Extended Graphite Supported Flower-like MnO2 as Bifunctional Materials for Supercapacitors and Glucose Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of MnO2/Extended Graphite Nanocomposite
2.3. Apparatus
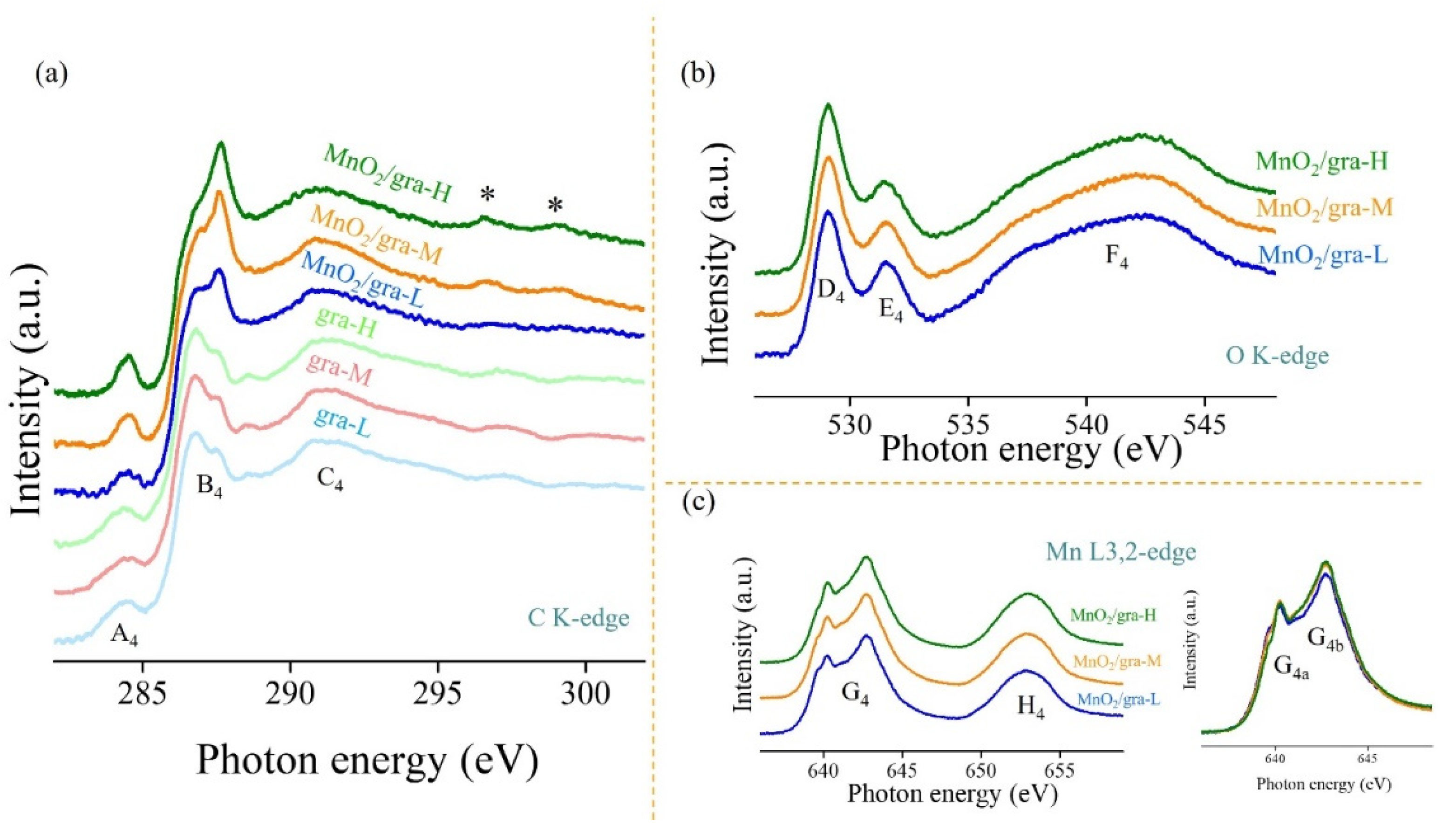
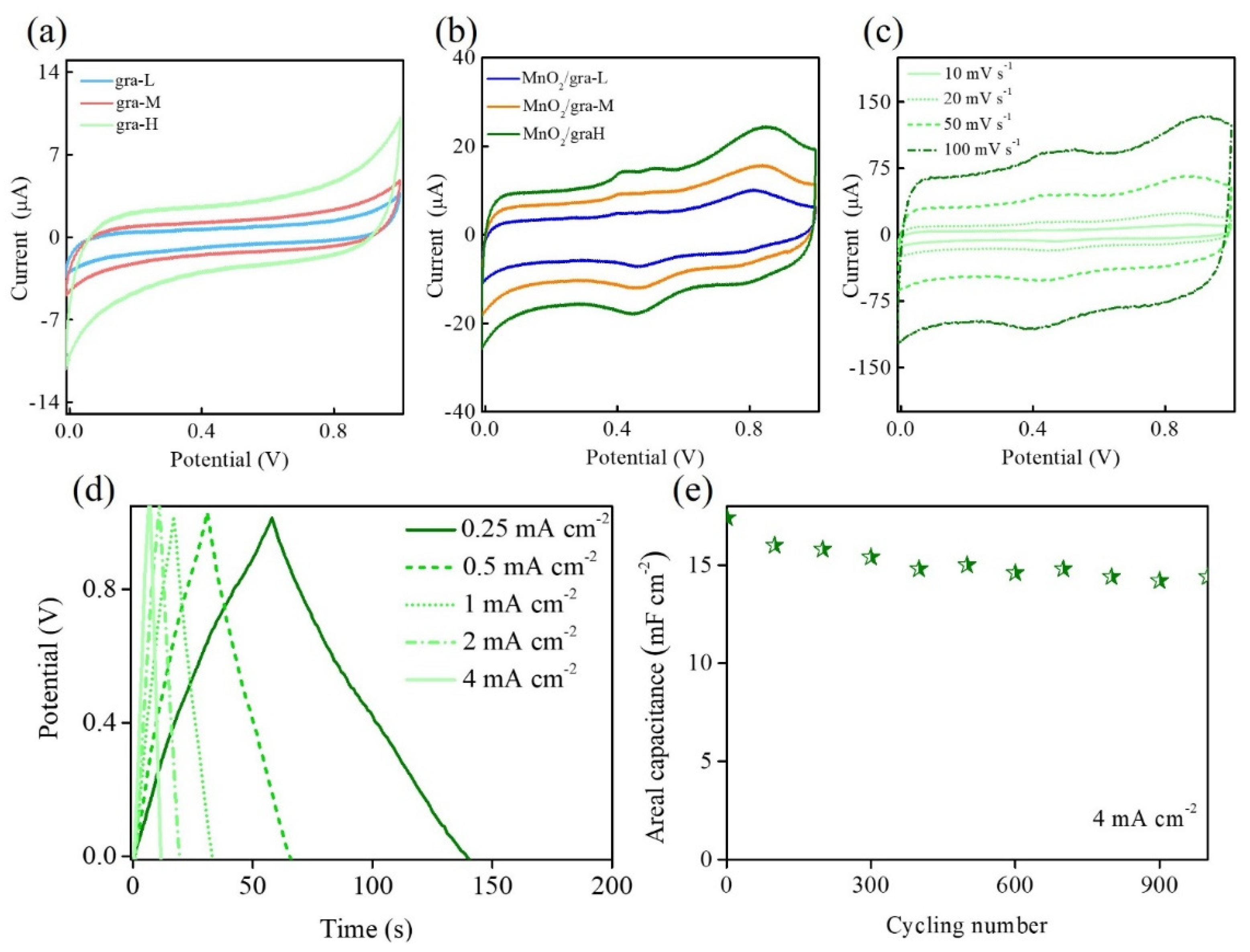
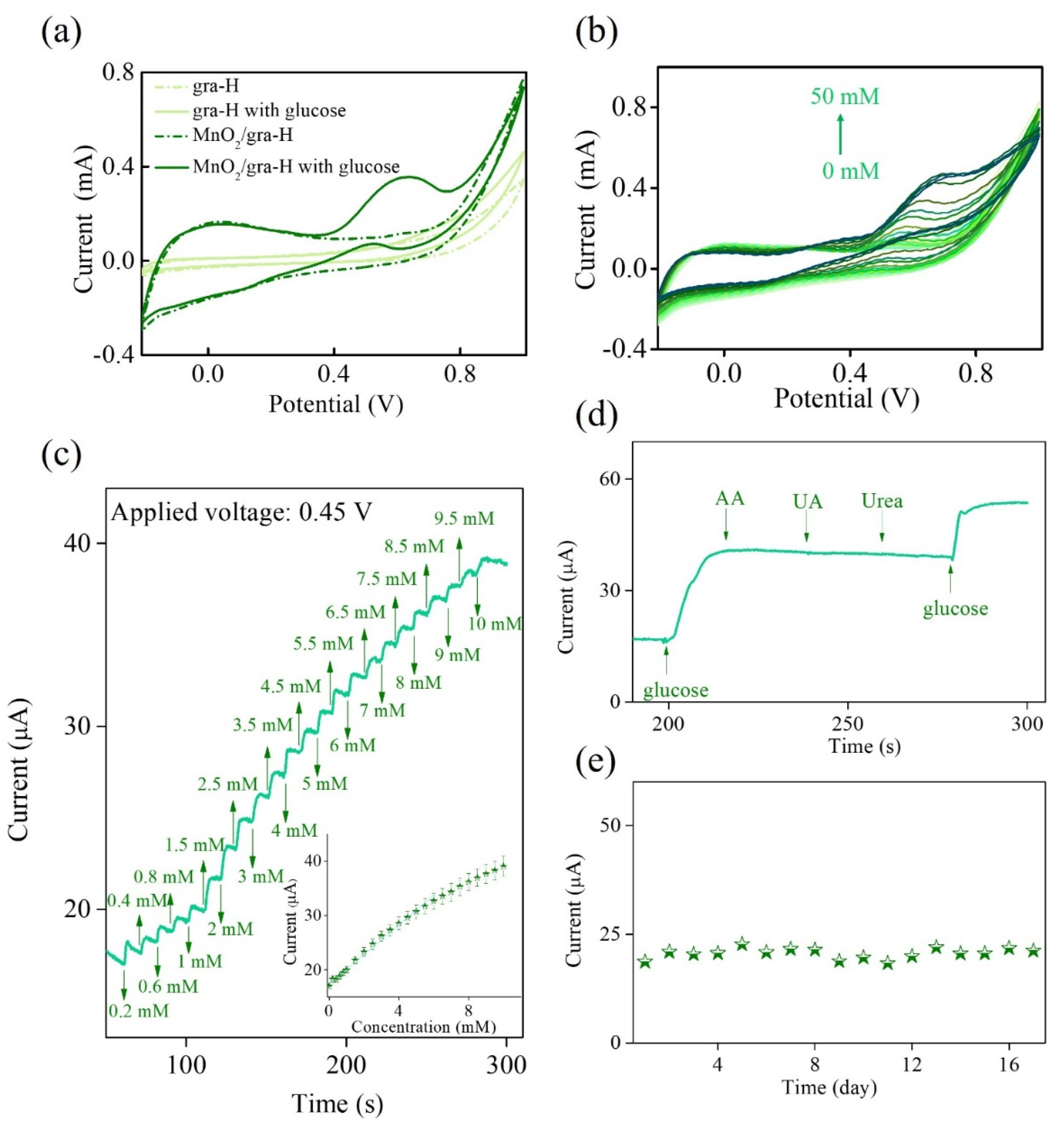
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Moha, W. Taiwan Health and Welfare Report; Ministry of Health and Welfare: Taipei, Taiwan, 2018. [Google Scholar]
- Yang, X.; Qiao, Z.; Liu, F.; Yang, S.; Zhang, L.; Cao, B. In-depth study of electrochemical capacitor performance of MnO2 during phase transition from Ramsdellite-MnO2 to Birnessite-MnO2. Electrochimica Acta 2018, 280, 77–85. [Google Scholar] [CrossRef]
- Ponnusamy, R.; Venkatesan, R.; Kandasamy, M.; Chakraborty, B.; Rout, C.S. MnO2 polymorph selection for non-enzymatic glucose detection: An integrated experimental and density functional theory investigation. Appl. Surf. Sci. 2019, 487, 1033–1042. [Google Scholar] [CrossRef]
- Jeon, H.; Jeong, J.; Hong, S.B.; Yang, M.; Park, J.; Kim, D.H.; Hwang, S.Y.; Choi, B.G. Facile and fast microwave-assisted fabrication of activated and porous carbon cloth composites with graphene and MnO2 for flexible asymmetric supercapacitors. Electrochim. Acta 2018, 280, 9–16. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, L.; Zeng, Y.; Cao, X.; Huang, H.; Liu, J.; Chen, X.; Wei, S.; Gan, L.; Yang, P. Three-dimensional hierarchical porous carbon derived from resorcinol formaldehyde-zinc tatrate/poly (styrene-maleic anhydride) for high performance su-percapacitor electrode. J. Alloys Compd. 2021, 886, 161176. [Google Scholar] [CrossRef]
- Liu, M.; Shi, M.; Lu, W.; Zhu, D.; Li, L.; Gan, L. Core–shell reduced graphene oxide/MnOx@ carbon hollow nanospheres for high performance supercapacitor electrodes. Chem. Eng. J. 2017, 313, 518–526. [Google Scholar] [CrossRef]
- Xiong, C.; Lin, X.; Liu, H.; Li, M.; Li, B.; Jiao, S.; Zhao, W.; Duan, C.; Dai, L.; Ni, Y. Fabrication of 3D Expanded Graphite-Based (MnO2 Nanowalls and PANI Nanofibers) Hybrid as Bifunctional Material for High-Performance Supercapacitor and Sensor. J. Electrochem. Soc. 2019, 166, A3965–A3971. [Google Scholar] [CrossRef]
- Xiong, C.; Li, M.; Zhao, W.; Duan, C.; Ni, Y. Flexible N-doped reduced graphene oxide/carbon nanotube-MnO2 film as a multifunctional material for high-performance supercapacitors, catalysts and sensors. J. Mater. 2020, 6, 523–531. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Robust Nanocomposite of Nitrogen-Doped Reduced Graphene Oxide and MnO2 Nanorods for High-Performance Supercapacitors and Nonenzymatic Peroxide Sensors. ACS Sustain. Chem. Eng. 2018, 6, 10489–10504. [Google Scholar] [CrossRef]
- Kabir, H.; Gyan, I.O.; Cheng, I.F. Electrochemical modification of a pyrolytic graphite sheet for improved negative electrode performance in the vanadium redox flow battery. J. Power Sources 2017, 342, 31–37. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, J.-Y.; Kabtamu, D.M.; Lin, G.-Y.; Hsu, N.-Y.; Chou, Y.-S.; Wei, H.-J.; Wang, C.-H. High efficiency of CO2-activated graphite felt as electrode for vanadium redox flow battery application. J. Power Sources 2017, 364, 1–8. [Google Scholar] [CrossRef]
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S.; Panda, S. Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals. Adv. Mater. 2018, 30, 1702149. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bai, L.; Wen, X.; Guan, J. Ultrafine Mn3O4 nanoparticles supported on functionalized-graphite towards efficient pho-tochemical and electrochemical water oxidation catalysis. Int. J. Hydrog. Energy 2018, 43, 15807–15814. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, L.; Yu, Z.; Jiang, R.; Huang, J.; Hou, Y.; Yang, F.; Zhang, B.; Zhang, R.; Zhang, Y. Adjustable anchoring of Ni/Co cations by oxygen-containing functional groups on functionalized graphite paper and accelerated mass/electron transfer for overall water splitting. Catal. Sci. Technol. 2020, 10, 2627–2643. [Google Scholar] [CrossRef]
- Yue, L.; Jia, D.; Tang, J.; Zhang, A.; Liu, F.; Chen, T.; Barrow, C.; Yang, W.; Liu, J. Improving the rate capability of ultrathin NiCo-LDH nanoflakes and FeOOH nanosheets on surface electrochemically modified graphite fibers for flexible asymmetric supercapacitors. J. Colloid Interface Sci. 2020, 560, 237–246. [Google Scholar] [CrossRef]
- Chang, H.-W.; Lu, Y.-R.; Chen, J.-L.; Chen, C.-L.; Lee, J.-F.; Chen, J.-M.; Tsai, Y.-C.; Chang, C.-M.; Yeh, P.-H.; Chou, W.-C.; et al. Nanoflaky MnO2/functionalized carbon nanotubes for supercapacitors: An in situ X-ray absorption spectroscopic investigation. Nanoscale 2015, 7, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, X. The effect of oxygen–containing functional groups on the H2 adsorption of graphene–based nanomaterials: Experiment and theory. Int. J. Hydrogen Energy 2018, 43, 5668–5679. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Sastikumar, D.; Manivannan, S. Effect of functional groups on dielectric, optical gas sensing properties of graphene oxide and reduced graphene oxide at room temperature. RSC Adv. 2015, 5, 10816–10825. [Google Scholar] [CrossRef]
- Gong, L.; Yin, B.; Li, L.-P.; Yang, M.-B. Nylon-6/Graphene composites modified through polymeric modification of graphene. Compos. Part B Eng. 2015, 73, 49–56. [Google Scholar] [CrossRef]
- Jung, J.Y.; Hong, Y.L.; Kim, J.-G.; Kim, M.J.; Kim, Y.-K.; Kim, N.D. New insight of tailor-made graphene oxide for the formation of atomic Co-N sites toward hydrogen evolution reaction. Appl. Surf. Sci. 2021, 563, 150254. [Google Scholar] [CrossRef]
- Radovic, L.R.; Mora-Vilches, C.V.; Salgado-Casanova, A.J.; Buljan, A. Graphene functionalization: Mechanism of carboxyl group formation. Carbon 2018, 130, 340–349. [Google Scholar] [CrossRef]
- Ahmad, H.; Fan, M.; Hui, D. Graphene oxide incorporated functional materials: A review. Compos. Part B: Eng. 2018, 145, 270–280. [Google Scholar] [CrossRef]
- Yang, D.; Yu, Q.; Gao, L.; Mao, L.; Yang, J.-H. The additive effect of graphene in nickel phosphate/graphene composite and enhanced activity for electrochemical oxidation of methanol. Appl. Surf. Sci. 2017, 416, 503–510. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Li, Q.; Shou, Q.; Cheng, J.; Zhang, L.; Nelson, B.J.; Zhang, X. Transition metal oxide and graphene nano-composites for high-performance electrochemical capacitors. Phys. Chem. Chem. Phys. 2012, 14, 16331–16337. [Google Scholar] [CrossRef] [PubMed]
- Christy, M.; Jang, H.; Nahm, K.S. Cobaltite oxide nanosheets anchored graphene nanocomposite as an efficient oxygen re-duction reaction (ORR) catalyst for the application of lithium-air batteries. J. Power Sources 2015, 288, 451–460. [Google Scholar]
- Makgopa, K.; Ejikeme, P.M.; Jafta, C.J.; Raju, K.; Zeiger, M.; Presser, V.; Ozoemena, K.I. A high-rate aqueous symmetric pseudocapacitor based on highly graphitized onion-like carbon/birnessite-type manganese oxide nanohybrids. J. Mater. Chem. A 2015, 3, 3480–3490. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, M.Q.; Yu, Y.; Liu, H.; Lu, S.; Bao, S.-J.; Xu, M. Assembling Hollow Cobalt Sulfide Nanocages Array on Graphene-like Manganese Dioxide Nanosheets for Superior Electrochemical Capacitors. ACS Appl. Mater. Interfaces 2017, 9, 35040–35047. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ma, Y.-L.; Li, C.-E. The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite. Sci. Total. Environ. 2019, 651, 580–590. [Google Scholar] [CrossRef]
- Song, L.; Li, C.; Chen, W.; Liu, B.; Zhao, Y. Highly efficient MnO2/reduced graphene oxide hydrogel motors for organic pol-lutants removal. J. Mater. Sci. 2020, 55, 1984–1995. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; Sun, L.; Wu, H.; Wang, J.; Zhao, Y.; Yan, L.; Luo, Y.; Jiang, K.; Li, Q.; et al. MnO2 nanoparticles anchored on carbon nanotubes with hybrid supercapacitor-battery behavior for ultrafast lithium storage. Carbon 2018, 139, 145–155. [Google Scholar] [CrossRef]
- Lee, S.-W.; Bak, S.-M.; Lee, C.-W.; Jaye, C.; Fischer, D.A.; Kim, B.-K.; Yang, X.-Q.; Nam, K.-W.; Kim, K.-B. Structural Changes in Reduced Graphene Oxide upon MnO2 Deposition by the Redox Reaction between Carbon and Permanganate Ions. J. Phys. Chem. C 2014, 118, 2834–2843. [Google Scholar] [CrossRef]
- Wu, L.; Klie, R.; Zhu, Y.; Jooss, C. Experimental confirmation of Zener-polaron-type charge and orbital ordering in Pr1− xCax MnO3. Phys. Rev. B 2007, 76, 174210. [Google Scholar] [CrossRef]
- Céspedes, E.; Laguna-Marco, M.; Jiménez-Villacorta, F.; Chaboy, J.; Boada, R.; Guglieri, C.; Andrés, A.d.; Prieto, C. On the origin of the magnetism of Mn–Zn–O systems: Structural, electronic, and magnetic study of exotic MnO2− δ/ZnO thin films. J. Phys. Chem. C 2011, 115, 24092–24101. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-W.; Huang, Y.-C.; Chen, J.-L.; Chen, C.-L.; Chen, J.-M.; Wei, D.-H.; Chou, W.-C.; Dong, C.-L.; Tsai, Y.-C. Soft X-ray absorption spectroscopic investigation of MnO2/graphene nanocomposites used in supercapacitor. Catal. Today 2021, 45, 17120–17127. [Google Scholar] [CrossRef]
- Durai, G.; Kuppusami, P.; Maiyalagan, T.; Ahila, M.; Kumar, P.V. Supercapacitive properties of manganese nitride thin film electrodes prepared by reactive magnetron sputtering: Effect of different electrolytes. Ceram. Int. 2019, 45, 17120–17127. [Google Scholar] [CrossRef]
- Achour, A.; Guerra, A.; Moulaï, F.; Islam, M.; Hadjersi, T.; Ahmad, I.; Parvez, S.; Boukherroub, R.; Pireaux, J.-J. MnOx thin film based electrodes: Role of surface point defects and structure towards extreme enhancement in specific capacitance. Mater. Chem. Phys. 2019, 242, 122487. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wu, J.; Shu, X.; Yu, C.; Cui, J.; Qin, Y.; Zhang, Y.; Ajayan, P.M.; Wu, Y. Remarkable supercapacitive performance of TiO2 nanotube arrays by introduction of oxygen vacancies. Chem. Eng. J. 2017, 313, 1071–1081. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Yang, C.; Hu, W. Sol–gel synthesis of nanoporous NiCo2O4 thin films on ITO glass as high-performance supercapacitor electrodes. Ceram. Int. 2016, 42, 11411–11416. [Google Scholar] [CrossRef]
- Prasad, K.; Rajasekhara Reddy, G.; Rajesh, M.; Babu, P.R.; Shanmugam, G.; Sushma, N.J.; Pratap Reddy, M.S.; Deva Prasad Raju, B.; Mallikarjuna, K. Electrochemical performance of 2D-hierarchical sheet-Like ZnCo2O4 microstructures for superca-pacitor applications. Crystals 2020, 10, 566. [Google Scholar] [CrossRef]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Mirzaei, H.; Nasiri, A.A.; Mohamadee, R.; Yaghoobi, H.; Khatami, M.; Azizi, O.; Zaimy, M.A.; Azizi, H. Direct growth of ternary copper nickel cobalt oxide nanowires as binder-free electrode on carbon cloth for nonenzymatic glucose sensing. Microchem. J. 2018, 142, 343–351. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Xiong, Y.; Liu, X.; Nsabimana, A.; Bo, X.; Guo, L. Bimetallic MCo (M=Cu, Fe, Ni, and Mn) nanoparticles doped-carbon nanofibers synthetized by electrospinning for nonenzymatic glucose detection. Sens. Actuators B Chem. 2015, 207, 614–622. [Google Scholar] [CrossRef]
- Xiao, F.; Li, Y.; Gao, H.; Ge, S.; Duan, H. Growth of coral-like PtAu–MnO2 binary nanocomposites on free-standing graphene paper for flexible nonenzymatic glucose sensors. Biosens. Bioelectron. 2013, 41, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Huang, L.-H.; Chen, S.-M. Electrochemical synthesis of mixed-valence manganese/copper hybrid composite using graphene oxide and multi-walled carbon nanotubes for nonenzymatic glucose sensor. J. Electroanal. Chem. 2014, 735, 36–42. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Miao, Z.; Ma, M.; Du, X.; Lin, J.; Han, B.; Takahashi, S.; Anzai, J.-I.; Chen, Q. Dual-function amperometric sensors based on poly(diallydimethylammoniun chloride)-functionalized reduced graphene oxide/manganese dioxide/gold nanoparticles nanocomposite. Sens. Actuators B Chem. 2016, 222, 663–673. [Google Scholar] [CrossRef]



| Samples | Fitted Results of C 1s XPS Spectra | |||
|---|---|---|---|---|
| C-C sp2 (%) | C-C sp3 (%) | C-OH (%) | O-C-O/O-C=O (%) | |
| gra-L | 28.3 | 37.0 | 32.5 | 2.2 |
| gra-M | 12.6 | 42.4 | 41.4 | 3.6 |
| gra-H | 10.1 | 43.9 | 42.3 | 3.7 |
| MnO2/gra-L | 53.5 | 23.5 | 19.3 | 3.7 |
| MnO2/gra-M | 42.9 | 35.3 | 17.0 | 4.8 |
| MnO2/gra-H | 22.5 | 52.3 | 18.8 | 6.4 |
| Samples | Fitted Results of O 1s XPS Spectra | |||
|---|---|---|---|---|
| Mn-O-Mn (%) | Mn-OH (%) | H-O-H (%) | C=O (%) | |
| MnO2/gra-L | 51.1 | 26.0 | 13.5 | 9.4 |
| MnO2/gra-M | 59.5 | 22.9 | 9.5 | 8.1 |
| MnO2/gra-H | 61.5 | 22.2 | 8.5 | 7.8 |
| Samples | Fitted Results of Mn 2p XPS Spectra | ||||||
|---|---|---|---|---|---|---|---|
| Mn3+ 2p3/2 (%) | Mn3+ 2p1/2 (%) | Mn4+ 2p3/2 (%) | Mn4+ 2p1/2 (%) | Sat. 2p3/2 (%) | Sat. 2p1/2 (%) | Mn3+/Mn4+ Ratio | |
| MnO2/gra-L | 47.1 | 20.2 | 17.2 | 10.3 | 3.1 | 2.1 | 2.4 |
| MnO2/gra-M | 41.9 | 18.2 | 20.3 | 14.2 | 3.2 | 2.0 | 1.7 |
| MnO2/gra-H | 39.4 | 16.2 | 22.7 | 16.4 | 3.1 | 2.2 | 1.4 |
| Electrode Materials | Electrolyte | Current Density | Areal Capacitance (mF cm−2) | Reference |
|---|---|---|---|---|
| Mn3N2 | Na2SO4 | 1.00 mA cm−2 | 74.0 | [36] |
| MnOx | Na2SO4 | 0.25 mA cm−2 | 19.3 | [37] |
| TiO2 | Na2SO4 | 2.00 mV s−1 | 23.2 | [38] |
| NiCo2O4 | KOH | 0.25 mA cm−2 | 28.0 | [39] |
| ZnCo2O4 | KOH | 0.01 mA cm−2 | 16.1 | [40] |
| MnO2/gra-H | Na2SO4 | 0.25 mA cm−2 | 20.4 | This work |
| Electrode Materials | Applied Potential (V vs. Ag/AgCl) | Linear Range (mM) | Sensitivity (μA mM−1cm−2) | Detection Limit (mM) | Reference |
|---|---|---|---|---|---|
| MnCo–carbon nanofibers/Nafion | 0.60 | 0.5–7.0 | 36 | 0.050 | [43] |
| PtAu–MnO2 | 0.00 | 0.1–30.0 | 59 | 0.020 | [44] |
| MnCu/MWCNT/GO | −0.05 | 1.0–32.0 | 59 | 0.001 | [45] |
| PDDA-RGO/MnO2/AuNPs | 0.60 | 0.02–0.85 | 84 | 0.002 | [46] |
| MnO2/gra-H | 0.45 | Up to 5.0 | 43 | 0.080 | This work |
| Added (mM) | Found by Electrochemical Sensing (mM) | Recovery (%) | RSD (%) | Found by Mass Spectrometry (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 1.02 | 102 | 5.2 | 0.94 | 94 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-W.; Dong, C.-L.; Chen, Y.-H.; Xu, Y.-Z.; Huang, T.-C.; Chen, S.-C.; Liu, F.-J.; Lai, Y.-H.; Tsai, Y.-C. Extended Graphite Supported Flower-like MnO2 as Bifunctional Materials for Supercapacitors and Glucose Sensing. Nanomaterials 2021, 11, 2881. https://doi.org/10.3390/nano11112881
Chang H-W, Dong C-L, Chen Y-H, Xu Y-Z, Huang T-C, Chen S-C, Liu F-J, Lai Y-H, Tsai Y-C. Extended Graphite Supported Flower-like MnO2 as Bifunctional Materials for Supercapacitors and Glucose Sensing. Nanomaterials. 2021; 11(11):2881. https://doi.org/10.3390/nano11112881
Chicago/Turabian StyleChang, Han-Wei, Chung-Li Dong, Yan-Hua Chen, Yuan-Zhang Xu, Tzu-Chi Huang, Song-Chi Chen, Feng-Jiin Liu, Yin-Hung Lai, and Yu-Chen Tsai. 2021. "Extended Graphite Supported Flower-like MnO2 as Bifunctional Materials for Supercapacitors and Glucose Sensing" Nanomaterials 11, no. 11: 2881. https://doi.org/10.3390/nano11112881
APA StyleChang, H.-W., Dong, C.-L., Chen, Y.-H., Xu, Y.-Z., Huang, T.-C., Chen, S.-C., Liu, F.-J., Lai, Y.-H., & Tsai, Y.-C. (2021). Extended Graphite Supported Flower-like MnO2 as Bifunctional Materials for Supercapacitors and Glucose Sensing. Nanomaterials, 11(11), 2881. https://doi.org/10.3390/nano11112881