Noncovalent Functionalization of Single-Walled Carbon Nanotubes with a Photocleavable Polythiophene Derivative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Dispersants for SWCNT
2.3. Dispersal of SWCNTs in THF
2.4. Preparation of PC56T44/SWCNT Composite Films
2.5. Characterization
3. Results and Discussion
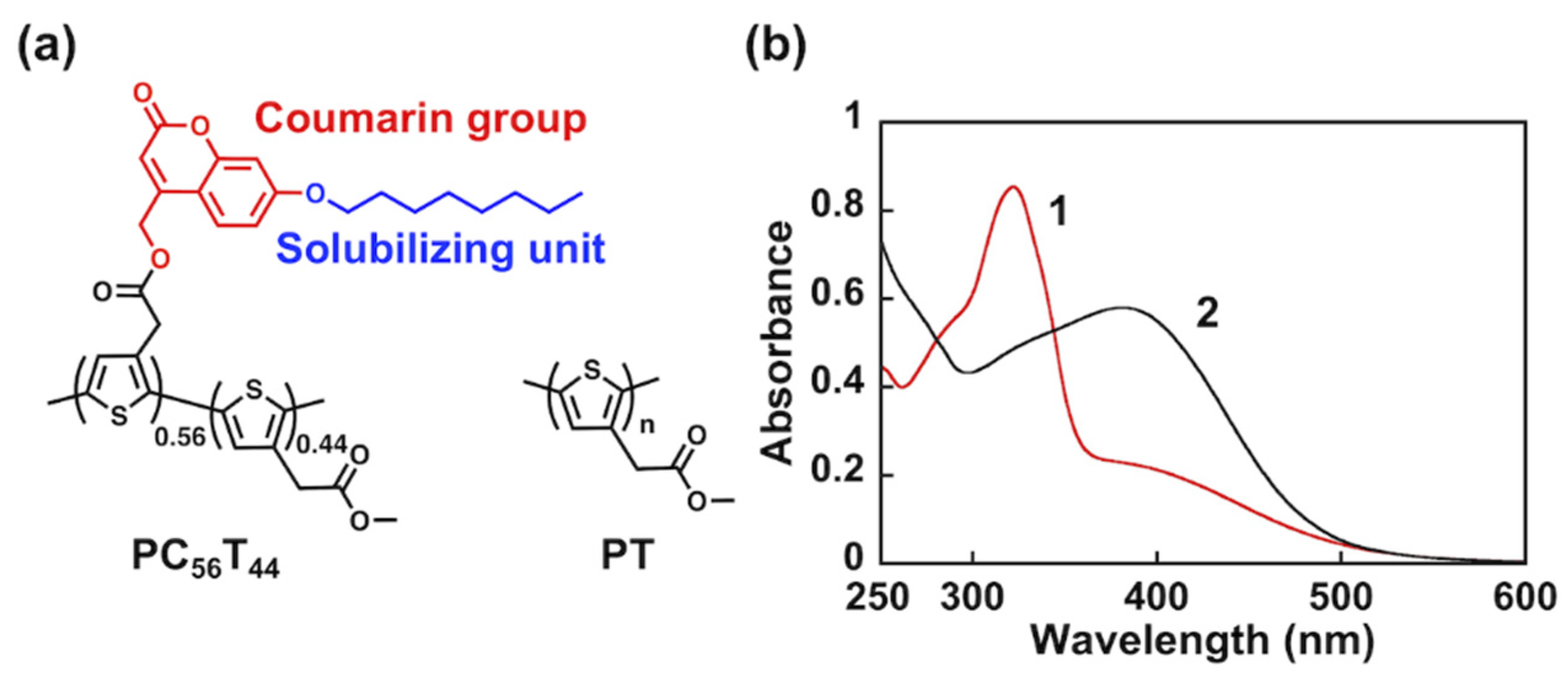
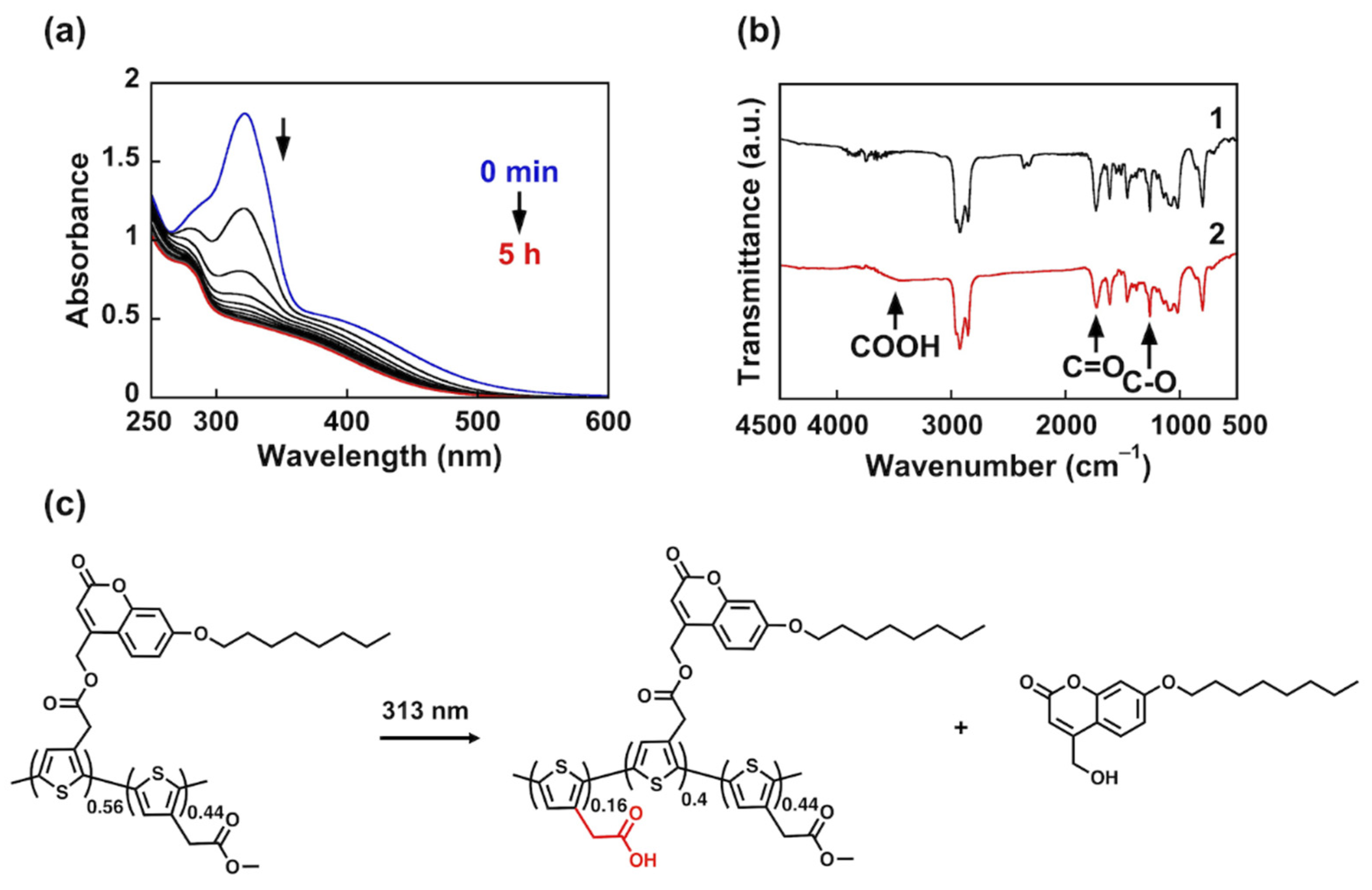
3.1. Photocleavage of PC56T44
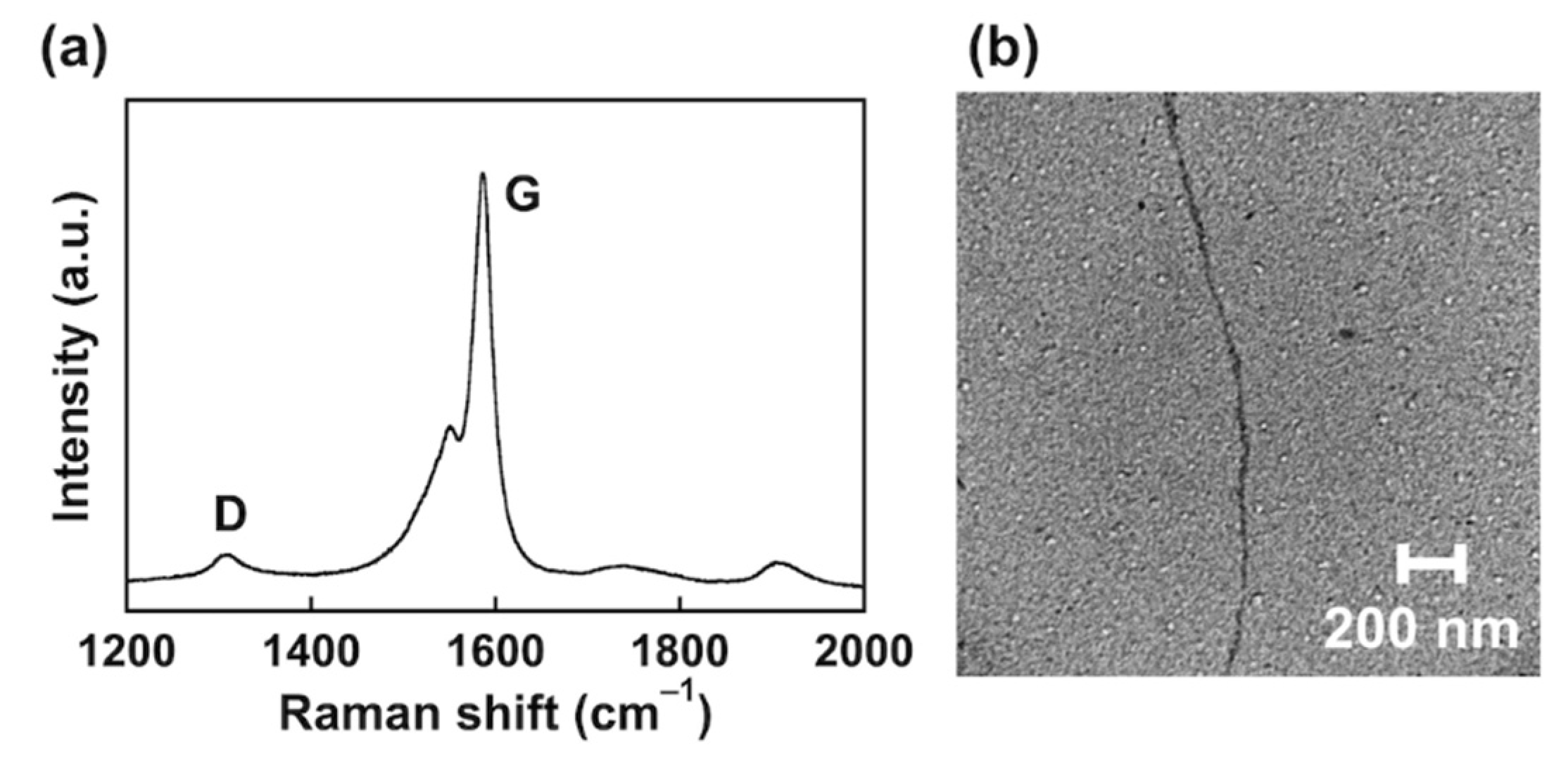
3.2. Dispersion of SWCNT Using Noncovalent Functionalization
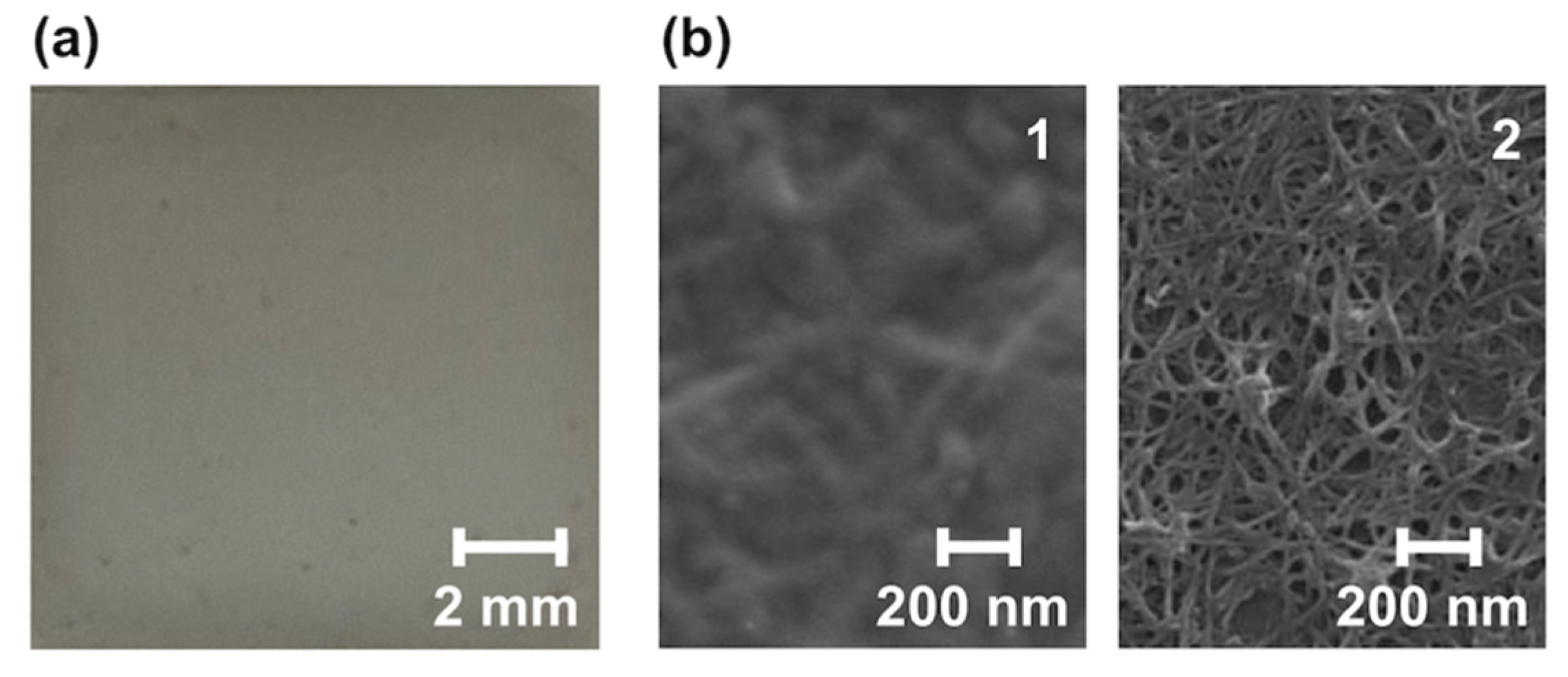
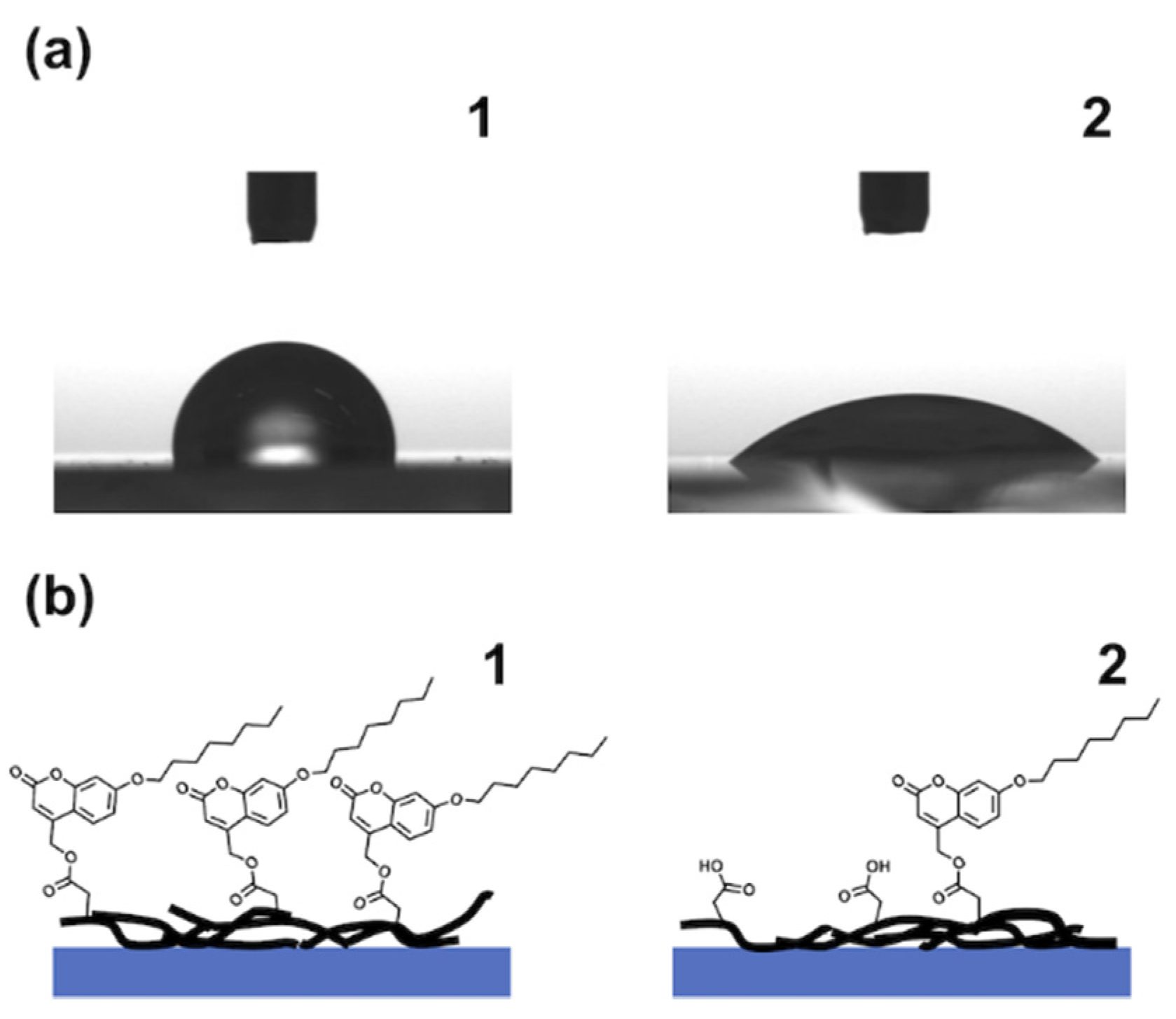
3.3. Morphological Change at the Surface of the Solution-Processed SWCNT Film
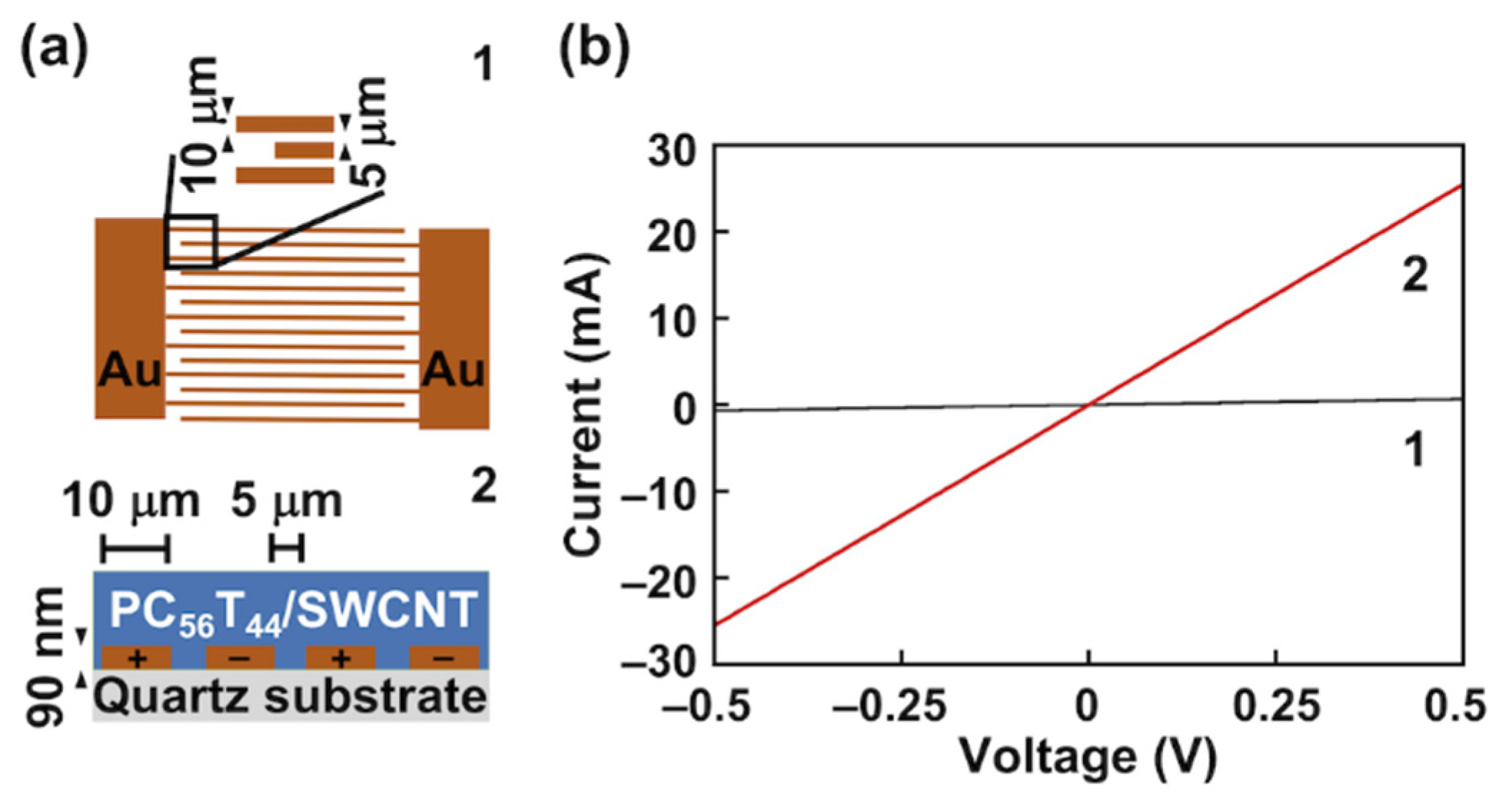
3.4. Modulation of the Electrical Conductivity of the PC56T44/SWCNT Composite Film upon Photocleavage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon nanotubes and related nanomaterials: Critical advances and challenges for synthesis toward mainstream commercial applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batmunkh, M.; Shearer, C.J.; Bat-Erdene, M.; Biggs, M.J.; Shapter, J.G. Single-walled carbon nanotubes enhance the efficiency and stability of mesoscopic perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 19945–19954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Shastry, T.A.; Xie, Y.; Bernardi, M.; Jasion, D.; Luck, K.A.; Marks, T.J.; Grossman, J.C.; Ren, S.; Hersam, M.C. Polychiral semiconducting carbon nanotube–fullerene solar cells. Nano Lett. 2014, 14, 5308–5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hills, G.; Lau, C.; Wright, A.; Fuller, S.; Bishop, M.D.; Srimani, T.; Kanhaiya, P.; Ho, R.; Amer, A.; Stein, Y.; et al. Modern microprocessor built from complementary carbon nanotube transistors. Nature 2019, 572, 595–602. [Google Scholar] [CrossRef]
- Dekker, C. How we made the carbon nanotube transistor. Nat. Electron. 2018, 1, 518. [Google Scholar] [CrossRef]
- Huang, W.; Toshimitsu, F.; Ozono, K.; Matsumoto, M.; Borah, A.; Motoishi, Y.; Park, K.H.; Jang, J.W.; Fujigaya, T. Thermoelectric properties of dispersant-free semiconducting single-walled carbon nanotubes sorted by a flavin extraction method. Chem. Commun. 2019, 55, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Dörling, B.; Ryan, J.D.; Craddock, J.D.; Sorrentino, A.; Basaty, A.E.; Gomez, A.; Garriga, M.; Pereiro, E.; Anthony, J.E.; Weisenberger, M.C.; et al. Photoinduced p- to n-type switching in thermoelectric polymer-carbon nanotube composites. Adv. Mater. 2016, 28, 2782–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Lee, D.-M.; Jung, Y.; Park, J.; Lee, H.S.; Kim, Y.-K.; Park, C.R.; Jeong, H.S.; Kim, S.M. Direct spinning and densification method for high-performance carbon nanotube fibers. Nat. Commun. 2019, 10, 2962. [Google Scholar] [CrossRef] [Green Version]
- Fujigaya, T.; Nakashima, N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Tech. Adv. Mater. 2015, 16, 024802. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Stoddart, J.F. Noncovalent functionalization of single-walled carbon nanotubes. Acc. Chem. Res. 2009, 42, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, P.; Barnes, B.; Fortner, J.; Wang, Y. Cleanly removable surfactant for carbon nanotubes. Chem. Mater. 2021, 33, 4551–4557. [Google Scholar] [CrossRef]
- Kawamoto, M.; He, P.; Ito, Y. Green processing of carbon nanomaterials. Adv. Mater. 2017, 29, 1602423. [Google Scholar] [CrossRef]
- Samanta, S.K.; Fritsch, M.; Scherf, U.; Gomulya, W.; Bisri, S.Z.; Loi, M.A. Conjugated polymer-assisted dispersion of single-wall carbon nanotubes: The power of polymer wrapping. Acc. Chem. Res. 2014, 47, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Barnes, B.; Huang, Z.; Wang, Z.; Zheng, M.; Wang, Y. Beyond color: The new carbon ink. Adv. Mater. 2021, 33, 2005890. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Shimano, S.; Salikolimi, K.; Isoshima, T.; Kakefuda, Y.; Mori, T.; Taguchi, Y.; Ito, Y.; Kawamoto, M. Noncovalent modification of single-walled carbon nanotubes using thermally cleavable polythiophenes for solution-processed thermoelectric films. ACS Appl. Mater. Interfaces 2019, 11, 4211–4218. [Google Scholar] [CrossRef]
- Luo, J.; Uprety, R.; Naro, Y.; Chou, C.; Nguyen, D.P.; Chin, J.W.; Deiters, A. Genetically encoded optochemical probes for simultaneous fluorescence reporting and light activation of protein function with two-photon excitation. J. Am. Chem. Soc. 2014, 136, 15551–15558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, H.; Furuta, T.; Tsien, R.Y.; Okamoto, H. Photo-mediated gene activation using caged RNA/DNA in zebrafish embryos. Nat. Genet. 2001, 28, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, J.R.; Kodama, K.; Hirose, T.; Ito, Y.; Kawamoto, M. Photocleavage behavior of a polythiophene derivative containing a coumarin unit. Polym. J. 2021; 1–8, in press. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Mechanism of photocleavage of (coumarin-4-yl)methyl esters. J. Phys. Chem. A 2007, 111, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.R.; Goldoni, F.; Schenning, A.P.H.J.; Meijer, E.W. Non-ionic polythiophenes: A non-aggregating folded structure in water. Chem. Commun. 2005, 44, 5503–5505. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Tanaka, T.; Kataura, H. Adsorbability of single-wall carbon nanotubes onto agarose gels affects the quality of the metal/semiconductor separation. J. Phys. Chem. C 2011, 115, 21723–21729. [Google Scholar] [CrossRef]
- Graf, A.; Zakharko, Y.; Schießl, S.P.; Backes, C.; Pfohl, M.; Flavel, B.S.; Zaumseil, J. Large scale, selective dispersion of long single-walled carbon nanotubes with high photoluminescence quantum yield by shear force mixing. Carbon 2016, 105, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.M.; Richter, E.; Bandow, S.; Chase, B.; Eklund, P.C.; Williams, K.A.; Fang, S.; Subbaswamy, K.R.; Menon, M.; Thess, A.; et al. Diameter-selective raman scattering from vibrational modes in carbon nanotubes. Science 1997, 275, 187–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhai, J.; Jiang, L. Wetting and anti-wetting on aligned carbon nanotube films. Soft Matter. 2006, 2, 811–821. [Google Scholar] [CrossRef]
- Li, Z.; He, P.; Chong, H.; Furube, A.; Seki, K.; Yu, H.-H.; Tajima, K.; Ito, Y.; Kawamoto, M. Direct aqueous dispersion of carbon nanotubes using nanoparticle-formed fullerenes and self-assembled formation of p/n heterojunctions with polythiophene. ACS Omega 2017, 2, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhao, H.; Bai, Y.; Zhang, T.; Lupinacci, A.S.; Minor, A.M.; Liu, G. Solvent processed conductive polymer with single-walled carbon nanotube composites. J. Mater. Res. 2015, 30, 3403–3411. [Google Scholar] [CrossRef]
- Kim, H.J.; Koizhaiganova, R.B.; Karim, M.R.; Lee, G.H.; Vasudevan, T.; Lee, M.S. Synthesis and characterization of poly(3-octylthiophene)/single wall carbon nanotube composites for photovoltaic applications. J. Appl. Polym. Sci. 2010, 118, 1386–1394. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muralidhar, J.R.; Kodama, K.; Hirose, T.; Ito, Y.; Kawamoto, M. Noncovalent Functionalization of Single-Walled Carbon Nanotubes with a Photocleavable Polythiophene Derivative. Nanomaterials 2022, 12, 52. https://doi.org/10.3390/nano12010052
Muralidhar JR, Kodama K, Hirose T, Ito Y, Kawamoto M. Noncovalent Functionalization of Single-Walled Carbon Nanotubes with a Photocleavable Polythiophene Derivative. Nanomaterials. 2022; 12(1):52. https://doi.org/10.3390/nano12010052
Chicago/Turabian StyleMuralidhar, Jyorthana Rajappa, Koichi Kodama, Takuji Hirose, Yoshihiro Ito, and Masuki Kawamoto. 2022. "Noncovalent Functionalization of Single-Walled Carbon Nanotubes with a Photocleavable Polythiophene Derivative" Nanomaterials 12, no. 1: 52. https://doi.org/10.3390/nano12010052
APA StyleMuralidhar, J. R., Kodama, K., Hirose, T., Ito, Y., & Kawamoto, M. (2022). Noncovalent Functionalization of Single-Walled Carbon Nanotubes with a Photocleavable Polythiophene Derivative. Nanomaterials, 12(1), 52. https://doi.org/10.3390/nano12010052





