Carbon-Based Nanocomposite Smart Sensors for the Rapid Detection of Mycotoxins
Abstract
:1. Introduction
2. Carbon-Based Functional Nanomaterials
2.1. Antibody-Functionalized CNMs
2.2. Aptamer-Functionalized CNMs
2.3. MIPs Decorated CNMs
2.4. Carbon-Based Nanocomposites
2.4.1. Nanostructured Nobel Metal-Doped CNMs
2.4.2. CNMs Support Metal Oxide Nanoparticles
2.4.3. Others
3. CNM-Based Smart Sensor for the Detection of Mycotoxins
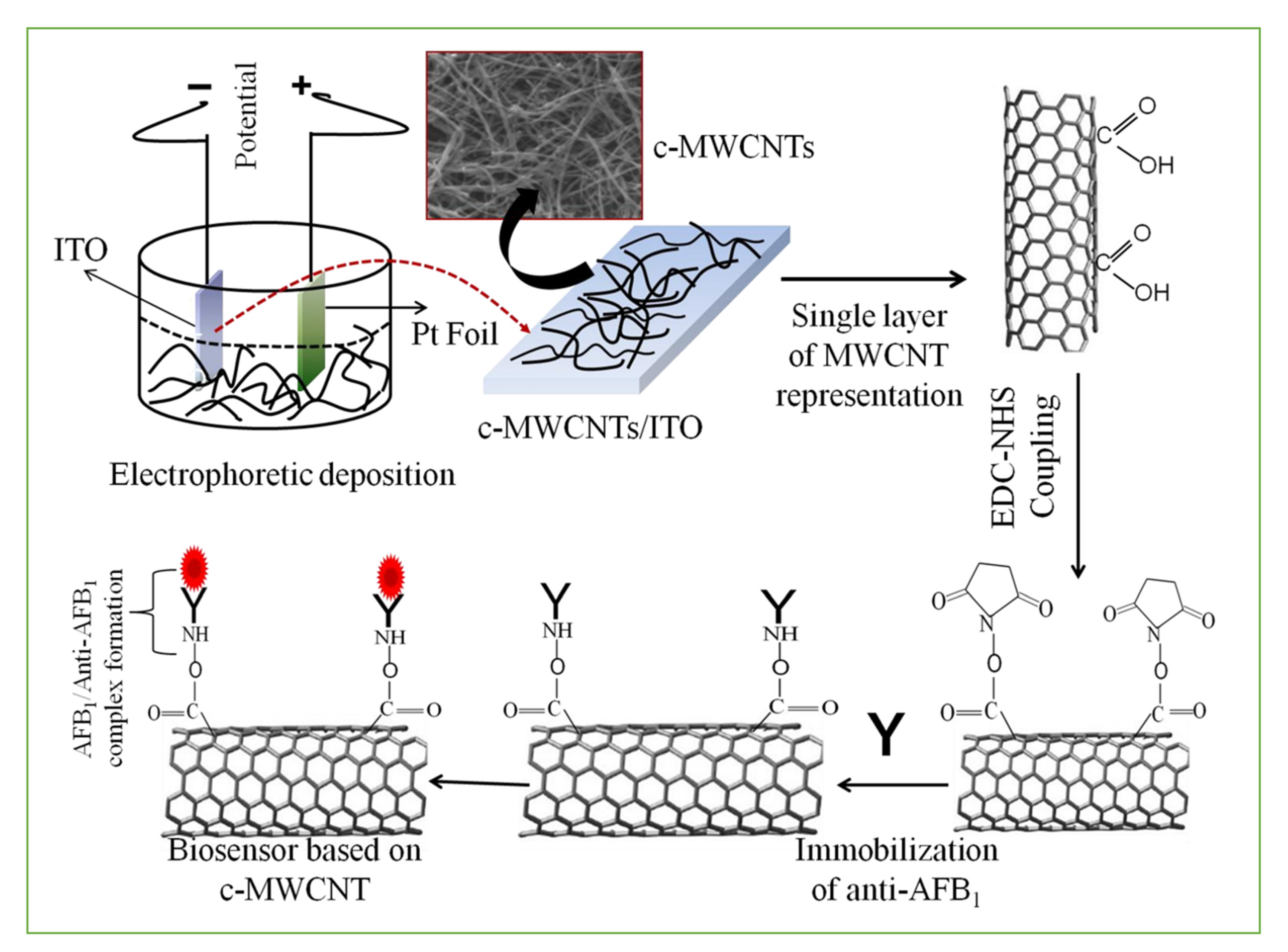
3.1. Smart Sensors Based on Antibodies
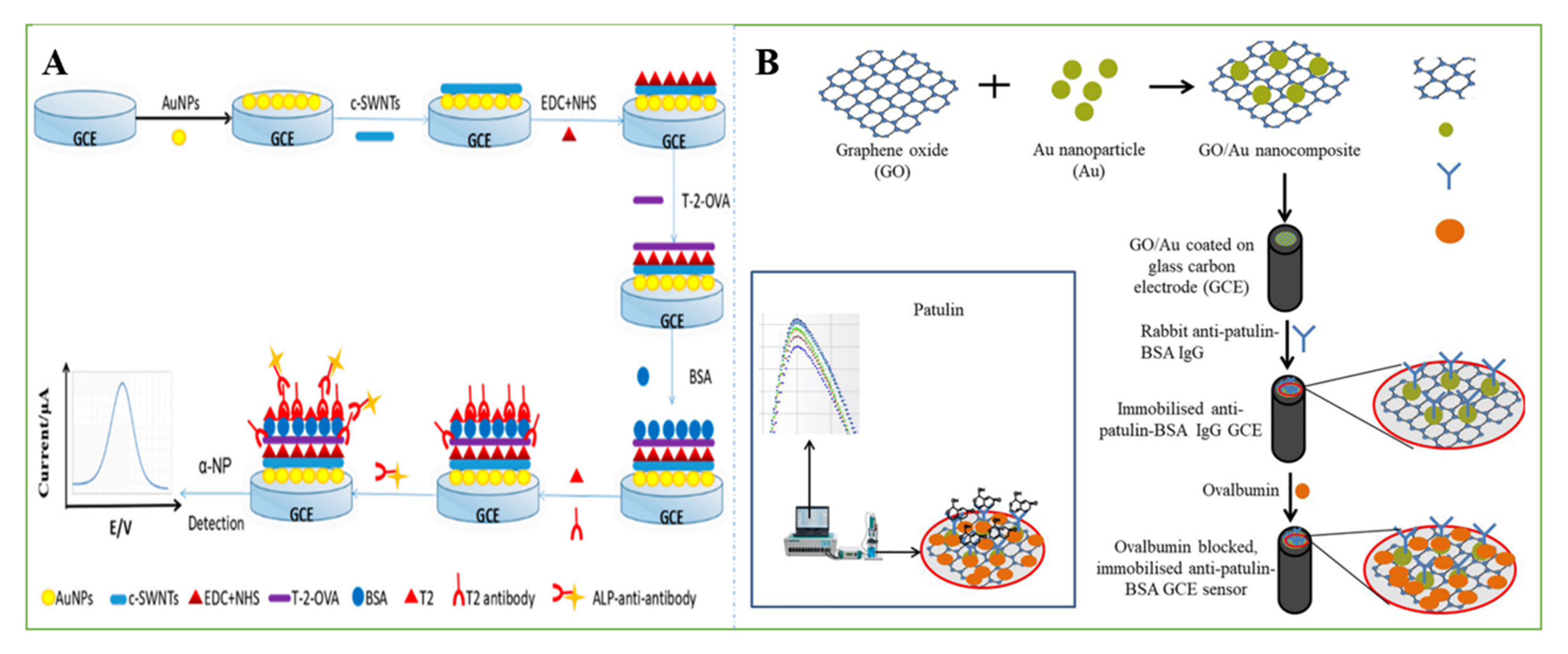
3.2. Smart Sensors Based on Aptamers
3.2.1. Optical Aptasensors
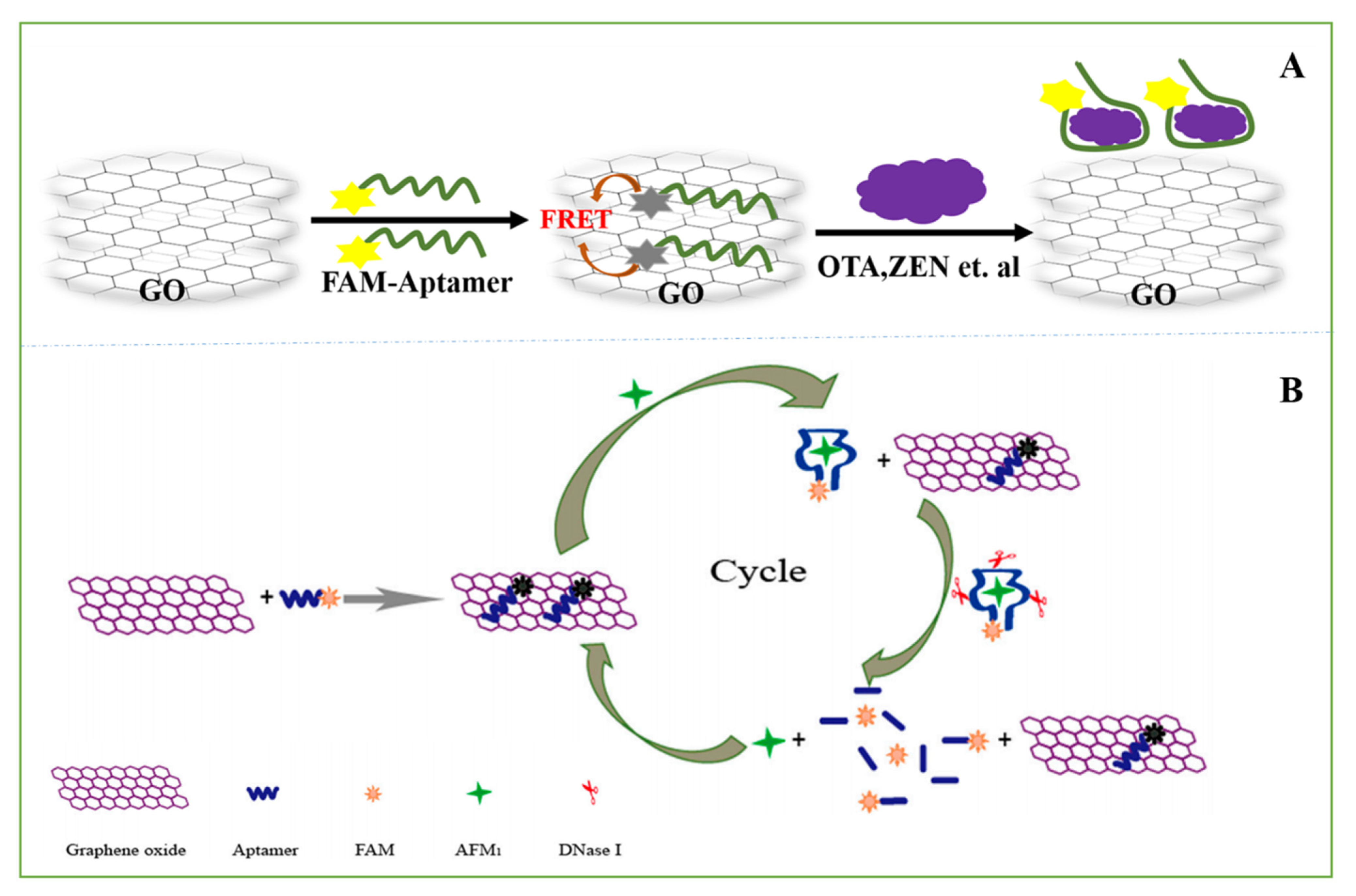
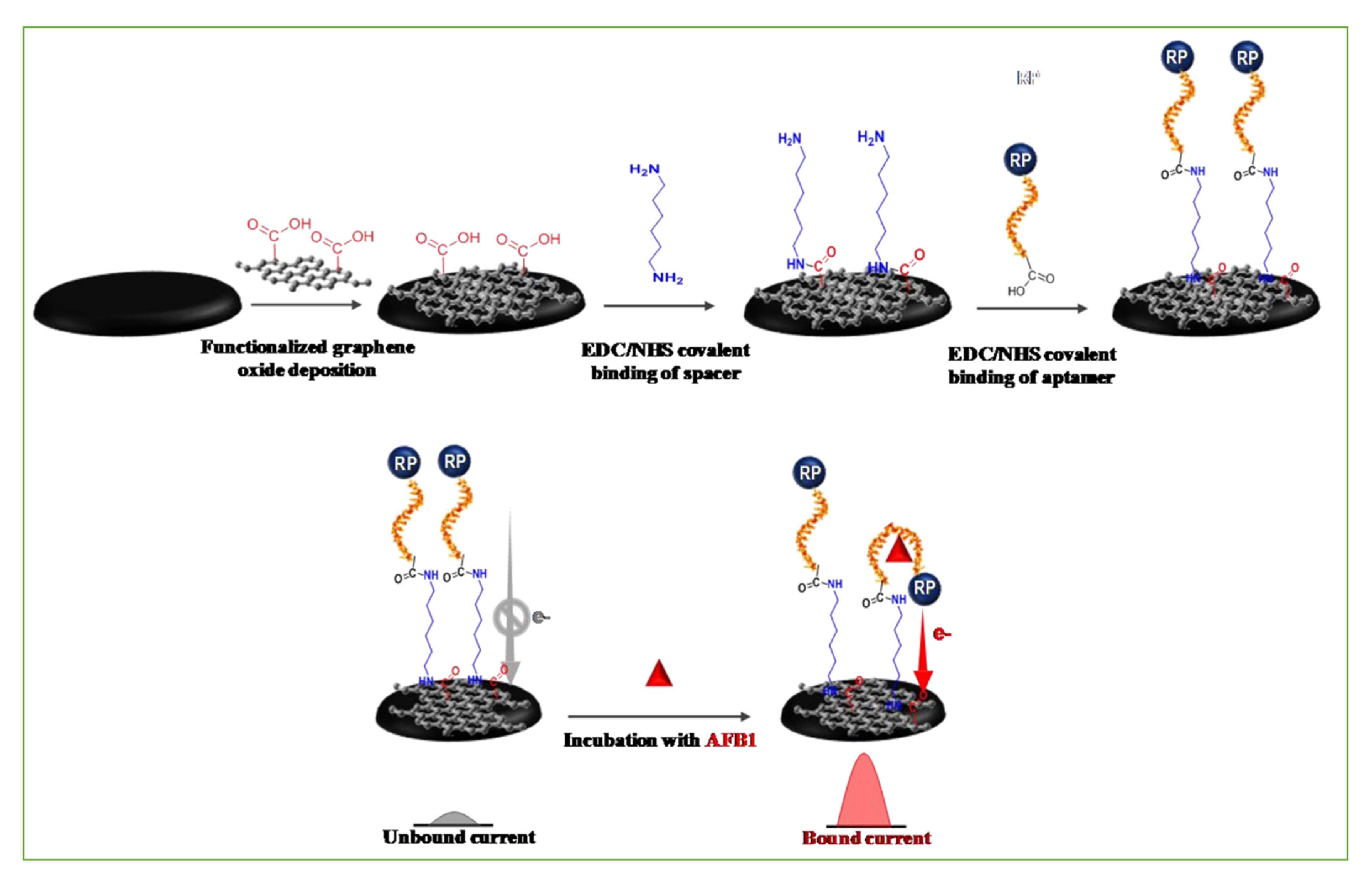
3.2.2. Electrochemical Aptasensors
3.3. Smart Sensors Based on MIPs
3.4. Others
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brase, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.; Bennett, J.W. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, J.I.; Miller, J.D. A concise history of mycotoxin research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Horky, P.; Skalickova, S.; Baholet, D.; Skladanka, J. Nanoparticles as a solution for eliminating the risk of mycotoxins. Nanomaterials 2018, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotech. 2016, 37, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Singh, J.; Sachdev, T.; Basu, T.; Malhotra, B.D. Recent advances in mycotoxins detection. Biosens. Bioelectron. 2016, 81, 532–545. [Google Scholar] [CrossRef]
- Seymour, S.G. Determination of mycotoxins in human foods. Chem. Soc. Rev. 2008, 37, 2468–2477. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, F.; Fang, Y.; Li, P.; Zhao, Y.; Shen, F.; Zou, Y.; Hu, Q. Comparison of concentration and health risks of 9 fusarium mycotoxins in commercial whole wheat flour and refined wheat flour by multi-IAC-HPLC. Food Chem. 2018, 275, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.G.-M.; Crapnell, R.D.; Banks, C.E. Electroanalytical overview: Electrochemical sensing platforms for food and drink safety. Biosensors 2021, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.A.D.; Heneine, L.G.D.; Matencio, T.; Messaddeq, Y. Recent trends in the electroanalytical detection of food fraud. Biosens. Bioelectron. 2019, 5, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Escarpa, A.; González, M.C.; López, M.Á. Agricultural and Food Electroanalysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Mònica, C.; Diana, G.; Beatriz, P.-S. Novel nanobiotechnological concepts in electrochemical biosensors for the analysis of toxins. Analyst 2012, 137, 1055. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Srivastava, S.; Ali, M.A.; Singh, C. Nanomaterial-based biosensors for food toxin detection. Appl. Biochem. Biotechnol. 2014, 174, 880–896. [Google Scholar] [CrossRef]
- Unwin, P.R.; Güell, A.G.; Zhang, G. Nanoscale electrochemistry of sp2 carbon materials: From graphite and graphene to carbon nanotubes. Acc. Chem. Res. 2016, 49, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Liu, X.; Lv, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-nanomaterial-based flexible batteries for wearable electronics. Adv. Mater. 2019, 31, 1800716. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; David, R.M.; Fissan, H.; Morris, H.; Shatkin, J.A.; Stintz, M.; Zepp, R.; Brouwer, D. Potential release scenarios for carbon nanotubes used in composites Potential release scenarios for carbon nanotubes used in composites. Environ. Int. 2013, 59, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goud, K.Y.; Reddy, K.K.; Satyanarayana, M.; Kummari, S.; Gobi, K.V. A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Microchim. Acta 2019, 187, 1–32. [Google Scholar] [CrossRef]
- Richter, M.; Heumüller, T.; Matt, G.J.; Heiss, W.; Brabec, C.J. Carbon photodetectors: The versatility of carbon allotropes. Adv. Energy Mater. 2017, 7, 1601574. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.H. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv. Mater. 2018, 30, 1802368. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Srivastava, S.; Ali, M.A.; Gupta, T.K.; Sumana, G.; Srivastava, A.; Mathur, R.B.; Malhotra, B.D. Carboxylated multiwalled carbon nanotubes based biosensor for aflatoxin detection. Sens. Actuators B Chem. 2013, 185, 258–264. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.-R.; Wang, W.-C.; Xue, J.; Huang, Y.-L.; Yang, X.-X.; Tan, B.; Zhou, X.-P.; Shao, C.; Ding, S.-J.; et al. A novel electrochemical immunosensor for highly sensitive detection of aflatoxin B1 in corn using single-walled carbon nanotubes/chitosan. Food Chem. 2016, 192, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Althagafi, I.I.; Ahmed, S.A.; El-Said, W.A. Fabrication of gold/graphene nanostructures modified ITO electrode as highly sensitive electrochemical detection of Aflatoxin B1. PLoS ONE 2019, 14, e0210652. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wen, Y.; Wang, W.; Zhao, Z.; Han, Y.; Tang, K.; Wang, D. Nanobody-based electrochemical competitive immunosensor for the detection of AFB1 through AFB1-HCR as signal amplifier. Microchim. Acta 2020, 187, 352. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, Q.; Lin, Y.; Tang, D.; Chen, G.; Tang, D. Simple and sensitive detection of aflatoxin B1 within five minute using a non-conventional competitive immunosensing mode. Biosens. Bioelectron. 2015, 74, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Jauset-Rubio, M.; El-Shahawi, M.S.; Bashammakh, A.S.; Alyoubi, A.O.; O′Sullivan, C.K. Advances in aptamers-based lateral flow assays. Trends Anal. Chem. 2017, 97, 385–398. [Google Scholar] [CrossRef]
- Meng, H.; Liu, H.; Kuai, H.; Peng, R.; MO, L.; Zhang, X. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Bulbul, G.; Hayat, A.; Andreescu, S. A generic amplification strategy for electrochemical aptasensors using a non-enzymatic nanoceria tag. Nanoscale 2015, 7, 13230–13238. [Google Scholar] [CrossRef] [PubMed]
- Alhamoud, Y.; Li, Y.; Zhou, H.; Al-Wazer, R.; Gong, Y.; Zhi, S.; Yang, D. Label-free and highly-sensitive detection of Ochratoxin A using one-pot synthesized reduced graphene oxide/gold nanoparticles-based impedimetric aptasensor. Biosensors 2021, 11, 87. [Google Scholar] [CrossRef]
- Miao, H.; Wang, L.; Zhuo, Y.; Zhou, Z.; Yang, X. Label-free fluorimetric detection of CEA using carbon dots derived from tomato juice. Biosens. Bioelectron. 2016, 86, 83–89. [Google Scholar] [CrossRef]
- Rahimi, F.; Roshanfekr, H.; Peyman, H. Ultra-sensitive electrochemical aptasensor for label-free detection of Aflatoxin B1 in wheat flour sample using factorial design experiments. Food Chem. 2020, 343, 128436. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Wang, X.; Duan, Y. Amplified fluorescent aptasensor through catalytic recycling for highly sensitive detection of ochratoxin A. Biosens. Bioelectron. 2015, 65, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ren, J.; Wang, J.; Wang, E. Single-walled carbon nanotubes based quenching of free FAM-aptamer for selective determination of ochratoxin A. Talanta 2011, 85, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Duan, F.; Cui, P.; Yang, Y.; Liu, X.; Hou, X. A molecularly-imprinted polymer decorated on graphene oxide for the selective recognition of quercetin. New Carbon Mater. 2018, 33, 529–543. [Google Scholar] [CrossRef]
- Mazzotta, E.; Turco, A.; Chianella, I.; Guerreiro, A.; Piletsky, S.A.; Malitesta, C. Solid-phase synthesis of electroactive nanoparticles of molecularly imprinted polymers. A novel platform for indirect electrochemical sensing applications. Sens. Actuators B Chem. 2016, 229, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Radi, A.E.; Eissa, A.; Wahdan, T. Molecularly Imprinted Impedimetric Sensor for Determination of Mycotoxin Zearalenone. Electroanalysis 2020, 32, 1788–1794. [Google Scholar] [CrossRef]
- Xu, L.; Fang, G.; Pan, M.; Wang, X.; Wang, S. One-pot synthesis of carbon dots-embedded molecularly imprinted polymer for specific recognition of sterigmatocystin in grains. Biosens. Bioelectron. 2016, 77, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.G.; Castro, M.; Machado, S.; Barroso, M.F.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor for ochratoxin A detection in food samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Beheshti, H.R.; Boroushaki, M.T. Ultra-sensitive molecularly imprinted electrochemical sensor for patulin detection based on a novel assembling strategy using Au@Cu-MOF/N-GQDs. Sens. Actuators B Chem. 2020, 318, 128219. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Gu, H.; Rapole, S.B.; Wang, Q.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal. Environ. Sci. Technol. 2012, 46, 977–985. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Sandhyarani, N. Carbon nanostructures as immobilization platform for DNA: A review on current progress in electrochemical DNA sensors. Biosens. Bioelectron. 2017, 97, 226–237. [Google Scholar] [CrossRef]
- He, K.; Zeng, Z.; Chen, A.; Zeng, G.; Xiao, R.; Xu, P.; Huang, Z.; Shi, J.; Hu, L.; Chen, G. Advancement of Ag-graphene based nanocomposites: An overview of synthesis and its applications. Small 2018, 14, 1800871. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Boukherroub, R.; Szunerits, S. Gold-graphene nanocomposites for sensing and biomedical applications. J. Mater. Chem. B 2015, 3, 4301–4324. [Google Scholar] [CrossRef]
- Tiwari, I.; Gupta, M.; Pandey, C.M.; Mishra, V. Gold nanoparticle decorated graphene sheet-polypyrrole based nanocomposite: Its synthesis, characterization and genosensing application. Dalton Trans. 2015, 44, 15557–15566. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Pandey, M.K.; Sumana, G. Electrochemical Aflatoxin B1 immunosensor based on the use of graphene quantum dots and gold nanoparticles. Microchim. Acta 2019, 186, 592. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, P.; Cheng, Y.; Chen, Y.; Peng, S.; Zhu, Z.Q. Enzymatic oxydate-triggered Ag NPs etching: A novel signal-on photoelectrochemical immunosensing platform based on Ag@AgCl nanocubes loaded RGO plasmonic heterostructure. Biosens. Bioelectron. 2019, 130, 125–131. [Google Scholar] [CrossRef]
- He, B.; Lu, X. An electrochemical aptasensor based on tetrahedral DNA nanostructures as a signal probe carrier platform for sensitive detection of patulin. Anal. Chim. Acta 2020, 1138, 123–131. [Google Scholar] [CrossRef]
- He, X.; Li, H.; Liu, Y.; Huang, H.; Kang, Z.; Lee, S.T. Water soluble carbon nanoparticles: Hydrothermal synthesis and excellent photoluminescence properties. Colloids Surf. B Biointerfaces 2011, 87, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, X.; Ma, S.; Li, L.; You, T. Quantification of zearalenone in mildewing cereal crops using an innovative photoelectrochemical aptamer sensing strategy based on ZnO-NGQDs composites. Food Chem. 2020, 322, 126778. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Xin, S.; Yang, Z.; Li, Y.; Zhang, D.; Yao, P. Facile synthesis of MoS2/reduced graphene oxide@polyaniline for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 21373–21380. [Google Scholar] [CrossRef]
- Chu, Y.; Cai, B.; Ma, Y.; Zhao, M.; Ye, Z.; Huang, J. Highly sensitive electrochemical detection of circulating tumor DNA based on thin-layer MoS2/graphene composites. RSC Adv. 2016, 6, 22673–22678. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Li, J.; Liu, Y.-M. Electrochemical sensing based on layered MoS2–graphene composites. Sens. Actuators B Chem. 2013, 178, 671–677. [Google Scholar] [CrossRef]
- Geleta, G.S.; Zhao, Z.; Wang, Z. A novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite-based electrochemical aptasensor for detection of aflatoxin B1. Analyst 2018, 143, 1644–1649. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Peng, D.; Xie, S.; Chen, D.; Pan, Y.; Tao, Y.; Yuan, Z. Construction of electrochemical immunosensor based on gold-nanoparticles/carbon nanotubes/chitosan for sensitive determination of T-2 toxin in feed and swine meat. Int. J. Mol. Sci. 2018, 19, 3895. [Google Scholar] [CrossRef] [Green Version]
- Dunne, L.; Daly, S.; Baxter, A.; Haughey, S.; O’Kennedy, R. Surface plasmon resonance-based immunoassay for the detection of Aflatoxin B1 using single-chain antibody fragments. Spectrosc. Lett. 2005, 38, 229–245. [Google Scholar] [CrossRef]
- Parker, C.O.; Tothill, I.E. Development of an electrochemical immunosensor for aflatoxin M1 in milk with focus on matrix interference. Biosens. Bioelectron. 2009, 24, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, L.; Liu, X.; Ren, Y.; Xu, K.; Zhang, N.; Sun, X.; Yang, X.; Ren, X.; Wei, Q. A dual-mode PCT electrochemical immunosensor with CuCo2S4 bimetallic sulfides as enhancer. Biosens. Bioelectron. 2020, 163, 112280. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Bondili, J.S.; Brahman, P.K. Development of proof of concept for prostate cancer detection: An electrochemical immunosensor based on fullerene-C60 and copper nanoparticles composite film as diagnostic tool. Mater. Today Chem. 2020, 16, 100257. [Google Scholar] [CrossRef]
- Abera, B.D.; Falco, A.; Ibba, P.; Cantarella, G.; Petti, L.; Lugli, P. Development of flexible dispense-printed electrochemical immunosensor for aflatoxin M1 detection in milk. Sensors 2019, 19, 3912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Song, X.; Wang, D.; Kim, M. Development of an immuno-electrochemical glass carbon electrode sensor based on graphene oxide/gold nanocomposite and antibody for the detection of patulin. Food Chem. 2020, 342, 128257. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Marquette, C.A.; Dutta, P.; Sumana, G. Integrated graphene quantum dot decorated functionalized nanosheet biosensor for mycotoxin detection. Anal. Bioanal. Chem. 2020, 412, 7029–7041. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Z.; Tan, X.; Xiao, X.; Wang, P.; Wu, L.; Shao, K.; Yin, W.; Han, H. Ultrasensitive detection of aflatoxin B1 by SERS aptasensor based on exonuclease-assisted recycling amplification. Biosens. Bioelectron. 2017, 97, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Niazi, S.; Yu, Y.; Mohsin, A.; Mushtaq, B.S.; Iqbal, M.W.; Rehman, A.; Akhtar, W.; Wang, Z. Aptamer induced multicolored AuNCs-WS2”turn on” FRET nano platform for dual-color simultaneous detection of aflatoxinB1 and zearalenone. Anal. Chem. 2019, 91, 14085–14092. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Shen, P.; Liu, R.; Li, Y.; Xu, J.; Zheng, Q.; Zhang, Y.; Li, J.; Zheng, T. Aptamer fluorescence signal recovery screening for multiplex mycotoxins in cereal samples based on photonic crystal microsphere suspension array. Sens. Actuators B Chem. 2017, 248, 351–358. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Ma, X.; Xia, Y.; Wang, H.; Wang, Z.; Zhang, Q. Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal. Chem. 2012, 84, 6263–6270. [Google Scholar] [CrossRef]
- Mok, W.; Li, Y. Recent progress in nucleic acid aptamer-based biosensors and bioassays. Sensors 2008, 8, 7050–7084. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Zhang, W.; Zhao, S.; Wang, Z. Screening and development of DNA aptamers as capture probes for colorimetric detection of patulin. Anal. Biochem. 2016, 508, 58–64. [Google Scholar] [CrossRef]
- Wu, H.; Liu, R.; Kang, X.; Liang, C.; Lv, L.; Guo, Z. Fluorometric aptamer assay for ochratoxin A based on the use of single walled carbon nanohorns and exonuclease III-aided amplification. Microchim. Acta 2017, 185, 27. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, B.; Liu, L.; Tian, W. A Label-free fluorescent aptasensor for turn-on monitoring Ochratoxin A based on AIE-active probe and graphene oxide. Chem. Res. Chin. Univ. 2018, 34, 363–368. [Google Scholar] [CrossRef]
- Tian, J.; Wei, W.; Wang, J.; Ji, S.; Chen, G.; Lu, J. Fluorescence resonance energy transfer aptasensor between nanoceria and graphene quantum dots for the determination of ochratoxin A. Anal. Chim. Acta 2018, 1000, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, K.; Zhao, H.; Liu, H.; Wang, K.; Xia, K. Fluorometric aptamer-based determination of ochratoxin A based on the use of graphene oxide and RNase H-aided amplification. Microchim. Acta 2018, 185, 347. [Google Scholar] [CrossRef]
- Joo, M.; Baek, S.H.; Cheon, S.A.; Chun, H.S.; Choi, S.W.; Park, T.J. Development of aflatoxin B1 aptasensor based on wide-range fluorescence detection using graphene oxide quencher. Colloids Surf. B Biointerfaces 2017, 154, 27–32. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, F.; Chen, M.; Zhu, Y.; Xiao, J.; Yang, H.; Chen, X. Rapid and visual detection of aflatoxin B1 in foodstuffs using aptamer/G-quadruplex DNAzyme probe with low background noise. Food Chem. 2019, 271, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Hayat, A.; Satyanarayana, M.; Kumar, V.S.; Catanante, G.; Gobi, K.V.; Marty, J.L. Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim. Acta 2017, 184, 4401–4408. [Google Scholar] [CrossRef]
- Ma, L.; Guo, T.; Pan, S.; Zhang, Y. A fluorometric aptasensor for patulin based on the use of magnetized graphene oxide and DNase I-assisted target recycling amplification. Microchim. Acta 2018, 185, 487. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wu, S.; Duan, N.; Chen, J.; Zheng, Z.; Wang, Z. An ultrasensitive aptasensor for Ochratoxin A using hexagonal core/shell upconversion nanoparticles as luminophores. Biosens. Bioelectron. 2017, 91, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Khoshfetrat, S.M.; Bagheri, H.; Mehrgardi, M.A. Visual electrochemiluminescence biosensing of aflatoxin M1 based on luminol-functionalized, silver nanoparticle-decorated graphene oxide. Biosens. Bioelectron. 2018, 100, 382–388. [Google Scholar] [CrossRef]
- Beheshti-Marnani, A.; Hatefi-Mehrjardi, A.; Es’haghi, Z. A sensitive biosensing method for detecting of ultra-trace amounts of AFB1 based on “Aptamer/reduced graphene oxide” nano-bio interaction. Colloids Surf. B Biointerfaces 2019, 175, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Taghdisi, S.M. Amperometric aptasensor for ochratoxin A based on the use of a gold electrode modified with aptamer, complementary DNA, SWCNTs and the redox marker Methylene Blue. Microchim. Acta 2017, 184, 1151–1159. [Google Scholar] [CrossRef]
- Loo, A.H.; Bonanni, A.; Pumera, M. Mycotoxin aptasensing amplification by using inherently electroactive graphene-oxide nanoplatelet labels. ChemElectroChem 2015, 2, 743–747. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, Y.; Yang, C.; Guo, Q.; Nie, G. Simple “signal-on” photoelectrochemical aptasensor for ultrasensitive detecting AFB1 based on electrochemically reduced graphene oxide/poly(5-formylindole)/Au nanocomposites. Biosens. Bioelectron. 2019, 134, 42–48. [Google Scholar] [CrossRef]
- Zhong, H.; Yu, C.; Gao, R.; Chen, J.; Yu, Y.; Geng, Y.; Wen, Y.; He, J. A novel sandwich aptasensor for detecting T-2 toxin based on rGO-TEPA-Au@Pt nanorods with a dual signal amplification strategy. Biosens. Bioelectron. 2019, 144, 111635. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Y.; Zheng, Y.-T.; Zhang, H.-B.; He, C.-H.; Wu, W.-D.; Zhang, H.-B. DNA electrochemical aptasensor for detecting fumonisins B1 based on graphene and thionine nanocomposite. Electroanalysis 2015, 27, 1097–1103. [Google Scholar] [CrossRef]
- He, B.; Yan, X. A “signal-on” voltammetric aptasensor fabricated by hcPt@AuNFs/PEI-rGO and Fe3O4NRs/rGO for the detection of zearalenone. Sens. Actuators B Chem. 2019, 290, 477–483. [Google Scholar] [CrossRef]
- Ma, L.; Bai, L.; Zhao, M.; Zhou, J.; Chen, Y.; Mu, Z. An electrochemical aptasensor for highly sensitive detection of zearalenone based on PEI-MoS2-MWCNTs nanocomposite for signal enhancement. Anal. Chim. Acta 2019, 1060, 71–78. [Google Scholar] [CrossRef]
- Kaur, N.; Bharti, A.; Batra, S.; Rana, S.; Rana, S.; Bhalla, A.; Prabhakar, N. An electrochemical aptasensor based on graphene doped chitosan nanocomposites for determination of Ochratoxin A. Microchem. J. 2019, 144, 102–109. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhou, Y.; Xing, Y.; Zhang, G.M.; Zhang, Y.; Zhang, C.H.; Lei, P.; Dong, C.; Deng, X.; He, Y.; et al. A Label-free aptasensor based on Aptamer/NH2 Janus particles for ultrasensitive electrochemical detection of Ochratoxin A. Talanta 2019, 199, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.L.; Zhang, Y.F.; Sun, G.P.; Wang, X.N.; Tang, D. Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer-graphene oxide nanosheets and DNase I-based target recycling reaction. Biosens. Bioelectron. 2017, 89, 659–665. [Google Scholar] [CrossRef]
- Jiang, L.; Qian, J.; Yang, X.; Yan, Y.; Liu, Q.; Wang, K.; Wang, K. Amplified impedimetric aptasensor based on gold nanoparticles covalently bound graphene sheet for the picomolar detection of ochratoxin A. Anal. Chim. Acta 2014, 806, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Jiang, L.; Yang, X.; Yan, Y.; Mao, H.; Wang, K. Highly sensitive impedimetric aptasensor based on covalent binding of gold nanoparticles on reduced graphene oxide with good dispersity and high density. Analyst 2014, 139, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yue, S.; Zhang, W.; Li, X. Development of an electrochemical aptasensor using Au octahedra and graphene for signal amplification. Anal. Methods 2020, 12, 317–323. [Google Scholar] [CrossRef]
- Le, V.T.; Vasseghian, Y.; Dragoi, E.-N.; Moradi, M.; Khaneghah, A.M. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luong, J.H.T. Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites. Carbon 2015, 84, 519–550. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, B.; Zhang, L.; Zhang, J.; Ma, T.; Zhang, J.; Fu, X.; Tian, W. Folic acid-functionalized mesoporous silica nanospheres hybridized with AIE luminogens for targeted cancer cell imaging. Nanoscale 2013, 5, 2065–2072. [Google Scholar] [CrossRef]
- Ma, K.; Wang, H.; Li, H.; Wang, S.; Li, X.; Xu, B.; Tian, W. A label-free aptasensor for turn-on fluorescent detection of ATP based on AIE-active probe and water-soluble carbon nanotubes. Sens. Actuators B Chem. 2016, 230, 556–558. [Google Scholar] [CrossRef]
- Ma, K.; Li, X.; Xu, B.; Tian, W. A sensitive and selective “turn-on” fluorescent probe for Hg2+ based on thymine–Hg2+–thymine complex with an aggregation-induced emission feature. Anal. Methods 2014, 6, 2338–2342. [Google Scholar] [CrossRef]
- Li, X.; Zhu, S.; Xu, B.; Ma, K.; Zhang, J.; Yang, B.; Tian, W. Self-assembled graphene quantum dots induced by cytochrome c: A novel biosensor for trypsin with remarkable fluorescence enhancement. Nanoscale 2013, 5, 7776–7779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, B.; Lu, H.; Wang, Z.; Zhang, J.; Zhang, Y.; Dong, Y.; Ma, K.; Wen, S.; Tian, W. Label-free fluorescence turn-on detection of Pb2+ based on AIE-active quaternary ammonium salt of 9,10-distyrylanthracene. Anal. Methods 2013, 5, 438–441. [Google Scholar] [CrossRef]
- Li, X.; Ma, K.; Lu, H.; Xu, B.; Wang, Z.; Zhang, Y.; Gao, Y.; Yan, L.; Tian, W. Highly sensitive determination of ssDNA and real-time sensing of nuclease activity and inhibition based on the controlled self-assembly of a 9,10-distyrylanthracene probe. Anal. Bioanal. Chem. 2014, 406, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, B.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Lv, H.; Wen, S.; Li, B.; Ye, L.; et al. Piezochromic luminescence based on the molecular aggregation of 9,10-Bis((E)-2-(pyrid-2-yl)vinyl)anthracene. Angewandte Chemie 2012, 51, 10782–10785. [Google Scholar] [CrossRef]
- Wang, H.B.; Ou, L.J.; Huang, K.J.; Wen, X.G.; Wang, L.L.; Liu, Y.M. A sensitive biosensing strategy for DNA detection based on graphene oxide and T7 exonuclease assisted target recycling amplification. Can. J. Chem. 2013, 91, 1266–1271. [Google Scholar] [CrossRef]
- Liu, H.; Li, L.; Wang, Q.; Duan, L.; Tang, B. Graphene Fluorescence Switch-Based Cooperative Amplification: A Sensitive and Accurate Method to Detection MicroRNA. Anal. Chem. 2014, 86, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, J.; Lv, M.; Wang, J.; Gao, J.; Lu, J.; Li, Y.; Huang, Q.; Hu, J.; Fan, C. A graphene-based sensor array for high-precision and adaptive target identification with ensemble aptamers. J. Am. Chem. Soc. 2012, 134, 13843–13849. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wen, F.; Qiao, Q.; Zheng, N.; Saive, M.; Fauconnier, M.-L.; Wang, J. A novel graphene oxide-based aptasensor for amplified fluorescent detection of aflatoxin M1 in milk powder. Sensors 2019, 19, 3840. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Lu, Q.; Hou, Y.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Nanosensor composed of nitrogen-doped carbon dots and gold nanoparticles for highly selective detection of cysteine with multiple signals. Anal. Chem. 2015, 87, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.F.C.; da Silva, J.C.G.E.; Leitão, J.M.M. Carbon dots from tryptophan doped glucose for peroxynitrite sensing. Anal. Chim. Acta 2014, 852, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, Y.; Wu, Y.; Weng, B.; Liu, Y.; Lu, Z.; Li, C.M.; Yu, C. Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosens. Bioelectron. 2016, 78, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Crecchio, C.; Stotzky, G. Binding of DNA on humic acids: Effect on transformation of Bacillus subtilis and resistance to DNase. Soil Biol. Biochem. 1998, 30, 1061–1067. [Google Scholar] [CrossRef]
- Guo, M.; Hou, Q.; Waterhouse, G.I.N.; Hou, J.; Ai, S.; Li, X. A simple aptamer-based fluorescent aflatoxin B1 sensor using humic acid as quencher. Talanta 2019, 205, 120131. [Google Scholar] [CrossRef]
- Abdulbari, H.A.; Basheer, E.A.M. Electrochemical biosensors: Electrode development, materials, design, and fabrication. ChemBioEng Rev. 2017, 4, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Hynek, D.; Trnkova, L.; Hemzal, D.; Marik, M.; Kizek, R.; Hubalek, J. Electrochemical determination of adenine using a glassy carbon electrode modified with graphene oxide and polyaniline. Microchim. Acta 2016, 183, 1299–1306. [Google Scholar] [CrossRef]
- Lv, Y.; Tan, T.; Svec, F. Molecular imprinting of proteins in polymers attached to the surface of nanomaterials for selective recognition of biomacromolecules. Biotechnol. Adv. 2013, 31, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Haginaka, J. Molecularly imprinted polymers as affinity-based separation media for sample preparation. J. Sep. Sci. 2009, 32, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ji, K.; Yao, L.; Xue, X.; Wen, W.; Zhang, X.; Wang, S. Molecularly imprinted photoelectrochemical sensor for fumonisin B1 based on GO-CdS heterojunction. Biosens. Bioelectron. 2018, 127, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pi, F.; Zhang, H.; Sun, J.; Zhang, Y.; Sun, X. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens. Bioelectron. 2017, 98, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Haldorai, Y.; Khan, I.; Kang, S.-M.; Kwak, C.H.; Gandhi, S.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Bioreceptor-free, sensitive and rapid electrochemical detection of patulin fungal toxin, using a reduced graphene oxide@SnO2 nanocomposite. Mater. Sci. Eng. C 2020, 113, 110916. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, A.R. Fabrication of a novel and high-performance amperometric sensor for highly sensitive determination of ochratoxin A in juice samples. Talanta 2018, 188, 225–231. [Google Scholar] [CrossRef]

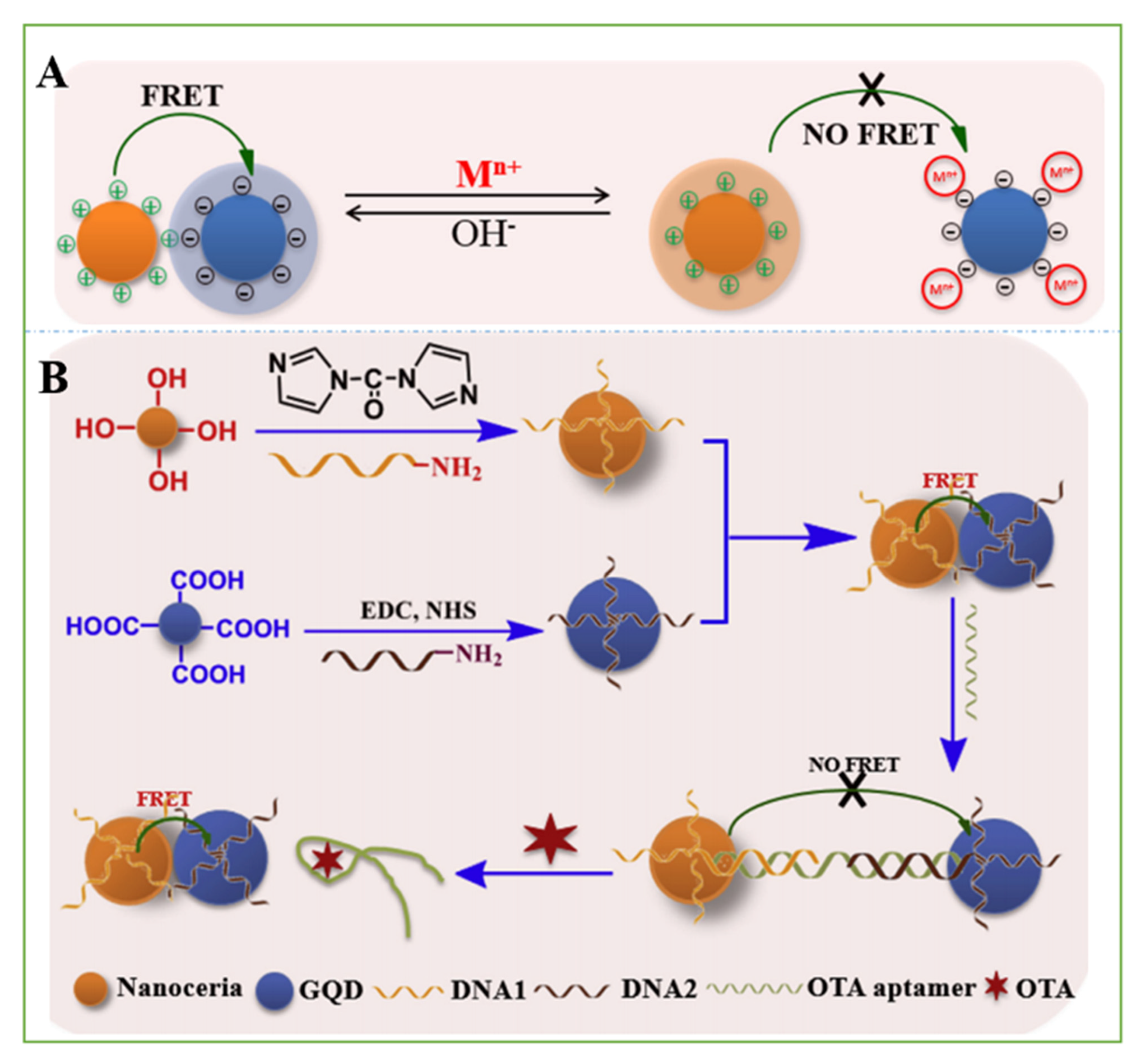
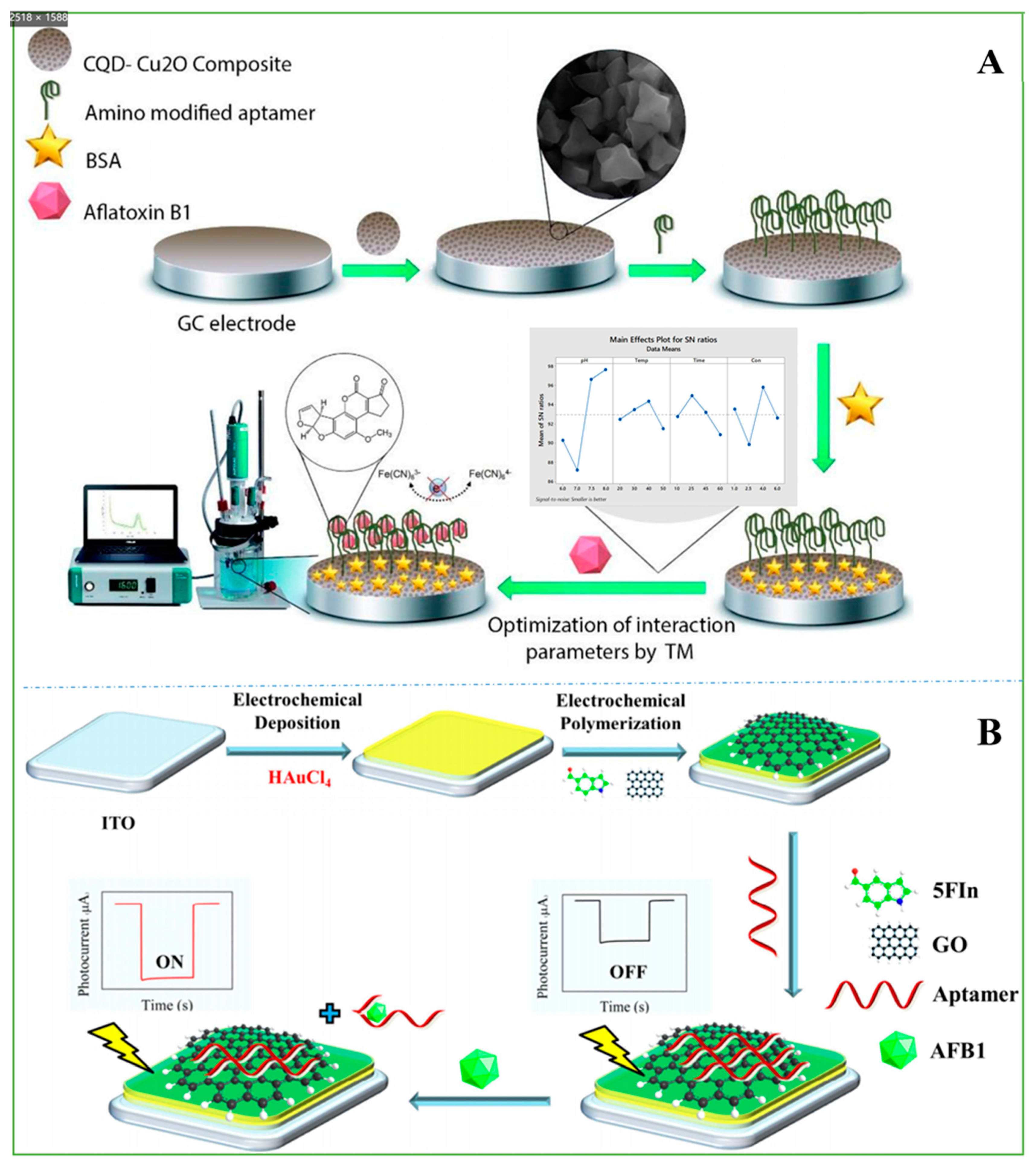
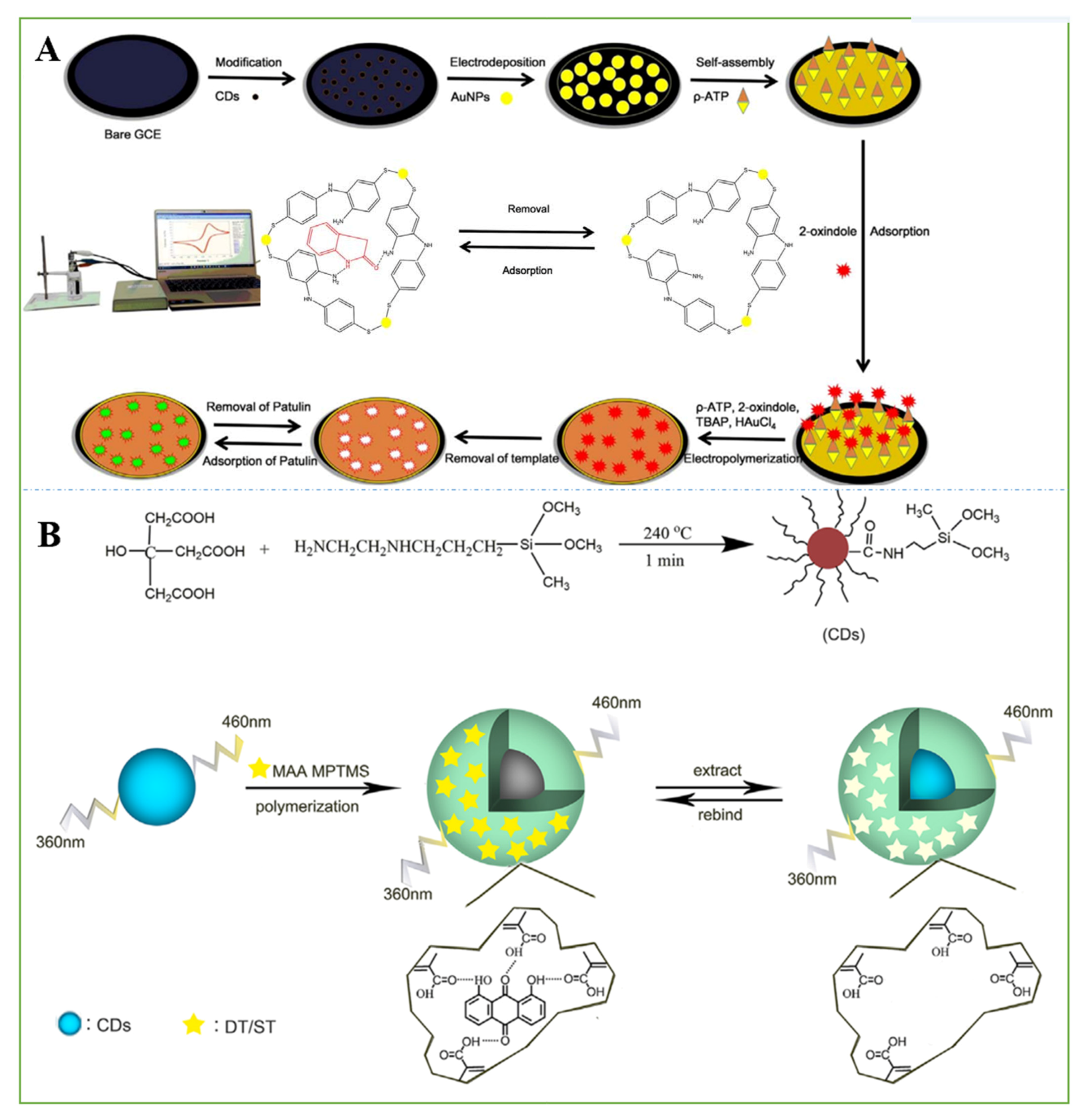
| Materials | Method | Mycotoxin | Samples | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|---|
| Au nanodots/rGO | layer-by-layer electrochemical deposition | AFB1 | peanut | up to 300 ppb | 6.9 pg/mL | [27] |
| MWCNTs/WS2 | drop-casting | AFB1 | corn | 0.5 to 10 ng mL−1 | 68 fg mL−1 | [28] |
| rGO/Au | one-pot hydrothermal | OTA | red wine | 1 pg/mL−10 ng/mL | 0.34 pg/mL | [34] |
| GQDs/AuNOs | chemical conjugation | AFB1 | maize | 0.1 to 2.5 ng mL−1 | 0.11 ng mL−1 | [53] |
| N-GQDs/Au@Cu-MOF | electropolymerization | patulin | apple juice | 0.001 to 70.0 ng mL−1 | 0.0007 ng mL−1 | [44] |
| rGO/Ag@AgCl | in-situ synthesis | OTA | red wine | 0.05 to 300 nM | 0.01 nM | [54] |
| rGO/Fe3O4NPs | in-situ synthesis | patulin | apple juice | 5 × 10−8 to 0.5 μg mL−1 | 30.4 fg mL−1 | [55] |
| CQDs-Cu2O | in-situ crystallization | AFB1 | wheat flour | 3 ag mL−1–1.9 µg mL−1 | 0.9 ± 0.04 ag ml−1 | [36] |
| NGQDs/ZnO | in-situ synthesis | ZEN | cereal crops | 1.0 × 10−13–1.0 × 10−7 g mL−1 | 3.3 × 10−14 g mL−1 | [57] |
| rGO/MoS2 | in-situ synthesis | AFB1 | wine | 0.01 fg mL−1 to 1.0 fg mL−1 | 0.002 fg mL−1 | [61] |
| CSWNTs/Au NPs | electrodeposition | T2 | swine meat | 0.01 to 100 µg L−1 | 0.13 µg L−1 | [62] |
| Mycotoxin | Aptamer Sequence (5′-3′) | Materials | Method | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|---|
| PAT | GGCCCGCCAACCCGCATCATCTACACTGATATTTTACCTT | GO | colorimetric | 50–2500 pg mL−1 | 48 pg mL−1 | [76] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | SWCNTs | fluorescence | 25–200 nM | 24.1 nM | [38] |
| OTA | TCCCTTTACGCCTTTTTGATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | SWCNHs | fluorescence | 10–1000 nM | 4.2 nM | [77] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | Nano-graphite | fluorescence | 2.0–50.0 μM | 20 nM | [37] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | GO | fluorescence | 10–200 nM | 0.324 nM | [78] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | GQDs | fluorescence | 0.01–20 ng mL−1 | 2.5 pg mL−1 | [79] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | GO | fluorescence | 0.08–200 ng mL−1 | 0.08 ng mL−1 | [80] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGG | GO | FRET | 0.05–100 ng mL−1 | 20 pg mL−1 | [74] |
| FB1 | ATACCAGCTTATTCAATTAATCGCATTACCTTATACCAG CTTATTCAATTACGTCTGCACATACCAGCTTATTCAATT AGATAGTAAGTGCAATCT | GO | FRET | 0.1–500 ng mL−1 | 100 pg mL−1 | [74] |
| AFB1 | AAAAAAAAAAGTTGGGCACGTGTTGTCTCTCTGTGTCTCGTGCCCTTCGCTAGGCCCACA | GO | fluorescence | up to 300 ppb | 4.5 ng mL−1 | [81] |
| AFB1 | GTTGGGCACGTGTTGTCTCTCTGTGTCTCGTGCCCT TCGCTAGGCCC | MWCNTs | fluorescence | 0.5–15 ng mL−1 | 20 pg mL−1 | [82] |
| ZON | AGCAGCACAGAGGTCAGATGTCATCTATCTATGGTACATTACTATCTGTAATGTGATATGCCTATGCGTGCTACCGTGAA | fGO | fluorescence | 0.5–64 ng mL−1 | 0.5 ng mL−1 | [83] |
| PAT | GGCCCGCCAACCCGCATCATCTACACTGATATTTTACCT | rGO-Fe3O4 | fluorescence | 0.5–30 ng mL−1 | 0.28 ng mL−1 | [84] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | GO | Luminescence | 0.001–250 ng mL−1 | 1 pg mL−1 | [85] |
| OTA | ATCCGTCACACCTGCTCTGACGCTGGGGTCGACCCGGAG AAATGCATTCCCCTGTGGTGTTGGCTCCCGTAT | GO-L-Ag NPs | ECL | 10–200 ng mL−1 | 0.05ng mL−1 | [86] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | GO | CV | 0.15–180 nM | 1000 pM | [34] |
| AFB1 | GTTGGGCACGTCTTGTCTCTCTGTGTCTCGTGCCCTTCGCTACGCCCACA | rGO | DPV | 0.5 nM–4 μM | 0.07 nM | [87] |
| AFB1 | TGGGGTTTTGGTGGCGGGTGGTGTACGGGCGAGGG | FGO | DPV | 0.05–6.0 ng mL−1 | 0.05 ng mL−1 | [32] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | SWCNTs | DPV | 0–45 nM | 58 pM | [88] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | GONPs | DPV | 310 fM–310 pM | 310 fM | [89] |
| AFM1 | ATCCGTCACACCTGCTCTGACGCTGGGGTCGACCCGGAG AAATGCATTCCCCTGTGGTGTTGGCTCCCGTAT | GO-L-Ag NPs | ECL | 5–150 ng mL−1 | 10 pg mL−1 | [86] |
| AFB1 | GTTGGGCACGTGTTGTCTCTCTGTGTCTCGTGCCCT TCGCTAGGCCCACA | erGO | PEC | 10 pg mL−1–100 ng mL−1 | 2 pg mL−1 | [90] |
| T2 | GTATATCAAGCATCGCGTGTTTACACATGCGAGAGGTGAA | rGO | Chronoamperometry | 10 fg mL−1–100 ng mL−1 | 1.79 fg mL−1 | [91] |
| FB1 | ATACCAGCTTATTCAATTAATCGCATTACCTTATACCAGCTTATTCAATTACGTCTGCACATACCAGCTTATTCAATTAGATAGTAAGTGCAATCT | GSTH | CV | 1–10−6 pg mL−1 | 1 pg m L−1 | [92] |
| ZEN | TCATCTATCTATGGTACATTACTATCTGTAATGTGATATG | rGO | DPV | 0.5 pg mL−1–50 ng mL−1 | 0.105 pg mL−1 | [93] |
| ZEN | TCATCTATCTATGGTACATTACTATCTGTAATGTGATATG | MWCNTs | CV | 0.5 pg mL−1–50 ng mL−1 | 0.17 pg mL−1 | [94] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | Graphene | DPV | 0.01–1000 × 10−6 ng mL−1 | 1 × 10−7 ng mL−1 | [95] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | Carboxylated graphene | DPV | 10 fmol L−1–10 nmol L−1 | 3.3 fmol L−1 | [96] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | GO | DPV | 0.01–50 ng mL−1 | 5.6 pg mL−1 (ppt) | [97] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | Au NPs–rGO | EIS | 0.001–50ng mL−1 | 0.3 pg mL−1 | [98] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | Au NPs–rGO | EIS | 0.1–200 ng mL−1 | 0.03 ng mL−1 | [99] |
| OTA | GATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA | graphene | DPV | 0.001–5 ng mL−1 | 0.13 pgmL−1 | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, X.; Zhang, W.; Meng, F.; Wang, X.; Qin, Y.; Zhang, M. Carbon-Based Nanocomposite Smart Sensors for the Rapid Detection of Mycotoxins. Nanomaterials 2021, 11, 2851. https://doi.org/10.3390/nano11112851
Ma X, Li X, Zhang W, Meng F, Wang X, Qin Y, Zhang M. Carbon-Based Nanocomposite Smart Sensors for the Rapid Detection of Mycotoxins. Nanomaterials. 2021; 11(11):2851. https://doi.org/10.3390/nano11112851
Chicago/Turabian StyleMa, Xiaoli, Xinbo Li, Wenrui Zhang, Fanxing Meng, Xin Wang, Yanan Qin, and Minwei Zhang. 2021. "Carbon-Based Nanocomposite Smart Sensors for the Rapid Detection of Mycotoxins" Nanomaterials 11, no. 11: 2851. https://doi.org/10.3390/nano11112851
APA StyleMa, X., Li, X., Zhang, W., Meng, F., Wang, X., Qin, Y., & Zhang, M. (2021). Carbon-Based Nanocomposite Smart Sensors for the Rapid Detection of Mycotoxins. Nanomaterials, 11(11), 2851. https://doi.org/10.3390/nano11112851






