Exploiting the Potential of Supported Magnetic Nanomaterials as Fenton-Like Catalysts for Environmental Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Nanostructured Catalysts
2.2.1. Preparation of Sterically Stabilized Magnetite
2.2.2. Preparation of Poly(Ethyleneimine)-Coated Magnetite and Poly(Acrylic Acid)-Coated Magnetite Nanoparticles
2.2.3. Preparation of Silica-Coated Magnetite Nanoparticles
2.2.4. Preparation of the Fe3O4@PAA/SBA15 Nanocomposite
2.3. Characterization Methods
2.4. Selection of Nanoparticles and Nanomaterials as Catalysts in Fenton-Type Reactions for Dye Oxidation
2.5. Experimental Design for Estrogen Degradation
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Preliminary Screening of Nanocatalysts for RB19 and MG Removal
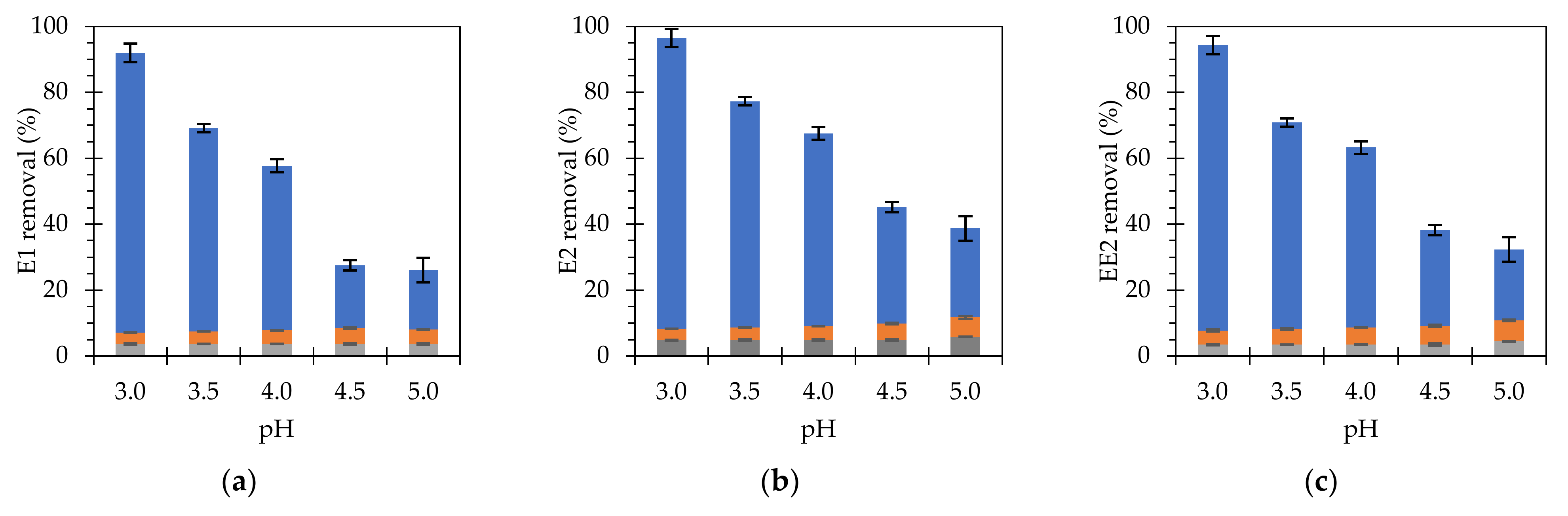
3.3. Heterogeneous Fenton removal for Estrogens Using Fe3O4@PAA/SBA15
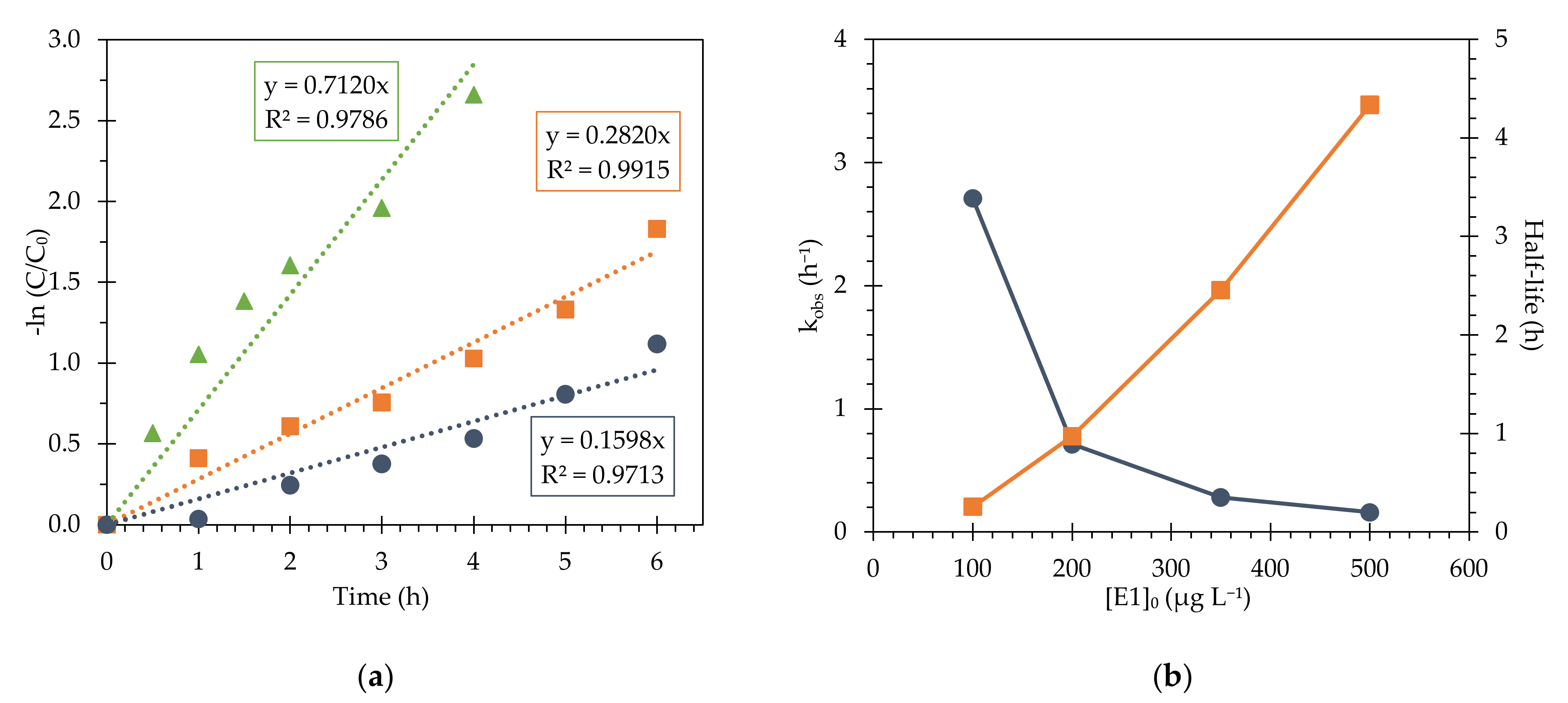
3.4. Determination of Kinetic Parameters for Estrogen Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Souza Celente, G.; Colares, G.S.; da Silva Araújo, P.; Machado, Ê.L.; Lobo, E.A. Acute ecotoxicity and genotoxicity assessment of two wastewater treatment units. Environ. Sci. Pollut. Res. 2020, 27, 10520–10527. [Google Scholar] [CrossRef]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Crisp, T.M.; Clegg, E.D.; Cooper, R.L.; Wood, W.P.R.; Anderson, D.G.; Baetcke, K.P.R.; Hoffmann, J.L.; Morrow, M.S.; Rodier, D.J.; Schaeffer, J.E.; et al. Environmental endocrine disruption: An effects assessment and analysis. Environ. Health Perspect. 1998, 106, 11–56. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Ryu, J.; Oh, J.; Choi, B.-G.; Snyder, S.A. Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han River (Seoul, South Korea). Sci. Total Environ. 2010, 408, 636–643. [Google Scholar] [CrossRef]
- Giannakis, S.; Gamarra Vives, F.A.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone Sci. Eng. 2019, 41, 3–16. [Google Scholar] [CrossRef]
- Teixeira, S.; Gurke, R.; Eckert, H.; Kühn, K.; Fauler, J.; Cuniberti, G. Photocatalytic degradation of pharmaceuticals present in conventional treated wastewater by nanoparticle suspensions. J. Environ. Chem. Eng. 2016, 4, 287–292. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Velichkova, F.; Julcour-Lebigue, C.; Koumanova, B.; Delmas, H. Heterogeneous Fenton oxidation of paracetamol using iron oxide (nano) particles. J. Environ. Chem. Eng. 2013, 1, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Abu-Dief, A.M.; Nassar, I.F.; Elsayed, W.H. Magnetic NiFe2O4 nanoparticles: Efficient, heterogeneous and reusable catalyst for synthesis of acetylferrocene chalcones and their anti-tumour activity. Appl. Organomet. Chem. 2016, 30, 917–923. [Google Scholar] [CrossRef]
- El-Remaily, M.A.; Abu-Dief, A.M. CuFe2O4 nanoparticles: An efficient heterogeneous magnetically separable catalyst for synthesis of some novel propynyl-1H-imidazoles derivatives. Tetrahedron 2015, 71, 2579–2584. [Google Scholar] [CrossRef]
- Mehdaoui, R.; El Ghali, A.; Cheikhrouhou, W.; Beyou, E.; Baouab, M.H.V. Fe3O4 nanoparticles coated by new functionalized tetraaza-2,3 dialdehyde micro-crystalline cellulose: Synthesis, characterization, and catalytic application for degradation of Acid Yellow 17. Iran. Polym. J. (Engl. Ed.) 2017, 26, 597–613. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.P.; Mansur, H.S. Advanced Functional Nanostructures based on Magnetic Iron Oxide Nanomaterials for Water Remediation: A Review. Water Res. 2021, 190, 116693. [Google Scholar] [CrossRef] [PubMed]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; Diniz da Costa, J.C. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 2015, 4, 4594. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Xiang, J.; Lin, J.; Xiang, L.; Chen, S.; Yan, B.; Fan, H.; Zhang, S.; Shi, X. Sustainable Advanced Fenton-like Catalysts Based on Mussel-Inspired Magnetic Cellulose Nanocomposites to Effectively Remove Organic Dyes and Antibiotics. ACS Appl. Mater. Interfaces 2020, 12, 51952–51959. [Google Scholar] [CrossRef]
- Lin, J.; Chen, S.; Xiao, H.; Zhang, J.; Lan, J.; Yan, B.; Zeng, H. Ultra-efficient and stable heterogeneous iron-based Fenton nanocatalysts for degrading organic dyes at neutral pH via a chelating effect under nanoconfinement. Chem. Commun. 2020, 56, 6571–6574. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Fernández, L.; Vargas-Osorio, Z.; Vázquez-Vázquez, C.; Piñeiro, Y.; Rivas, J.; Feijoo, G.; Moreira, M.T. Reusable Fe3O4/SBA15 Nanocomposite as an Efficient Photo-Fenton Catalyst for the Removal of Sulfamethoxazole and Orange II. Nanomaterials 2021, 11, 533. [Google Scholar] [CrossRef]
- Atashin, H.; Malakooti, R. Magnetic iron oxide nanoparticles embedded in SBA-15 silica wall as a green and recoverable catalyst for the oxidation of alcohols and sulfides. J. Saudi Chem. Soc. 2017, 21, S17–S24. [Google Scholar] [CrossRef] [Green Version]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Davila-Ibanez, A.B.; Salgueirino, V.; Martinez-Zorzano, V.; Mariño-Fernández, R.; García-Lorenzo, A.; Maceira-Campos, M.; Muñoz-Ubeda, M.; Junquera, E.; Aicart, E.; Rivas, J.; et al. Magnetic Silica Nanoparticle Cellular Uptake and Cytotoxicity Regulated by Electrostatic Polyelectrolytes–DNA Loading at Their Surface. ACS Nano 2012, 6, 747–759. [Google Scholar] [CrossRef]
- Colilla, M.; Balas, F.; Manzano, M.; Vallet-Regí, M. Novel method to enlarge the surface area of SBA-15. Chem. Mater. 2007, 19, 3099–3101. [Google Scholar] [CrossRef]
- Actor, Q.A. Advances in Nanotechnology Research and Application; ScholaryEditions: Atlanta, GA, USA, 2012; ISBN 9781464990472. [Google Scholar]
- Osorio, Z.V.; Pineiro, Y.; Vazquez, C.; Abreu, C.R.; Perez, M.A.A.; Quintela, M.A.L.; Rivas, J. Magnetic nanocomposites based on mesoporous silica for biomedical applications. Int. J. Nanotechnol. 2016, 13, 648. [Google Scholar] [CrossRef]
- Couto, D.; Freitas, M.; Vilas-Boas, V.; Dias, I.; Porto, G.; Lopez-Quintela, M.A.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E. Interaction of polyacrylic acid coated and non-coated iron oxide nanoparticles with human neutrophils. Toxicol. Lett. 2014, 225, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar]
- Moldes-Diz, Y.; Gamallo, M.; Eibes, G.; Vargas-Osorio, Z.; Vazquez-Vazquez, C.; Feijoo, G.; Lema, J.M.; Moreira, M.T. Development of a Superparamagnetic Laccase Nanobiocatalyst for the Enzymatic Biotransformation of Xenobiotics. J. Environ. Eng. 2018, 144, 04018007. [Google Scholar] [CrossRef]
- Fernández, L.; González-Rodríguez, J.; Gamallo, M.; Vargas-Osorio, Z.; Vázquez-Vázquez, C.; Piñeiro, Y.; Rivas, J.; Feijoo, G.; Moreira, M.T. Iron oxide-mediated photo-Fenton catalysis in the inactivation of enteric bacteria present in wastewater effluents at neutral pH. Environ. Pollut. 2020, 266, 115181. [Google Scholar] [CrossRef]
- Wang, J.; Shao, X.; Zhang, Q.; Ma, J.; Ge, H. Preparation and photocatalytic application of magnetic Fe2O3/SBA-15 nanomaterials. J. Mol. Liq. 2018, 260, 304–312. [Google Scholar] [CrossRef]
- Nahar, Y.; Rahman, M.A.; Hossain, M.K.; Sharafat, M.K.; Karim, M.R.; Elaissari, A.; Ochiai, B.; Ahmad, H.; Rahman, M.M. A facile one-pot synthesis of poly(acrylic acid)-functionalized magnetic iron oxide nanoparticles for suppressing reactive oxygen species generation and adsorption of biocatalyst. Mater. Res. Express 2020, 7, 016102. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, X.; Lv, G.; Zhai, Y.; Yang, Z.; Zhu, Y.; Li, H.; Wang, F. Influence of zeolite carriers on the dyes degradation for framework Fe-doped zeolite catalysts. J. Sol-Gel Sci. Technol. 2019, 91, 54–62. [Google Scholar] [CrossRef]
- Aliyan, H.; Fazaeli, R.; Jalilian, R. Fe3O4@mesoporous SBA-15: A magnetically recoverable catalyst for photodegradation of malachite green. Appl. Surf. Sci. 2013, 276, 147–153. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, J.; Gao, L.; Zhang, Y.; Gu, N.; Feng, J.; Yang, D.; Zhu, J.; Yan, X. Decomposing phenol by the hidden talent of ferromagnetic nanoparticles. Chemosphere 2008, 73, 1524–1528. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zeng, D.; Zhang, B.; Hassan, M.; Li, P.; Qi, C.; He, Y. Enhanced catalytic activation of photo-Fenton process by Cu0·5Mn0·5Fe2O4 for effective removal of organic contaminants. Chemosphere 2020, 247, 125780. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, Y.; Kim, S. Investigation of the reaction pathway of OH radicals produced by Fenton oxidation in the conditions of wastewater treatment. Water Sci. Technol. 2001, 44, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, J.; Bai, Y.; Li, T.; Xue, G. Enhanced degradation of Acid Red 73 by using cellulose-based hydrogel coated Fe3O4 nanocomposite as a Fenton-like catalyst. Int. J. Biol. Macromol. 2020, 152, 242–249. [Google Scholar] [CrossRef]
- Pukdee-Asa, M.; Su, C.C.; Ratanatamskul, C.; Lu, M.C. Degradation of azo dye by the fluidised-bed Fenton process. Color. Technol. 2012, 128, 28–35. [Google Scholar] [CrossRef]
- De Laat, J.; Gallard, H. Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: Mechanism and kinetic modeling. Environ. Sci. Technol. 1999, 33, 2726–2732. [Google Scholar] [CrossRef]
- Ding, X.; Gutierrez, L.; Croue, J.P.; Li, M.; Wang, L.; Wang, Y. Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison. Chemosphere 2020, 253, 126655. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hu, Y.; Xiao, J.; Huang, Y.; Wang, M.; Yang, H.; Zou, J.; Yuan, B.; Ma, J. Enhanced diclofenac elimination in Fe(II)/peracetic acid process by promoting Fe(III)/Fe(II) cycle with ABTS as electron shuttle. Chem. Eng. J. 2021, 420, 129692. [Google Scholar] [CrossRef]
- Guo, B.; Xu, T.; Zhang, L.; Li, S. A heterogeneous fenton-like system with green iron nanoparticles for the removal of bisphenol A: Performance, kinetics and transformation mechanism. J. Environ. Manag. 2020, 272, 111047. [Google Scholar] [CrossRef]
- Xin, S.; Ma, B.; Liu, G.; Ma, X.; Zhang, C.; Ma, X.; Gao, M.; Xin, Y. Enhanced heterogeneous photo-Fenton-like degradation of tetracycline over CuFeO2/biochar catalyst through accelerating electron transfer under visible light. J. Environ. Manag. 2021, 285, 112093. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, V.; Bingham, J.-P.; Kan, E. Heterogeneous Fenton degradation of bisphenol A by carbon nanotube-supported Fe3O4. Sep. Purif. Technol. 2014, 133, 388–395. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Y.; Gao, Z.; Liu, B.; Sun, C. Transformation and reduction of androgenic activity of 17α-methyltestosterone in Fe3O4/MWCNTs–H2O2 system. Appl. Catal. B Environ. 2012, 127, 167–174. [Google Scholar] [CrossRef]
- Oliveira, H.G.; Ferreira, L.H.; Bertazzoli, R.; Longo, C. Remediation of 17-α-ethinylestradiol aqueous solution by photocatalysis and electrochemically-assisted photocatalysis using TiO2 and TiO2/WO3 electrodes irradiated by a solar simulator. Water Res. 2015, 72, 305–314. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H.; Chen, L.; Wang, X. Molecularly imprinted TiO2 hybridized magnetic Fe3O4 nanoparticles for selective photocatalytic degradation and removal of estrone. RSC Adv. 2014, 4, 45266–45274. [Google Scholar] [CrossRef]
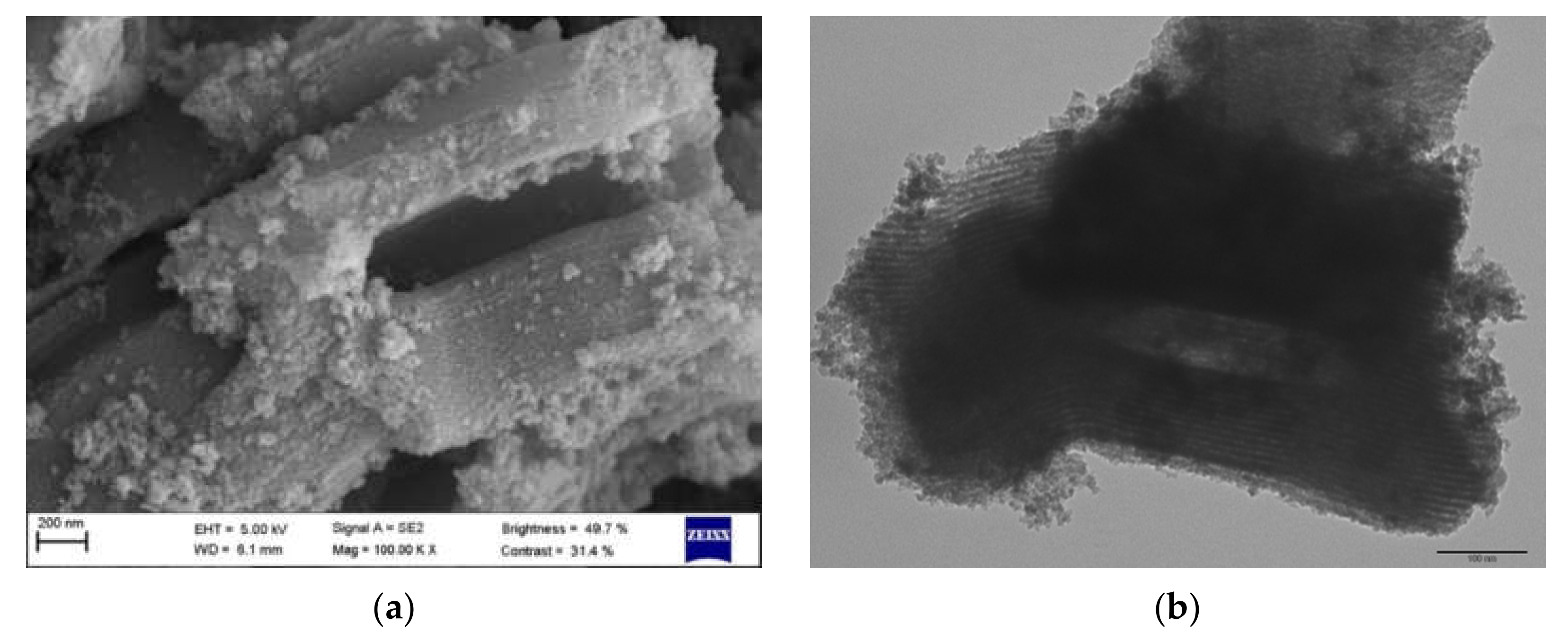
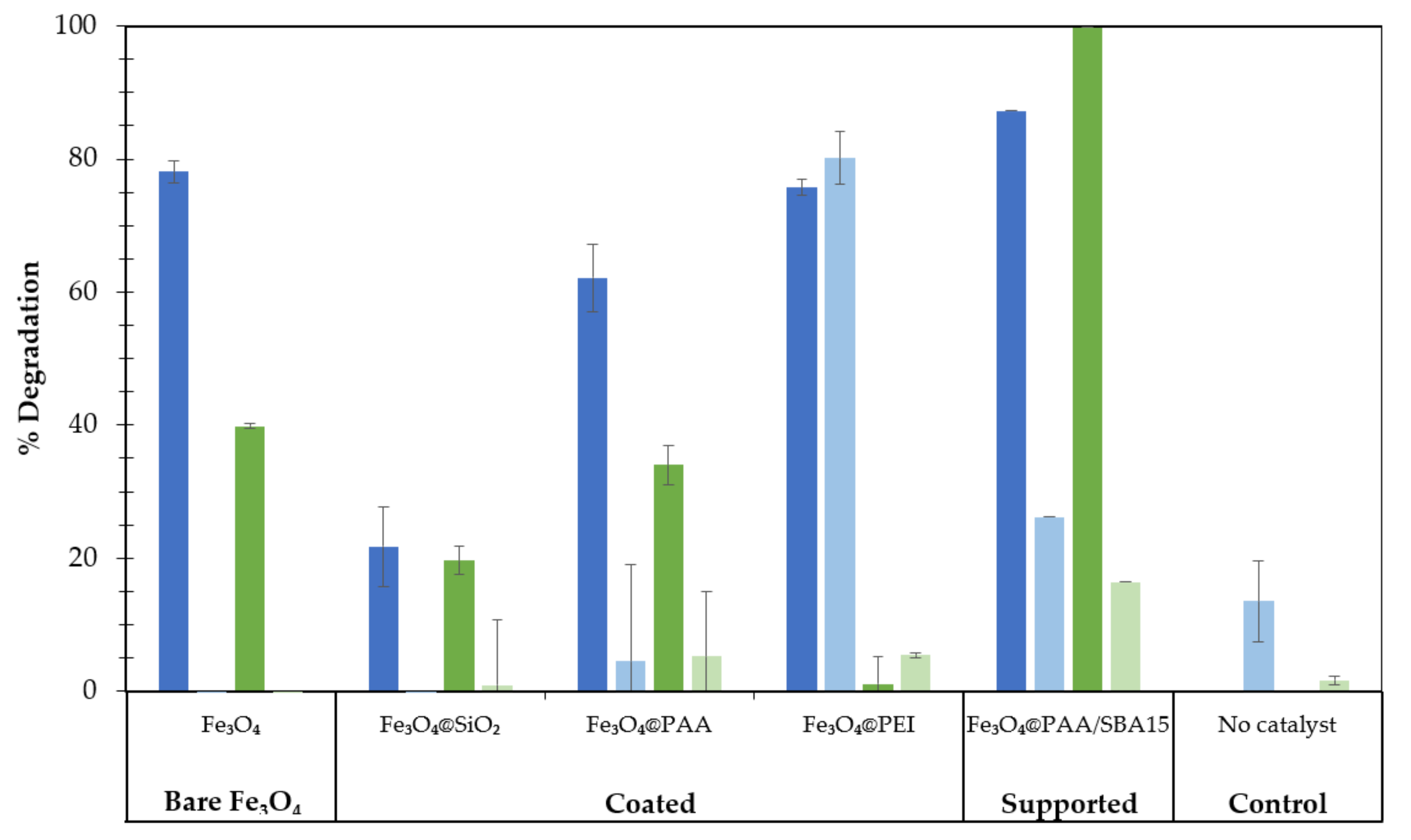
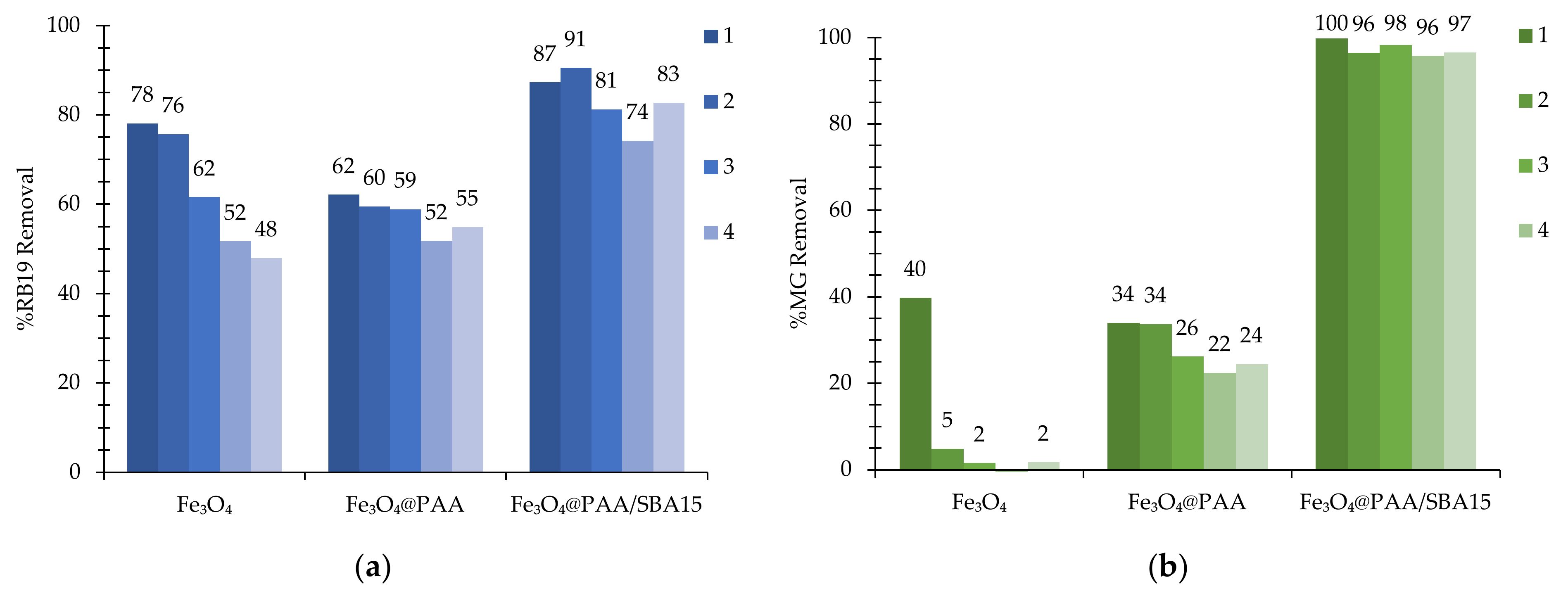
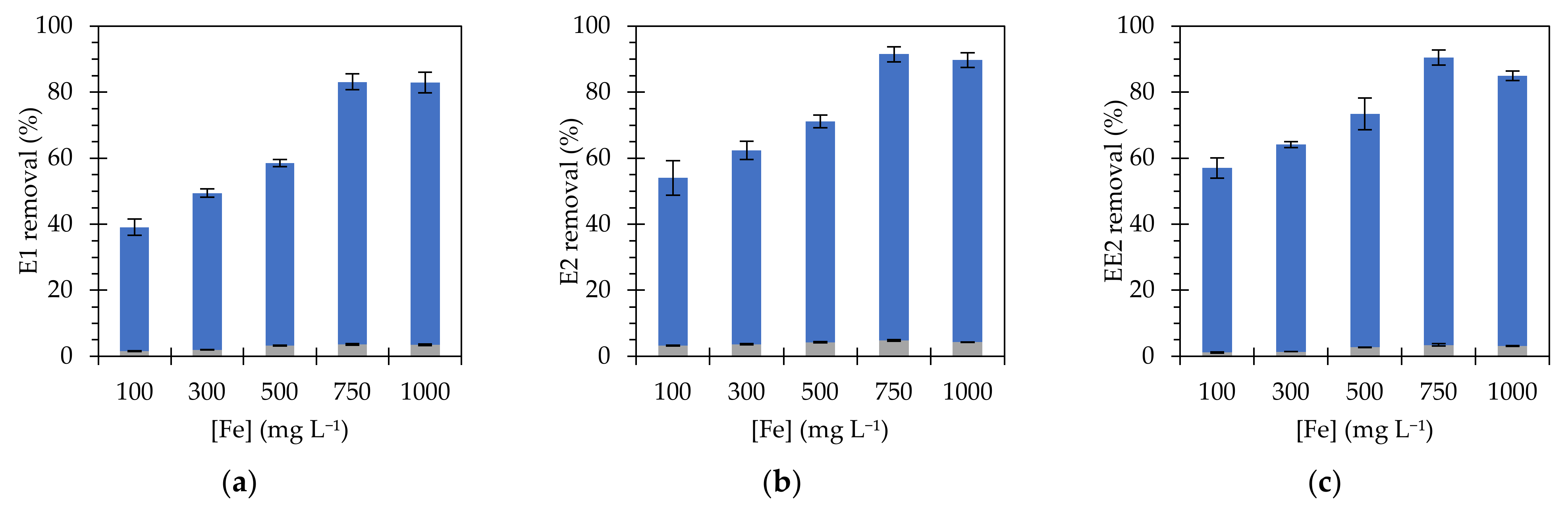

| Catalyst | Size (nm) | SBET 1 (m2 g−1) | PSDFT 2 (nm) | ZP 3 (mV) |
|---|---|---|---|---|
| Fe3O4 | 8.2 ± 3.8 | - | - | −11.54 |
| Fe3O4@PEI | 10.9 ± 3.0 | - | - | 29.94 |
| Fe3O4@PAA | 7.6 ± 2.7 | - | - | −4.53 |
| Fe3O4@SiO2 | 20.2 ± 4.2 | - | - | −14.28 |
| Fe3O4@PAA/SBA15 | >1000 | 276.9 | 7.59 | −1.47 |
| Ref. | Pollutant | MNP | Concentration (µg L−1) | kobs (h−1) | t1/2 (h) | R2 |
|---|---|---|---|---|---|---|
| This work | E1 | Fe3O4@PAA/SBA15 | 500 | 0.160 ± 0.011 | 4.34 ± 0.30 | 0.9713 |
| 350 | 0.282 ± 0.011 | 2.46 ± 0.09 | 0.9915 | |||
| 200 | 0.712 ± 0.043 | 0.97 ± 0.06 | 0.9791 | |||
| 100 | 2.708 ± 0.241 | 0.26 ± 0.02 | 0.9769 | |||
| This work | E2 | Fe3O4@PAA/SBA15 | 500 | 0.228 ± 0.016 | 3.03 ± 0.21 | 0.9729 |
| 350 | 0.366 ± 0.012 | 1.89 ± 0.06 | 0.9931 | |||
| 200 | 0.898 ± 0.060 | 0.77 ± 0.05 | 0.9741 | |||
| 100 | 2.613 ± 0.037 | 0.27 ± 0.00 | 0.9717 | |||
| This work | EE2 | Fe3O4@PAA/SBA15 | 500 | 0.214 ± 0.011 | 3.24 ± 0.17 | 0.9840 |
| 350 | 0.361 ± 0.015 | 1.92 ± 0.08 | 0.9895 | |||
| 200 | 0.909 ± 0.054 | 0.76 ± 0.04 | 0.9796 | |||
| 100 | 3.211 ± 0.271 | 0.22 ± 0.02 | 0.9791 | |||
| [45] | BPA | GS-Fe | 25 | 1.338 | - | 0.9994 |
| 50 | 1.050 | - | 0.9972 | |||
| 75 | 0.468 | - | 0.9985 | |||
| [46] | TC | CuFeO2/biochar | 20 | 0.272 | - | - |
| [47] | BPA | Fe3O4@MWCNT | 70 | 0.330 | - | - |
| [48] | MT | Fe3O4@MWCNT | 212 | 0.396 | - | 0.9420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, J.; Gamallo, M.; Conde, J.J.; Vargas-Osorio, Z.; Vázquez-Vázquez, C.; Piñeiro, Y.; Rivas, J.; Feijoo, G.; Moreira, M.T. Exploiting the Potential of Supported Magnetic Nanomaterials as Fenton-Like Catalysts for Environmental Applications. Nanomaterials 2021, 11, 2902. https://doi.org/10.3390/nano11112902
González-Rodríguez J, Gamallo M, Conde JJ, Vargas-Osorio Z, Vázquez-Vázquez C, Piñeiro Y, Rivas J, Feijoo G, Moreira MT. Exploiting the Potential of Supported Magnetic Nanomaterials as Fenton-Like Catalysts for Environmental Applications. Nanomaterials. 2021; 11(11):2902. https://doi.org/10.3390/nano11112902
Chicago/Turabian StyleGonzález-Rodríguez, Jorge, María Gamallo, Julio J. Conde, Zulema Vargas-Osorio, Carlos Vázquez-Vázquez, Yolanda Piñeiro, José Rivas, Gumersindo Feijoo, and Maria Teresa Moreira. 2021. "Exploiting the Potential of Supported Magnetic Nanomaterials as Fenton-Like Catalysts for Environmental Applications" Nanomaterials 11, no. 11: 2902. https://doi.org/10.3390/nano11112902








