Underwater Light Manipulation by the Benthic Diatom Ctenophora pulchella: From PAR Efficient Collection to UVR Screening
Abstract
1. Introduction
2. Materials and Methods
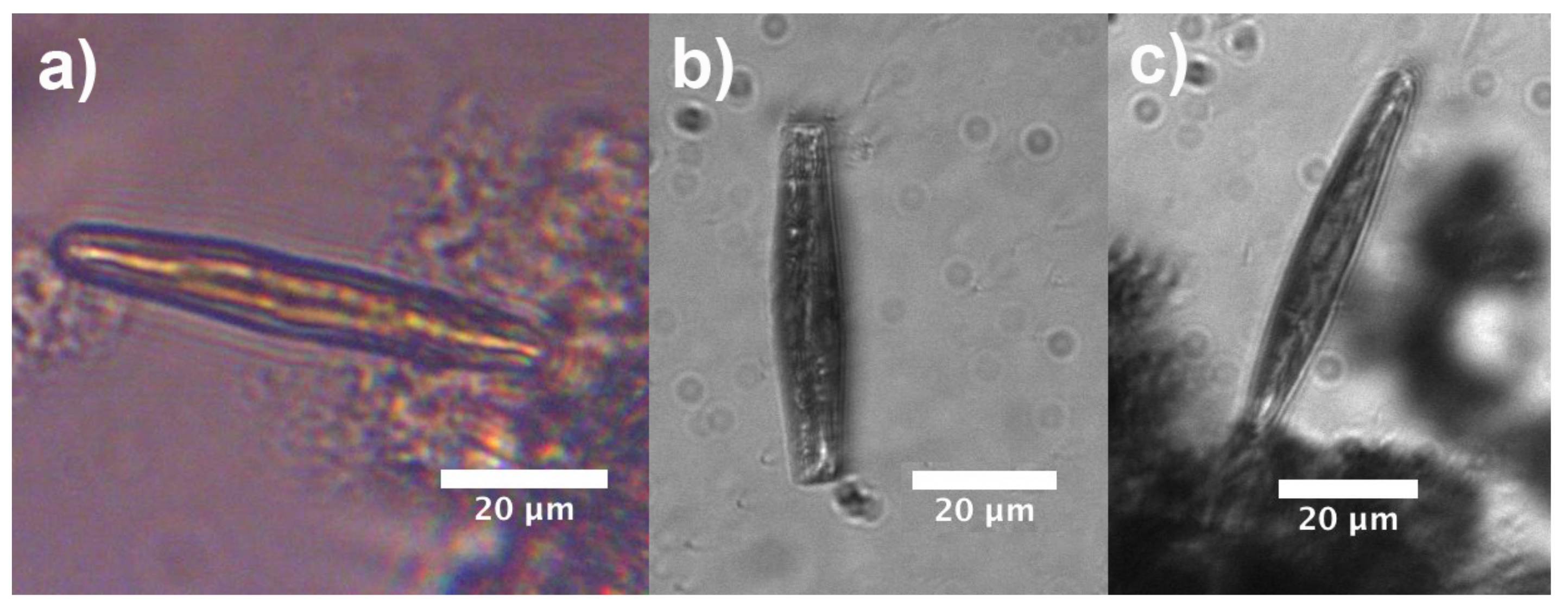
2.1. Sampling, Culture Conditions, Frustule Cleaning and Microscopy
2.2. UVR Treatments
2.3. Growth Rate
2.4. Chlorophyll Fluorescence Measurements
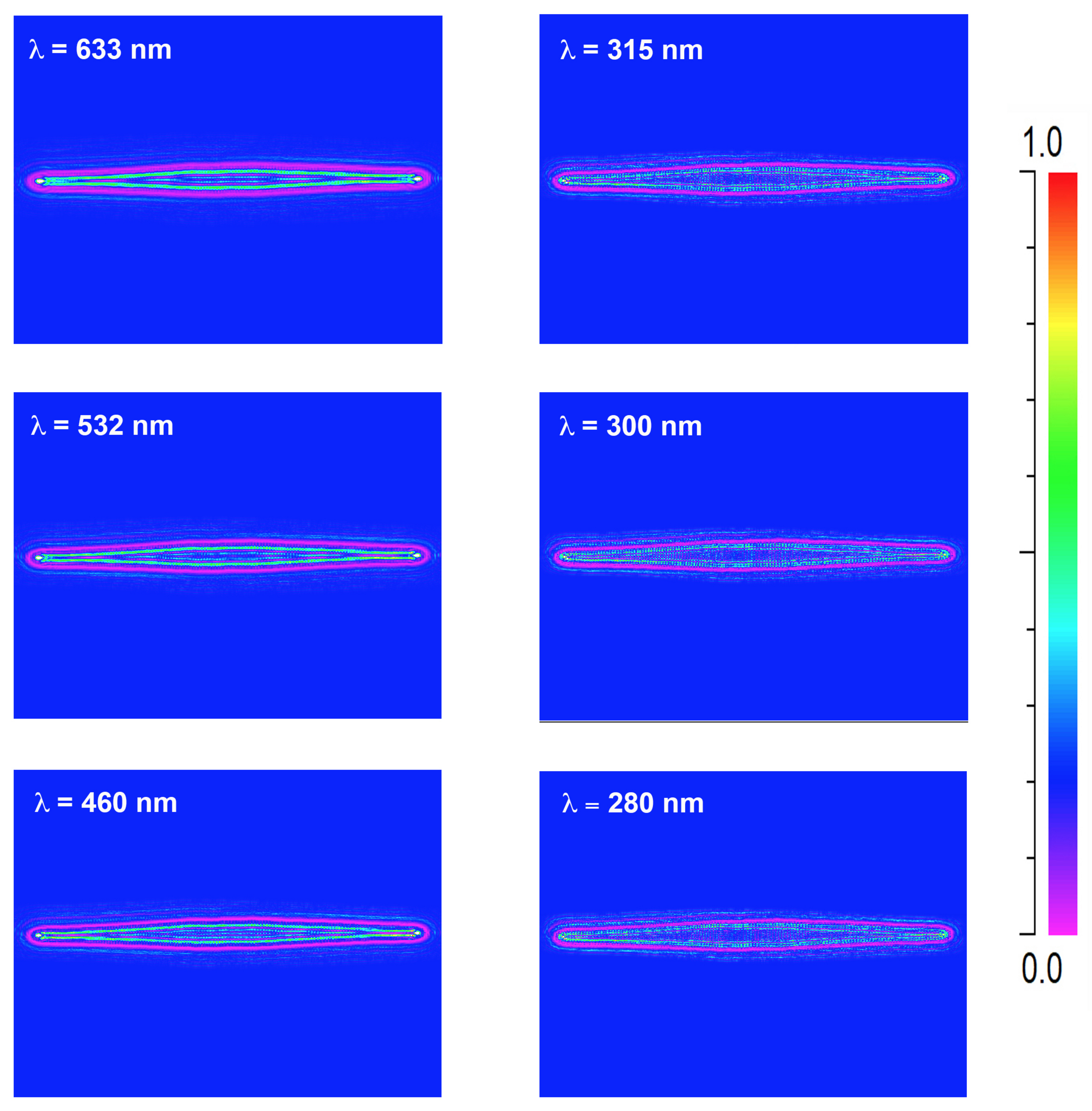
2.5. Numerical Simulations
2.6. Transmission Imaging
2.7. Digital Holography
2.7.1. Hologram Acquisition
2.7.2. Amplitude and Phase Reconstruction
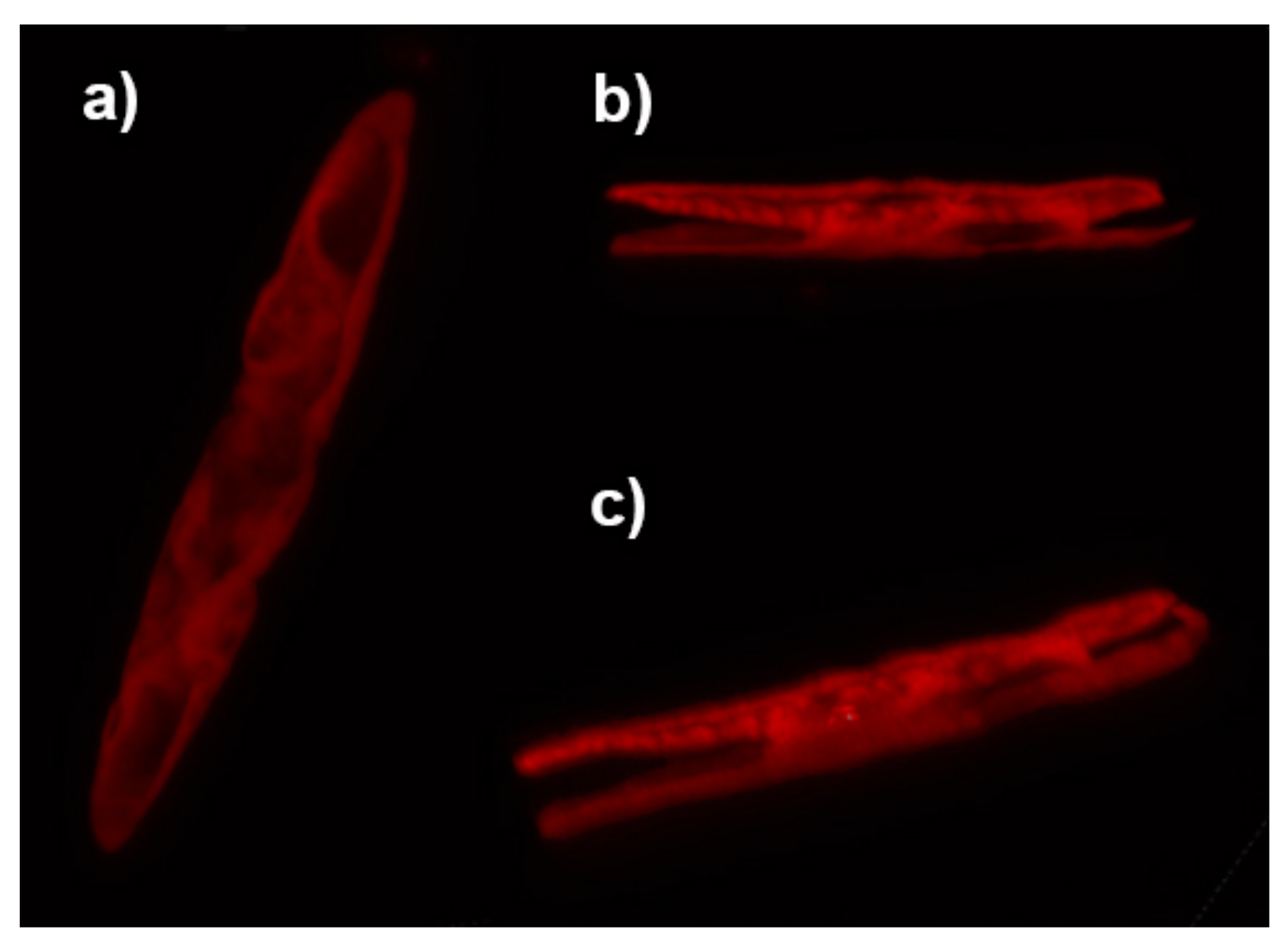
2.8. Fluorescence Imaging and Photoluminescence Spectroscopy
3. Results and Discussion
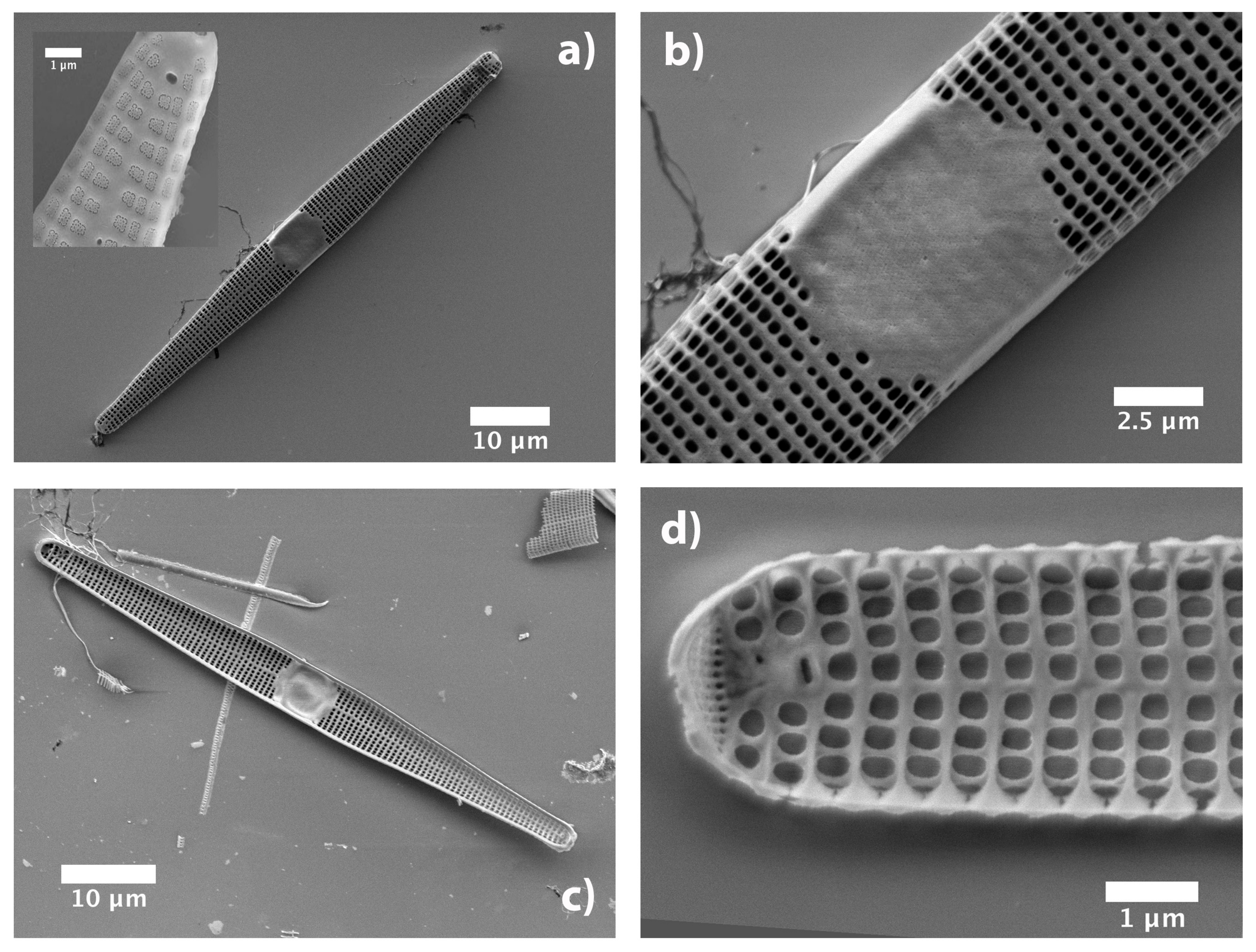
3.1. Morphological Characterization of the Frustule
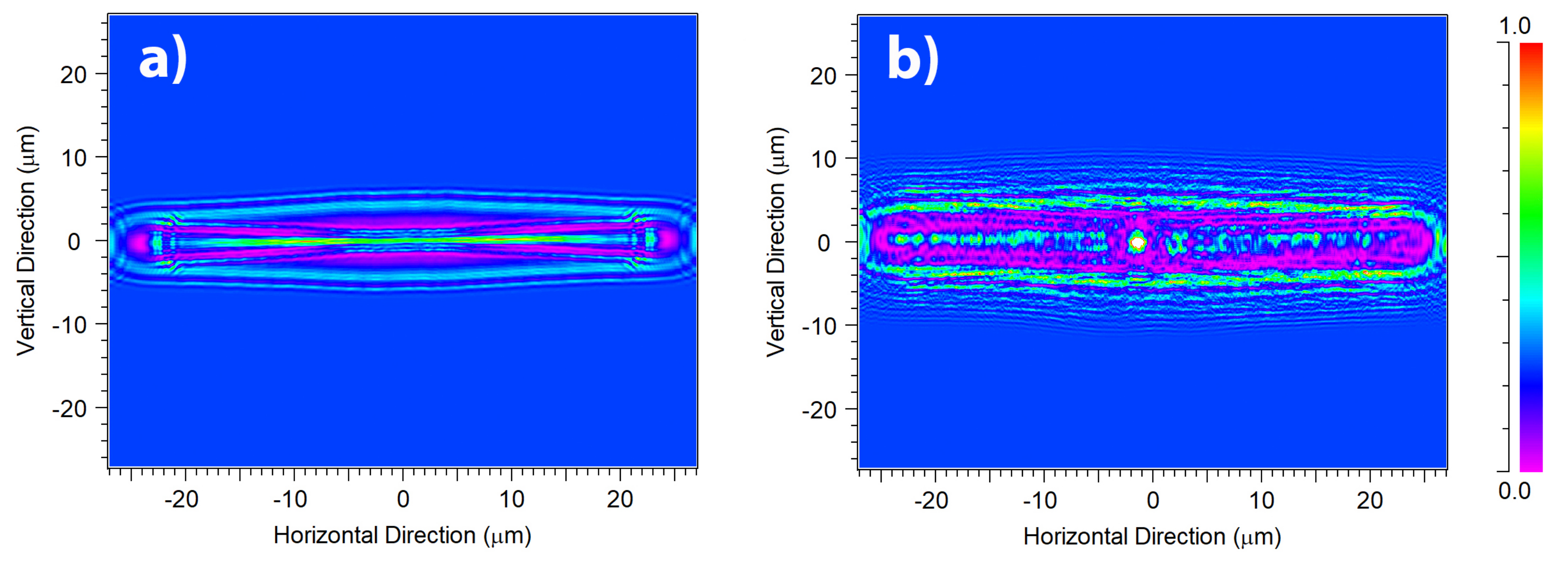
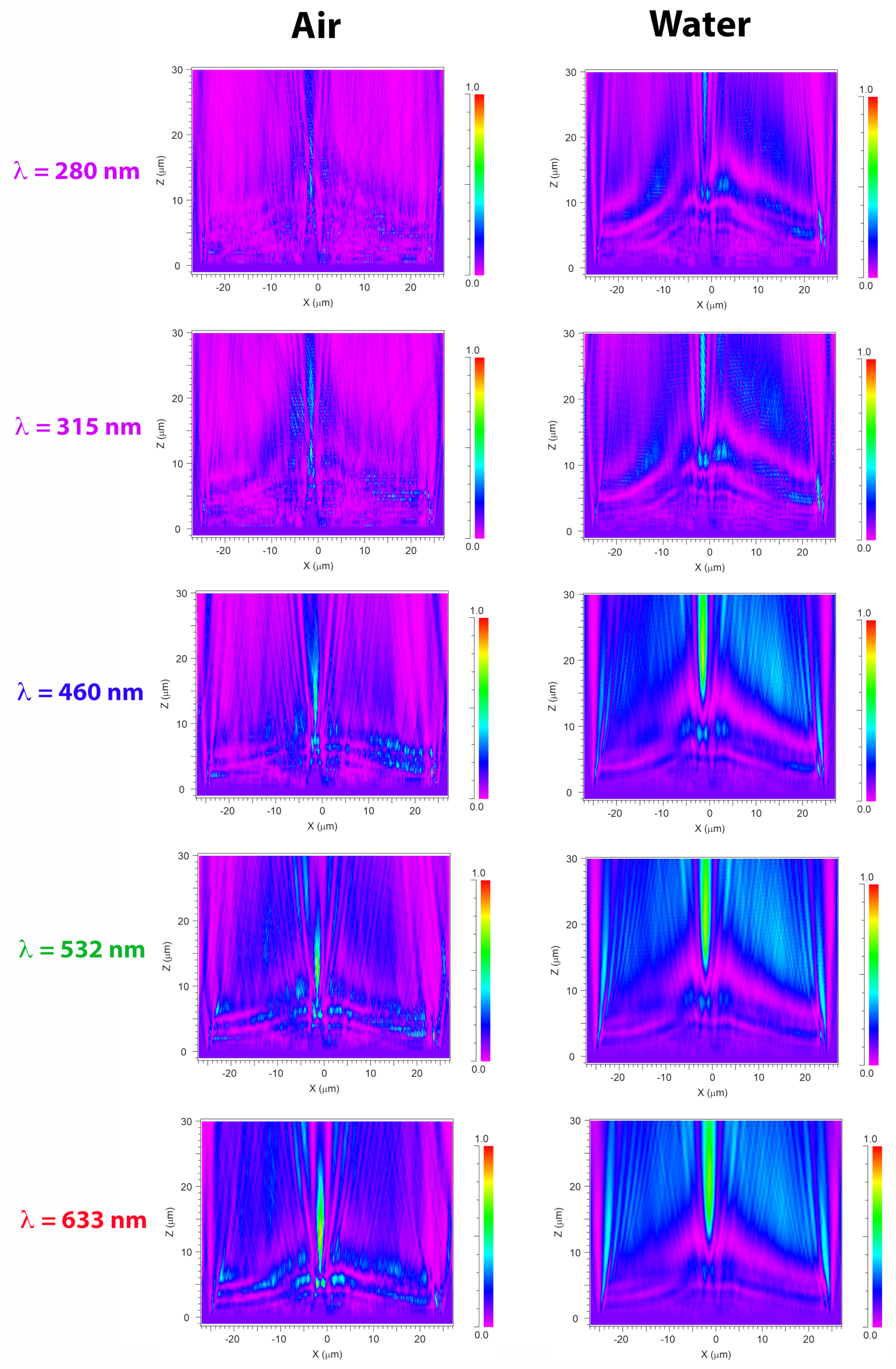
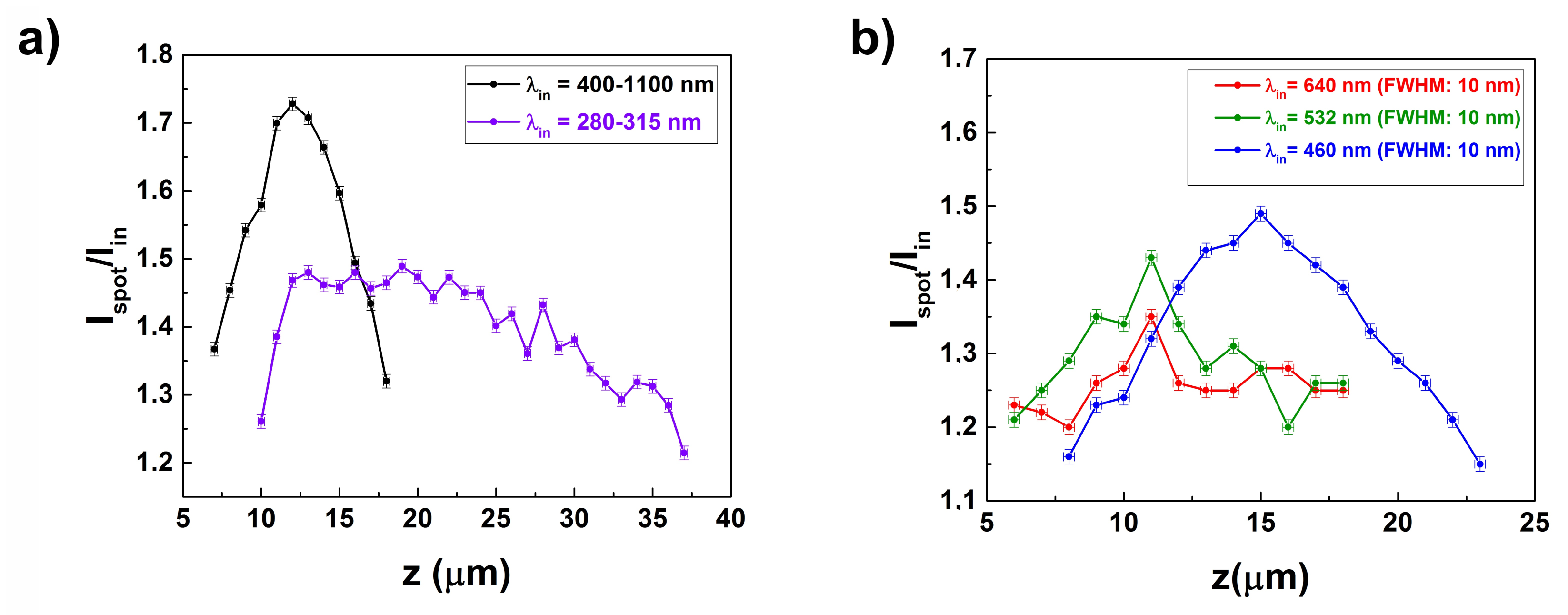
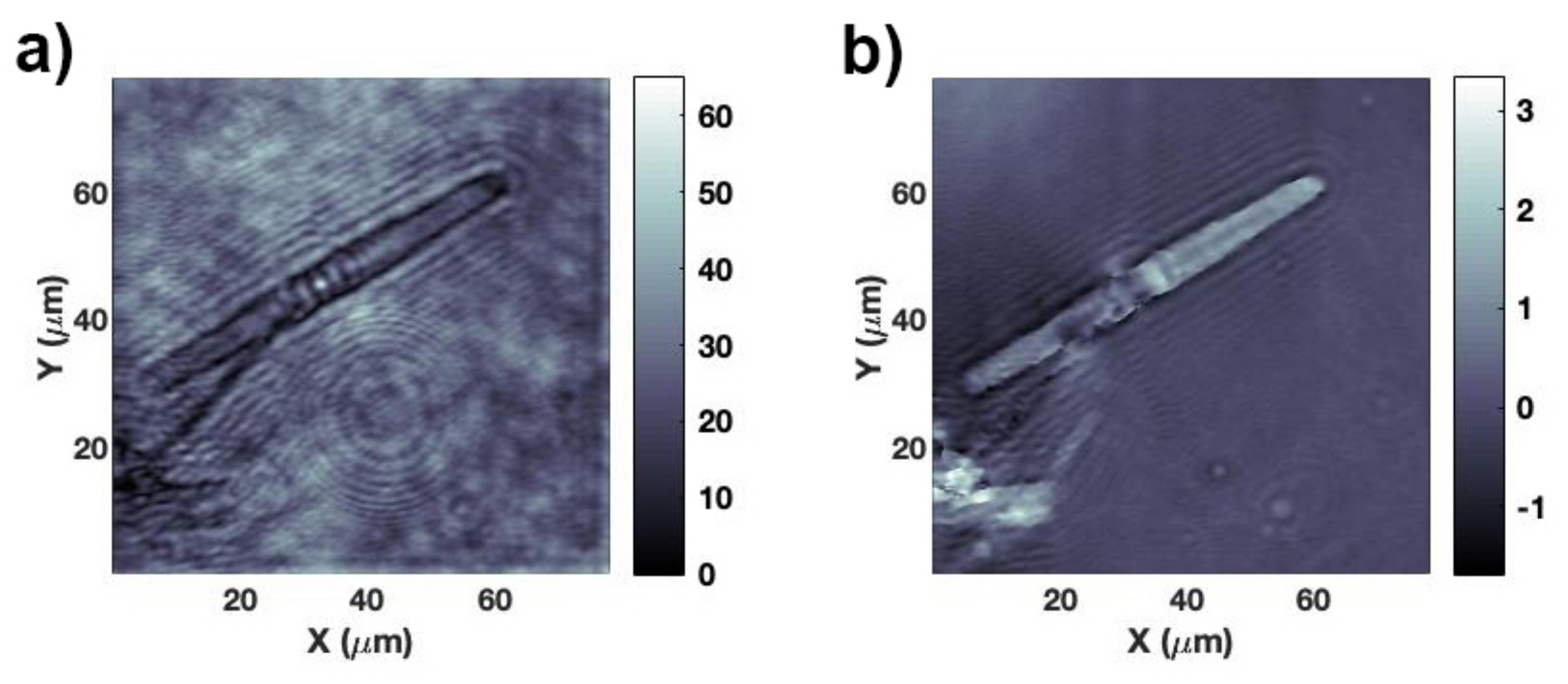
3.2. Light Propagation through a Single Valve
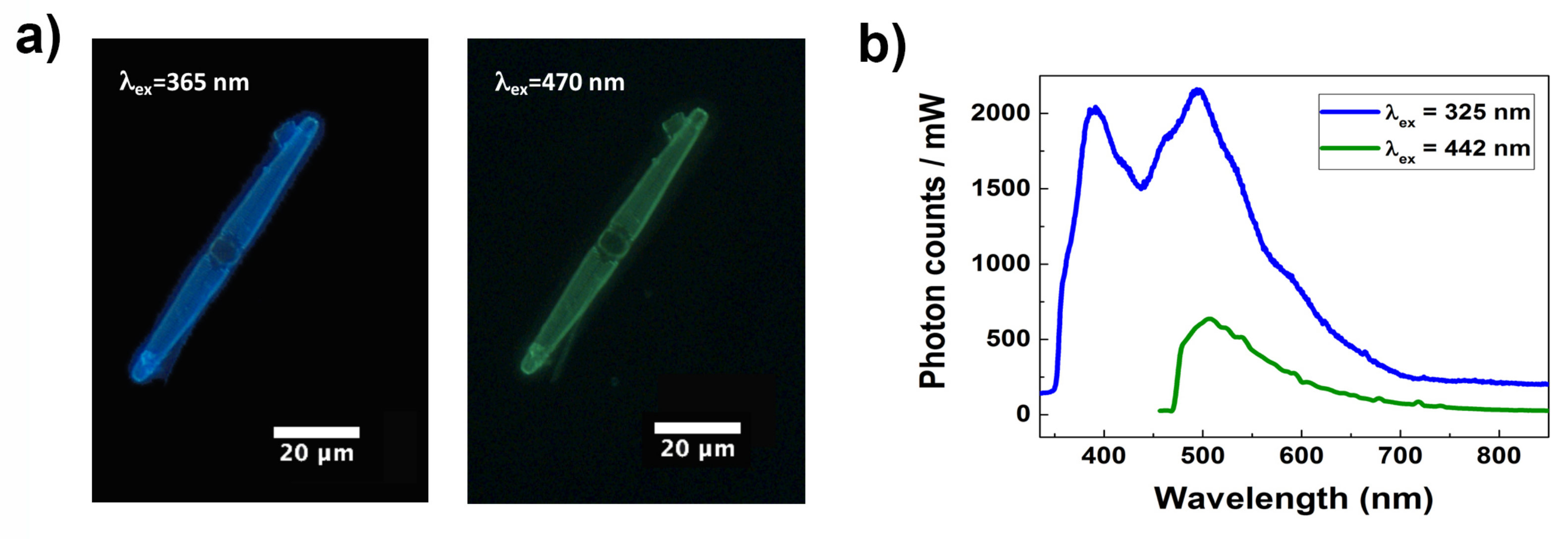
3.3. Photoluminescence Properties of the Frustule
3.4. Interaction of Living Cells with Optical Radiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UVR | Ultra-violet radiation |
| VIS | Visible radiation |
| NIR | Near-infrared radiation |
| PAR | Photosynthetic active radiation |
| PER | Photo-enzymatic repair photoreactivation |
| MAA | Mycosporine-like amino acid |
| HPLC | High performance liquid chromatography |
| rETR | Relative electron transport rate |
| PFR | Photon fluence rate |
| WA-BPM | Wide-angle beam propagation method |
| CAD | Computer-aided design |
| FWHM | Full width at half maximum |
| CMOS | Complementary metal-oxide semiconductor |
| CCD | Charged-coupled device |
| SEM | Scanning electron microscopy |
| CSLM | Confocal laser scanning microscopy |
References
- Seckbach, J.; Kociolek, P. The Diatom World; Springer Science & Business Media: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK; New York, NY, USA, 2011; Volume 19. [Google Scholar]
- Leblanc, K.; Queguiner, B.; Diaz, F.; Cornet, V.; Michel-Rodriguez, M.; de Madron, X.D.; Bowler, C.; Malviya, S.; Thyssen, M.; Grégori, G.; et al. Nanoplanktonic diatoms are globally overlooked but play a role in spring blooms and carbon export. Nat. Commun. 2018, 9, 953. [Google Scholar] [CrossRef]
- Hildebrand, M.; Lerch, S.J.; Shrestha, R.P. Understanding diatom cell wall silicification—Moving forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef]
- Nelson, D.M.; Tréguer, P.; Brzezinski, M.A.; Leynaert, A.; Quéguiner, B. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Smol, J.P.; Stoermer, E.F. The Diatoms: Applications for the Environmental and Earth Sciences; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Zgłobicka, I.; Gluch, J.; Liao, Z.; Werner, S.; Guttmann, P.; Li, Q.; Bazarnik, P.; Plocinski, T.; Witkowski, A.; Kurzydlowski, K.J. Insight into diatom frustule structures using various imaging techniques. Sci. Rep. 2021, 11, 14555. [Google Scholar] [CrossRef] [PubMed]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- De Tommasi, E.; Gielis, J.; Rogato, A. Diatom frustule morphogenesis and function: A multidisciplinary survey. Mar. Genom. 2017, 35, 1–18. [Google Scholar] [CrossRef]
- Losic, D.; Mitchell, J.G.; Lal, R.; Voelcker, N.H. Rapid fabrication of micro-and nanoscale patterns by replica molding from diatom biosilica. Adv. Funct. Mater. 2007, 17, 2439–2446. [Google Scholar] [CrossRef]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.S.; Mitchell, J.G. Functional morphology of diatom frustule microstructures: Hydrodynamic control of Brownian particle diffusion and advection. Aquat. Microb. Ecol. 2001, 24, 287–295. [Google Scholar] [CrossRef]
- Hale, M.S.; Mitchell, J.G. Effects of particle size, flow velocity, and cell surface microtopography on the motion of submicrometer particles over diatoms. Nano Lett. 2002, 2, 657–663. [Google Scholar] [CrossRef]
- Waite, A.; Fisher, A.; Thompson, P.A.; Harrison, P.J. Sinking rate versus cell volume relationships illuminate sinking rate control mechanisms in marine diatoms. Mar. Ecol. Prog. Ser. 1997, 157, 97–108. [Google Scholar] [CrossRef]
- De Tommasi, E. Light manipulation by single cells: The case of diatoms. J. Spectrosc. 2016, 2016, 2490128. [Google Scholar] [CrossRef]
- De Stefano, L.; Rea, I.; Rendina, I.; De Stefano, M.; Moretti, L. Lensless light focusing with the centric marine diatom Coscinodiscus walesii. Opt. Express 2007, 15, 18082–18088. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, E.; Rea, I.; Mocella, V.; Moretti, L.; De Stefano, M.; Rendina, I.; De Stefano, L. Multi-wavelength study of light transmitted through a single marine centric diatom. Opt. Express 2010, 18, 12203–12212. [Google Scholar] [CrossRef]
- Ferrara, M.A.; Dardano, P.; De Stefano, L.; Rea, I.; Coppola, G.; Rendina, I.; Congestri, R.; Antonucci, A.; De Stefano, M.; De Tommasi, E. Optical properties of diatom nanostructured biosilica in Arachnoidiscus sp: Micro-optics from mother nature. PLoS ONE 2014, 9, e103750. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, T.; Landwehr, S.; El Rharbi-Kucki, M.; Sumper, M. Diatoms as living photonic crystals. Appl. Phys. B 2004, 78, 257–260. [Google Scholar] [CrossRef]
- Goessling, J.W.; Wardley, W.P.; Lopez-Garcia, M. Highly Reproducible, Bio-Based Slab Photonic Crystals Grown by Diatoms. Adv. Sci. 2020, 7, 1903726. [Google Scholar] [CrossRef]
- Rogato, A.; De Tommasi, E. Physical, Chemical, and Genetic Techniques for Diatom Frustule Modification: Applications in Nanotechnology. Appl. Sci. 2020, 10, 8738. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Mirliss, M.J. Diatomaceous Earth filtration for drinking water. In National Drinking Water Clearing House Fact Sheet; West Virginia University: Morgantown, WV, USA, 2001; Volume 1. [Google Scholar]
- Verma, K. Role of diatoms in the world of forensic science. J. Forensic Res. 2013, 4, 181–184. [Google Scholar] [CrossRef]
- De Tommasi, E.; De Luca, A.; Lavanga, L.; Dardano, P.; De Stefano, M.; De Stefano, L.; Langella, C.; Rendina, I.; Dholakia, K.; Mazilu, M. Biologically enabled sub-diffractive focusing. Opt. Express 2014, 22, 27214–27227. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, V.W.; Cai, Y.; Berrigan, J.D.; Marder, S.R.; Perry, J.W.; Sandhage, K.H. Biologically enabled syntheses of freestanding metallic structures possessing subwavelength pore arrays for extraordinary (surface plasmon-mediated) infrared transmission. Adv. Funct. Mater. 2012, 22, 2550–2559. [Google Scholar] [CrossRef]
- Managò, S.; Zito, G.; Rogato, A.; Casalino, M.; Esposito, E.; De Luca, A.C.; De Tommasi, E. Bioderived three-dimensional hierarchical nanostructures as efficient surface-enhanced raman scattering substrates for cell membrane probing. ACS Appl. Mater. Interfaces 2018, 10, 12406–12416. [Google Scholar] [CrossRef]
- Kumari, S.; Min, K.H.; Kanth, B.K.; Jang, E.K.; Pack, S.P. Production of TiO2-deposited Diatoms and Their Applications for Photo-catalytic Degradation of Aqueous Pollutants. Biotechnol. Bioprocess Eng. 2020, 25, 758–765. [Google Scholar] [CrossRef]
- De Stefano, L.; Rotiroti, L.; De Stefano, M.; Lamberti, A.; Lettieri, S.; Setaro, A.; Maddalena, P. Marine diatoms as optical biosensors. Biosens. Bioelectron. 2009, 24, 1580–1584. [Google Scholar] [CrossRef]
- Gale, D.K.; Gutu, T.; Jiao, J.; Chang, C.H.; Rorrer, G.L. Photoluminescence detection of biomolecules by antibody-functionalized diatom biosilica. Adv. Funct. Mater. 2009, 19, 926–933. [Google Scholar] [CrossRef]
- Toster, J.; Iyer, K.S.; Xiang, W.; Rosei, F.; Spiccia, L.; Raston, C.L. Diatom frustules as light traps enhance DSSC efficiency. Nanoscale 2013, 5, 873–876. [Google Scholar] [CrossRef]
- Bandara, T.; Furlani, M.; Albinsson, I.; Wulff, A.; Mellander, B.E. Diatom frustules enhancing the efficiency of gel polymer electrolyte based dye-sensitized solar cells with multilayer photoelectrodes. Nanoscale Adv. 2020, 2, 199–209. [Google Scholar] [CrossRef]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Taté, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 3393–3403. [Google Scholar] [CrossRef]
- Managò, S.; Tramontano, C.; Delle Cave, D.; Chianese, G.; Zito, G.; De Stefano, L.; Terracciano, M.; Lonardo, E.; De Luca, A.C.; Rea, I. SERS Quantification of Galunisertib Delivery in Colorectal Cancer Cells by Plasmonic-Assisted Diatomite Nanoparticles. Small 2021, 17, 2101711. [Google Scholar] [CrossRef] [PubMed]
- Ellegaard, M.; Lenau, T.; Lundholm, N.; Maibohm, C.; Friis, S.M.M.; Rottwitt, K.; Su, Y. The fascinating diatom frustule—Can it play a role for attenuation of UV radiation? J. Appl. Phycol. 2016, 28, 3295–3306. [Google Scholar] [CrossRef]
- Su, Y.; Lenau, T.A.; Gundersen, E.; Kirkensgaard, J.J.; Maibohm, C.; Pinti, J.; Ellegaard, M. The UV filtering potential of drop-casted layers of frustules of three diatom species. Sci. Rep. 2018, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.E.; Ouyang, L.; Elfwing, A.; Hedblom, M.; Wulff, A.; Inganäs, O. Diatom frustules protect DNA from ultraviolet light. Sci. Rep. 2018, 8, 5138. [Google Scholar] [CrossRef]
- De Tommasi, E.; Congestri, R.; Dardano, P.; De Luca, A.C.; Managò, S.; Rea, I.; De Stefano, M. UV-shielding and wavelength conversion by centric diatom nanopatterned frustules. Sci. Rep. 2018, 8, 16285. [Google Scholar] [CrossRef]
- Karentz, D.; Cleaver, J.E.; Mitchell, D.L. Cell Survival Characteristics and Molecular Responses of Antarctic Phytoplankton to Ultraviolet-B Radiation 1. J. Phycol. 1991, 27, 326–341. [Google Scholar] [CrossRef]
- Smith, R.C.; Prezelin, B.; Baker, K.; Bidigare, R.; Boucher, N.; Coley, T.; Karentz, D.; MacIntyre, S.; Matlick, H.; Menzies, D.; et al. Ozone depletion: Ultraviolet radiation and phytoplankton biology in Antarctic waters. Science 1992, 255, 952–959. [Google Scholar] [CrossRef]
- Kiefer, J. Biological Radiation Effects; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Jeffrey, S.; MacTavish, H.; Dunlap, W.; Vesk, M.; Groenewoud, K. Occurrence of UVA-and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Mar. Ecol. Prog. Ser. 1999, 189, 35–51. [Google Scholar] [CrossRef]
- Xiong, F.; Komenda, J.; Kopeckỳ, J.; Nedbal, L. Strategies of ultraviolet-B protection in microscopic algae. Physiol. Plant. 1997, 100, 378–388. [Google Scholar] [CrossRef]
- Wulff, A.; Nilsson, C.; Sundbäck, K.; Odmark, S.Å.W.S. UV radiation effects on microbenthos—A four month field experiment. Aquat. Microb. Ecol. 1999, 19, 269–278. [Google Scholar] [CrossRef]
- Wulff, A.; Roleda, M.Y.; Zacher, K.; Wiencke, C. UV radiation effects on pigments, photosynthetic efficiency and DNA of an Antarctic marine benthic diatom community. Aquat. Biol. 2008, 3, 167–177. [Google Scholar] [CrossRef][Green Version]
- Wulff, A.; Zacher, K.; Hanelt, D.; Al-Handal, A.; Wiencke, C. UV radiation—A threat to Antarctic benthic marine diatoms? Antarct. Sci. 2008, 20, 13–20. [Google Scholar] [CrossRef]
- Wulff, A.; Wängberg, S.Å.k.; Sundbäck, K.; Nilsson, C.; Underwood, G.J. Effects of UVB radiation on a marine microphytobenthic community growing on a sand-substratum under different nutrient conditions. Limnol. Oceanogr. 2000, 45, 1144–1152. [Google Scholar] [CrossRef]
- Zacher, K.; Hanelt, D.; Wiencke, C.; Wulff, A. Grazing and UV radiation effects on an Antarctic intertidal microalgal assemblage: A long-term field study. Polar Biol. 2007, 30, 1203–1212. [Google Scholar] [CrossRef][Green Version]
- Sundbäck, K.; Nilsson, C.; Odmark, S.; Wulff, A. Does ambient UV-B radiation influence marine diatom-dominated microbial mats? A case study. Aquat. Microb. Ecol. 1996, 11, 151–159. [Google Scholar] [CrossRef][Green Version]
- Underwood, G.J.; Nilsson, C.; Sundbäck, K.; Wulff, A. Short-term effects of UVB radiation on chlorophyll fluorescence, biomass, pigments, and carbohydrate fractions in a benthic diatom mat. J. Phycol. 1999, 35, 656–666. [Google Scholar] [CrossRef]
- Sliney, D.H. Radiometric quantities and units used in photobiology and photochemistry: Recommendations of the Commission Internationale de l’Eclairage (International Commission on Illumination). Photochem. Photobiol. 2007, 83, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Helbling, E.W.; Villafane, V.E.; Buma, A.G.; Andrade, M.; Zaratti, F. DNA damage and photosynthetic inhibition induced by solar ultraviolet radiation in tropical phytoplankton (Lake Titicaca, Bolivia). Eur. J. Phycol. 2001, 36, 157–166. [Google Scholar] [CrossRef]
- Vass, I.; Szilárd, A.; Sicora, C. Adverse effects of UV-B light on the structure and function of the photosynthetic apparatus. Section XIII: Photosynthesis under environmental stress conditions. In Handbook of Photosynthesis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hadley, G.R. Wide-angle beam propagation using Padé approximant operators. Opt. Lett. 1992, 17, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Hadley, G.R. Multistep method for wide-angle beam propagation. Opt. Lett. 1992, 17, 1743–1745. [Google Scholar] [CrossRef]
- Yu, L.; Cai, L. Iterative algorithm with a constraint condition for numerical reconstruction of a three-dimensional object from its hologram. J. Opt. Soc. Am. A 2001, 18, 1033–1045. [Google Scholar] [CrossRef]
- Goodman, J.W. Introduction to Fourier Optics; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Nazarathy, M.; Shamir, J. Fourier optics described by operator algebra. J. Opt. Soc. Am. 1980, 70, 150–159. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light; Pergamon Press: Oxford, UK, 2013. [Google Scholar]
- Rodríguez-de Marcos, L.V.; Larruquert, J.I.; Méndez, J.A.; Aznárez, J.A. Self-consistent optical constants of SiO2 and Ta2O5 films. Opt. Mater. Express 2016, 6, 3622–3637. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Gutu, T.; Jiao, J.; Chang, C.H.; Rorrer, G.L. Photoluminescence of silica nanostructures from bioreactor culture of marine diatom Nitzschia frustulum. J. Nanosci. Nanotechnol. 2008, 8, 2392–2398. [Google Scholar] [CrossRef] [PubMed]
- Itoh, C.; Tanimura, K.; Itoh, N. Optical studies of self-trapped excitons in SiO2. J. Phys. C Solid State Phys. 1988, 21, 4693. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- De Stefano, L.; Rendina, I.; De Stefano, M.; Bismuto, A.; Maddalena, P. Marine diatoms as optical chemical sensors. Appl. Phys. Lett. 2005, 87, 233902. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Mulders, K.J.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Bhowmik, A.; Pilon, L. Can spherical eukaryotic microalgae cells be treated as optically homogeneous? J. Opt. Soc. Am. A 2016, 33, 1495–1503. [Google Scholar] [CrossRef]
- Dauchet, J.; Blanco, S.; Cornet, J.F.; Fournier, R. Calculation of the radiative properties of photosynthetic microorganisms. J. Quant. Spectrosc. Radiat. Transf. 2015, 161, 60–84. [Google Scholar] [CrossRef]
- Lehmuskero, A.; Chauton, M.S.; Boström, T. Light and photosynthetic microalgae: A review of cellular-and molecular-scale optical processes. Prog. Oceanogr. 2018, 168, 43–56. [Google Scholar] [CrossRef]
- Goold, H.; Beisson, F.; Peltier, G.; Li-Beisson, Y. Microalgal lipid droplets: Composition, diversity, biogenesis and functions. Plant Cell Rep. 2015, 34, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Nojima, D.; Yoshino, T.; Tanaka, T. Structure and properties of oil bodies in diatoms. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160408. [Google Scholar] [CrossRef]
- Wang, Z.T.; Ullrich, N.; Joo, S.; Waffenschmidt, S.; Goodenough, U. Algal lipid bodies: Stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot. Cell 2009, 8, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Siaut, M.; Cuine, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylides, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Boyle, N.R.; Page, M.D.; Liu, B.; Blaby, I.K.; Casero, D.; Kropat, J.; Cokus, S.J.; Hong-Hermesdorf, A.; Shaw, J.; Karpowicz, S.J.; et al. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J. Biol. Chem. 2012, 287, 15811–15825. [Google Scholar] [CrossRef] [PubMed]
- Hemschemeier, A.; Casero, D.; Liu, B.; Benning, C.; Pellegrini, M.; Happe, T.; Merchant, S.S. Copper response regulator1—Dependent and—Independent responses of the Chlamydomonas reinhardtii transcriptome to dark anoxia. Plant Cell 2013, 25, 3186–3211. [Google Scholar] [CrossRef]


| Photosynthetic Activity | Specific | |||||
|---|---|---|---|---|---|---|
| Growth Rate | ||||||
| (Rel. Units) | (Mol Photons ) | % | NPQ | () | ||
| Start | 0.470 (0.015) | 93 (11) | 610 (30) | 0.15 (0.06) | 0.59 (0.08) | |
| 7 days treatment: | ||||||
| Control | 0.541 (0.019) | 100 (10) | 610 (30) | 0.16 (0.07) | 0.56 (0.02) | 0.091 (0.005) |
| PAR | 0.34 (0.04) | 119 (13) | 680 (30) | 0.17 (0.05) | 1.12 (0.15) | 0.118 (0.014) |
| PA | 0.29 (0.05) | 149 (14) | 700 (30) | 0.21 (0.09) | 1.00 (0.08) | 0.080 (0.003) |
| PAB | 0.28 (0.03) | 118 (14) | 690 (30) | 0.13 (0.06) | 1.00 (0.09) | 0.085 (0.008) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Tommasi, E.; Rea, I.; Ferrara, M.A.; De Stefano, L.; De Stefano, M.; Al-Handal, A.Y.; Stamenković, M.; Wulff, A. Underwater Light Manipulation by the Benthic Diatom Ctenophora pulchella: From PAR Efficient Collection to UVR Screening. Nanomaterials 2021, 11, 2855. https://doi.org/10.3390/nano11112855
De Tommasi E, Rea I, Ferrara MA, De Stefano L, De Stefano M, Al-Handal AY, Stamenković M, Wulff A. Underwater Light Manipulation by the Benthic Diatom Ctenophora pulchella: From PAR Efficient Collection to UVR Screening. Nanomaterials. 2021; 11(11):2855. https://doi.org/10.3390/nano11112855
Chicago/Turabian StyleDe Tommasi, Edoardo, Ilaria Rea, Maria Antonietta Ferrara, Luca De Stefano, Mario De Stefano, Adil Y. Al-Handal, Marija Stamenković, and Angela Wulff. 2021. "Underwater Light Manipulation by the Benthic Diatom Ctenophora pulchella: From PAR Efficient Collection to UVR Screening" Nanomaterials 11, no. 11: 2855. https://doi.org/10.3390/nano11112855
APA StyleDe Tommasi, E., Rea, I., Ferrara, M. A., De Stefano, L., De Stefano, M., Al-Handal, A. Y., Stamenković, M., & Wulff, A. (2021). Underwater Light Manipulation by the Benthic Diatom Ctenophora pulchella: From PAR Efficient Collection to UVR Screening. Nanomaterials, 11(11), 2855. https://doi.org/10.3390/nano11112855










