A Water-Soluble Polyacid Polymer Based on Hydrophilic Metal–Organic Frameworks Using Amphoteric Carboxylic Acid Ligands as Linkers for Hydroxycamptothecin Loading and Release In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of Hydrophilic MOF
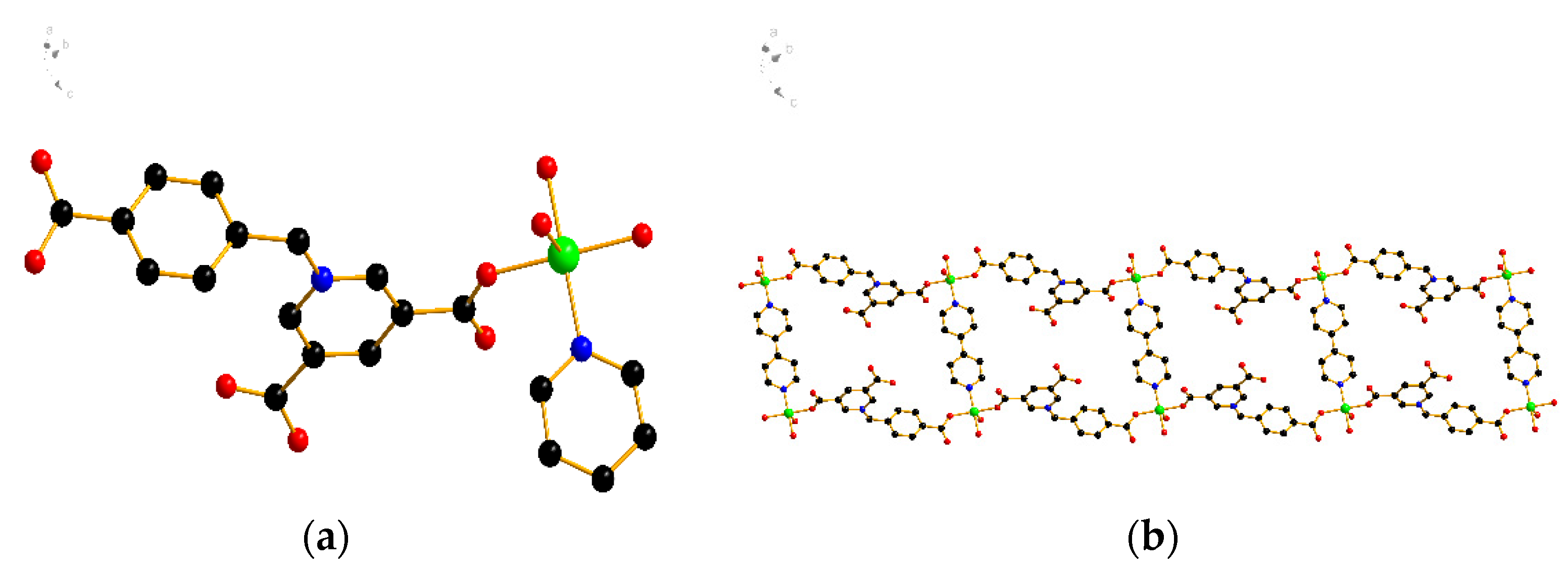
2.3. X-ray Crystallographic Study
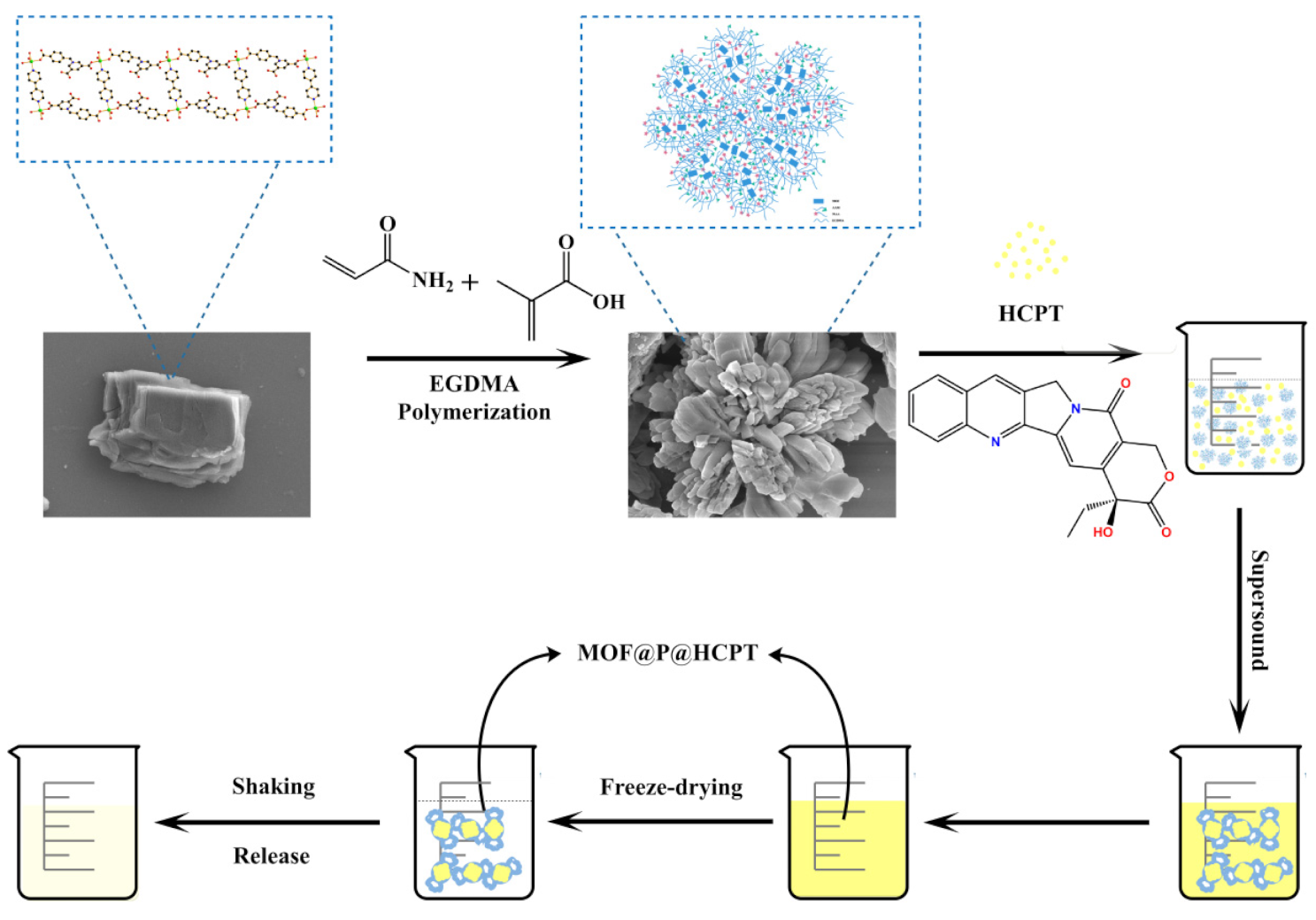
2.4. Synthesis of Water-Soluble Composites (MOF@P)
2.5. Drug Loading on Water-Soluble Composites (MOF@P)
2.6. Drug Release In Vitro under pH-Controlled Conditions
2.7. Cytotoxicity Assay
3. Results
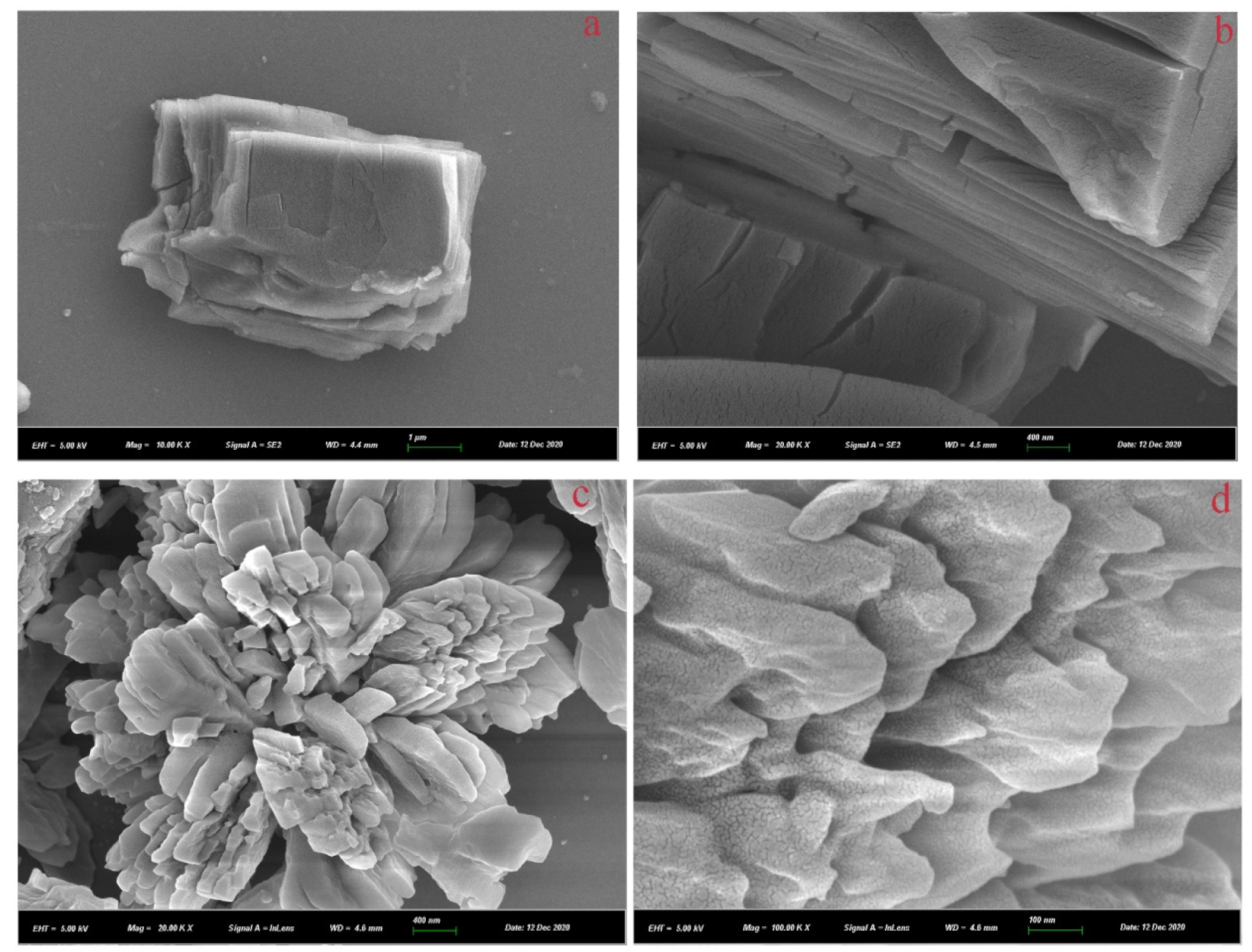
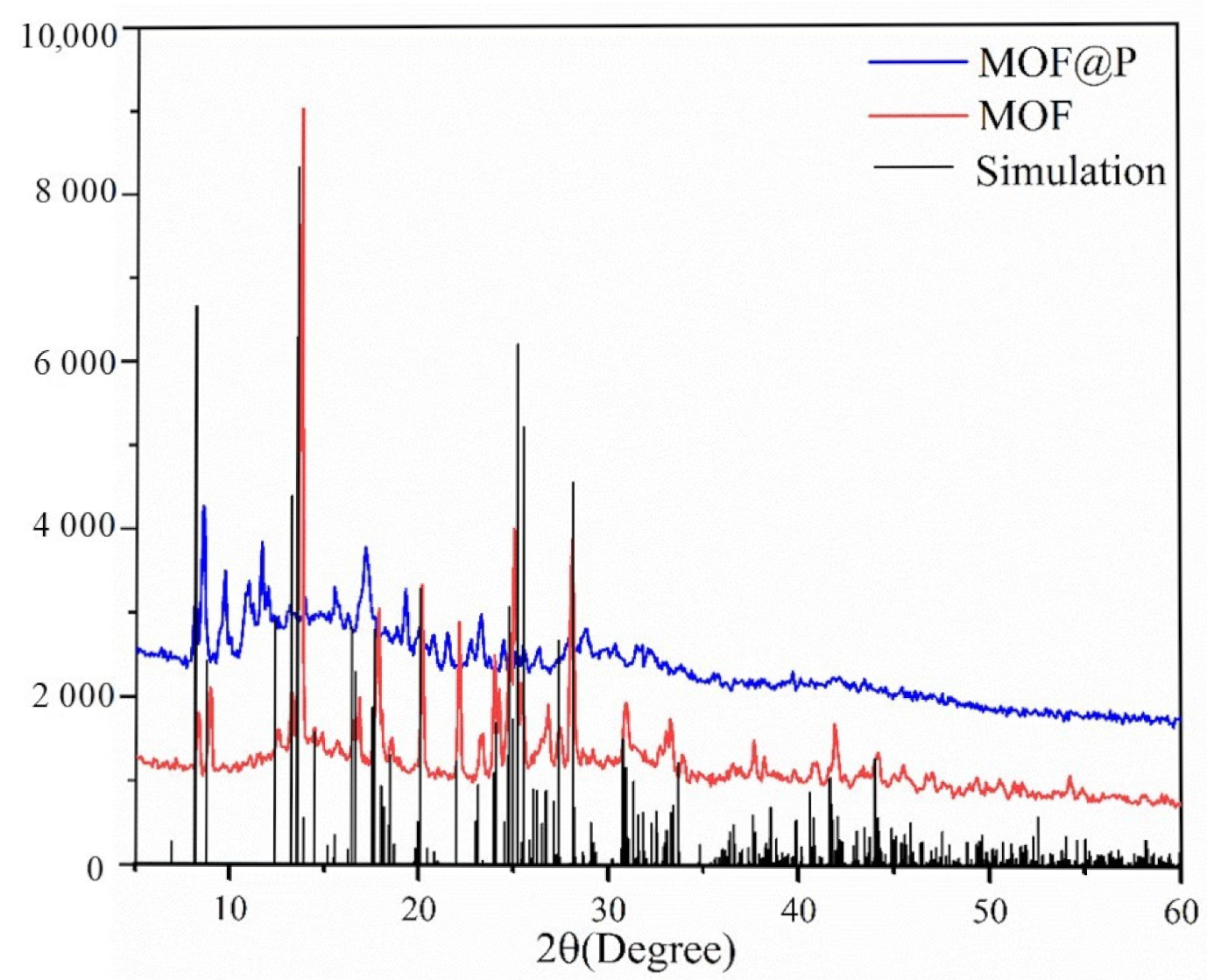
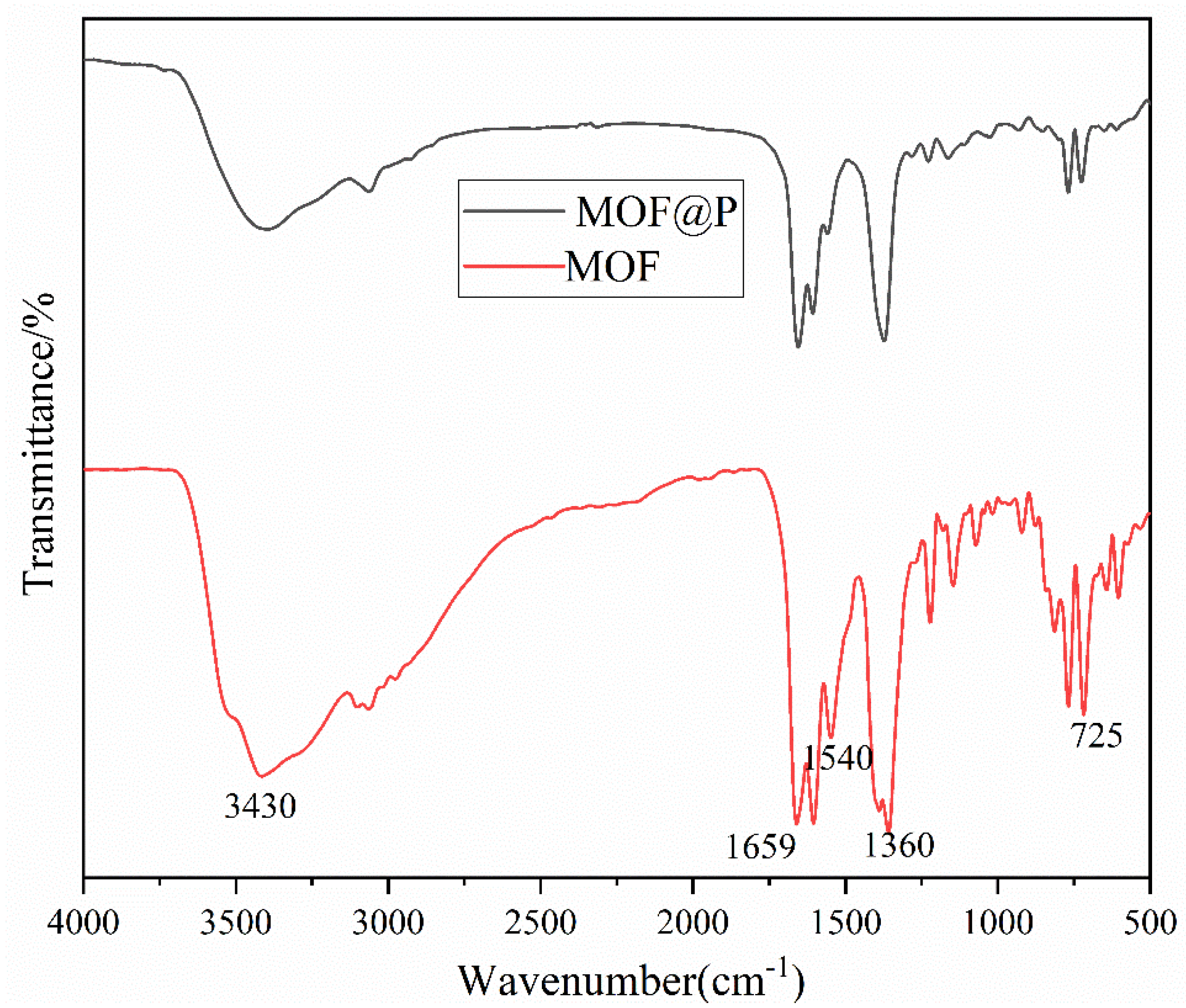
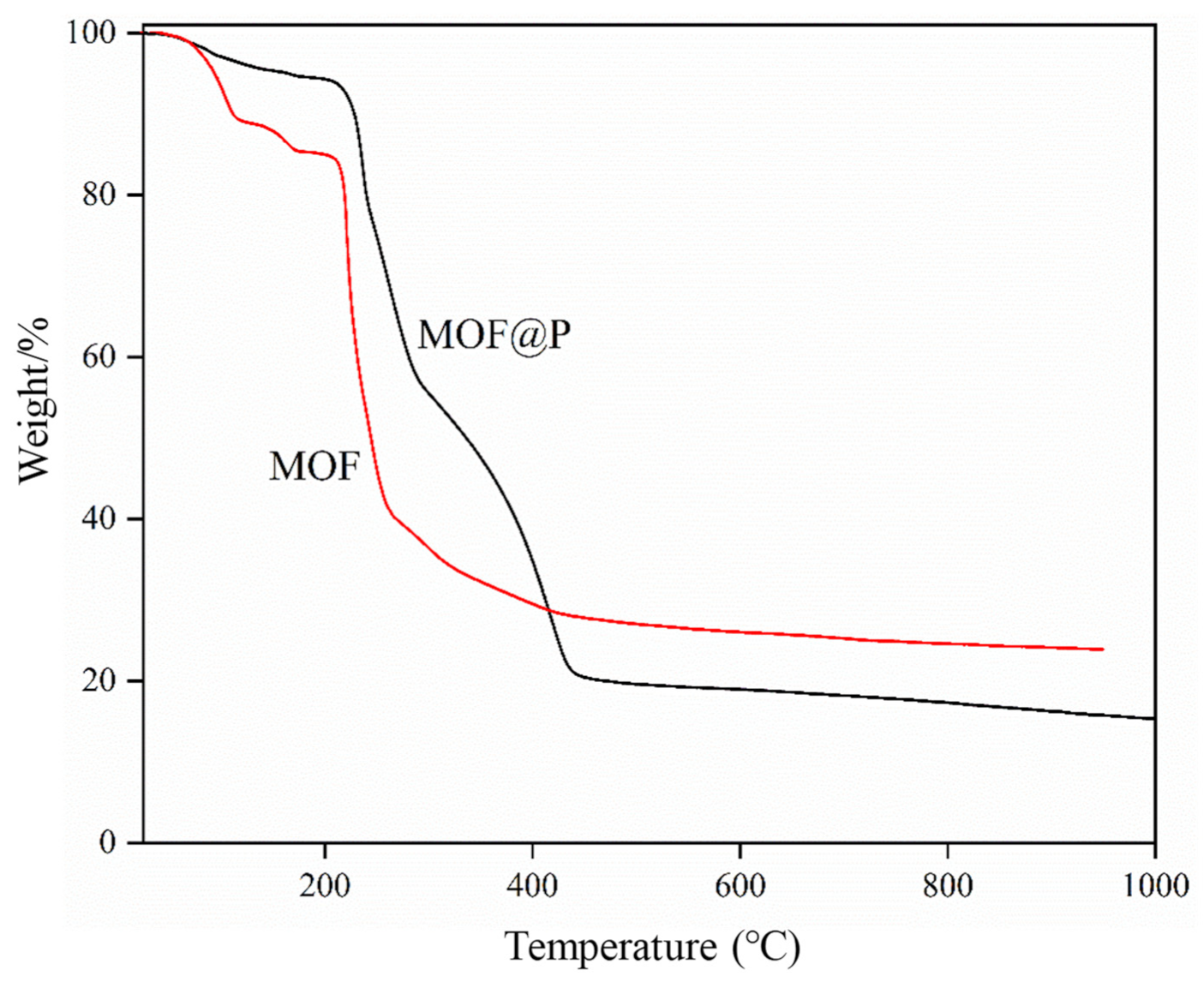
3.1. Characterization of the Synthesized Materials
3.2. Optimization of the MOF@P synthesis Parameters
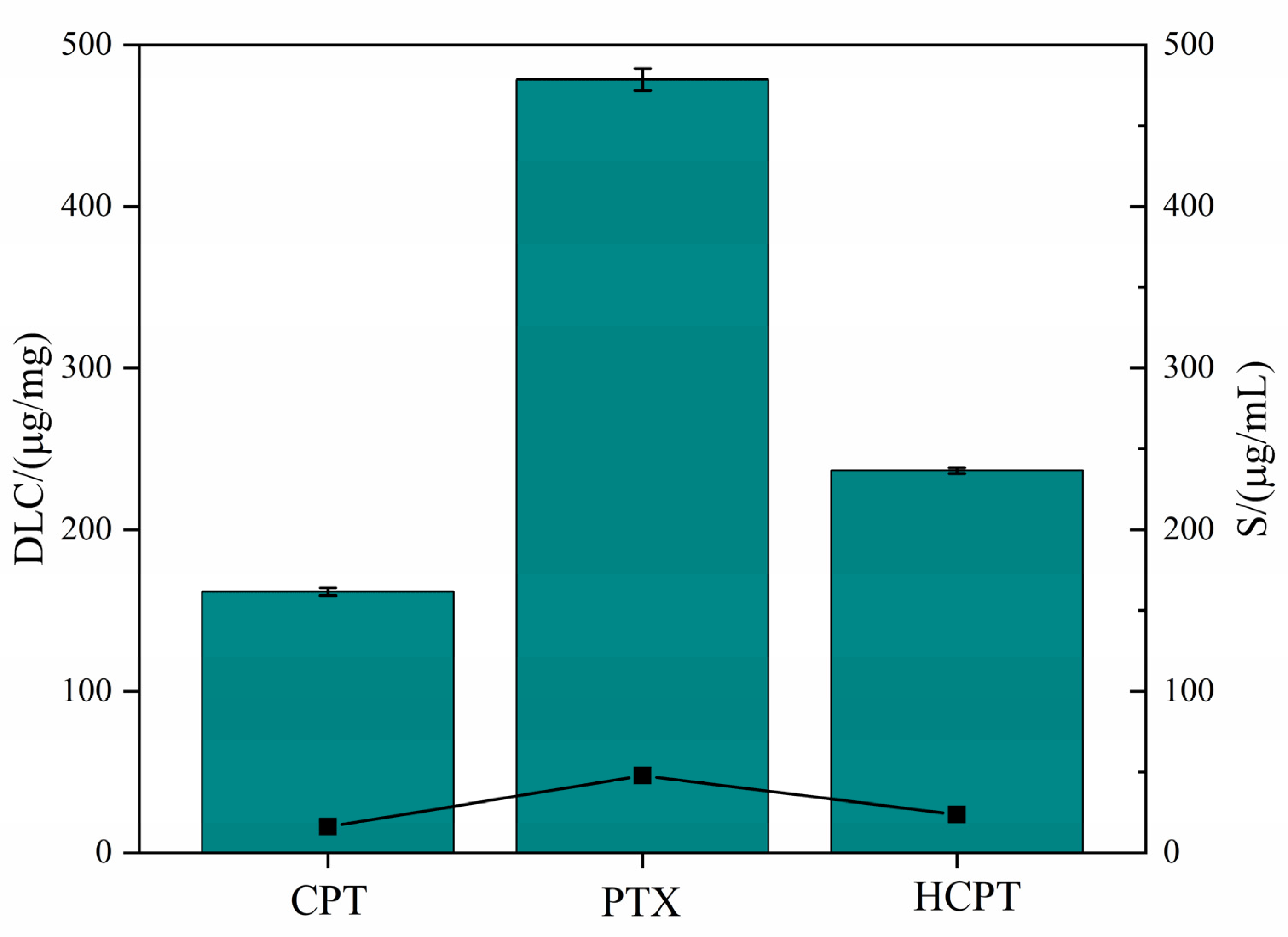
3.3. Drug Solubilization Research
3.4. Stability of Drug-Loaded MOF@P
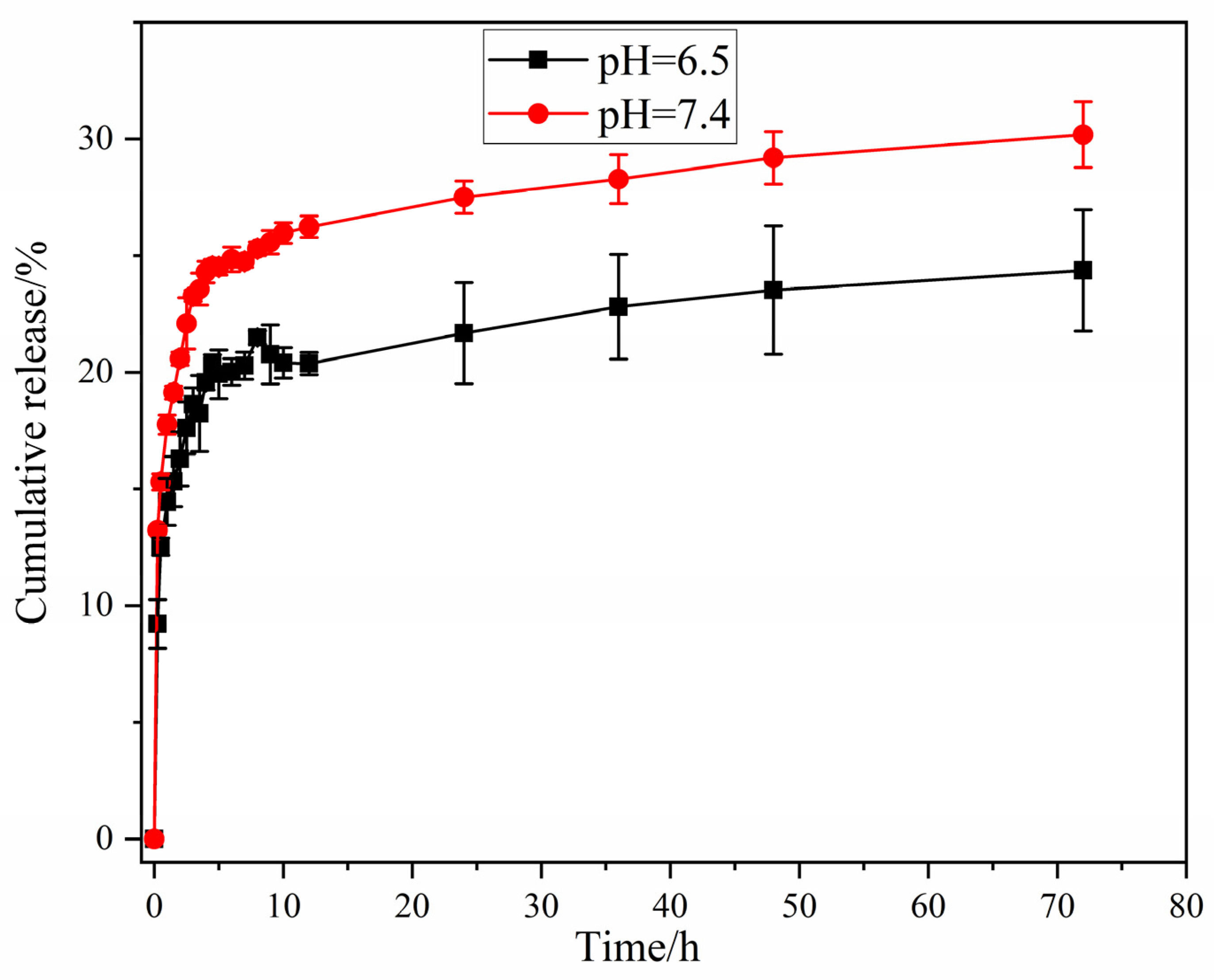
3.5. In Vitro Release
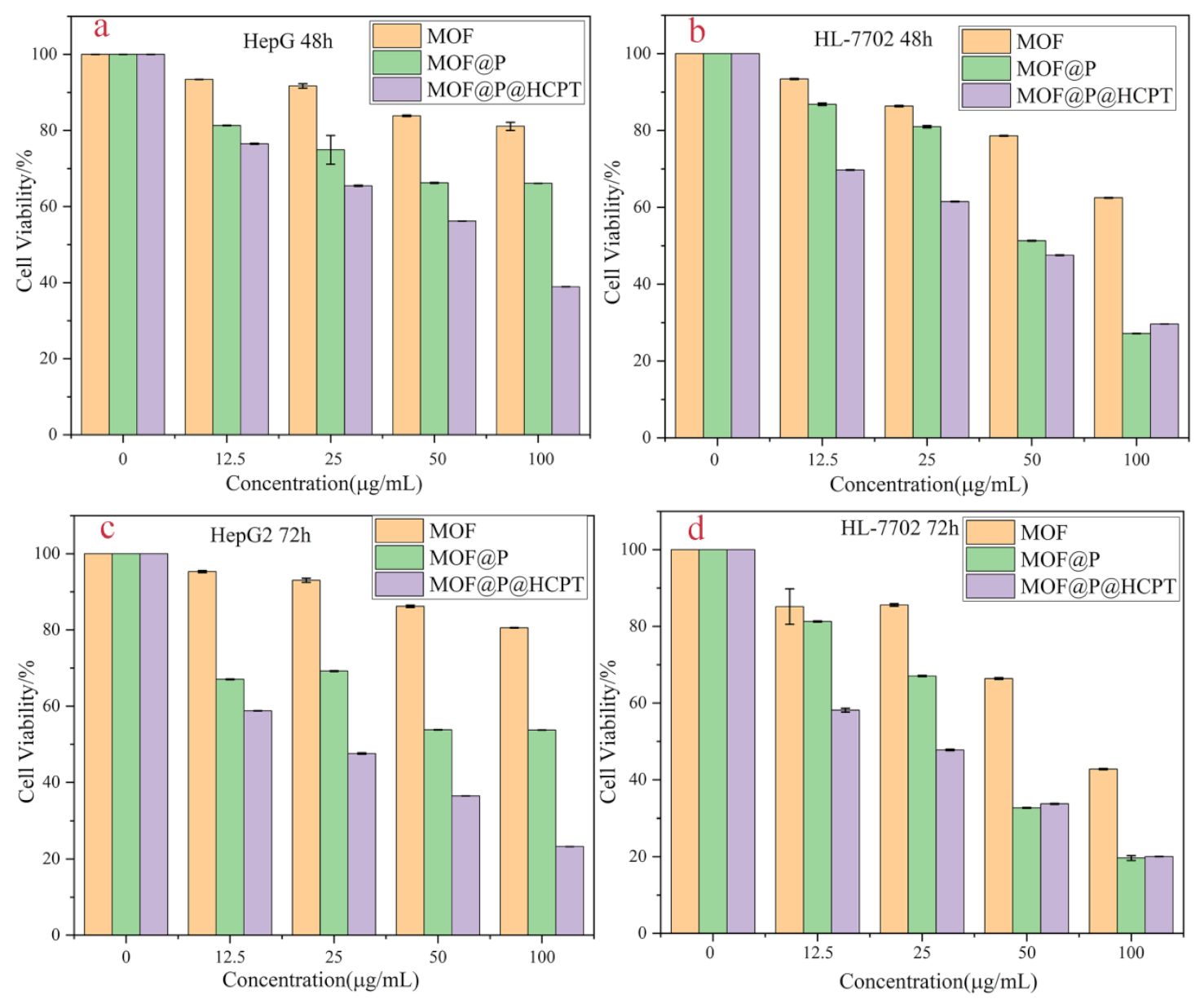
3.6. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guo, S.; Zheng, J.; Dong, J.; Guo, N.; Jing, L.; Yue, X.; Yan, X.; Wang, Y.; Dai, Z. Iron/dextran sulfate multilayered microcapsules for controlled release of 10-hydroxycamptothecin. Int. J. Biol. Macromol. 2011, 49, 409–415. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Sun, S.; Liu, B.; Wu, N.; Cao, X. The effect of 10-hydroxycamptothecine in preventing fibroblast proliferation and epidural scar adhesion after laminectomy in rats. Eur. J. Pharmacol. 2008, 593, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Lin, S.-Y.; Hsieh, T.-F.; Chan, S.-A. Thermal behavior and thermal decarboxylation of 10-hydroxycamptothecin in the solid state. J. Pharm. Biomed. Anal. 2007, 43, 457–463. [Google Scholar] [CrossRef]
- Yuan, Z.F.; Tang, Y.M.; Xu, X.J.; Li, S.S.; Zhang, J.Y. 10-Hydroxycamptothecin induces apoptosis in human neuroblastoma SMS-KCNR cells through p53, cytochrome c and caspase 3 pathways. Neoplasma 2016, 63, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.; Wang, Y.; Kokot, S. Study of the interaction between 10-hydroxycamptothecine and DNA with the use of ethidium bromide dye as a fluorescence probe. Sens. Actuators B Chem. 2011, 156, 290–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Q.; Yin, L.; Ma, L.; Tang, L.; Cheng, J. Chain-shattering polymeric therapeutics with on-demand drug-release capability. Angew. Chem. Int. Ed. 2013, 52, 6435–6439. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Schellinger, J.G.; Chu, D.S.H.; Pun, S.H. Neuron-Targeted Copolymers with Sheddable Shielding Blocks Synthesized Using a Reducible, RAFT-ATRP Double-Head Agent. J. Am. Chem. Soc. 2012, 134, 16554–16557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çırpanlı, Y.; Allard, E.; Passirani, C.; Bilensoy, E.; Lemaire, L.; Çalış, S.; Benoit, J.-P. Antitumoral activity of camptothecin-loaded nanoparticles in 9L rat glioma model. Int. J. Pharm. 2010, 403, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Q.; Ye, W.-L.; Huan, M.-L.; Cheng, Y.; Liu, D.-Z.; Cui, H.; Liu, M.; Zhang, B.-L.; Mei, Q.-B.; Zhou, S.-Y. Mitochondria and nucleus delivery of active form of 10-hydroxycamptothecin with dual shell to precisely treat colorectal cancer. Nanomedicine 2019, 14, 1011–1032. [Google Scholar] [CrossRef]
- Yang, L.; Hong, J.; Di, J.; Guo, Y.; Han, M.; Liu, M.; Wang, X. 10-Hydroxycamptothecin (HCPT) nanosuspensions stabilized by mPEG1000-HCPT conjugate: High stabilizing efficiency and improved antitumor efficacy. Int. J. Nanomed. 2017, ume 12, 3681–3695. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Liu, J.; Li, X.; Xu, Y.; Liu, D.; He, H.; Wang, Y.; Tang, X. Hydroxycamptothecin (HCPT)-loaded PEGlated lipid–polymer hybrid nanoparticles for effective delivery of HCPT: QbD-based development and evaluation. Drug Deliv. Transl. Res. 2021, 1–19. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Huang, J.; Qiu, S.; Zhong, H.; Liu, D.; Liu, J. Cyclodextrin-Based Metal-Organic Frameworks (CD-MOFs) in Pharmaceutics and Biomedicine. Pharmaceutics 2018, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Jalvandi, J.; White, M.; Gao, Y.; Truong, Y.B.; Padhye, R.; Kyratzis, I.L. Polyvinyl alcohol composite nanofibres containing conjugated levofloxacin-chitosan for controlled drug release. Mater. Sci. Eng. C 2017, 73, 440–446. [Google Scholar] [CrossRef]
- Kiadeh, S.Z.H.; Ghaee, A.; Farokhi, M.; Nourmohammadi, J.; Bahi, A.; Ko, F.K. Electrospun pectin/modified copper-based metal–organic framework (MOF) nanofibers as a drug delivery system. Int. J. Biol. Macromol. 2021, 173, 351–365. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, G.; Zeng, L.; Song, X.; Bian, X.-W. Nanoscaled Metal-Organic Frameworks for Biosensing, Imaging, and Cancer Therapy. Adv. Health Mater. 2018, 7, e1800022. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, A.; Darpandeep, A.; Xuan, Z.; Wolfgang, V.; Kim, R.D.; Mario, W. Switching of Adsorption Properties in a Zwitterionic Metal–Organic Framework Triggered by Photogenerated Radical Triplets. Chem. Mater. 2016, 28, 7825–7832. [Google Scholar] [CrossRef]
- Leroux, M.; Mercier, N.; Allain, M.; Dul, M.C.; Dittmer, J.; Kassiba, A.H.; Bellat, J.P.; Weber, G.; Bezverkhyy, I. Porous Coordination Polymer Based on Bipyridinium Carboxylate Linkers with High and Reversible Ammonia Uptake. Inorg. Chem. 2016, 55, 8587–8594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, M.; Nakamura, K.; Horike, S.; Hijikata, Y.; Yanai, N.; Fukushima, T.; Kim, J.; Kato, K.; Takata, M.; Watanabe, D.; et al. Design of Flexible Lewis Acidic Sites in Porous Coordination Polymers by using the Viologen Moiety. Angew. Chem. Int. Ed. 2012, 51, 8369–8372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Q.; Yang, S.-P.; Ding, N.-N.; Qin, L.; Qiu, G.-H.; Chen, J.-X.; Zhang, W.-H.; Chen, W.-H.; Hor, T.S.A. A zwitterionic 1D/2D polymer co-crystal and its polymorphic sub-components: A highly selective sensing platform for HIV ds-DNA sequences. Dalton Trans. 2016, 45, 5092–5100. [Google Scholar] [CrossRef]
- Xie, B.-P.; Qiu, G.-H.; Sun, B.; Yang, Z.-F.; Zhang, W.-H.; Chen, J.-X.; Jiang, Z.-H. Synchronous sensing of three conserved sequences of Zika virus using a DNAs@MOF hybrid: Experimental and molecular simulation studies. Inorg. Chem. Front. 2018, 6, 148–152. [Google Scholar] [CrossRef]
- Zhai, L.-Y.; Li, M.-X.; Pan, W.-L.; Chen, Y.; Li, M.-M.; Pang, J.-X.; Zheng, L.; Chen, J.-X.; Duan, W.-J. In Situ Detection of Plasma Exosomal MicroRNA-1246 for Breast Cancer Diagnostics by a Au Nanoflare Probe. ACS Appl. Mater. Interfaces 2018, 10, 39478–39486. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Li, R.-T.; Zhang, M.-M.; Wu, K.-Y.; Li, H.-H.; Huang, N.-H.; Sun, B.; Chen, J.-X. Phenanthroline-linked berberine dimer and fluorophore-tagged DNA conjugate for the selective detection of microRNA-185: Experimental and molecular docking studies. Anal. Chim. Acta 2019, 1051, 153–159. [Google Scholar] [CrossRef]
- Hu, P.-P.; Liu, N.; Wu, K.-Y.; Zhai, L.-Y.; Xie, B.-P.; Sun, B.; Duan, W.-J.; Zhang, W.-H.; Chen, J.-X. Successive and Specific Detection of Hg(2+) and I(-) by a DNA@MOF Biosensor: Experimental and Simulation Studies. Inorg. Chem. 2018, 57, 8382–8389. [Google Scholar] [CrossRef]
- Xie, B.-P.; Chai, J.; Fan, C.; Ouyang, J.-H.; Duan, W.-J.; Sun, B.; Chen, J.; Yuan, L.-X.; Xu, X.-Q.; Chen, J.-X. Water-Stable Silver-Based Metal–Organic Frameworks of Quaternized Carboxylates and Their Antimicrobial Activity. ACS Appl. Bio Mater. 2020, 3, 8525–8531. [Google Scholar] [CrossRef]
- Chen, M.; Wu, K.-Y.; Pan, W.-L.; Huang, N.-H.; Li, R.-T.; Chen, J.-X. Selective and recyclable tandem sensing of PO43− and Al3+ by a water-stable terbium-based metal–organic framework. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 247, 119084. [Google Scholar] [CrossRef] [PubMed]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, X.; Huang, Y.; Zhu, Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.; Pellois, J.-P.; Zhou, H.-C. Enzyme-MOF Nanoreactor Activates Nontoxic Paracetamol for Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 5725–5730. [Google Scholar] [CrossRef]
- Neisi, Z.; Ansari-Asl, Z.; Jafarinejad-Farsangi, S.; Tarzi, M.E.; Sedaghat, T.; Nobakht, V. Synthesis, characterization and biocompatibility of polypyrrole/Cu(II) metal-organic framework nanocomposites. Colloids Surf. B Biointerfaces 2019, 178, 365–376. [Google Scholar] [CrossRef]
- Cai, X.; Deng, X.; Xie, Z.; Shi, Y.; Pang, M.; Lin, J. Controllable synthesis of highly monodispersed nanoscale Fe-soc-MOF and the construction of Fe-soc-MOF@polypyrrole core-shell nanohybrids for cancer therapy. Chem. Eng. J. 2018, 358, 369–378. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Tiwari, A.; Wang, S.; Turner, A.; Piletsky, S. ‘On/off’-switchable catalysis by a smart enzyme-like imprinted polymer. J. Catal. 2011, 278, 173–180. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Wang, N.; Ma, X.; Niu, D.; Jiang, B.; Liu, L.; Huang, W.; Yang, W.; Zhou, Z. Synthesis of magnetic molecularly imprinted polymer particles for selective adsorption and separation of dibenzothiophene. Microchim. Acta 2012, 179, 123–130. [Google Scholar] [CrossRef]
- Qin, L.; He, X.-W.; Yuan, X.; Li, W.-Y.; Zhang, Y.-K. Molecularly imprinted beads with double thermosensitive gates for selective recognition of proteins. Anal. Bioanal. Chem. 2011, 399, 3375–3385. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Tang, L.; Li, R.-S.; Huang, Y.-P.; Liu, Z.-S. Water-compatible molecularly imprinted polymers prepared using metal–organic gel as porogen. RSC Adv. 2015, 5, 84601–84609. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Zhong, J.; Lu, Z.; Wang, G.; Sun, M.; Jiang, Y.; Zou, P.; Wang, X.; Zhao, Q.; et al. Molecularly imprinted electrochemical sensor based on biomass carbon decorated with MOF-derived Cr2O3 and silver nanoparticles for selective and sensitive detection of nitrofurazone. Chem. Eng. J. 2020, 398, 125664. [Google Scholar] [CrossRef]
- Chen, J.-X.; Chen, M.; Ding, N.-N.; Chen, W.-H.; Zhang, W.-H.; Hor, A.; Young, D.J. Transmetalation of a Dodecahedral Na9 Aggregate-Based Polymer: A Facile Route to Water Stable Cu(II) Coordination Networks. Inorg. Chem. 2014, 53, 7446–7454. [Google Scholar] [CrossRef] [PubMed]
- Qing, W.; Wang, Y.; Wang, Y.; Zhao, D.; Liu, X.; Zhu, J. The modified nanocrystalline cellulose for hydrophobic drug delivery. Appl. Surf. Sci. 2016, 366, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Clarkson, C.R.; Atwa, M.; Debuhr, C.; Ghanizadeh, A.; Birss, V.I. Wetting dynamics of nanoliter water droplets in nanoporous media. J. Colloid Interface Sci. 2020, 589, 411–423. [Google Scholar] [CrossRef]
- Qian, K.; Fang, G.; Wang, S. A novel core–shell molecularly imprinted polymer based on metal–organic frameworks as a matrix. Chem. Commun. 2011, 47, 10118–10120. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Mo, C.-E.; Huang, Y.-P.; Liu, Z.-S. Preparation of Liquid Crystalline Molecularly Imprinted Polymer Coated Metal Organic Framework for Capecitabine Delivery. Part. Part. Syst. Charact. 2018, 36. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; He, Y.; Zhao, Y.; Ma, P.; Wang, J. A propionate-functionalized polyoxovanadate K2[V10O16(OH)6(CH3CH2CO2)6]·20H2O: As catalyst for degradation of methylene blue. J. Mol. Struct. 2019, 1195, 184–188. [Google Scholar] [CrossRef]
- Morgan, M.T.; Carnahan, M.A.; Immoos, C.E.; Ribeiro, A.A.; Finkelstein, S.; Lee, S.J.; Grinstaff, M.W. Dendritic Molecular Capsules for Hydrophobic Compounds. J. Am. Chem. Soc. 2003, 125, 15485–15489. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Pal, T.; Kim, H.; Kim, T.W.; Biswas, G.; Lee, D.; Singh, T.; Murthy, A.S.N.; Kim, W.; Kim, K.; et al. Preparation of a Camptothecin-conjugated Molecular Carrier and its Cytotoxic Effect Toward Human Colorectal Carcinoma In Vitro. Bull. Korean Chem. Soc. 2018, 39, 1385–1393. [Google Scholar] [CrossRef]
- Yang, Q.; Zu, C.; Li, W.; Wu, W.; Ge, Y.; Wang, L.; Wang, L.; Li, Y.; Zhao, X. Enhanced Water Solubility and Oral Bioavailability of Paclitaxel Crystal Powders through an Innovative Antisolvent Precipitation Process: Antisolvent Crystallization Using Ionic Liquids as Solvent. Pharmaceutics 2020, 12, 1008. [Google Scholar] [CrossRef]
- Sezgin, Z.; Yüksel, N.; Baykara, T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, V.; Kinnear, C.; Moniatte, M.; Rothen-Rutishauser, B.; Clift, M.J.D.; Fink, A. Surface charge of polymer coated SPIONs influences the serum protein adsorption, colloidal stability and subsequent cell interaction in vitro. Nanoscale 2013, 5, 3723–3732. [Google Scholar] [CrossRef] [Green Version]
- Trimaille, T.; Mondon, K.; Gumy, R. Novel polymeric miclles for hydrophobie drug delivery based on biodegradable poly(hexyl-substituted lactides). Int. J. Pharm. 2006, 319, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, X.Z.; Cheng, C. Self-assembled, thermosensitive micelles of a star block copolymer based on PMMA and PNIPAAm for contolled drug delivery. Biomaterials 2006, 28, 99–107. [Google Scholar] [CrossRef]
- Kataoka, K.; Matsumoto, T.; Yokoyama, M. Doxorubicin loaded poly (ethyleneglycol)-poly(β-benzyl-L-aspartate) copolymer micells their pharmacetical haracteristics and biological significance. J. Control. Release 2000, 64, 143–1531. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Lin, Q.; Wu, H.; Jia, S.; Shi, Y. One-Step Synthesis of Nitrogen-Doped Hydrophilic Mesoporous Carbons from Chitosan-Based Triconstituent System for Drug Release. Nanoscale Res. Lett. 2019, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Narisawa, S.; Nagata, M.; Hirakawa, Y.; Kobayashi, M.; Yoshino, H. An Organic Acid-Induced Sigmoidal Release System for Oral Controlled-Release Preparations. 2. Permeability Enhancement of Eudragit RS Coating Led by the Physicochemical Interactions with Organic Acid. J. Pharm. Sci. 1996, 85, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.I.; Alonso-Morales, N.; Baeza, J.A.; Rodríguez, J.J.; Gilarranz, M.A. High load drug release systems based on carbon porous nanocapsule carriers. Ibuprofen case study. J. Mater. Chem. B 2020, 8, 5293–5304. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH-and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef]
- Saxena, A.; Kaloti, M.; Bohidar, H. Rheological properties of binary and ternary protein–polysaccharide co-hydrogels and comparative release kinetics of salbutamol sulphate from their matrices. Int. J. Biol. Macromol. 2011, 48, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, M.; Shen, R.; Shao, S.; Chen, L.; Gong, W.; Shan, L.; Gao, C. Development of metoprolol tartrate-loaded sustained-release pellets: Effect of talc on the mechanism of drug release. Pharm. Dev. Technol. 2016, 23, 664–673. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Zhu, J.; Liu, W.; Khan, Z.H.; Liu, X. Molecularly Imprinting Polymers (MIP) Based on Nitrogen Doped Carbon Dots and MIL-101(Fe) for Doxorubicin Hydrochloride Delivery. Nanomaterials 2020, 10, 1655. [Google Scholar] [CrossRef] [PubMed]


| The Key Crystallographic Information of MOF | |
|---|---|
| Empirical formula | C20H19CuN2O10 |
| Formula weight | 510.91 |
| Temperature/K | 296 |
| Crystal system | triclinic |
| Space group | P-1 |
| a/Å | 7.2164 (7) |
| b/Å | 11.6530 (11) |
| c/Å | 13.4323 (11) |
| α/° | 108.287 (3) |
| β/° | 93.184 (3) |
| γ/° | 102.297 (4) |
| Volume/Å3 | 1038.75 (17) |
| Z | 2 |
| ρcalcg/cm3 | 1.633 |
| μ/mm−1 | 1.114 |
| F (000) | 524.0 |
| Crystal size/mm3 | 0.17 × 0.14 × 0.06 |
| Bond Lengths (Å) | |||
| Cu1-O1 | 1.9365 (16) | Cu1-O2W | 2.2092 (18) |
| Cu1-O1W | 1.9869 (16) | Cu1-N2 | 2.028 (2) |
| Cu1-O51 | 1.9654 (17) | ||
| Bond Angles (°) | |||
| O1-Cu1-O1W | 85.77 (7) | O1W-Cu1-N2 | 172.49 (7) |
| O1-Cu1-O51 | 172.24 (7) | O51-Cu1-O1W | 91.04 (7) |
| O1-Cu1-O2W | 87.34 (7) | O51-Cu1-O2W | 99.91 (8) |
| O1-Cu1-N2 | 93.34 (8) | O51-Cu1-N2 | 88.91 (7) |
| O1W-Cu1-O2W | 93.12 (7) | N2-Cu1-O2W | 94.28 (7) |
| nEGDMA (mmol) | DLE (%) | DLC (μg/mg) |
|---|---|---|
| 0.50 | 14.2 | 141.5 (2.13) a |
| 0.75 | 23.1 | 231.4 (0.73) |
| 1.00 | 23.8 | 237.8 (0.50) |
| 1.25 | 21.4 | 214.0 (0.82) |
| 1.50 | 16.4 | 164.3 (0.64) |
| nMAA:nAAm | DLE (%) | DLC (μg/mg) |
|---|---|---|
| 1:4 | 17.6 | 176.0 (5.52) a |
| 2:3 | 23.5 | 235.3 (1.95) |
| 1:1 | 23.8 | 237.8 (1.05) |
| 3:2 | 20.3 | 202.5 (2.86) |
| 4:1 | 18.5 | 184.6 (4.56) |
| Sample | MOF | MOF@P | MOF@P@HCPT | |
|---|---|---|---|---|
| Parameter | ||||
| Zeta potential (mV) | 0.31 (0.21) a | −0.70 (0.024) | −4.58 (0.15) | |
| Size (nm)/PDI | 1285.7/0.192 (62.57) | 68.14/0.163 (9.00) | 278.29/0.144 (17.76) | |
| Condition | Korsmeyer–Peppas | Sigmoidal Model | |||||
|---|---|---|---|---|---|---|---|
| K | n | R2 | ks | Rs | t50 | R2 | |
| pH = 6.5 | 14.29 | 0.14 | 0.75 | 1.16 | 21.03 | 0.716 | 0.85 |
| pH = 7.4 | 18.47 | 0.13 | 0.90 | 1.17 | 26.10 | 0.666 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Liu, W.; Wu, X.; Zhu, J.; Zhou, D.; Liu, X. A Water-Soluble Polyacid Polymer Based on Hydrophilic Metal–Organic Frameworks Using Amphoteric Carboxylic Acid Ligands as Linkers for Hydroxycamptothecin Loading and Release In Vitro. Nanomaterials 2021, 11, 2854. https://doi.org/10.3390/nano11112854
Shi Y, Liu W, Wu X, Zhu J, Zhou D, Liu X. A Water-Soluble Polyacid Polymer Based on Hydrophilic Metal–Organic Frameworks Using Amphoteric Carboxylic Acid Ligands as Linkers for Hydroxycamptothecin Loading and Release In Vitro. Nanomaterials. 2021; 11(11):2854. https://doi.org/10.3390/nano11112854
Chicago/Turabian StyleShi, Yuqiong, Wei Liu, Xiangrong Wu, Jinhua Zhu, Danyang Zhou, and Xiuhua Liu. 2021. "A Water-Soluble Polyacid Polymer Based on Hydrophilic Metal–Organic Frameworks Using Amphoteric Carboxylic Acid Ligands as Linkers for Hydroxycamptothecin Loading and Release In Vitro" Nanomaterials 11, no. 11: 2854. https://doi.org/10.3390/nano11112854
APA StyleShi, Y., Liu, W., Wu, X., Zhu, J., Zhou, D., & Liu, X. (2021). A Water-Soluble Polyacid Polymer Based on Hydrophilic Metal–Organic Frameworks Using Amphoteric Carboxylic Acid Ligands as Linkers for Hydroxycamptothecin Loading and Release In Vitro. Nanomaterials, 11(11), 2854. https://doi.org/10.3390/nano11112854





