Silver Nanoparticle-Mediated Synthesis of Fluorescent Thiolated Gold Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Quasi-Spherical Ag NPs
2.3. Synthesis of Au NCs
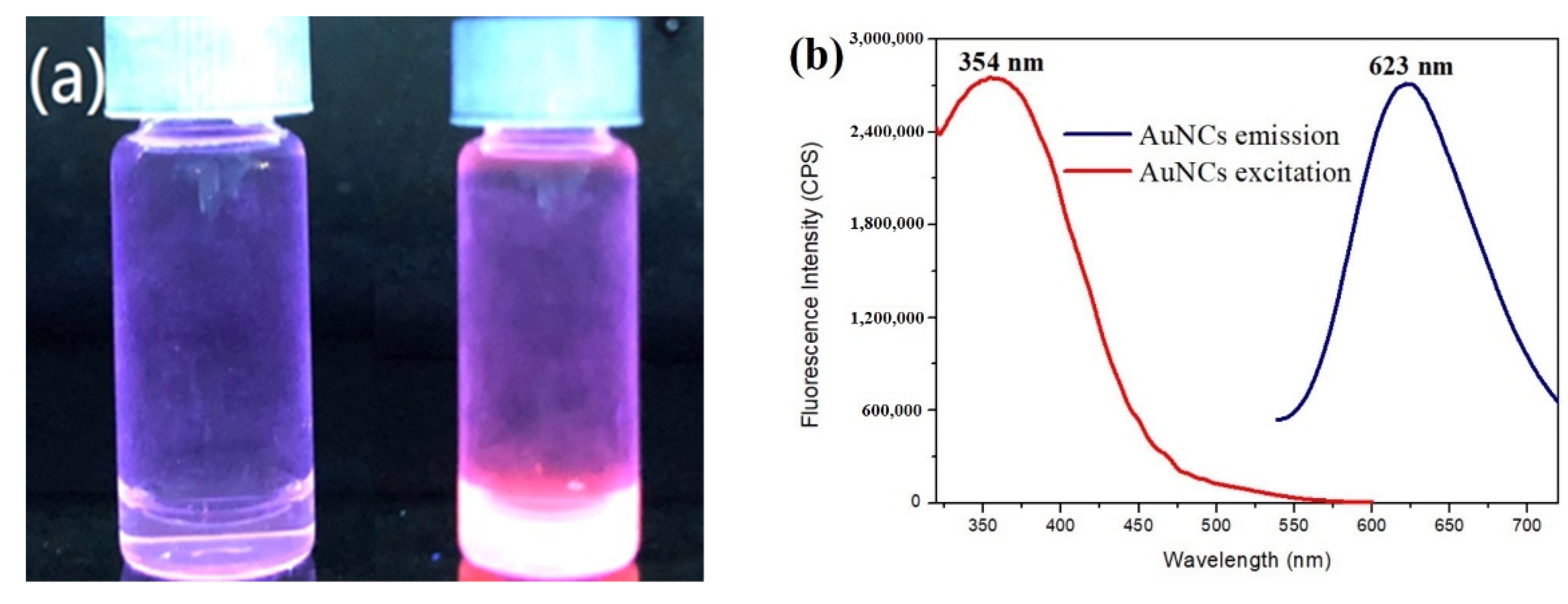
2.4. Fluorescence Spectroscopy
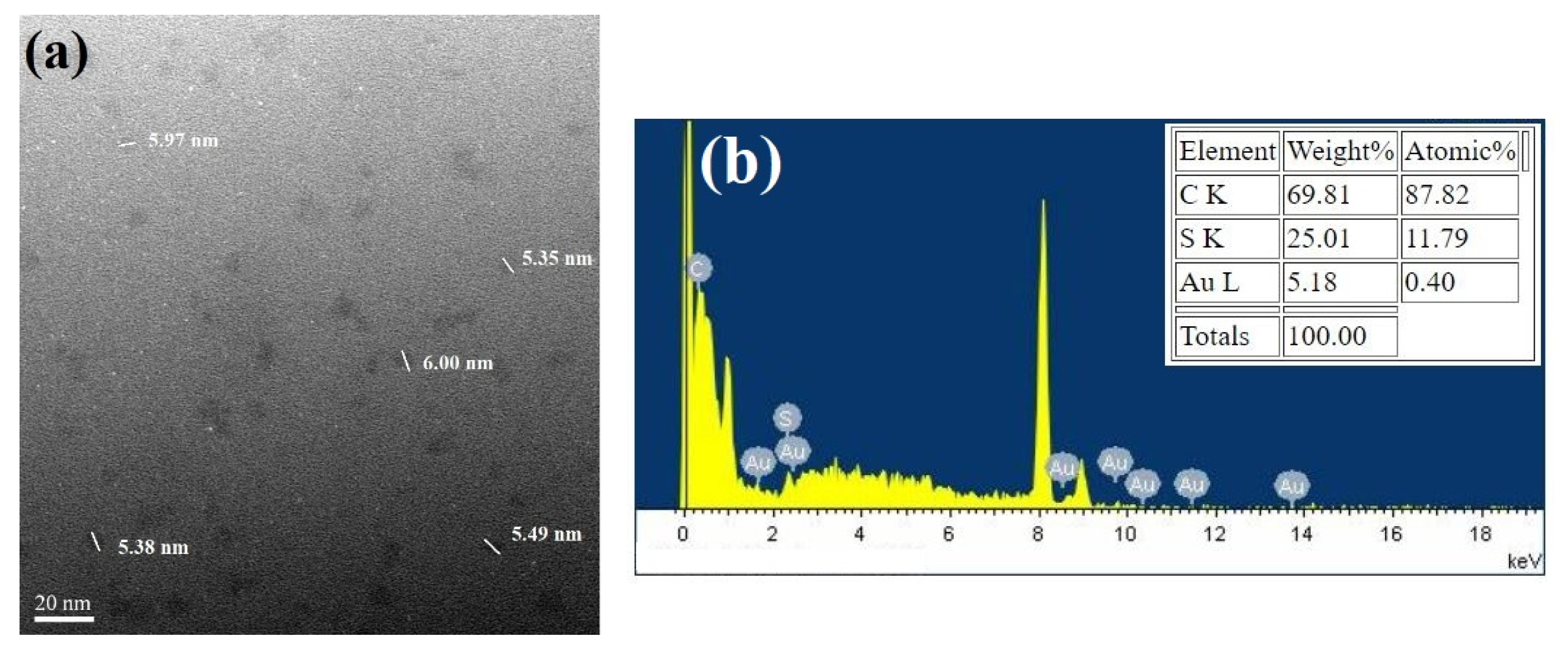
2.5. TEM Imaging of Ag NPs and Au NCs
2.6. Quantum Yields
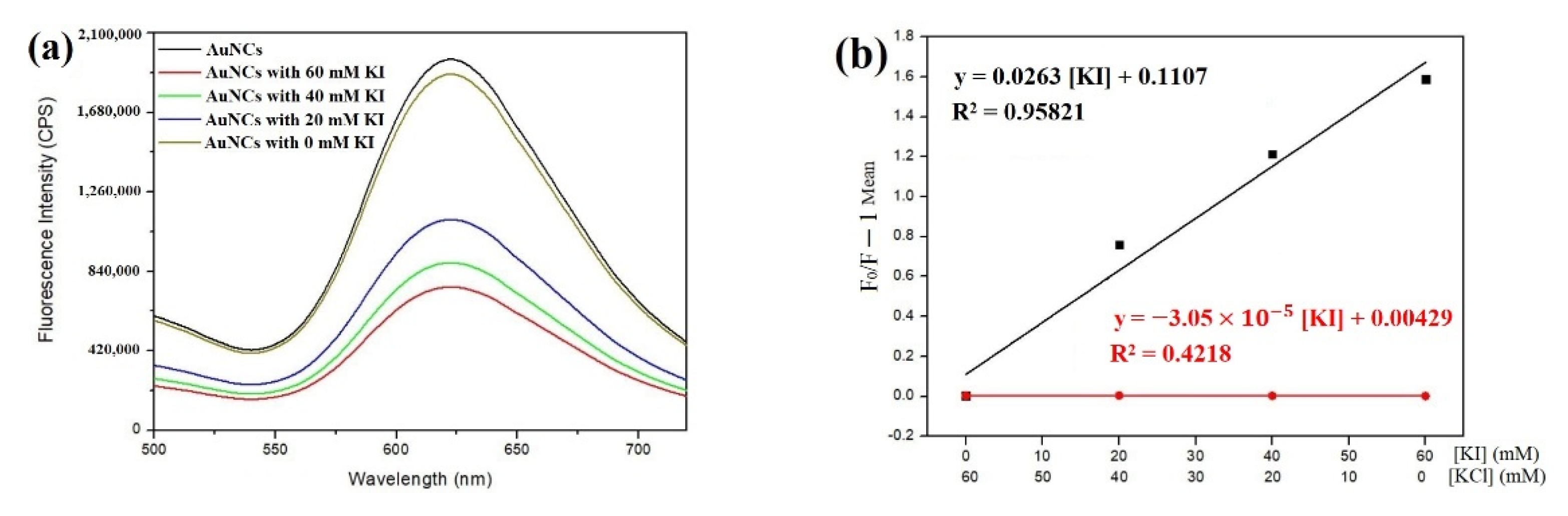
2.7. Collisional Quenching by Iodide Ions and Other Quenchers
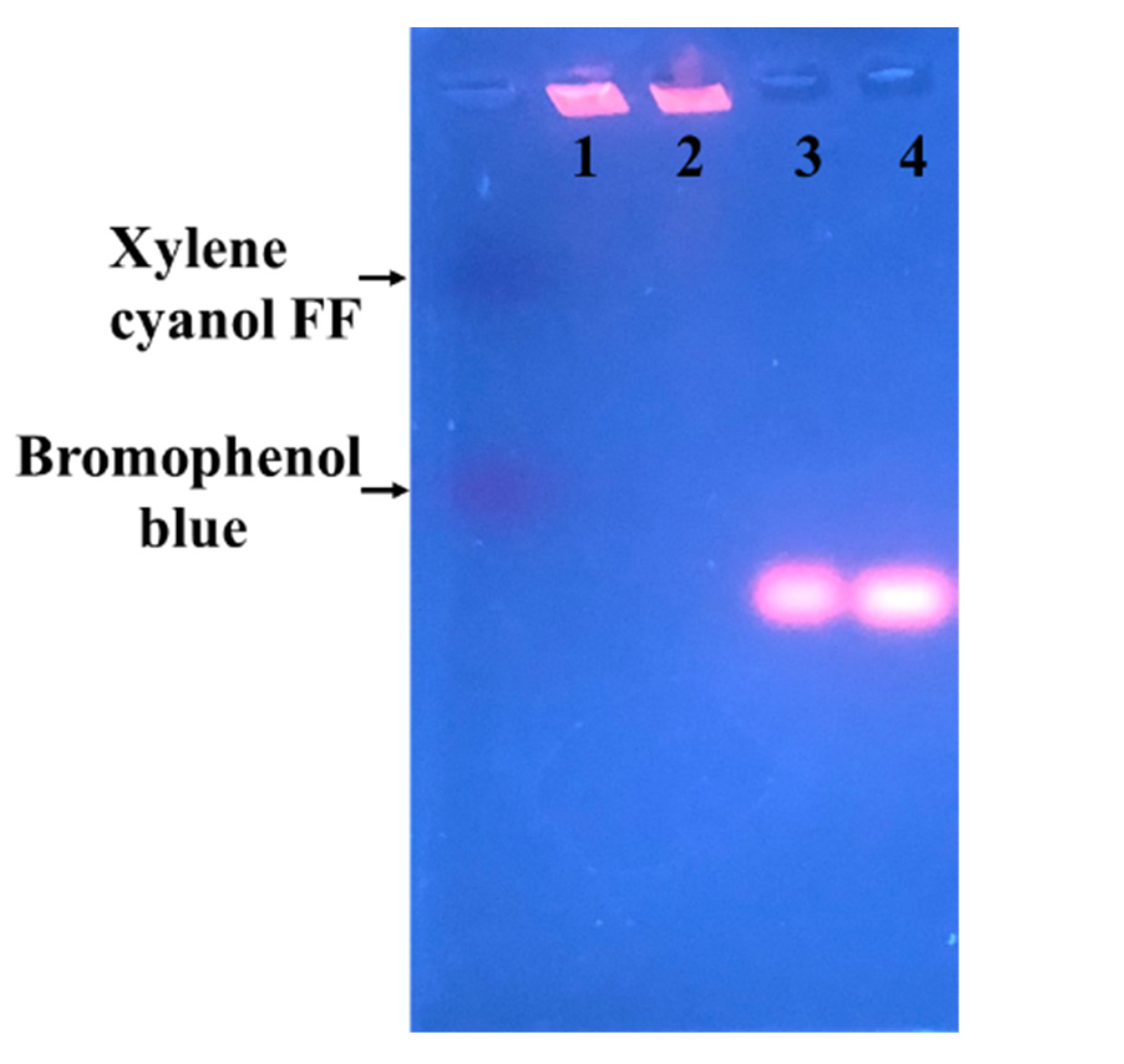
2.8. Electrophoresis
3. Results
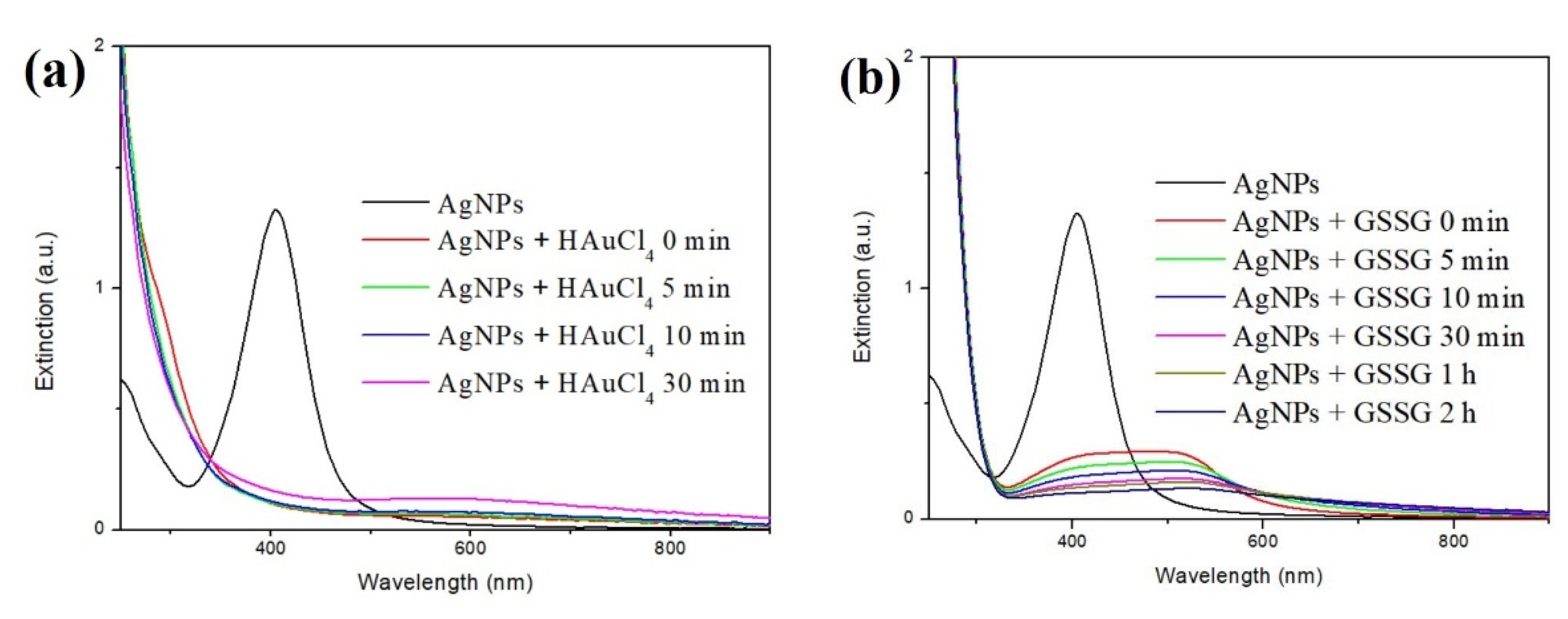
3.1. Synthesis of Quasi-Spherical Silver Nanoparticles (Ag NPs)
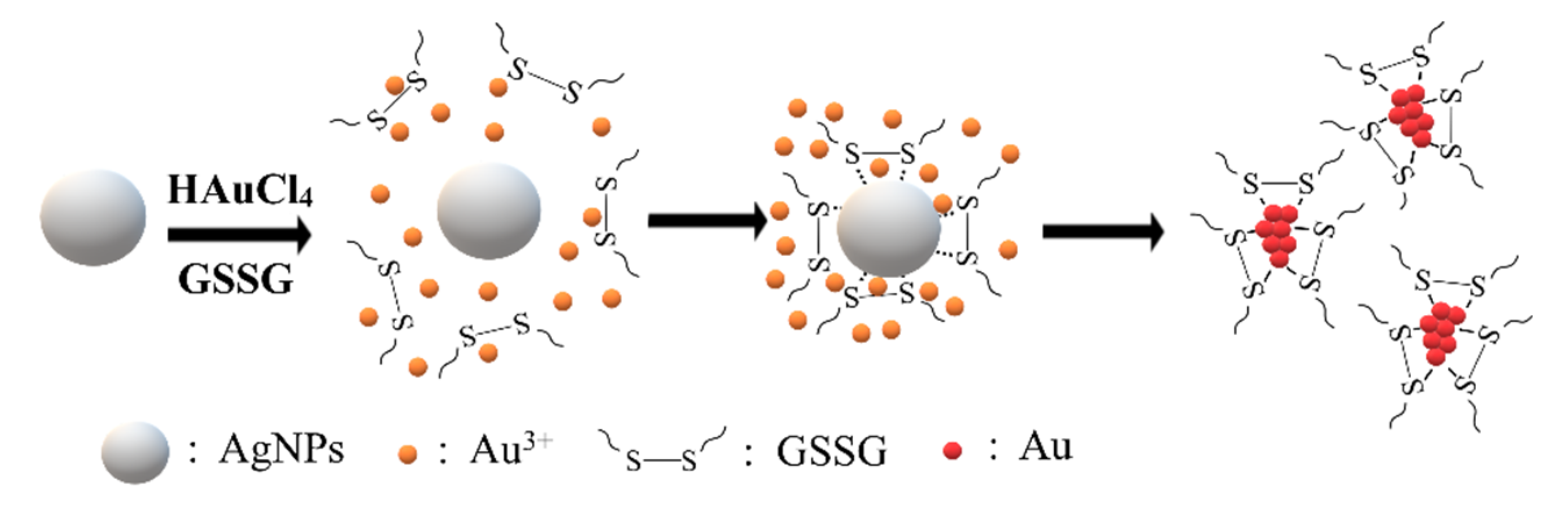
3.2. Synthesis of Gold Nanoclusters Using GSSG as Capping Ligands
3.3. The Role of Ag NPs in the Synthesis of Au NCs
3.4. Using Dithiothreitol as Capping Ligands
3.5. Electrophoretic Mobiliy of Glutathione-Protected and DTT-Protected Au NCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qu, X.; Li, Y.; Li, L.; Wang, Y.; Liang, J.; Liang, J. Fluorescent Gold Nanoclusters: Synthesis and Recent Biological Application. J. Nanomater. 2015, 2015, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2014, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Fedrigo, S.; Harbich, W.; Buttet, J. Optical response of Ag2, Ag3, Au2, and Au3 in argon matrices. J. Chem. Phys. 1993, 99, 5712–5717. [Google Scholar] [CrossRef]
- Morishita, K.; MacLean, J.L.; Liu, B.; Jiang, H.; Liu, J. Correlation of photobleaching, oxidation and metal induced fluorescence quenching of DNA-templated silver nanoclusters. Nanoscale 2013, 5, 2840–2849. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-Y.; Wei, X.-R.; Chen, Y.; Wang, H.-F.; Ge, J.-F.; Xu, Y.-J.; Ren, Z.-G.; Braunstein, P.; Lang, J.-P. Tetradecanuclear and Octadecanuclear Gold(I) Sulfido Clusters: Synthesis, Structures, and Luminescent Selective Tracking of Lysosomes in Living Cells. Inorg. Chem. 2019, 58, 3690–3697. [Google Scholar] [CrossRef]
- Roy, S.; Baral, A.; Bhattacharjee, R.; Jana, B.; Datta, A.; Ghosh, S.; Banerjee, A. Preparation of multi-coloured different sized fluorescent gold clusters from blue to NIR, structural analysis of the blue emitting Au7cluster, and cell-imaging by the NIR gold cluster. Nanoscale 2014, 7, 1912–1920. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, M.; Chen, X.; Wang, M.; Chen, H. Atomically Monodispersed and Fluorescent Sub-Nanometer Gold Clusters Created by Biomolecule-Assisted Etching of Nanometer-Sized Gold Particles and Rods. Chem. Eur. J. 2009, 15, 4944–4951. [Google Scholar] [CrossRef]
- Duan, H.; Nie, S. Etching Colloidal Gold Nanocrystals with Hyperbranched and Multivalent Polymers: A New Route to Fluorescent and Water-Soluble Atomic Clusters. J. Am. Chem. Soc. 2007, 129, 2412–2413. [Google Scholar] [CrossRef]
- Weare, W.W.; Reed, S.M.; Warner, M.G.; Hutchison, J.E. Improved Synthesis of Small (dCORE ≈ 1.5 nm) Phosphine-Stabilized Gold Nanoparticles. J. Am. Chem. Soc. 2000, 122, 12890–12891. [Google Scholar] [CrossRef]
- Briant, C.E.; Theobald, B.R.C.; White, J.W.; Bell, L.K.; Mingos, D.M.P.; Welch, A.J. Synthesis and X-ray structural characterization of the centred icosahedral gold cluster compound [Aul3(PMe2Ph)10Cl2](PF6)3; the realization of a theoretical prediction. J. Chem. Soc. Chem. Commun. 1981, 5, 201–202. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Antoine, R. Supramolecular Gold Chemistry: From Atomically Precise Thiolate-Protected Gold Nanoclusters to Gold-Thiolate Nanostructures. Nanomaterials 2020, 10, 377. [Google Scholar] [CrossRef] [Green Version]
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 A Resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Eckenhoff, W.; Zhu, Y.; Pintauer, T.; Jin, R. Total Structure Determination of Thiolate-Protected Au38 Nanoparticles. J. Am. Chem. Soc. 2010, 132, 8280–8281. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, W.; Niihori, Y.; Sharma, S.; Negishi, Y. Precise synthesis, functionalization and application of thiolate-protected gold clusters. Co-Ord. Chem. Rev. 2016, 320–321, 238–250. [Google Scholar] [CrossRef]
- Hosier, C.A.; Anderson, I.D.; Ackerson, C.J. Acetylide-for-thiolate and thiolate-for-acetylide exchange on gold nanoclusters. Nanoscale 2020, 12, 6239–6242. [Google Scholar] [CrossRef]
- Pichugina, D.A.; Nikitina, N.A.; Kuz’menko, N.E. On the Mechanism of the Reaction between Thiolate-Protected Gold Clusters and Molecular Oxygen: What is Activated? J. Phys. Chem. C 2020, 124, 3080–3086. [Google Scholar] [CrossRef]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2009, 2, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Pu, K.-Y.; Liu, B. Fluorescent Conjugated Polyelectrolytes for Bioimaging. Adv. Funct. Mater. 2011, 21, 3408–3423. [Google Scholar] [CrossRef]
- Chan, P.-H.; Chen, Y.-C. Human Serum Albumin Stabilized Gold Nanoclusters as Selective Luminescent Probes forStaphylococcus aureusand Methicillin-ResistantStaphylococcus aureus. Anal. Chem. 2012, 84, 8952–8956. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Wu, H.-T.; Hsiao, Y.-H.; Lai, C.-W.; Shih, C.-W.; Peng, Y.-K.; Tang, K.-C.; Chang, H.-W.; Chien, Y.-C.; Hsiao, J.-K.; et al. Insulin-Directed Synthesis of Fluorescent Gold Nanoclusters: Preservation of Insulin Bioactivity and Versatility in Cell Imaging. Angew. Chem. Int. Ed. 2011, 50, 7056–7060. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Z.; Yang, L.; Tian, S.; Hou, C.; Lu, Y. Lysozyme-stabilized gold fluorescent cluster: Synthesis and application as Hg2+ sensor. Analyst 2010, 135, 1406–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.X.; Tzeng, W.C.; Huang, C.L. One-pot synthesis of icosahedral silver nanoparticles by using a photoassisted tartrate re-duction method under UV light with a wavelength of 310 nm. ChemPhysChem 2016, 17, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Bain, D.; Paramanik, B.; Patra, A. Silver (I)-Induced Conformation Change of DNA: Gold Nanocluster as a Spectroscopic Probe. J. Phys. Chem. C 2017, 121, 4608–4617. [Google Scholar] [CrossRef]
- Ding, H.; Chen, Z. Nanotheranostic Application of Fluorescent Protein-Gold Nanocluster Hybrid Materials: A Mini-review. Nanotheranostics 2021, 5, 461–471. [Google Scholar] [CrossRef]
- Hamman, B.D.; Chen, J.-C.; Johnson, E.E.; Johnson, A.E. The Aqueous Pore through the Translocon Has a Diameter of 40–60 Å during Cotranslational Protein Translocation at the ER Membrane. Cell 1997, 89, 535–544. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Tseng, T.-H.; Chen, B.-R.; Wu, Y.-R.; Huang, C.-L.; Chen, J.-C. Silver Nanoparticle-Mediated Synthesis of Fluorescent Thiolated Gold Nanoclusters. Nanomaterials 2021, 11, 2835. https://doi.org/10.3390/nano11112835
Chang C-Y, Tseng T-H, Chen B-R, Wu Y-R, Huang C-L, Chen J-C. Silver Nanoparticle-Mediated Synthesis of Fluorescent Thiolated Gold Nanoclusters. Nanomaterials. 2021; 11(11):2835. https://doi.org/10.3390/nano11112835
Chicago/Turabian StyleChang, Cheng-Yeh, Tzu-Hsien Tseng, Bo-Ru Chen, Yi-Ru Wu, Cheng-Liang Huang, and Jui-Chang Chen. 2021. "Silver Nanoparticle-Mediated Synthesis of Fluorescent Thiolated Gold Nanoclusters" Nanomaterials 11, no. 11: 2835. https://doi.org/10.3390/nano11112835
APA StyleChang, C.-Y., Tseng, T.-H., Chen, B.-R., Wu, Y.-R., Huang, C.-L., & Chen, J.-C. (2021). Silver Nanoparticle-Mediated Synthesis of Fluorescent Thiolated Gold Nanoclusters. Nanomaterials, 11(11), 2835. https://doi.org/10.3390/nano11112835





