Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Treatment Protocols
- Supporting materials: γ-Al2O3 in the form of cylindrical pellets were obtained from Engelhard (Engelhard de Meern B.V., The Netherlands) and ground into powder (ca. < 250 mesh). ACZ (80 wt% γ-Al2O3 – 20 wt% Ce0.5Zr0.5O2−δ) and CZ (Ce0.5Zr0.5O2−δ) supports were prepared by co-precipitation of the corresponding metal nitrate precursors, namely Al(NO3)3·9H2O, Zr(NO3)2·H2O and Ce(NO3)3·6H2O (99.5%; Alfa Aesar, Haverhill, MA, USA), followed by calcination for 1 h at 800 °C; details of the method can be found elsewhere [77].
- Supported Ir catalysts: The wet impregnation method was followed to prepare the low iridium loading (0.4–1.0 wt%, Table 1) catalysts supported on γ-Al2O3, ACZ and CZ as follows: powders of γ-Al2O3, ACZ, or CZ supports were impregnated under continuous stirring at 75 °C in an aqueous solution of IrCl3·H2O (Abcr GmbH & Co KG, Karlsruhe, Germany) of appropriate concentration to lead to a nominal Ir loading of 1 wt%. After water evaporation, these suspensions were dried at 110 °C for 12 h in air, then heated at 400 °C for 2 h under 50% H2/He flow (50 mL/min) followed by heating at 850 °C for 1 h under 1% H2/He flow (50 mL/min) in order to reduce the metal and yield the corresponding catalysts, hereafter referred to as “fresh”. It is worth noting that application of a reduction step directly after catalyst suspension drying was beneficial to the formation of small metallic Ir nanoparticles on the support surfaces [50,74,75], in contrast to methods which, instead of a reduction step at this stage of the preparation, use an oxidation one that results to the formation of larger Ir particles [78].
2.2. Materials Characterization
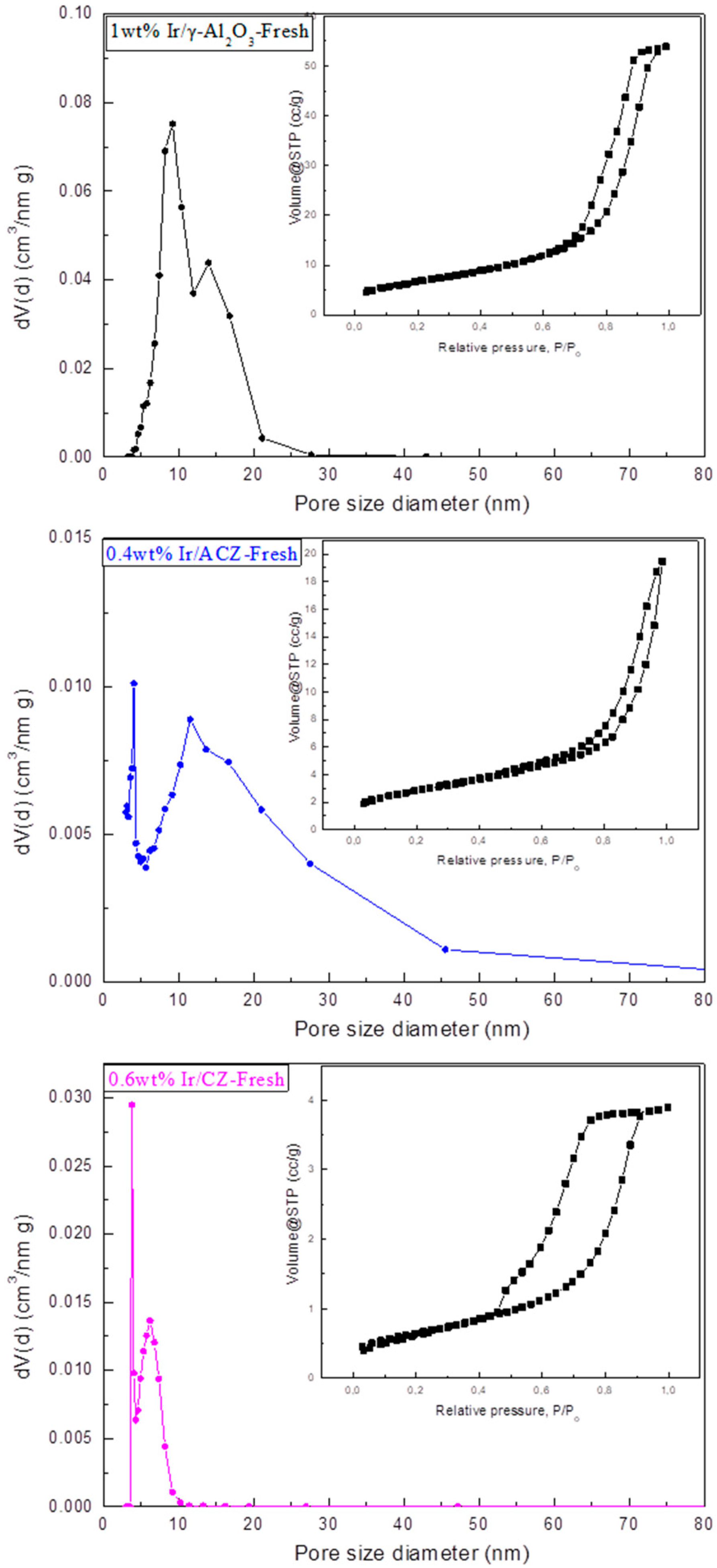
- Textural characteristics: A Quantachrome Nova 2200e instrument (Boynton Beach, FL, USA) was used to determine the BET surface areas of the samples through N2 adsorption–desorption isotherms at relative pressures in the range of 0.05–0.30 and a temperature of −196 °C. The samples were previously vacuum degassed at 350 °C for 12 h. The total pore volumes and pore size distributions were calculated by means of the BJH method from the N2 volume sorbed at the highest relative pressure.
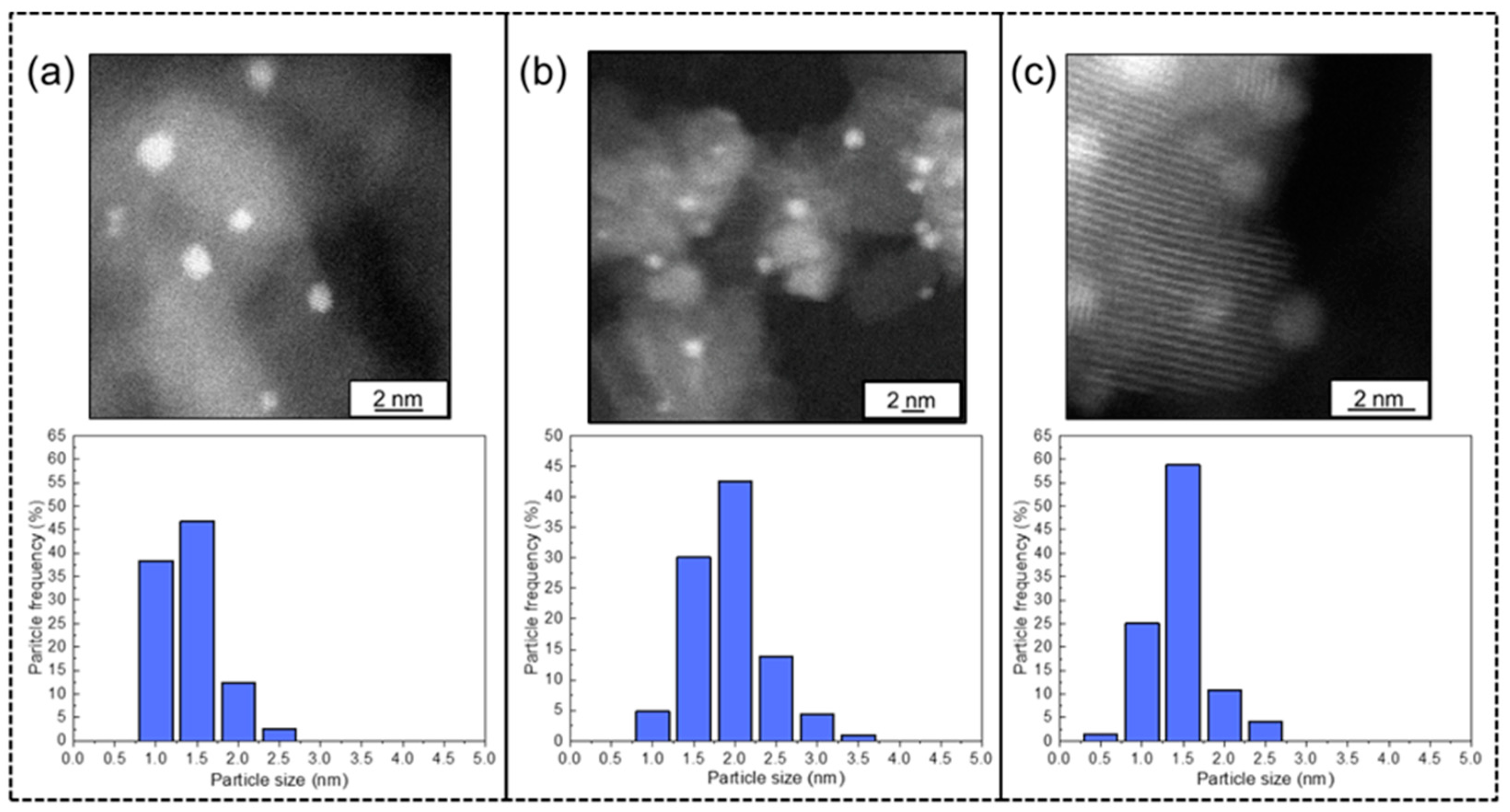
- Morphological characteristics: (i) Metal contents were determined by ICP-OES using a Thermo Scientific iCAP 7400 duo instrument (Waltham, MA, USA). The samples were microwave digested in 5 mL HNO3 (70%, Fisher, Waltham, MA, USA) and 100 mg NH4F (≥98.0%, Sigma Aldrich, Darmstadt, Germany) at 190 °C (CEM–MARS microwave reactor, Matthews, NC, USA ) and finally diluted in 10% aqueous HNO3; (ii) Aberration-corrected High resolution TEM images were obtained on a JEOL 2100F (Tokyo, Japan) operated at 200 kV; (iii) A Bruker D8 Advance diffractometer (Karlsruhe, Germany) using monochromated Cu Kα1 radiation (λ = 0.1542 nm) was used for Powder X-ray diffraction; (iv) The number of Ir surface sites and associated crystallite sizes were estimated via isothermal H2 chemisorption measurements, with a Quantachrome/ChemBet Pulsar TPR/TPD (Boynton Beach, Florida) chemisorption analyzer equipped with an Omnistar/Pfeiffer Vacuum (Aßlar, Germany) mass spectrometer as a detector. For this purpose, 100–300 mg of catalysts was loaded on the quartz U tube holder of the instrument and pretreated with a 1% H2/He mixture (15 mL/min) at 400 °C for 1 h, followed by flushing with N2 (15 mL/min) at 400 °C for 0.5 h, and cooling to room temperature under N2 flow. Then, pulses of pure hydrogen (280 μL) were injected until saturation and the total hydrogen uptake per gram of catalyst (chemisorbed H2, ) was measured. These values were used to calculate the hydrogen to metal ratio, H/Ir, (i.e., the dispersion, DIr) and the mean Ir crystallite size () using the following equations:where, is the H2-uptake in the chemisorption experiments (mL/g), Fs is the hydrogen to metal correlation factor (= 2 assuming one-to-one correlation of adsorbed H atoms with metal sites, i.e., H-Ir), MIr is the molecular weight of iridium (192.22 g/mol), Vmol is the molar volume of an ideal gas at room temperature and 1 atm pressure (24,450 mL/mol), XIr is the iridium content of the catalyst (gIr/gcat), is the Ir metal density (22.5 g/mL), is the area occupied by a surface Ir atom (0.12 nm2/atom), NAV = 6.023 × 1023 molecules/mol is the Avogadro number, and 1020 is a unit conversion factor when the units of parameters in Equation (2) are used as indicated herein.
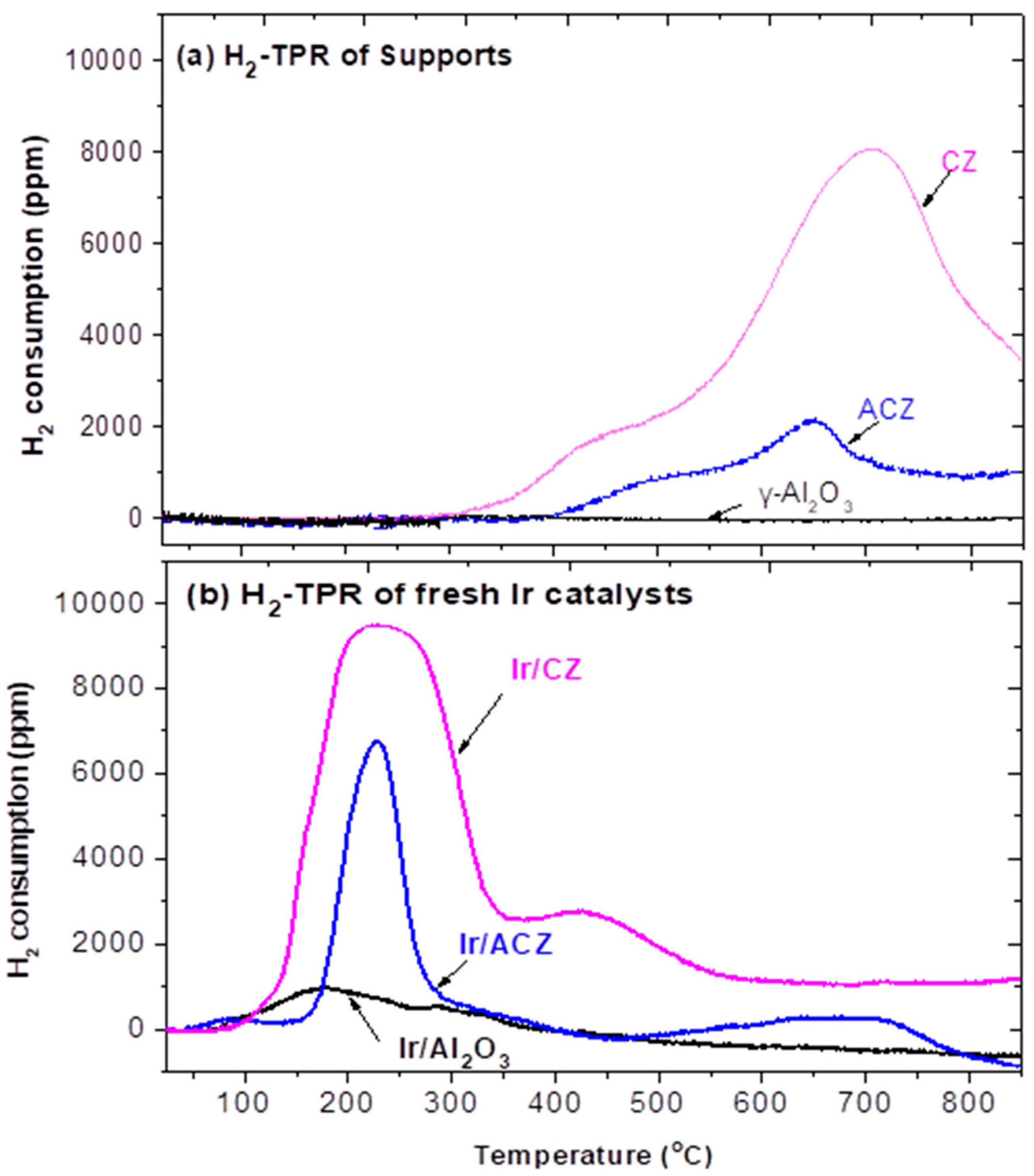
- Reducibility characteristics: H2-TPR measurements were conducted to obtain the reducibility profiles and determine the total oxygen storage capacity (OSC) of both the supports and the corresponding catalysts, using the same TPR/TPD apparatus employed for H2 chemisorption measurements. Typically, an amount of ca. 150 mg of the material (support or catalyst) was loaded into the quartz U-tube holder of the instrument. Prior to data acquisition, samples were oxidized in situ at 750 °C for 30 min using a flow of 20% O2/He mixture followed by cooling to 25 °C in the same flow. The tube was then purged with He for 10 min. The TPR measurements were then conducted with a 15 mL/min flow of 1% H2/He and a heating ramp of 10 °C/min up to ~850 °C.
2.3. Catalytic Activity Measurements
2.4. Carbon Deposition Measurements via TPO Experiments
3. Results and Discussion
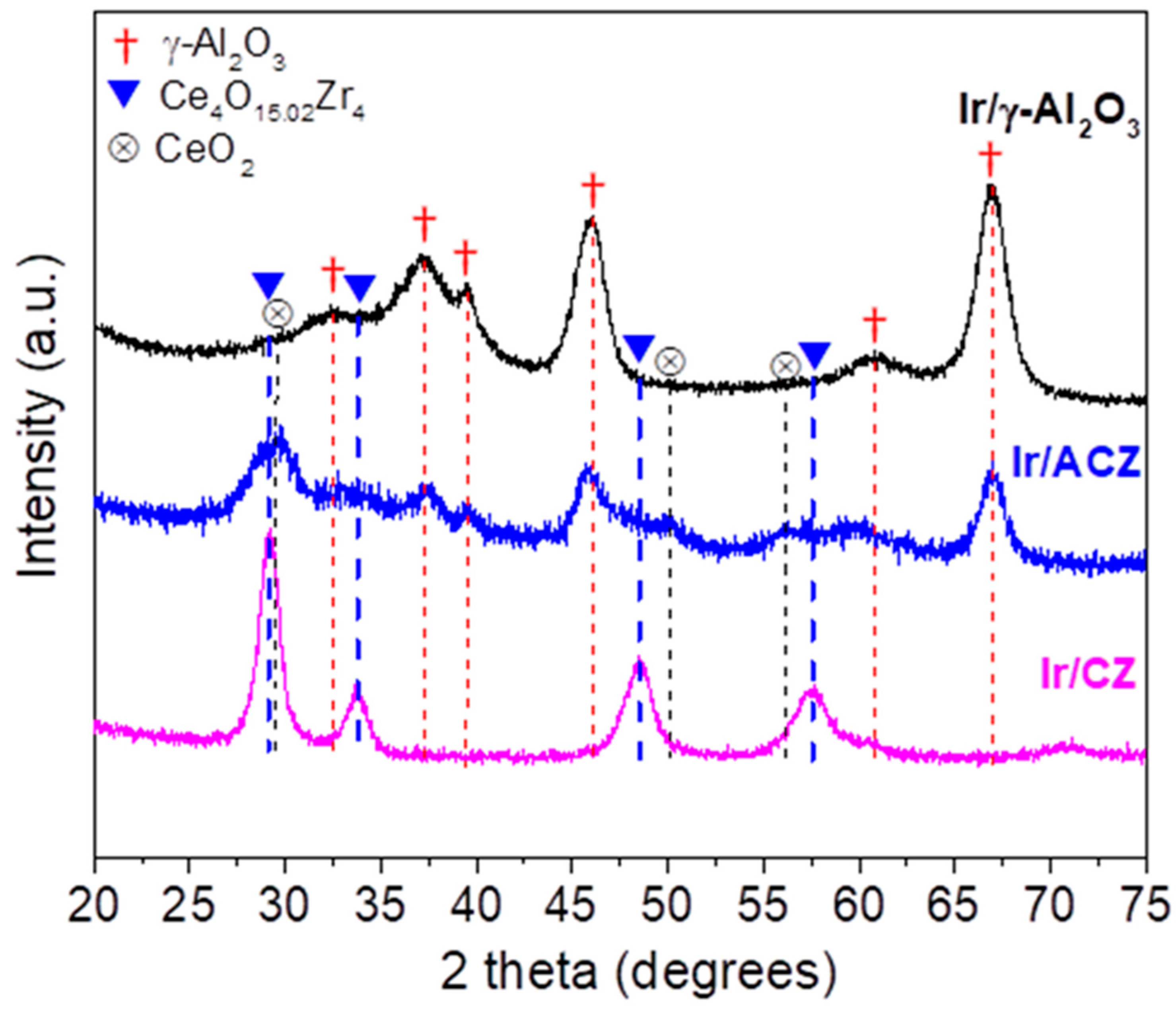
3.1. Materials Characterization Results
3.2. Evaluation of Catalytic Performance and Stability
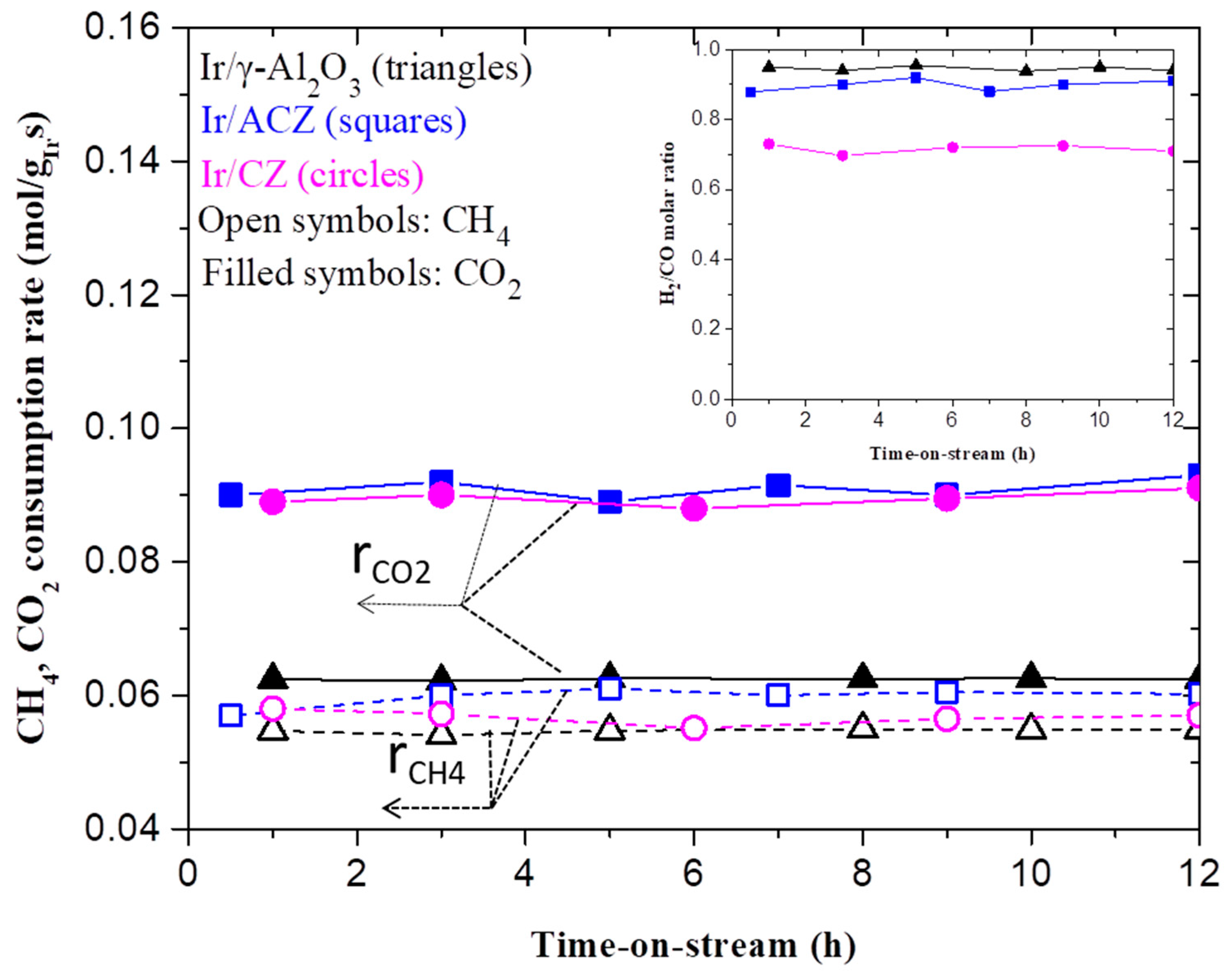
3.2.1. Time-on-Stream Stability and Catalytic Performance during DRM
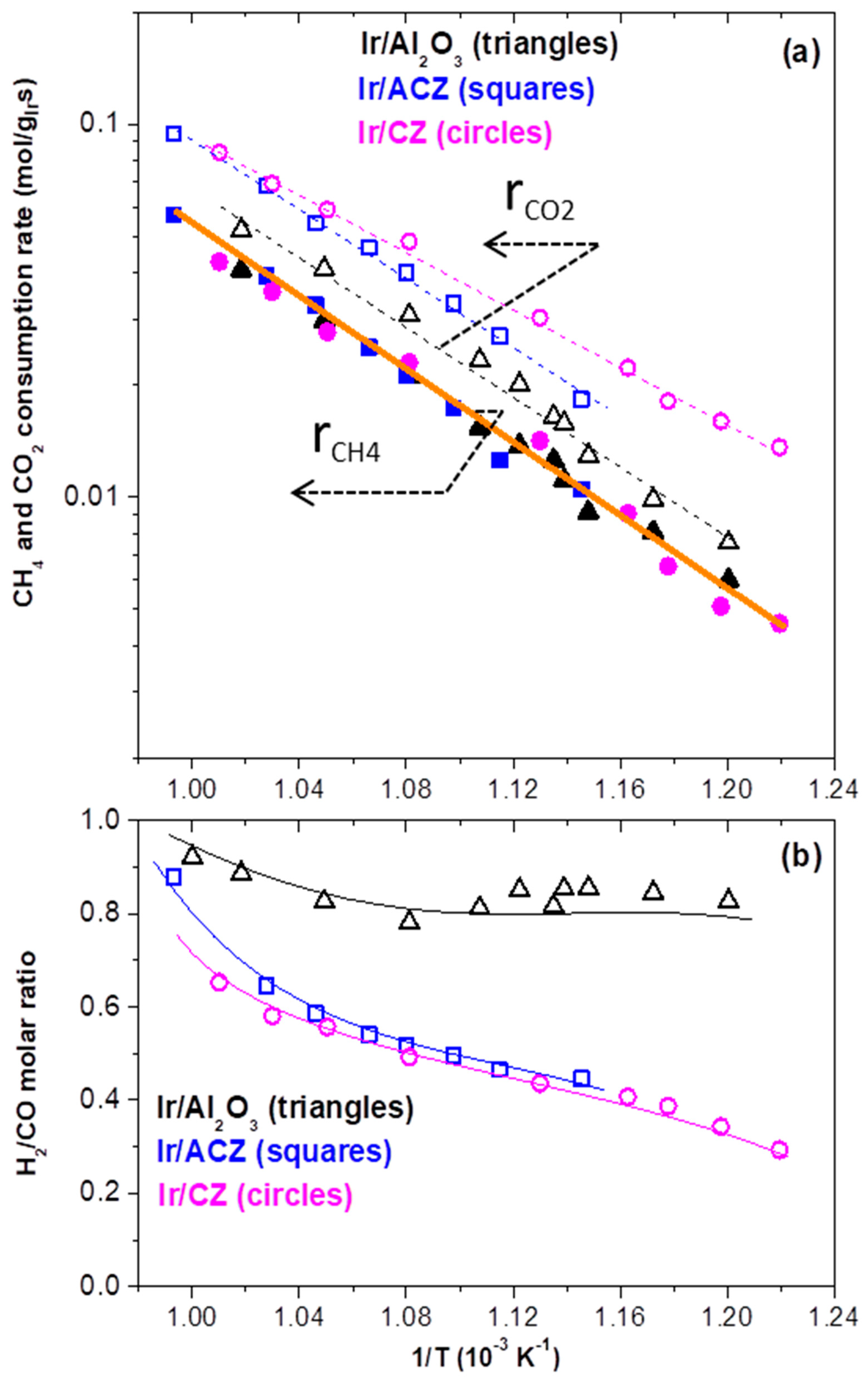
3.2.2. DRM Performance under Differential Conditions (Intrinsic Activity)
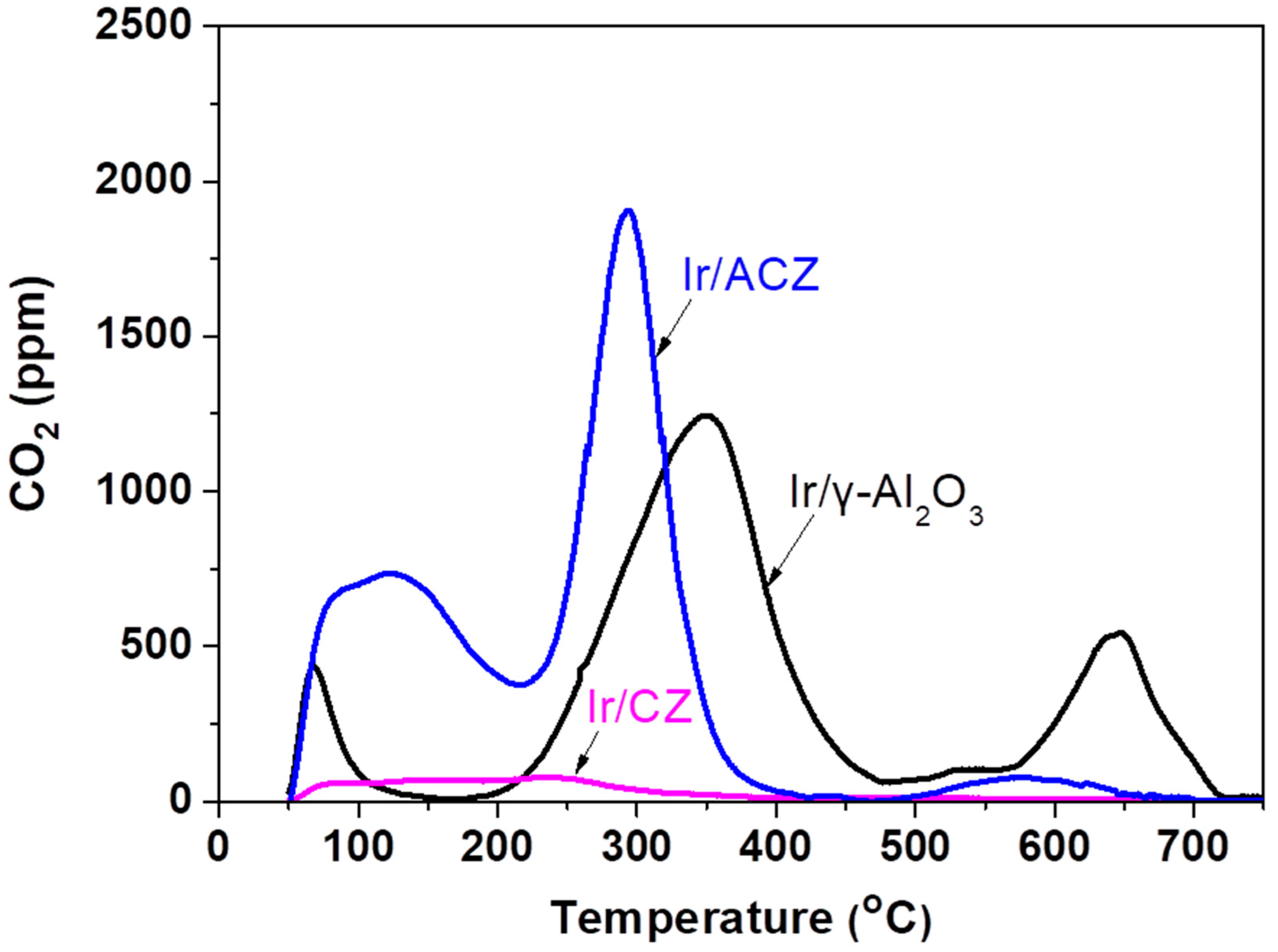
3.2.3. Carbon Deposition Measurements
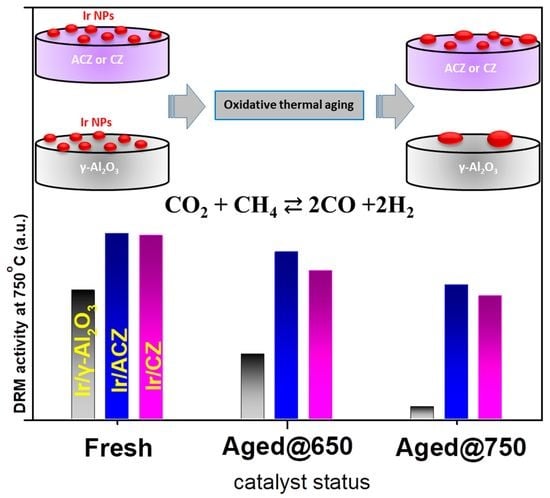
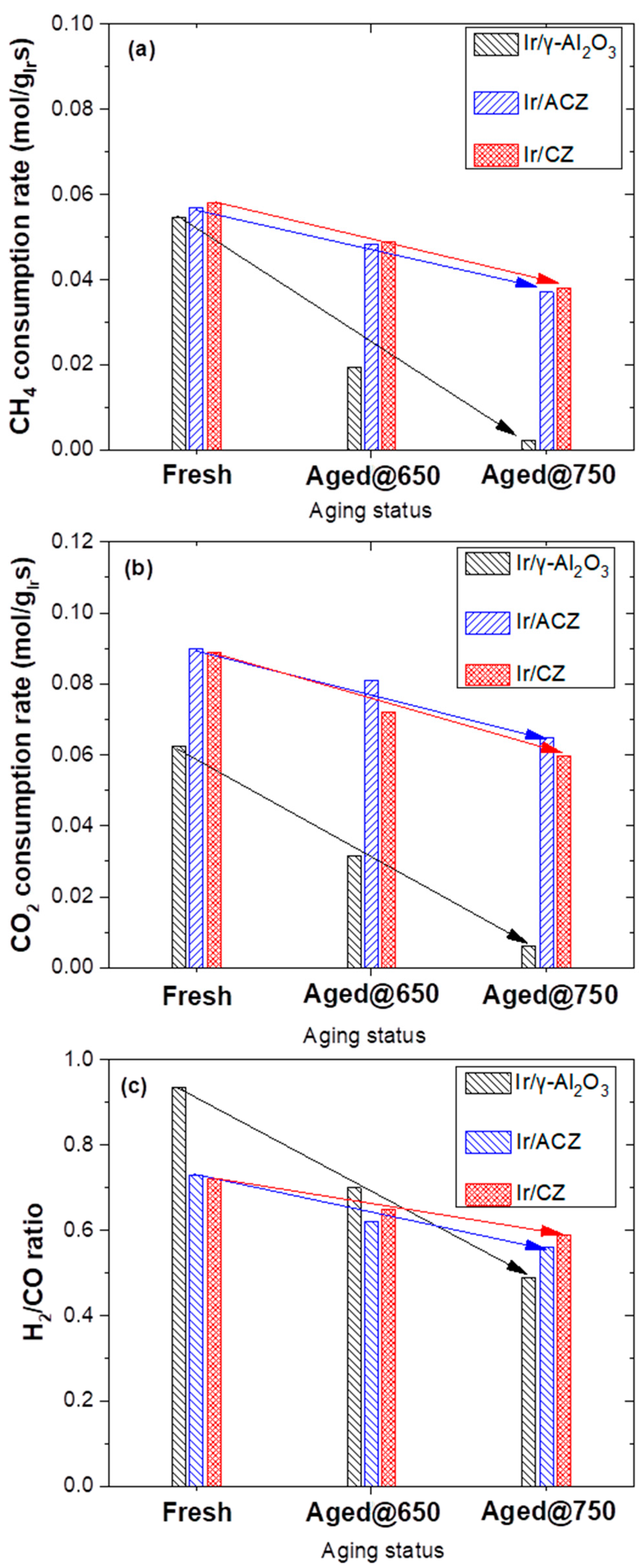
3.2.4. Evaluation of DRM Catalyst Stability after Exposure to Oxidative Thermal Aging
4. Conclusions
- The use of ACZ and CZ supports with sufficient OSC and surface oxygen vacancies substantially enhances the CO2 consumption rate but does not affect appreciably the CH4 consumption rate. This implies the occurrence of a bifunctional reaction mechanism according to which the oxygen vacancies and labile oxygen ions of the support promote dissociative CO2 adsorption and the production of a CO-enriched syngas. Such synergistic phenomena are absent on the γ-Al2O3-supported catalyst, which is characterized by negligible OSC and surface oxygen vacancies and, concomitantly, lower CO2 consumption rates. In addition, the apparent activation energy for CO2 consumption decreases with increasing OSC value of the support, thus favoring the DRM reaction, especially in the low-temperature region.
- All catalysts exhibit excellent time-on-stream stability regardless of the OSC of the support. This is attributed to the intrinsically low propensity of Ir for the formation and accumulation of carbon deposits and the predominance of the thermally stable metallic Ir phase under highly reducing DRM reaction conditions (CO+H2 reformate), which prevents particle agglomeration.
- The support OSC strongly affects the amount and type of carbon deposits accumulated on the catalyst surface following exposure to reaction conditions. The formation of graphitic carbon is significantly suppressed over Ir/ACZ, compared to Ir/γ-Al2O3, and is negligible for the Ir/CZ sample. Interestingly, the latter catalyst does not promote the accumulation of any type of carbon deposits during DRM, verifying the important role of labile O2− species of the support on the gasification rate of surface carbon species.
- Oxidative thermal aging experiments demonstrated that the OSC of the support is a key factor in preventing iridium particle growth (sintering) despite the fact that IrO2 is highly prone to agglomeration under such conditions. Thus, Ir/ACZ and Ir/CZ (but not Ir/γ-Al2O3) maintain their initial DRM activity, even after severe thermal aging. The spontaneous, thermally driven O2− back-spillover from the high oxygen ion lability supports to the Ir particle’s surface is responsible for this anti-sintering behavior.
- These advantageous features of iridium supported on high-oxygen storage capacity and lability supports indicate that such catalysts can be cost effective (low Ir-loading), stable (regardless of the oxidizing or reducing environments) and highly active, especially for the low-temperature DRM process, which remains a challenging and attractive industrial application.
Author Contributions
Funding
Conflicts of Interest
References
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Yentekakis, I.V.; Goula, G. Biogas management: Advanced utilization for production of renewable energy and added-value chemicals. Front. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Yentekakis, I.V.; Dong, F. Grand challenges for catalytic remediation in environmental and energy applications towards a cleaner and sustainable future. Front. Environ. Chem. 2020, 1, 5. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B Environ. 2019, 243, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Yentekakis, I.V. Open- and closed-circuit study of an intermediate temperature SOFC directly fueled with simulated biogas mixtures. J. Power Sources 2006, 160, 422–425. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Papadam, T.; Goula, G. Electricity production from wastewater treatment via a novel biogas-SOFC aided process. Solid State Ion. 2008, 179, 1521–1525. [Google Scholar] [CrossRef]
- Papadam, T.; Goula, G.; Yentekakis, I.V. Long-term operation stability tests of intermediate and high temperature Ni-based anodes’ SOFCs directly fueled with simulated biogas mixtures. Int. J. Hydrogen Energy 2012, 37, 16680–16685. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Barelli, L.; Ottaviano, A. Solid oxide fuel cell technology coupled with methane dry reforming: A viable option for high efficiency plant with reduced CO2 emissions. Energy 2014, 71, 118–129. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Botzolaki, G.; Goula, G.; Rontogianni, A.; Nikolaraki, E.; Chalmpes, N.; Zygouri, P.; Karakassides, M.; Gournis, D.; Charisiou, N.; Goula, M.; et al. CO2 Methanation on supported Rh nanoparticles: The combined effect of support oxygen storage capacity and Rh particle size. Catalysts 2020, 10, 944. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic dry reforming of methane: Insights from model systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Kahle, L.C.S.; Roussiere, T.; Maier, L.; Delgado, K.H.; Wasserschaff, G.; Schunk, S.A.; Deutschmann, O. Methane dry reforming at high temperature and elevated pressure: Impact of gas-phase reactions. Ind. Eng. Chem. Res. 2013, 52, 11920–11930. [Google Scholar] [CrossRef]
- Helveg, S.; Sehested, J.; Rostrup-Nielsen, J.R. Whisker carbon in perspective. Catal. Today 2011, 178, 42–46. [Google Scholar] [CrossRef]
- Helveg, S.; Lopez-Cartes, C.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Nørskov, J.K. Atomic-scale imaging of carbon nanofibre growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Rostrup-Nielsen, J.R.; Christiansen, L.J. Concepts in Syngas Manufacture; Imperial College Press: London, UK, 2011. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-based ceria, zirconia, and ceria–zirconia catalytic systems for low-temperature carbon dioxide reforming of methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [Green Version]
- Al-Fatesh, A.S.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. In situ auto-gasification of coke deposits over a novel Ni-Ce/W-Zr catalyst by sequential generation of oxygen vacancies for remarkably stable syngas production via CO2-reforming of methane. Appl. Catal. B Environ. 2021, 280, 119445. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E. Ni supported on La2O3+ZrO2 for dry reforming of methane: The impact of surface adsorbed oxygen species. Int. J. Hydrogen Energy 2021, 46, 3780–3788. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Toniolo, F.S.; Noronha, F.B. Embedded Ni nanoparticles in CeZrO2 as stable catalyst for dry reforming of methane. Appl. Catal. B Environ. 2020, 268, 118387. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X.E.; MacDonald, S.M.; Affrossman, S. Comparative study of carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 and conventional nickel-based catalysts. J. Phys. Chem. 1996, 100, 744–754. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Zavasnik, J.; Djinovic, P.; Efstathiou, A.M. Dry reforming of methane over Ni/Ce0.8Ti0.2O2-δ: The effect of Ni particle size on the carbon pathways studied by transient and isotopic techniques. Appl. Catal. B Environ. 2021, 296, 120321. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Damaskinos, C.M.; Djinovic, P.; Pintar, A.; Efstathiou, A.M. Dry reforming of CH4 over NiCo/Ce0.75Zr0.25O2-δ: The effect of Co on the site activity and carbon pathways studied by transient techniques. Appl. Catal. B Environ. 2021, 149, 106237. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.-J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B Environ. 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Yokota, S.; Okumura, K.; Niwa, M. Support effect of metal oxide on Rh catalysts in the CH4-CO2 reforming reaction. Catal. Lett. 2002, 84, 131–134. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Ibrahim, A.A.; Kurdi, A.N.; Abasaeed, A.E. Yttria modified ZrO2 supported Ni catalysts for CO2 reforming of methane: The role of Ce promoter. ACS Omega 2021, 6, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5 wt% Ni/Ce1−xMxO2−δ (M = Zr4+, Pr3+) catalysts. Catal. Today 2015, 259, 150–164. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, B.S.; Tian, Y.L. CO2 reforming of methane over Ni/Sm2O3–CaO catalyst prepared by a sol–gel technique. Catal. Commun. 2007, 8, 661–667. [Google Scholar] [CrossRef]
- Amin, M.H.; Mantri, K.; Newnham, J.; Tardio, J.; Bhargava, S.K. Highly stable ytterbium promoted Ni/γ-Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. B Environ. 2012, 119, 217–226. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- Li, M.; van Veen, A.C. Tuning the catalytic performance of Ni-catalysed dry reforming of methaneand carbon deposition via Ni-CeO2-x interaction. Appl. Catal. B Environ. 2018, 237, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Al-Fatesh, A.S.; Fakeeha, A.H.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Al-Zahrani, A.A.; Abasaeed, A.E.; Srivastava, V.K.; Kumar, R. Impact of ceria over WO3-ZrO2 supported Ni catalyst towards hydrogen production through dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 25015–25028. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Goula, G.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. The effect of WO3 modification of ZrO2 support on the Ni-catalyzed dry reforming of biogas reaction for syngas production. Front. Environ. Sci. 2017, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. Available online: https://science.sciencemag.org/content/367/6479/777/tab-pdf (accessed on 26 October 2021). [CrossRef] [PubMed]
- Ferreira-Aparicio, P.; Guerrero-Ruiz, A.; Rodriquez-Ramos, I. Comparative study at low and medium reaction temperatures of syngas production by methane reforming with carbon dioxide over silica and alumina supported catalysts. Appl. Catal. A Gen. 1998, 170, 177–187. [Google Scholar] [CrossRef]
- Barama, S.; Dupeyrat-Batiot, C.; Capron, M.; Bordes-Richard, E.; Bakhti-Mohammedi, O. Catalytic properties of Rh, Ni, Pd and Ce supported on Al-pillared montmorillonites in dry reforming of methane. Catal. Today 2009, 141, 385–392. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.F. Syngas production by methane reforming with carbon dioxide on noble metal catalysts. J. Nat. Gas. Chem. 2006, 15, 327–334. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry reforming of methane: Catalytic performance and stability of Ir catalysts supported on γ-Al2O3, Zr0.92Y0.08O2-δ (YSZ) or Ce0.9Gd0.1O2-δ (GDC) supports. Top. Catal. 2015, 58, 1228–1241. Available online: https://link.springer.com/10.1007/s11244-015-0490-x (accessed on 26 October 2021). [CrossRef]
- Djinovic, P.; Batista, J.; Pintar, A. Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh–CeO2 catalyst. Int. J. Hydrogen Energy 2012, 37, 2699–2707. [Google Scholar] [CrossRef]
- Großmann, K.; Dellermann, T.; Dillig, M.; Karl, J. Coking behavior of nickel and a rhodium based catalyst used in steam reforming for power-to-gas applications. Int. J. Hydrogen. Energy 2017, 42, 11150–11158. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Mancino, G. Effect of phosphorous addition to Rh-supported catalysts for the dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 23587–23598. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Fernandez-Garcia, M.; Guerrero-Ruiz, A.; Rodriguez-Ramos, I. Evaluation of the role of the metal–support interfacial centers in the dry reforming of methane on alumina-supported rhodium catalysts. J. Catal. 2000, 190, 296–308. [Google Scholar] [CrossRef]
- Nematollahi, A.; Rezaei, M.; Khajenoori, M. Combined dry reforming and partial oxidation of methane to synthesis gas on noble metal catalysts. Int. J. Hydrogen Energy 2011, 36, 2969–2978. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Alfaro, G.; Bimbela, F.; Gandia, L.M. Syngas production by means of biogas catalytic partial oxidation and dry reforming using Rh-based catalysts. Catal. Today 2018, 299, 280–288. [Google Scholar] [CrossRef]
- Jagodka, P.; Matus, K.; Sobota, M.; Lamacz, A. Dry Reforming of methane over carbon fibre-supported CeZrO2, Ni-CeZrO2, Pt-CeZrO2 and Pt-Ni-CeZrO2 catalysts. Catalysts 2021, 11, 563. [Google Scholar] [CrossRef]
- Araiza, D.G.; Arcos, D.G.; Gomez-Cortes, A.; Diaz, G. Dry reforming of methane over Pt-Ni/CeO2 catalysts: Effect of the metal composition on the stability. Catal. Today 2021, 360, 46–54. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, B.; Kattel, S.; Lee, J.H.; Yao, S.; Wu, Q.; Rui, N.; Gomez, E.; Liu, Z.; Xu, W.; et al. Dry reforming of methane over CeO2-supported Pt-Co catalysts with enhanced activity. Appl. Catal. B Environ. 2018, 236, 280–293. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Liland, S.E.; Regli, S.K.; Yang, J.; Rout, K.R.; Luo, J.; Ronning, M.; Ran, J.; Chen, D. Unraveling enhanced activity, selectivity, and coke resistance of Pt–Ni bimetallic clusters in dry reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Gubanova, E.L.; Sazonova, N.N.; Pokrovskaya, S.A.; Chumakova, N.A.; Mezentseva, N.V.; Bobin, A.S.; Gulyaev, R.V.; Ishchenko, A.V.; Krieger, T.A.; et al. Dry reforming of methane over Pt/PrCeZrO catalyst: Kinetic and mechanistic features by transient studies and their modeling. Catal. Today 2011, 171, 140–149. [Google Scholar] [CrossRef]
- Safariamin, M.; Tidahy, L.H.; Abi-Aad, E.; Siffert, S.; Aboukaïs, A. Dry reforming of methane in the presence of ruthenium-based catalysts. C. R. Chim. 2009, 12, 748–753. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Rui, N.; Li, X.; Lin, L.; Betancourt, L.E.; Su, D.; Xu, W.; Cen, J.; Attenkofer, K.; et al. Highly active ceria-supported Ru catalyst for the dry reforming of methane: In situ identification of Ruδ+–Ce3+ interactions for enhanced conversion. ACS Catal. 2019, 9, 3349–3359. [Google Scholar] [CrossRef]
- Derk, A.R.; Moore, G.M.; Sharma, S.; McFarland, E.W.; Metiu, H. Catalytic dry reforming of methane on ruthenium-doped ceria and ruthenium supported on ceria. Top. Catal. 2014, 57, 118–124. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Ren, Y.; Fu, Y.; Yue, B.; Tsang, S.C.E.; He, H. Morphology-dependent catalytic activity of Ru/CeO2 in dry reforming of methane. Molecules 2019, 24, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Moulijn, J.A.; van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable? What to do? Appl. Catal. A Gen. 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Goula, G.; Botzolaki, G.; Osatiashtiani, A.; Parlett, C.M.A.; Kyriakou, G.; Lambert, R.M.; Yentekakis, I.V. Oxidative thermal sintering and redispersion of Rh nanoparticles on supports with high oxygen ion lability. Catalysts 2019, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Fiedorow, R.M.J.; Chahar, B.S.; Wanke, S.E. The sintering of supported metal catalysts. II. Comparison of sintering rates of supported Pt, Ir, and Rh Catalysts in hydrogen and oxygen. J. Catal. 1978, 51, 193–202. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Datye, A.; Wang, Y. Atom trapping: A novel approach to generate thermally stable and regenerable single-atom catalysts. Natl. Sci. Rev. 2018, 5, 630–632. [Google Scholar] [CrossRef] [Green Version]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Hernandez, X.I.P.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Kampouri, S.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalysed decomposition of N2O. Appl. Catal. B Environ. 2016, 192, 357–364. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Kampouri, S.; Betsi-Argyropoulou, I.; Panagiotopoulou, P.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Ir-catalyzed nitrous oxide (N2O) decomposition: Effect of the Ir particle size and metal-support interactions. Catal. Lett. 2018, 148, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, M.; Takahashi, N.; Tanabe, T.; Nagai, Y.; Dohmae, K.; Aoki, Y.; Yoshida, T.; Shinjoh, H. Ideal Pt loading for a Pt/CeO2-based catalyst stabilized by Pt-O-Ce bond. Appl. Catal. B Environ. 2010, 99, 336–342. [Google Scholar] [CrossRef]
- Papavasiliou, A.; Tsetsekou, A.; Matsuka, V.; Konsolakis, M.; Yentekakis, I.V. An investigation of the role of Zr and La dopants into Ce1−x−yZrxLayOδ enriched γ-Al2O3 TWC washcoats. Appl. Catal. A Gen. 2010, 382, 73–84. [Google Scholar] [CrossRef]
- Pachatouridou, E.; Papista, E.; Iliopoulou, E.L.; Delimitis, A.; Goula, G.; Yentekakis, I.V.; Marnellos, G.E.; Konsolakis, M. Nitrous oxide decomposition over Al2O3 supported noble metals (Pt, Pd, Ir): Effect of metal loading and feed composition. J. Environ. Chem. Eng. 2015, 3, 815–821. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special references to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Chen, H.; Ye, Z.; Cui, X.; Shi, J.; Yan, D. A novel mesostructured alumina-ceria-zirconia tri-component nanocomposite with high thermal stability and its three-way catalysis. Micropor. Mesopor. Mater. 2011, 143, 368–374. [Google Scholar] [CrossRef]
- Ozawa, M.; Takahashi-Morita, M.; Kobayashi, K.; Haneda, M. Core-shell type ceria zirconia support for platinum and rhodium three way catalysts. Catal. Today 2017, 281, 482–489. [Google Scholar] [CrossRef]
- Pliangos, C.; Yentekakis, I.V.; Papadakis, V.G.; Vayenas, C.G.; Verykios, X.E. Support induced promotional effects on the activity of automotive exhaust catalysts: 1. The case of oxidation of light hydrocarbons (C2H4). Appl. Catal. B Environ. 1997, 14, 161–173. [Google Scholar] [CrossRef]
- Vayenas, C.G. Promotion, electrochemical promotion and metal–support interactions: Their common features. Catal. Lett. 2013, 143, 1085–1097. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Carbon deposition in steam reforming and methanation. Catal. Rev. Sci. Eng. 1982, 24, 67–112. [Google Scholar] [CrossRef]
- Swaan, H.M.; Kroll, V.C.H.; Martin, G.A.; Mirodatos, C. Deactivation of supported nickel catalysts during the reforming of methane by carbon dioxide. Catal. Today 1994, 21, 571–578. [Google Scholar] [CrossRef]
- Malaibari, Z.O.; Amin, A.; Croiset, E.; Epling, W. Performance characteristics of Mo-Ni/Al2O3 catalysts in LPG oxidative steam reforming for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 10061–10073. [Google Scholar] [CrossRef]

| Supports and Catalysts | Ir-Content (wt.%) a | SBET (cm2/g) | Total Pore Volume (cm3/g) | OSC (μmol O2/g) | DIr (H/Ir) | Mean Ir Particle Size (nm) b H2-chem/TEM | |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | − | 178 | 0.60 | 0 | − | − | − |
| Ir/γ-Al2O3 | 1.0 | 167 | 0.57 | 38 | 0.45 | 0.70 | 1.0/1.2 ± 0.3 |
| ACZ: 80 wt% Al2O3−20 wt% Ce0.5Zr0.5O2−δ | − | 149 | 0.29 | 110 | − | − | − |
| Ir/ACZ | 0.4 | 73 | 0.22 | 176 | 0.103 | 0.41 | 1.7/1.8 ± 0.5 |
| CZ: Ce0.5Zr0.5O2−δ | − | 22 | 0.05 | 557 | − | − | − |
| Ir/CZ | 0.6 | 17 | 0.04 | 601 | 0.231 | 0.61 | 1.2/1.3 ± 0.4 |
| Catalyst | Support t-OSC (μmol O2/g) | rCO2 Apparent Activation Energy (Ea) (kJ/mol) | Pre-Exponential Factor ( ) (mol CO2/ gIr·s) |
|---|---|---|---|
| Ir/γ-Al2O3 | 0 | 91.3 ± 0.4 | 4146.4 ± 1.7 |
| Ir/ACZ | 110 | 88.5 ± 0.3 | 3827.6 ± 1.3 |
| Ir/CZ | 557 | 73.5 ± 0.2 | 645.5 ± 1.2 |
| Catalyst | Total Amount of Carbon Deposited (μmol C/gcat) | Mean Carbon Deposition Rate (μmol C/gcat·h) |
|---|---|---|
| Ir/γ-Al2O3 | 324 | 108 |
| Ir/ACZ | 328 | 109 |
| Ir/CZ | 27 | 9 |
| Catalyst | SBET (m2/g) | Total Pore Volume (cm3/g) | (cm3/g) | DIr (H/Ir) | Mean Ir Particle Size (nm) a |
|---|---|---|---|---|---|
| Ir/γ-Al2O3-Aged@750 | 140 | 0.31 | 0.033 | 0.05 | 13.6 |
| Ir/ACZ-Aged@750 | 64 | 0.20 | 0.087 | 0.34 | 2.1 |
| Ir/CZ-Aged@750 | 16 | 0.04 | 0.169 | 0.44 | 1.6 |
| Catalyst | Support t-OSC (μmol O2/gsupp) | (%) 1st Aging 2nd Aging | (%) 1st Aging 2nd Aging | Ref. | ||
|---|---|---|---|---|---|---|
| Ir/γ-Al2O3 | 0 | 64.5 a | 95.7 b | 49.5 a | 90.2 b | This work |
| Ir/ACZ | 110 | 15.1 a | 35.1 b | 10.0 a | 27.8 b | This work |
| Ir/CZ | 557 | 15.5 a | 34.5 b | 19.1 a | 33.0 b | This work |
| Ir/γ-Al2O3 | 0 | 35.6 c | 68.8 d | 29.0 c | 58.1 d | [50] |
| Ir/YSZ | 5.7 | 28.3 c | 81.5 d | 14.7 c | 69.0 d | [50] |
| Ir/GDC | 186 | 5.9 c | 5.2 d | 0.0 c | 0.0 d | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaraki, E.; Goula, G.; Panagiotopoulou, P.; Taylor, M.J.; Kousi, K.; Kyriakou, G.; Kondarides, D.I.; Lambert, R.M.; Yentekakis, I.V. Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane. Nanomaterials 2021, 11, 2880. https://doi.org/10.3390/nano11112880
Nikolaraki E, Goula G, Panagiotopoulou P, Taylor MJ, Kousi K, Kyriakou G, Kondarides DI, Lambert RM, Yentekakis IV. Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane. Nanomaterials. 2021; 11(11):2880. https://doi.org/10.3390/nano11112880
Chicago/Turabian StyleNikolaraki, Ersi, Grammatiki Goula, Paraskevi Panagiotopoulou, Martin J. Taylor, Kalliopi Kousi, Georgios Kyriakou, Dimitris I. Kondarides, Richard M. Lambert, and Ioannis V. Yentekakis. 2021. "Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane" Nanomaterials 11, no. 11: 2880. https://doi.org/10.3390/nano11112880
APA StyleNikolaraki, E., Goula, G., Panagiotopoulou, P., Taylor, M. J., Kousi, K., Kyriakou, G., Kondarides, D. I., Lambert, R. M., & Yentekakis, I. V. (2021). Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane. Nanomaterials, 11(11), 2880. https://doi.org/10.3390/nano11112880