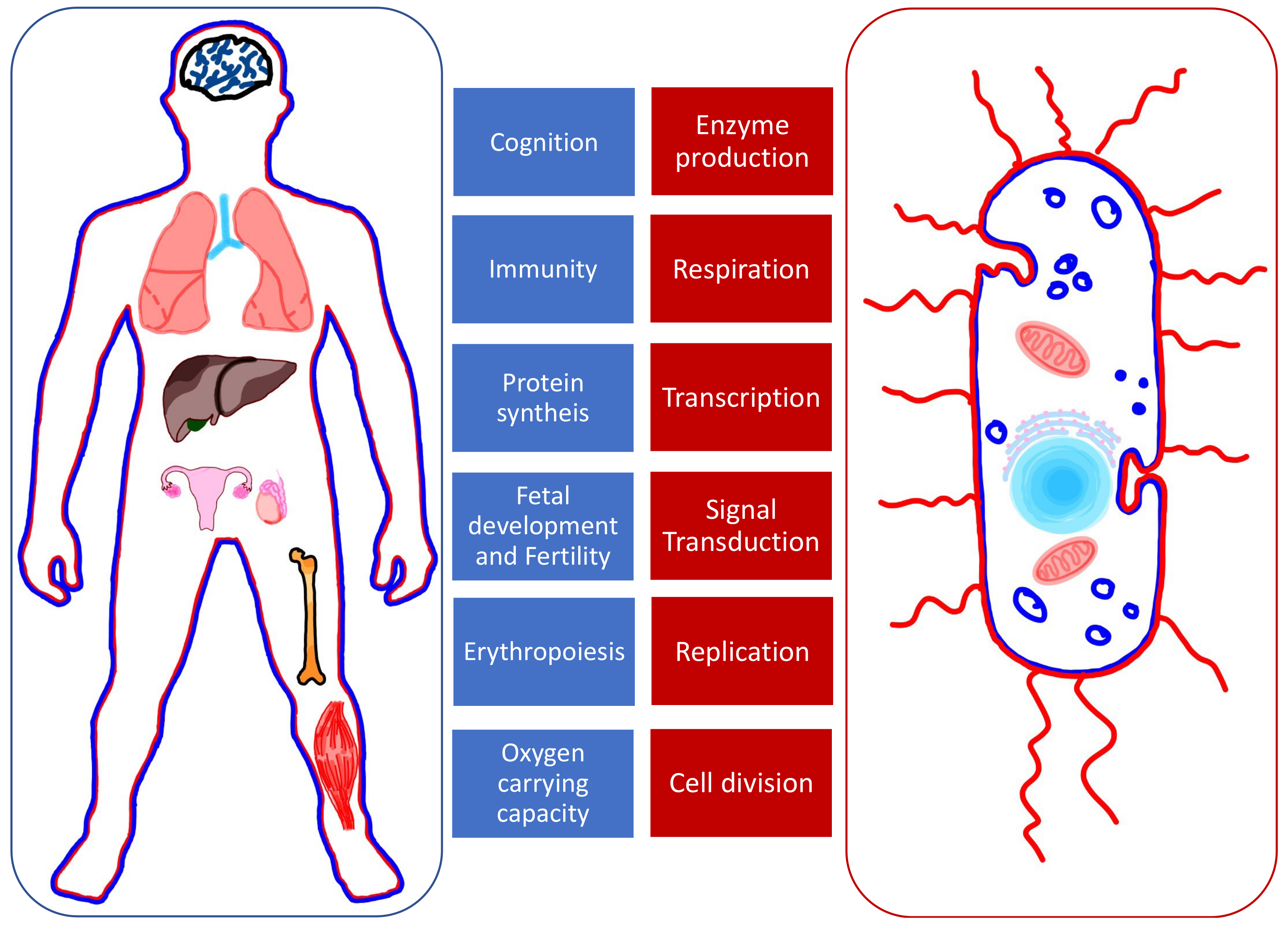
:1. Introduction
2. Iron Metabolism
2.1. Iron Uptake
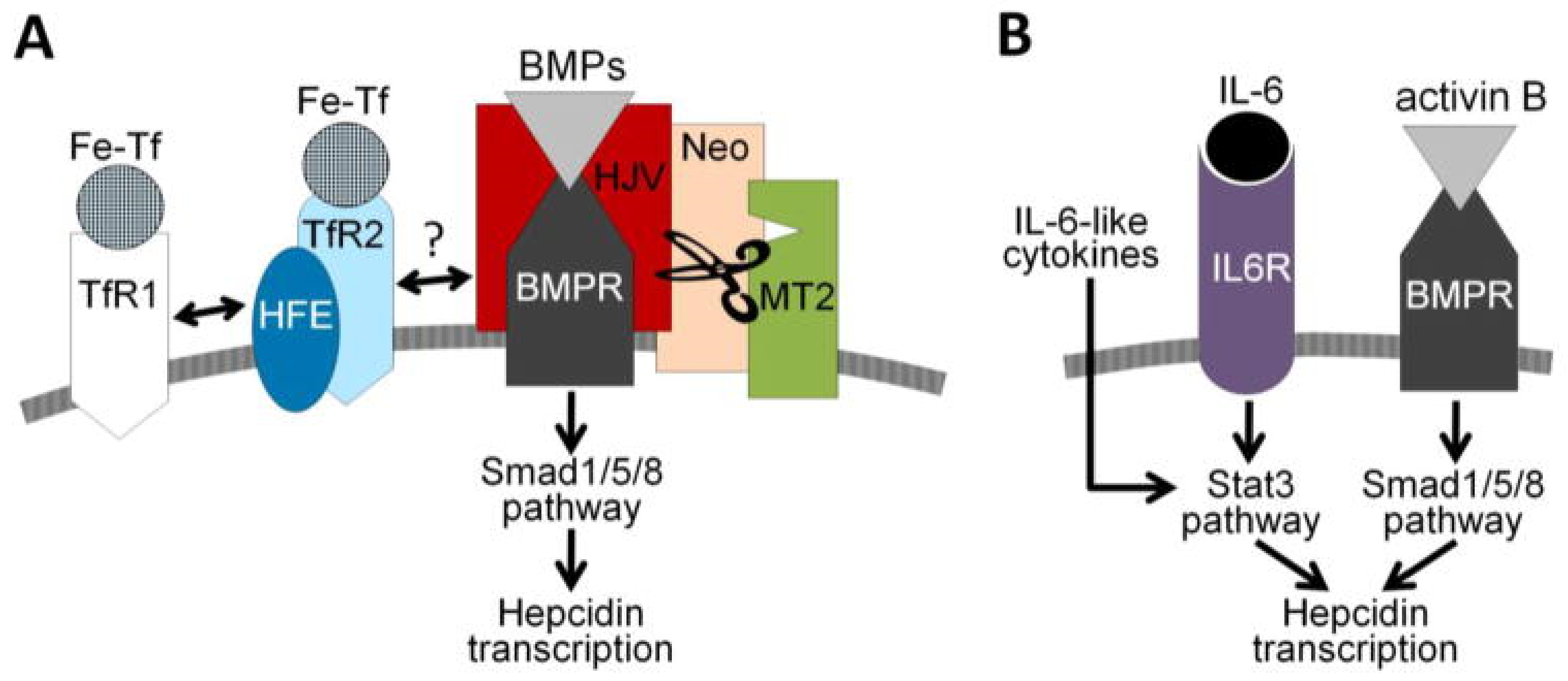
2.2. Iron Homeostasis
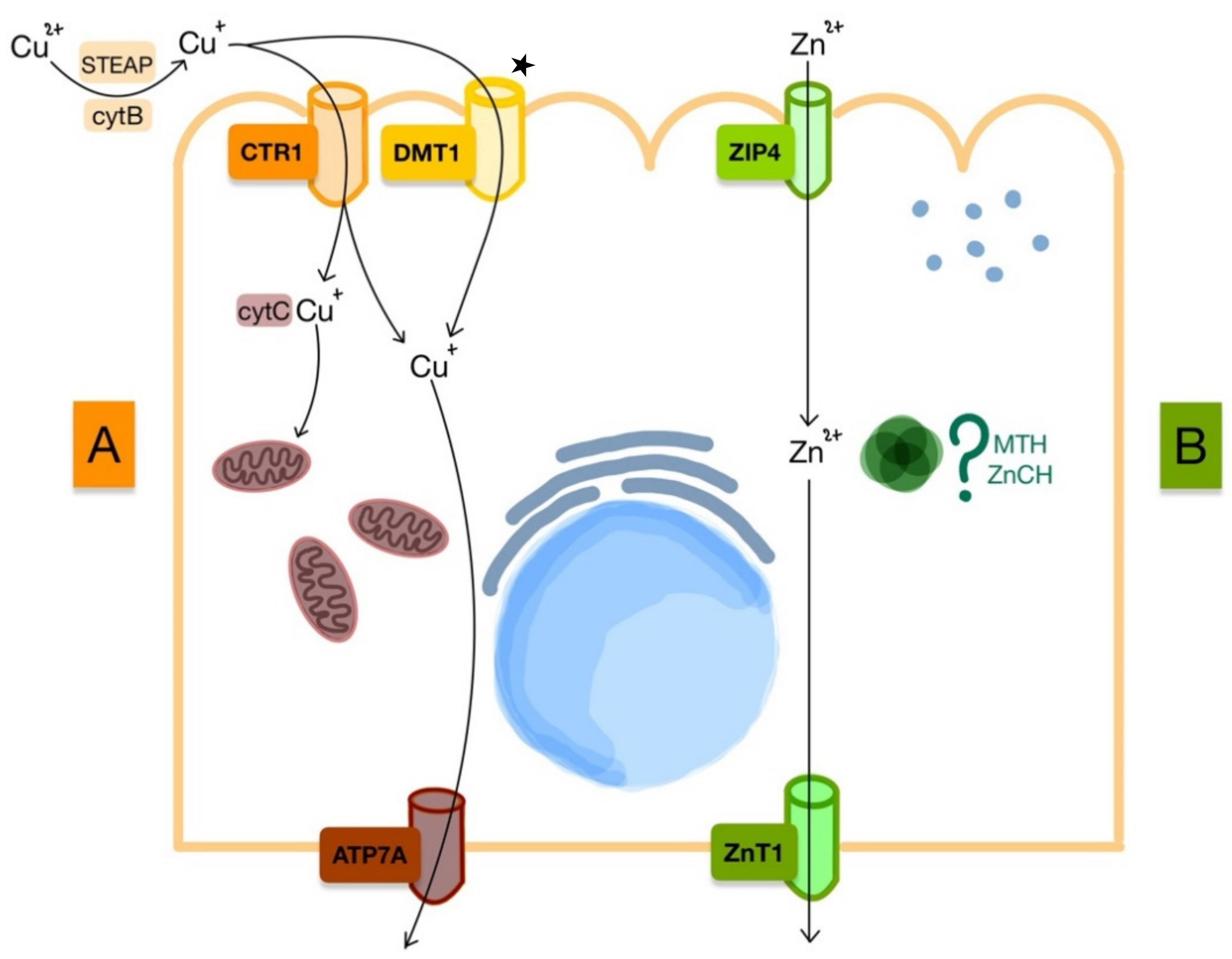
3. Copper and Zinc Metabolism
3.1. Copper
3.2. Zinc
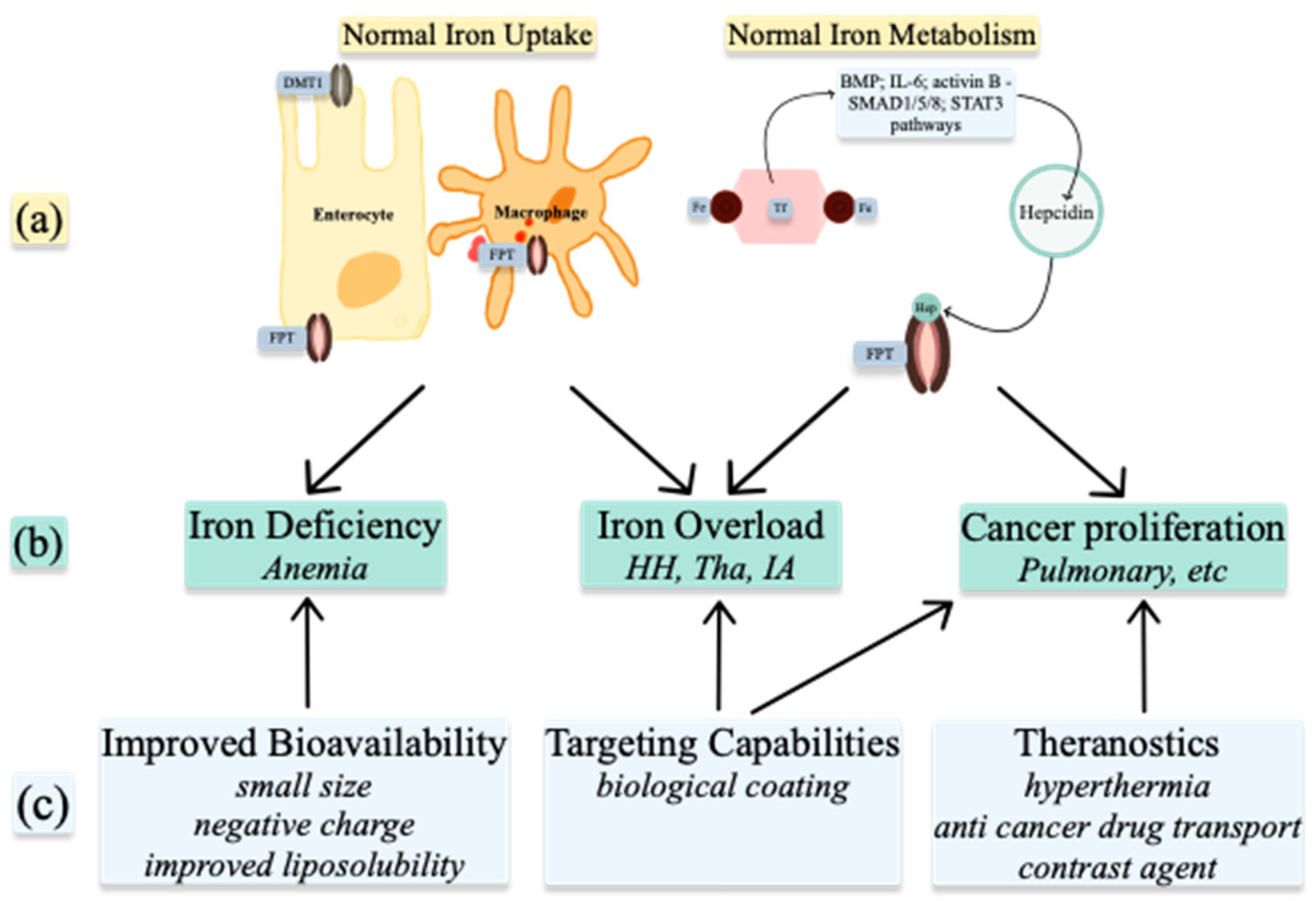
4. Medical Uses of Iron, Copper, and Zinc Nanoparticles
4.1. Nanoparticles to Improve Bioavailability
Iron Administration
4.2. Targeting Capabilities
4.2.1. Iron Chelation
4.2.2. Antimicrobial Treatment
4.2.3. Biological Sample Analysis
4.3. Theranostics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial Int. 2017, 21 (Suppl. 1), S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Garrick, M.D.; Collins, J.F. Animal Models of Normal and Disturbed Iron and Copper Metabolism. J. Nutr. 2019, 149, 2085–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Balla, J.; Balla, G.; Zarjou, A. Ferritin in Kidney and Vascular Related Diseases: Novel Roles for an Old Player. Pharmaceuticals 2019, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Roth, M.P.; Meynard, D.; Coppin, H. Regulators of hepcidin expression. Vitam. Horm 2019, 110, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kali, A.; Charles, M.V.P.; Seetharam, R.S.K. Hepcidin—A novel biomarker with changing trends. Pharmacogn. Rev. 2015, 9, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.E.; Umbreit, J.N. Iron absorption and transport-an update. Am. J. Hematol. 2000, 64, 287–298. [Google Scholar] [CrossRef]
- Yanatori, I.; Kishi, F. DMT1 and iron transport. Free Radic. Biol. Med. 2019, 133, 55–63. [Google Scholar] [CrossRef]
- Conrad, M.E.; Umbreit, J.N. Pathways of iron absorption. Blood Cells Mol. Dis. 2002, 29, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K. Function of the hemochromatosis protein HFE: Lessons from animal models. World J. Gastroenterol. 2008, 14, 6893–6901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, D. Anemia in the ICU: Anemia of chronic disease versus anemia of acute illness. Crit. Care Clin. 2012, 28, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [Green Version]
- Aisen, P. Transferrin receptor 1. Int. J. Biochem. Cell Biol. 2004, 36, 2137–2143. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Wu, Q.; Jiang, L.; Chen, L.; Yu, Y.; Zhang, P.; Huang, X.; Wang, J.; Ju, Z.; et al. Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 2020, 105, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, D.; Wang, X.T.; Lu, Y.P.; Zhu, L. The roles of hypoxia-inducible Factor-1 and iron regulatory protein 1 in iron uptake induced by acute hypoxia. Biochem. Biophys. Res. Commun. 2018, 507, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef] [Green Version]
- Jamnongkan, W.; Thanan, R.; Techasen, A.; Namwat, N.; Loilome, W.; Intarawichian, P.; Titapun, A.; Yongvanit, P. Upregulation of transferrin receptor-1 induces cholangiocarcinoma progression via induction of labile iron pool. Tumour Biol. 2017, 39, 1010428317717655. [Google Scholar] [CrossRef] [Green Version]
- Ruchala, P.; Nemeth, E. The pathophysiology and pharmacology of hepcidin. Trends Pharmacol. Sci. 2014, 35, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Herbison, C.E.; Thorstensen, K.; Chua, A.C.; Graham, R.M.; Leedman, P.; Olynyk, J.K.; Trinder, D. The role of transferrin receptor 1 and 2 in transferrin-bound iron uptake in human hepatoma cells. Am. J. Physiol. Cell Physiol. 2009, 297, C1567–C1575. [Google Scholar] [CrossRef] [Green Version]
- Layoun, A.; Samba-Mondonga, M.; Fragoso, G.; Calvé, A.; Santos, M.M. MyD88 Adaptor Protein Is Required for Appropriate Hepcidin Induction in Response to Dietary Iron Overload in Mice. Front. Physiol. 2018, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.S.; Anderson, S.A.; Wang, J.; Yang, F.; DeMaster, K.; Ahmed, R.; Nizzi, C.P.; Eisenstein, R.S.; Tsukamoto, H.; Enns, C.A. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood 2011, 117, 1687–1699. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Babitt, J.L. Hepcidin regulation in the anemia of inflammation. Curr. Opin. Hematol. 2016, 23, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Alfaro-Magallanes, V.M.; Babitt, J.L. Bone morphogenic proteins in iron homeostasis. Bone 2020, 138, 115495. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, S.; Longrois, D.; Montravers, P.; Beaumont, C. Hepcidin and anemia of the critically ill patient: Bench to bedside. Anesthesiology 2011, 114, 688–694. [Google Scholar] [CrossRef] [Green Version]
- Kanamori, Y.; Sugiyama, M.; Hashimoto, O.; Murakami, M.; Matsui, T.; Funaba, M. Regulation of hepcidin expression by inflammation-induced activin B. Sci. Rep. 2016, 6, 38702. [Google Scholar] [CrossRef] [Green Version]
- Besson-Fournier, C.; Gineste, A.; Latour, C.; Gourbeyre, O.; Meynard, D.; Martin, P.; Oswald, E.; Coppin, H.; Roth, M.P. Hepcidin upregulation by inflammation is independent of Smad1/5/8 signaling by activin B. Blood 2017, 129, 533–536. [Google Scholar] [CrossRef]
- Doguer, C.; Ha, J.H.; Collins, J.F. Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver. Compr. Physiol. 2018, 8, 1433–1461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Flores, S.R.; Ha, J.H.; Doguer, C.; Woloshun, R.R.; Xiang, P.; Grosche, A.; Vidyasagar, S.; Collins, J.F. Intestinal DMT1 Is Essential for Optimal Assimilation of Dietary Copper in Male and Female Mice with Iron-Deficiency Anemia. J. Nutr. 2018, 148, 1244–1252. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Absorption Mechanisms of Iron, Copper, and Zinc: An Overview. J. Nutr. Sci. Vitaminol. 2018, 64, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Cotin, G.; Blanco-Andujar, C.; Perton, F.; Asín, L.; de la Fuente, J.M.; Reichardt, W.; Schaffner, D.; Ngyen, D.V.; Mertz, D.; Kiefer, C.; et al. Unveiling the role of surface, size, shape and defects of iron oxide nanoparticles for theranostic applications. Nanoscale 2021, 13, 14552–14571. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials 2021, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramirez, S.; Jericó, C.; Muñoz, M. Perioperative anemia: Prevalence, consequences and pathophysiology. Transfus. Apher. Sci. 2019, 58, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Burton, B.N.; A’Court, A.M.; Brovman, E.Y.; Scott, M.J.; Urman, R.D.; Gabriel, R.A. Optimizing Preoperative Anemia to Improve Patient Outcomes. Anesthesiol. Clin. 2018, 36, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Filipescu, D.; Bănăţeanu, R.; Beuran, M.; Burcoş, T.; Corneci, D.; Cristian, D.; Diculescu, M.; Dobrotă, A.; Droc, G.; Isacoff, D.; et al. Perioperative Patient Blood Management Programme. Multidisciplinary recommendations from the Patient Blood Management Initiative Group. Rom. J. Anaesth. Intensive Care 2017, 24, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.F.; Frazer, D.M.; Faria, N.; Bruggraber, S.F.; Wilkins, S.J.; Mirciov, C.; Powell, J.J.; Anderson, G.J.; Pereira, D.I. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice. FASEB J. 2014, 28, 3671–3678. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Yang, Y.; Luo, Q.; Zhang, Y.; Zhou, C. One-Pot Facile Fabrication of Bioavailable Iron Nanoparticles with Good Biocompatibility for Anemia Therapy. Med. Sci. Monit. 2018, 24, 6449–6455. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine 2014, 10, 1877–1886. [Google Scholar] [CrossRef] [Green Version]
- Latunde-Dada, G.O.; Pereira, D.I.; Tempest, B.; Ilyas, H.; Flynn, A.C.; Aslam, M.F.; Simpson, R.J.; Powell, J.J. A nanoparticulate ferritin-core mimetic is well taken up by HuTu 80 duodenal cells and its absorption in mice is regulated by body iron. J. Nutr. 2014, 144, 1896–1902. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.I.; Mergler, B.I.; Faria, N.; Bruggraber, S.F.; Aslam, M.F.; Poots, L.K.; Prassmayer, L.; Lönnerdal, B.; Brown, A.P.; Powell, J.J. Caco-2 cell acquisition of dietary iron(III) invokes a nanoparticulate endocytic pathway. PLoS ONE 2013, 8, e81250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perfecto, A.; Elgy, C.; Valsami-Jones, E.; Sharp, P.; Hilty, F.; Fairweather-Tait, S. Mechanisms of Iron Uptake from Ferric Phosphate Nanoparticles in Human Intestinal Caco-2 Cells. Nutrients 2017, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.F.; Wu, C.C.; Liao, Y.J.; Jakfar, S.; Tang, Z.B.; Chen, J.K.; Lin, F.H. In Vitro and In Vivo Evaluations of Mesoporous Iron Particles for Iron Bioavailability. Int. J. Mol. Sci. 2019, 20, 5291. [Google Scholar] [CrossRef] [Green Version]
- Jahn, M.R.; Nawroth, T.; Fütterer, S.; Wolfrum, U.; Kolb, U.; Langguth, P. Iron oxide/hydroxide nanoparticles with negatively charged shells show increased uptake in Caco-2 cells. Mol. Pharm. 2012, 9, 1628–1637. [Google Scholar] [CrossRef]
- Shen, Y.; Posavec, L.; Bolisetty, S.; Hilty, F.M.; Nyström, G.; Kohlbrecher, J.; Hilbe, M.; Rossi, A.; Baumgartner, J.; Zimmermann, M.B.; et al. Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron. Nat. Nanotechnol. 2017, 12, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; He, J.; Fan, K.; Yan, X. Ferritin variants: Inspirations for rationally designing protein nanocarriers. Nanoscale 2019, 11, 12449–12459. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Hondow, N.; Pennycook, T.J.; Latunde-Dada, G.O.; Simpson, R.J.; Brown, A.P.; Pereira, D.I. A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomedicine 2014, 10, 1529–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathy, M.M.; Fahmy, H.M.; Balah, A.M.M.; Mohamed, F.F.; Elshemey, W.M. Magnetic nanoparticles-loaded liposomes as a novel treatment agent for iron deficiency anemia: In vivo study. Life Sci. 2019, 234, 116787. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable Nano Vehicles for Macromolecular Delivery of Transferrin and Lactoferrin to Specific Intracellular Compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Garcés, V.; Rodríguez-Nogales, A.; González, A.; Gálvez, N.; Rodríguez-Cabezas, M.E.; García-Martin, M.L.; Gutiérrez, L.; Rondón, D.; Olivares, M.; Gálvez, J.; et al. Bacteria-Carried Iron Oxide Nanoparticles for Treatment of Anemia. Bioconjug. Chem. 2018, 29, 1785–1791. [Google Scholar] [CrossRef] [Green Version]
- Alphandéry, E. Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef]
- Fütterer, S.; Andrusenko, I.; Kolb, U.; Hofmeister, W.; Langguth, P. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD). J. Pharm. Biomed. Anal. 2013, 86, 151–160. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Xu, X.; Lu, L.; Wang, J.; Wang, S.; Wang, Y.; Jin, Z.; Zhang, J.Z.; Jiang, Y. Fe(3)O(4)@ Astragalus Polysaccharide Core-Shell Nanoparticles for Iron Deficiency Anemia Therapy and Magnetic Resonance Imaging in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 10452–10461. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M.; Banjar, Z.M.; Hariri, A.H.; Hassan, A.H. Solid lipid nanoparticles loaded with iron to overcome barriers for treatment of iron deficiency anemia. Drug Des. Devel Ther. 2015, 9, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hou, L.; Jiao, X.; Ji, Y.; Zhu, X.; Zhang, Z. Transferrin-mediated fullerenes nanoparticles as Fe(2+)-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials 2015, 37, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.R.; Shukoor, I.; Tremel, W.; Wolfrum, U.; Kolb, U.; Nawroth, T.; Langguth, P. Hemin-coupled iron(III)-hydroxide nanoparticles show increased uptake in Caco-2 cells. J. Pharm. Pharmacol. 2011, 63, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Chiu, R.Y.; Tsuji, T.; Wang, S.J.; Wang, J.; Liu, C.T.; Kamei, D.T. Improving the systemic drug delivery efficacy of nanoparticles using a transferrin variant for targeting. J. Control. Release 2014, 180, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Dhaliwal, H.K.; Menon, A.V.; Chang, J.; Choi, J.E.; Amiji, M.M.; Kim, J. Site-specific intestinal DMT1 silencing to mitigate iron absorption using pH-sensitive multi-compartmental nanoparticulate oral delivery system. Nanomedicine 2019, 22, 102091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Flores, S.R.L.; Woloshun, R.R.; Yang, C.; Yin, L.; Xiang, P.; Xu, X.; Garrick, M.D.; Vidyasagar, S.; et al. Oral Gavage of Ginger Nanoparticle-Derived Lipid Vectors Carrying Dmt1 siRNA Blunts Iron Loading in Murine Hereditary Hemochromatosis. Mol. Ther. 2019, 27, 493–506. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.J.; Racie, T.; Westerman, M.; Fitzgerald, K.; Butler, J.S.; Fleming, M.D. Combination therapy with a Tmprss6 RNAi-therapeutic and the oral iron chelator deferiprone additively diminishes secondary iron overload in a mouse model of β-thalassemia intermedia. Am. J. Hematol. 2015, 90, 310–313. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.J.; Toudjarska, I.; Sendamarai, A.K.; Racie, T.; Milstein, S.; Bettencourt, B.R.; Hettinger, J.; Bumcrot, D.; Fleming, M.D. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood 2013, 121, 1200–1208. [Google Scholar] [CrossRef]
- Minchella, P.A.; Armitage, A.E.; Darboe, B.; Jallow, M.W.; Drakesmith, H.; Jaye, A.; Prentice, A.M.; McDermid, J.M. Elevated Hepcidin Is Part of a Complex Relation That Links Mortality with Iron Homeostasis and Anemia in Men and Women with HIV Infection. J. Nutr. 2015, 145, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.; Ortmann, E.; Besser, M.; Martin-Cabrera, P.; Richards, T.; Ghosh, M.; Bottrill, F.; Collier, T.; Klein, A.A. A prospective observational cohort study to identify the causes of anaemia and association with outcome in cardiac surgical patients. Heart 2015, 101, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Carver, P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect. 2018, 24, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Kumar, S.S.; Manikandan, M.; Saravanan, M. Photocatalytic properties and antimicrobial efficacy of Fe doped CuO nanoparticles against the pathogenic bacteria and fungi. Microb. Pathog. 2018, 122, 84–89. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Rehman, A.A. Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: As an effective antimicrobial and aflatoxin B1 adsorption agents. PLoS ONE 2020, 15, e0234964. [Google Scholar] [CrossRef]
- Kim, H.E.; Lee, H.J.; Kim, M.S.; Kim, T.; Lee, H.; Kim, H.H.; Cho, M.; Hong, S.W.; Lee, C. Differential Microbicidal Effects of Bimetallic Iron-Copper Nanoparticles on Escherichia coli and MS2 Coliphage. Environ. Sci. Technol 2019, 53, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Antonoglou, O.; Lafazanis, K.; Mourdikoudis, S.; Vourlias, G.; Lialiaris, T.; Pantazaki, A.; Dendrinou-Samara, C. Biological relevance of CuFeO(2) nanoparticles: Antibacterial and anti-inflammatory activity, genotoxicity, DNA and protein interactions. Mater. Sci. Eng C Mater. Biol. Appl. 2019, 99, 264–274. [Google Scholar] [CrossRef]
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave synthesis of nanoparticles and their antifungal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in Wistar rats. Pharmacol. Rep. 2019, 71, 509–516. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, W.; Huang, H.; Tang, Y.; Wang, W.; Meng, F.; Wang, H.; Zheng, Y. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227. [Google Scholar] [CrossRef] [PubMed]
- Abdel Maksoud, M.I.A.; El-Sayyad, G.S.; Ashour, A.H.; El-Batal, A.I.; Abd-Elmonem, M.S.; Hendawy, H.A.M.; Abdel-Khalek, E.K.; Labib, S.; Abdeltwab, E.; El-Okr, M.M. Synthesis and characterization of metals-substituted cobalt ferrite [M(x) Co((1 − x)) Fe(2)O(4); (M = Zn, Cu and Mn; x = 0 and 0.5)] nanoparticles as antimicrobial agents and sensors for Anagrelide determination in biological samples. Mater. Sci. Eng C Mater. Biol. Appl. 2018, 92, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M. Some new nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorg. Chem. 2016, 69, 140–152. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Zhou, L.; Li, J.; Xu, J.; Li, W.; Zhang, L.; Zhong, X.; Wang, T. Effects of Long-Term Exposure to Zinc Oxide Nanoparticles on Development, Zinc Metabolism and Biodistribution of Minerals (Zn, Fe, Cu, Mn) in Mice. PLoS ONE 2016, 11, e0164434. [Google Scholar] [CrossRef]
- Imaeda, T.; Nakada, T.A.; Abe, R.; Oda, S. Decreased total iron binding capacity upon intensive care unit admission predicts red blood cell transfusion in critically ill patients. PLoS ONE 2019, 14, e0210067. [Google Scholar] [CrossRef]
- Shah, A.; Fisher, S.A.; Wong, H.; Roy, N.B.; McKechnie, S.; Doree, C.; Litton, E.; Stanworth, S.J. Safety and efficacy of iron therapy on reducing red blood cell transfusion requirements and treating anaemia in critically ill adults: A systematic review with meta-analysis and trial sequential analysis. J. Crit. Care 2019, 49, 162–171. [Google Scholar] [CrossRef]
- Cenci, L.; Piotto, C.; Bettotti, P.; Maria Bossi, A. Study on molecularly imprinted nanoparticle modified microplates for pseudo-ELISA assays. Talanta 2018, 178, 772–779. [Google Scholar] [CrossRef]
- Cenci, L.; Andreetto, E.; Vestri, A.; Bovi, M.; Barozzi, M.; Iacob, E.; Busato, M.; Castagna, A.; Girelli, D.; Bossi, A.M. Surface plasmon resonance based on molecularly imprinted nanoparticles for the picomolar detection of the iron regulating hormone Hepcidin-25. J. Nanobiotechnol. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasocki, S.; Baron, G.; Driss, F.; Westerman, M.; Puy, H.; Boutron, I.; Beaumont, C.; Montravers, P. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med. 2010, 36, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Na, N.; Liu, T.; Huang, L.; He, D.; Hua, W.; Ouyang, J. Ultrasensitive detection of ferritin in human serum by Western blotting based on quantum dots-labeled avidin-biotin system. Proteomics 2011, 11, 3510–3517. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, Z.; Azizi, M.; Sadeghi Mohammadi, S.; Hashemi, N.; Naderi-Manesh, H. A novel iron quantum cluster confined in hemoglobin as fluorescent sensor for rapid detection of Escherichia coli. Talanta 2020, 218, 121137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, X.; Jiang, H.; Gu, J.; Yin, Y.; Shen, Z.; Xu, C.; Pu, Z.; Li, J.B.; Xu, G. Quantitative proteomic analysis of glycosylated proteins enriched from urine samples with magnetic ConA nanoparticles identifies potential biomarkers for small cell lung cancer. J. Pharm. Biomed. Anal. 2021, 206, 114352. [Google Scholar] [CrossRef]
- Xiao, X.; Li, H.; Pan, Y.; Si, P. Non-enzymatic glucose sensors based on controllable nanoporous gold/copper oxide nanohybrids. Talanta 2014, 125, 366–371. [Google Scholar] [CrossRef]
- Ledda, M.; Fioretti, D.; Lolli, M.G.; Papi, M.; Di Gioia, C.; Carletti, R.; Ciasca, G.; Foglia, S.; Palmieri, V.; Marchese, R.; et al. Biocompatibility assessment of sub-5 nm silica-coated superparamagnetic iron oxide nanoparticles in human stem cells and in mice for potential application in nanomedicine. Nanoscale 2020, 12, 1759–1778. [Google Scholar] [CrossRef]
- Oshtrakh, M.I. Applications of Mössbauer Spectroscopy in Biomedical Research. Cell Biochem. Biophys. 2019, 77, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Charlton, J.R.; Pearl, V.M.; Denotti, A.R.; Lee, J.B.; Swaminathan, S.; Scindia, Y.M.; Charlton, N.P.; Baldelomar, E.J.; Beeman, S.C.; Bennett, K.M. Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomedicine 2016, 12, 1735–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aşık, E.; Aslan, T.N.; Güray, N.T.; Volkan, M. Cellular uptake and apoptotic potential of rhenium labeled magnetic protein cages in MDA-MB-231 cells. Environ. Toxicol. Pharmacol. 2018, 63, 127–134. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, M.; Li, X.; Fan, K.; Xiao, J.; Li, Y.; Shi, H.; Wang, F.; Choi, H.S.; Cheng, D.; et al. Bioengineered Magnetoferritin Nanoprobes for Single-Dose Nuclear-Magnetic Resonance Tumor Imaging. ACS Nano 2016, 10, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Le Guével, X.; Daum, N.; Schneider, M. Synthesis and characterization of human transferrin-stabilized gold nanoclusters. Nanotechnology 2011, 22, 275103. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharm. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Deng, D.; Sun, J. Magnetoferritin: Process, Prospects, and Their Biomedical Applications. Int. J. Mol. Sci. 2019, 20, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piraux, H.; Hai, J.; Verbeke, P.; Serradji, N.; Ammar, S.; Losno, R.; Ha-Duong, N.T.; Hémadi, M.; El Hage Chahine, J.M. Transferrin receptor-1 iron-acquisition pathway—Synthesis, kinetics, thermodynamics and rapid cellular internalization of a holotransferrin-maghemite nanoparticle construct. Biochim. Biophys. Acta 2013, 1830, 4254–4264. [Google Scholar] [CrossRef]
- Li, J.; Xing, X.; Sun, B.; Zhao, Y.; Wu, Z. Metallofullerenol Inhibits Cellular Iron Uptake by Inducing Transferrin Tetramerization. Chem. Asian J. 2017, 12, 2646–2651. [Google Scholar] [CrossRef]
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Sivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Machado, J.F.; Sequeira, D.; Marques, F.; Piedade, M.F.M.; de Brito, M.J.V.; Garcia, M.H.; Fernandes, A.R.; Morais, T.S. New copper (i) complexes selective for prostate cancer cells. Dalton Trans. 2020, 49, 12273–12286. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, W.; Wang, S.; Liu, Y.; Pope, C. Phototoxicity of zinc oxide nanoparticle conjugatesin human ovarian cancer NIH: OVCAR-3 cells. J. Biomed. Nanotechnol. 2008, 4, 432–438. [Google Scholar] [CrossRef]
- Wiesmann, N.; Kluenker, M.; Demuth, P.; Brenner, W.; Tremel, W.; Brieger, J. Zinc overload mediated by zinc oxide nanoparticles as innovative anti-tumor agent. J. Trace Elem. Med. Biol. 2019, 51, 226–234. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C 2019, 100, 129–140. [Google Scholar] [CrossRef] [PubMed]

| Disorder | Implicated Divalent Ion | Levels on Ion |
|---|---|---|
| Hemochromatosis | Iron | |
| Thalassemia | Iron | |
| Inflammatory anemia | Iron | |
| Iron-deficient anemia | Iron | |
| Copper-deficient anemia | Copper | |
| Menkes syndrome | Copper | |
| Wilson disease | Copper | |
| Immunocompromise | Zinc | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabo, R.; Bodolea, C.; Mocan, T. Iron, Copper, and Zinc Homeostasis: Physiology, Physiopathology, and Nanomediated Applications. Nanomaterials 2021, 11, 2958. https://doi.org/10.3390/nano11112958
Szabo R, Bodolea C, Mocan T. Iron, Copper, and Zinc Homeostasis: Physiology, Physiopathology, and Nanomediated Applications. Nanomaterials. 2021; 11(11):2958. https://doi.org/10.3390/nano11112958
Chicago/Turabian StyleSzabo, Robert, Constantin Bodolea, and Teodora Mocan. 2021. "Iron, Copper, and Zinc Homeostasis: Physiology, Physiopathology, and Nanomediated Applications" Nanomaterials 11, no. 11: 2958. https://doi.org/10.3390/nano11112958
APA StyleSzabo, R., Bodolea, C., & Mocan, T. (2021). Iron, Copper, and Zinc Homeostasis: Physiology, Physiopathology, and Nanomediated Applications. Nanomaterials, 11(11), 2958. https://doi.org/10.3390/nano11112958







