Multi-Scale Photoacoustic Assessment of Wound Healing Using Chitosan–Graphene Oxide Hemostatic Sponge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biotoxicity Assessment
2.3. Interaction between Blood and Material
2.4. Animal Hemorrhage Model
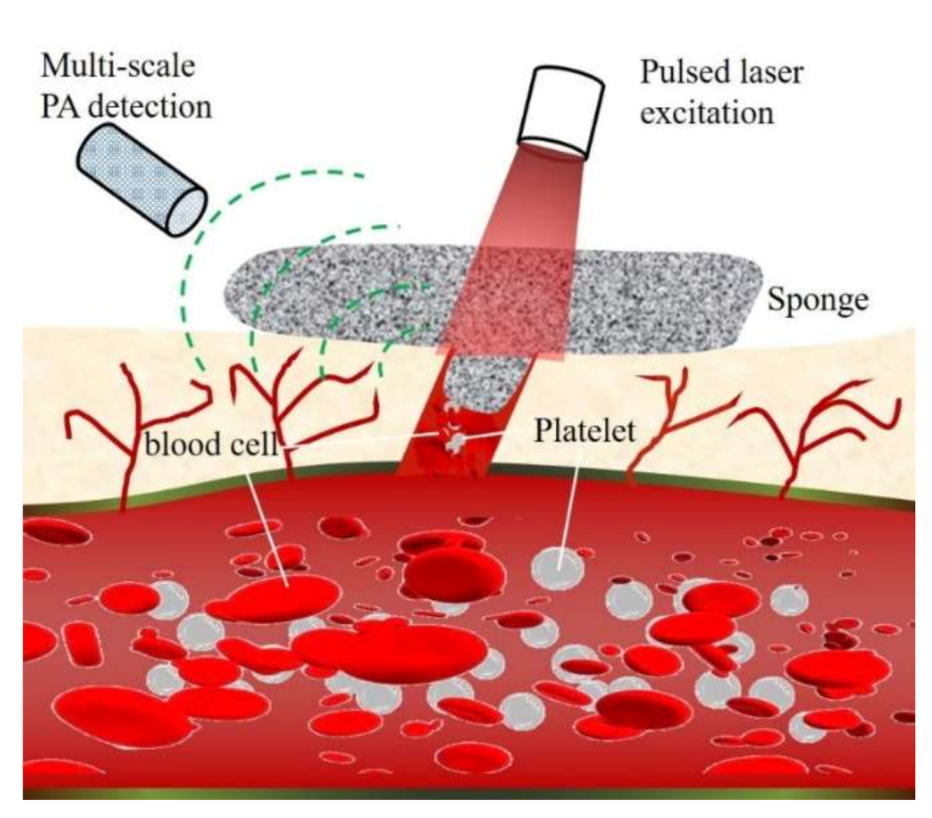
2.5. In Vitro and In Vivo Multi-Scale PA Imaging
3. Results and Discussion
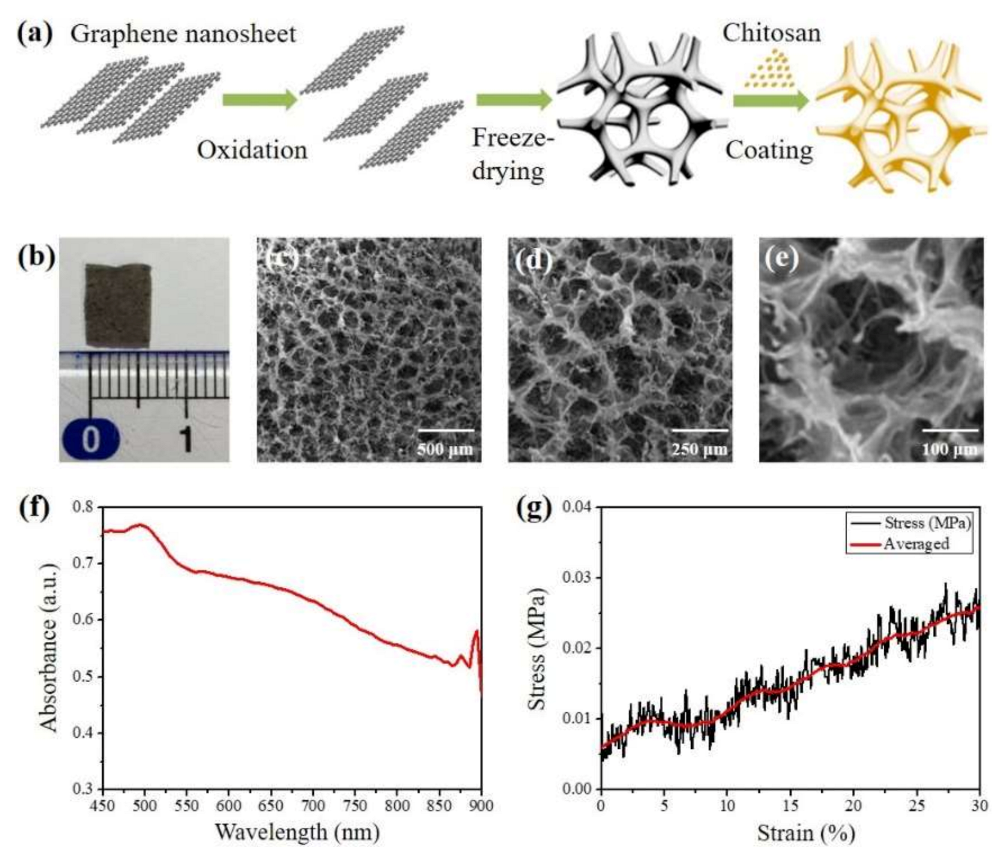
3.1. Synthesis and Characterization
3.2. Biotoxicity Evaluation
3.3. Hemostatic Performance Evaluation
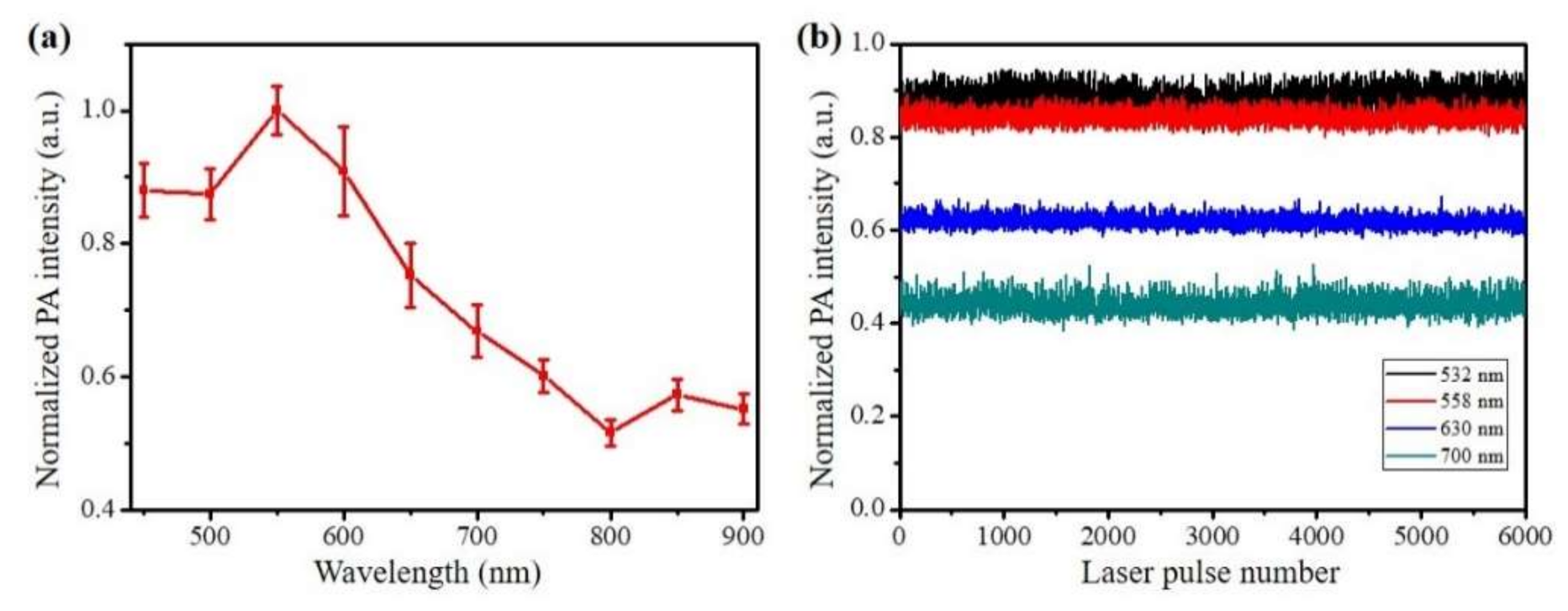
3.4. In Vitro PA Evaluation
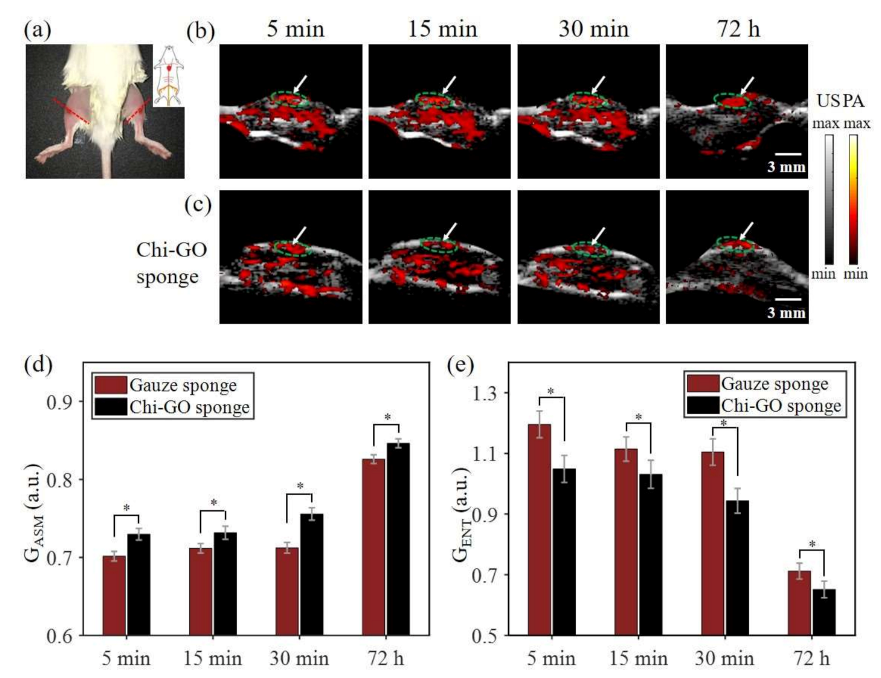
3.5. In Vivo PA Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achneck, H.E.; Sileshi, B.; Jamiolkowski, R.M.; Albala, D.M.; Shapiro, M.L.; Lawson, J.H. A comprehensive review of topical hemostatic agents efficacy and recommendations for use. Ann. Surg. 2010, 251, 217–228. [Google Scholar] [CrossRef]
- Behrens, A.M.; Sikorski, M.; Kofinas, P. Hemostatic strategies for traumatic and surgical bleeding. J. Biomed. Mater. Res. Part A 2014, 102, 4182–4194. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, R.F. The causes of death in conventional land warfare: Implications for combat casualty care research. Mil. Med. 1984, 149, 55–62. [Google Scholar] [CrossRef]
- Mannucci, P.M. Hemostatic drugs. N. Engl. J. Med. 1998, 339, 245–253. [Google Scholar] [CrossRef]
- Boffard, K.D.; Riou, B.; Warren, B.; Choong, P.; Rizoli, S.; Rossaint, R.; Axelsen, M.; Kluger, Y. Recombinant Factor VIIa as Adjunctive Therapy for Bleeding Control in Severely Injured Trauma Patients: Two Parallel Randomized, Placebo-Controlled, Double-Blind Clinical Trials. J. Trauma Inj. Infect. Crit. Care 2005, 59, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K.M.; Spazierer, D.; Urban, M.D.; Lin, L.; Redl, H.; Goppelt, A. Comparison of regenerated and non-regenerated oxi-dized cellulose hemostatic agents. Eur. Surg. 2013, 45, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Muench, T.R.; Kong, W.; Harmon, A.M. The performance of a hemostatic agent based on oxidized regenerated cellu-lose-polyglactin 910 composite in a liver defect model in immunocompetent and athymic rats. Biomaterials 2010, 31, 3649–3656. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Wu, G.S.; Mu, Y.Z.; Kong, M.; Jiang, C.Q.; Cheng, X.J.; Liu, Y.; Chen, X.G. Chitosan-coated diatom silica as hemostatic agent for hemor-rhage control. ACS Appl. Mater. Interfaces 2016, 8, 34234–34243. [Google Scholar] [CrossRef]
- Quan, K.; Li, G.; Tao, L.; Xie, Q.; Yuan, Q.; Wang, X. Diaminopropionic acid reinforced graphene sponge and its use for hemo-stasis. ACS Appl. Mater. Interfaces 2016, 8, 7666–7673. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. A Comparison of Chitosan and Collagen Sponges as Hemostatic Dressings. J. Bioact. Compat. Polym. 2006, 21, 39–54. [Google Scholar] [CrossRef]
- Levy, J.H.; Szlam, F.; Tanaka, K.A.; Sniecienski, R.M. Fibrinogen and hemostasis: A primary hemostatic target for the manage-ment of acquired bleeding. Anesth. Analg. 2012, 114, 261–274. [Google Scholar] [CrossRef]
- Ohta, S.; Nishiyama, T.; Sakoda, M.; Machioka, K.; Fuke, M.; Ichimura, S.; Inagaki, F.; Shimizu, A.; Hasegawa, K.; Kokudo, N.; et al. Development of carboxymethyl cellulose nonwoven sheet as a novel hemostatic agent. J. Biosci. Bioeng. 2015, 119, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Křížová, P.; Mášová, L.; Suttnar, J.; Salaj, P.; Dyr, J.; Homola, J.; Pecka, M. The influence of intrinsic coagulation pathway on blood platelets activation by oxidized cellulose. J. Biomed. Mater. Res. Part A 2007, 82, 274–280. [Google Scholar] [CrossRef]
- Arif, M.M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Khan, R.U.; Awais, S.M.; Butt, M.A. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S. Thrombin generation and fibrin clot structure. Blood Rev. 2007, 21, 131–142. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.B.; Kumar, R.; Keibler, M.A.; Hess, J.R.; Bochicchio, G.V.; Raghavan, S.R. A self-assembling hydrophobically modi-fied chitosan capable of reversible hemostatic action. Biomaterials 2011, 32, 3351–3357. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef]
- Pusateri, A.E.; McCarthy, S.J.; Gregory, K.W.; Harris, R.A.; Cardenas, L.; McManus, A.T.; Goodwin, C.W., Jr. Effect of a chitosan-based hemo-static dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J. Trauma 2003, 54, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: Experi-ence in current combat operations. J. Trauma 2006, 60, 655–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yan, L.; Chen, W.; Li, W. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym. 2011, 83, 653–658. [Google Scholar] [CrossRef]
- Chapman, W.C.; Wren, S.M.; Lebovic, G.S.; Malawer, M.; Sherman, R.; Block, J.E. Effective management of bleeding during tumor resection with a collagen-based hemostatic agent. Am. Surg. 2002, 68, 802–807. [Google Scholar]
- Maleki, A.; Paydar, R. Graphene oxide–chitosan bionanocomposite: A highly efficient nanocatalyst for the one-pot three-component synthesis of trisubstituted imidazoles under solvent-free conditions. RSC Adv. 2015, 5, 33177–33184. [Google Scholar] [CrossRef]
- Quan, K.; Li, G.; Luan, D.; Yuan, Q.; Tao, L.; Wang, X. Black hemostatic sponge based on facile prepared cross-linked graphene. Colloids Surf. B Biointerfaces 2015, 132, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, P.; Sun, H.; Wang, Z. Superhydrophobic Surface with Hierarchical Architecture and Bimetallic Composition for Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2014, 6, 22108–22115. [Google Scholar] [CrossRef]
- Tabish, T.A.; Pranjol, Z.I.; Horsell, D.W.; Rahat, A.A.M.; Whatmore, J.L.; Winyard, P.G.; Zhang, S. Graphene Oxide-Based Targeting of Extracellular Cathepsin D and Cathepsin L As A Novel Anti-Metastatic Enzyme Cancer Therapy. Cancers 2019, 11, 319. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2018, 31, e1805875. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Moon, H.; Kumar, D.; Kim, H.; Sim, C.; Chang, J.H.; Kim, J.M.; Kim, H.; Lim, D.K. Amplified photoacoustic performance and enhanced pho-tothermal stability of reduced graphene oxide coated gold nanorods for sensitive photoacoustic imaging. ACS Nano 2015, 9, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Hysi, E.; Fadhel, M.N.; Wang, Y.; Sebastian, J.A.; Giles, A.; Czarnota, G.J.; Exner, A.A.; Kolios, M.C. Photoacoustic imaging biomarkers for monitoring biophysical changes during nanobubble-mediated radiation treatment. Photoacoustics 2020, 20, 100201. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Manivasagan, P.; Quang Bui, N.; Oh, Y.O.; Lim, I.G.; Park, S.; Oh, J. Cytotoxic induction and photoacoustic imaging of breast cancer cells using astaxanthin-reduced gold nanoparticles. Nanomaterials 2016, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Lutzweiler, C.; Razansky, D. Optoacoustic Imaging and Tomography: Reconstruction Approaches and Outstanding Challenges in Image Performance and Quantification. Sensors 2013, 13, 7345–7384. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.L.X.; Feng, N.F.N.; Qu, Y.Q.Y.; Chen, D.C.D.; Shen, Y.S.Y.; Sun, M.S.M.; Lin, N.F.X. Compressed sensing in synthetic aperture photoacoustic tomography based on a linear-array ultrasound transducer. Chin. Opt. Lett. 2017, 15, 101102. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Liu, C.; Sheng, Z.; Gong, X.; Song, L.; Zhang, R.; Zheng, H.; Sun, M. Highly Sensitive Fluorescence and Photoacoustic Detection of Metastatic Breast Cancer in Mice Using Dual-Modal Nanoprobes. ACS Appl. Mater. Interfaces 2018, 10, 26064–26074. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.C.; Jo, S.H.; Dumani, D.; Yun, W.S.; Kim, K. Theragnostic glycol chitosan-conjugated gold nanoparticles for photoa-coustic imaging of regional lymph nodes and delivering tumor antigen to lymph nodes. Nanomaterials 2021, 11, 1700. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-G.; Kim, K.-S.; Kim, M.-H.; Ryu, S.-H. Investigation of Photoacoustic imaging for monitoring of Wound Healing under a Layer of Blood with Different Coagulation. In Proceedings of the 2011 3rd International Conference on Awareness Science and Technology (iCAST), Dalian, China, 27–30 September 2011; pp. 336–338. [Google Scholar]
- Padgett, M.E.; McCord, T.; McClung, J.M.; Kontos, C.D. Methods for Acute and Subacute Murine Hindlimb Ischemia. J. Vis. Exp. 2016, 112, e54166. [Google Scholar] [CrossRef]
- Liang, Y.; Jin, L.; Guan, B.-O.; Wang, L. 2 MHz multi-wavelength pulsed laser for functional photoacoustic microscopy. Opt. Lett. 2017, 42, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Lande, M.V.; Bhanodiya, P.; Jain, P. An Effective Content-Based Image Retrieval Using Color, Texture and Shape Feature. Adv. Intell. Syst. Comput. 2014, 243, 1163–1170. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Shih, M.F.; Shau, M.D.; Chang, M.Y.; Chiou, S.K.; Chang, J.K.; Cherng, J.Y. Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int. J. Pharm. 2006, 327, 117–125. [Google Scholar] [CrossRef]
- Hickman, D.S.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and advanced technologies for hemo-static management of bleeding. Adv. Mater. 2018, 30, 1700859. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Shen, Y.; Wang, L. Multi-Scale Photoacoustic Assessment of Wound Healing Using Chitosan–Graphene Oxide Hemostatic Sponge. Nanomaterials 2021, 11, 2879. https://doi.org/10.3390/nano11112879
Lin X, Shen Y, Wang L. Multi-Scale Photoacoustic Assessment of Wound Healing Using Chitosan–Graphene Oxide Hemostatic Sponge. Nanomaterials. 2021; 11(11):2879. https://doi.org/10.3390/nano11112879
Chicago/Turabian StyleLin, Xiangwei, Yajing Shen, and Lidai Wang. 2021. "Multi-Scale Photoacoustic Assessment of Wound Healing Using Chitosan–Graphene Oxide Hemostatic Sponge" Nanomaterials 11, no. 11: 2879. https://doi.org/10.3390/nano11112879
APA StyleLin, X., Shen, Y., & Wang, L. (2021). Multi-Scale Photoacoustic Assessment of Wound Healing Using Chitosan–Graphene Oxide Hemostatic Sponge. Nanomaterials, 11(11), 2879. https://doi.org/10.3390/nano11112879





