Preparation of W/O Hypaphorine–Chitosan Nanoparticles and Its Application on Promoting Chronic Wound Healing via Alleviating Inflammation Block
Abstract
:1. Introduction
2. Methods
2.1. Drugs and Chemicals
2.2. Determination of HYP
2.3. Preparation of HYP-NPS Nanoparticles and Hydrogels
2.4. Characterization of HYP-NPS Nanoparticles
2.5. Drug Release Assay In Vitro
2.6. Cell Cytotoxicity Test
2.7. Real-Time Quantitative PCR Analysis
2.8. Animal Experiment
2.9. In Vivo Wound Healing Study
2.10. Histopathology Study
2.11. Immunohistochemical (IHC) Staining
2.12. Western Blotting
2.13. Statistical Analysis
3. Results and Discussion
3.1. Physical–Chemical Characterization of HYP Nanoparticles (HYP-NPS)
3.2. FT-IR Analysis
3.3. Sustained Release Test
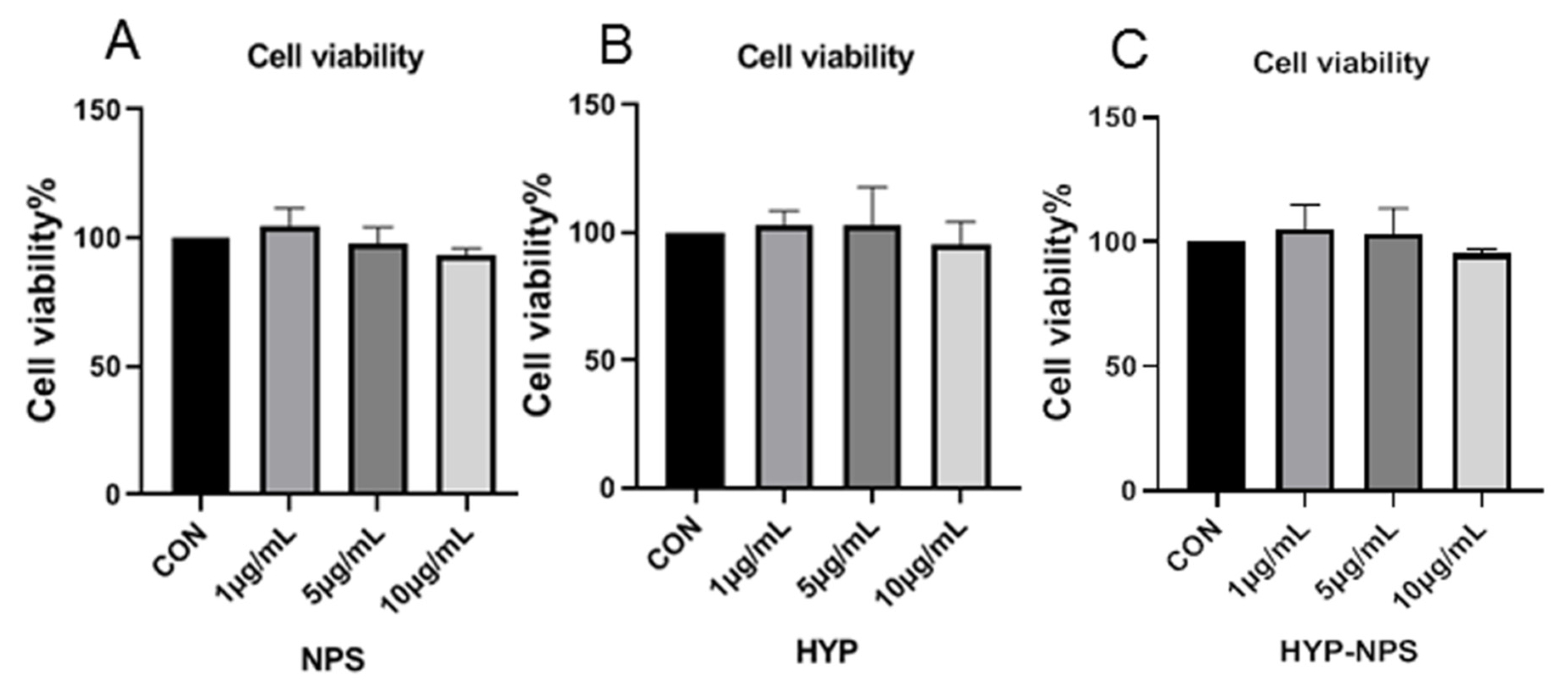
3.4. Cytocompatibility Assessment of HYP-NPS
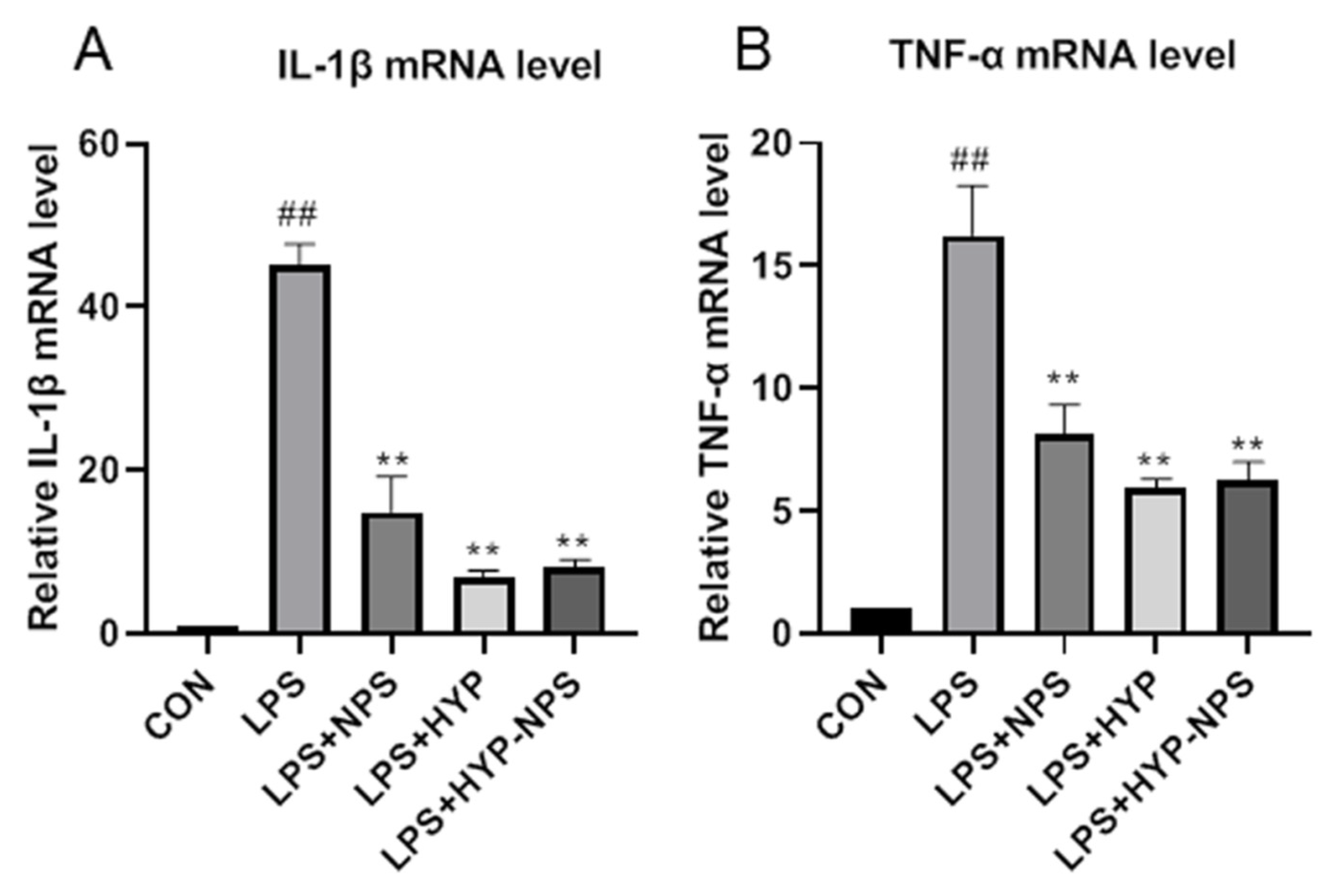
3.5. Effect of HYP-NPS on LPS-Induced RAW 264.7 Responses In Vitro
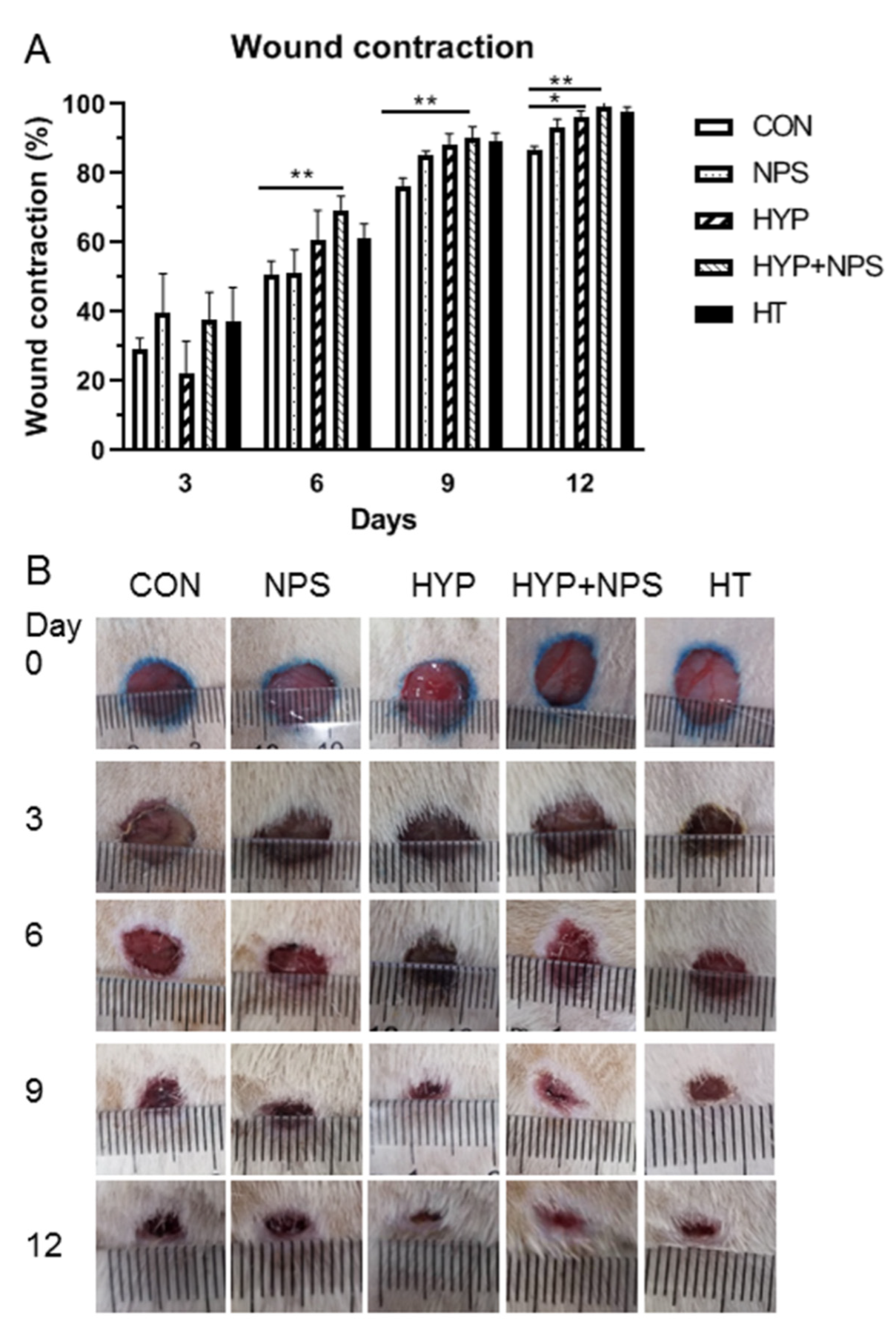
3.6. Effect of HYP-NPS on Wound Healing in Diabetic Rats
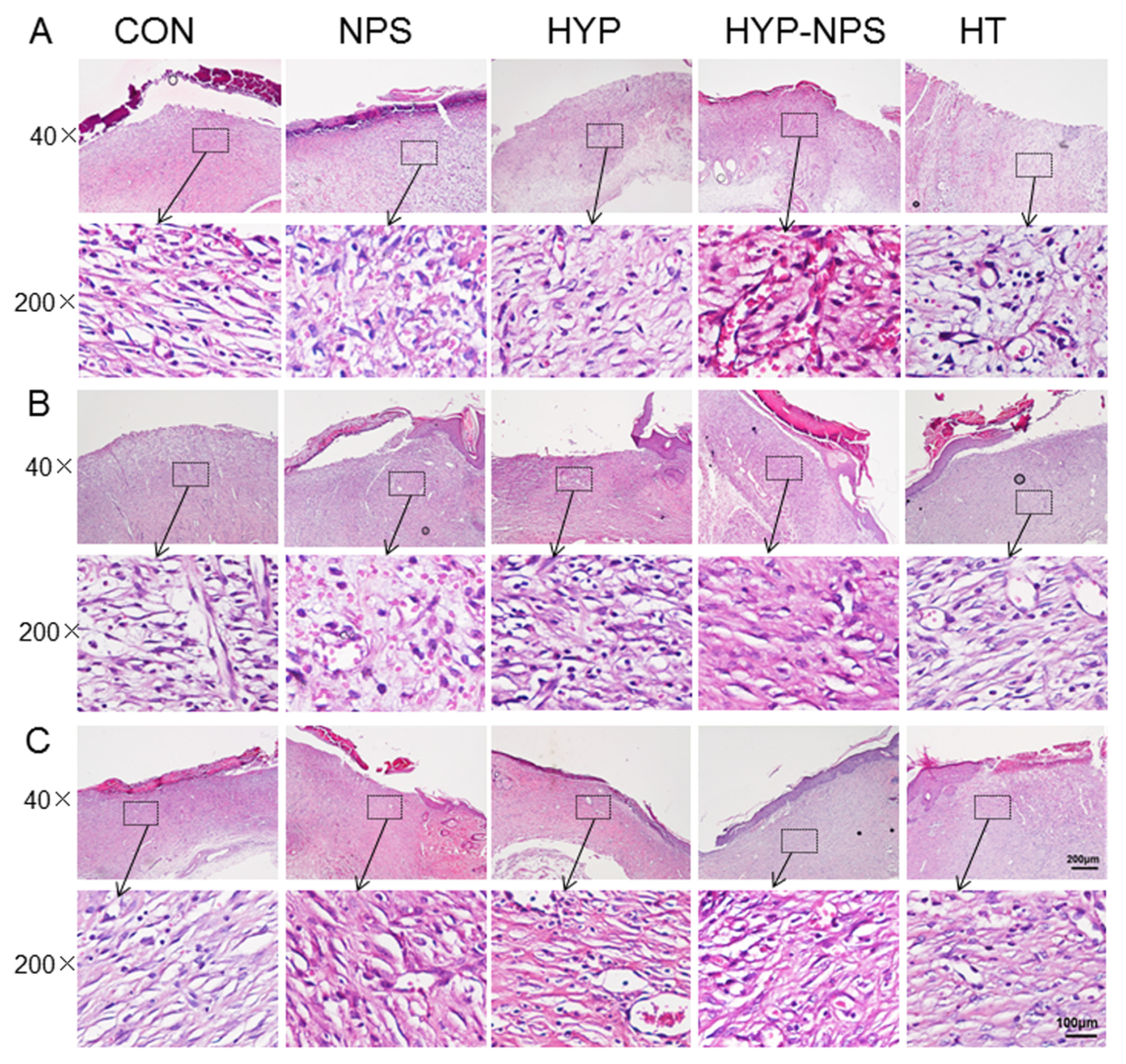
3.7. Histopathological Results
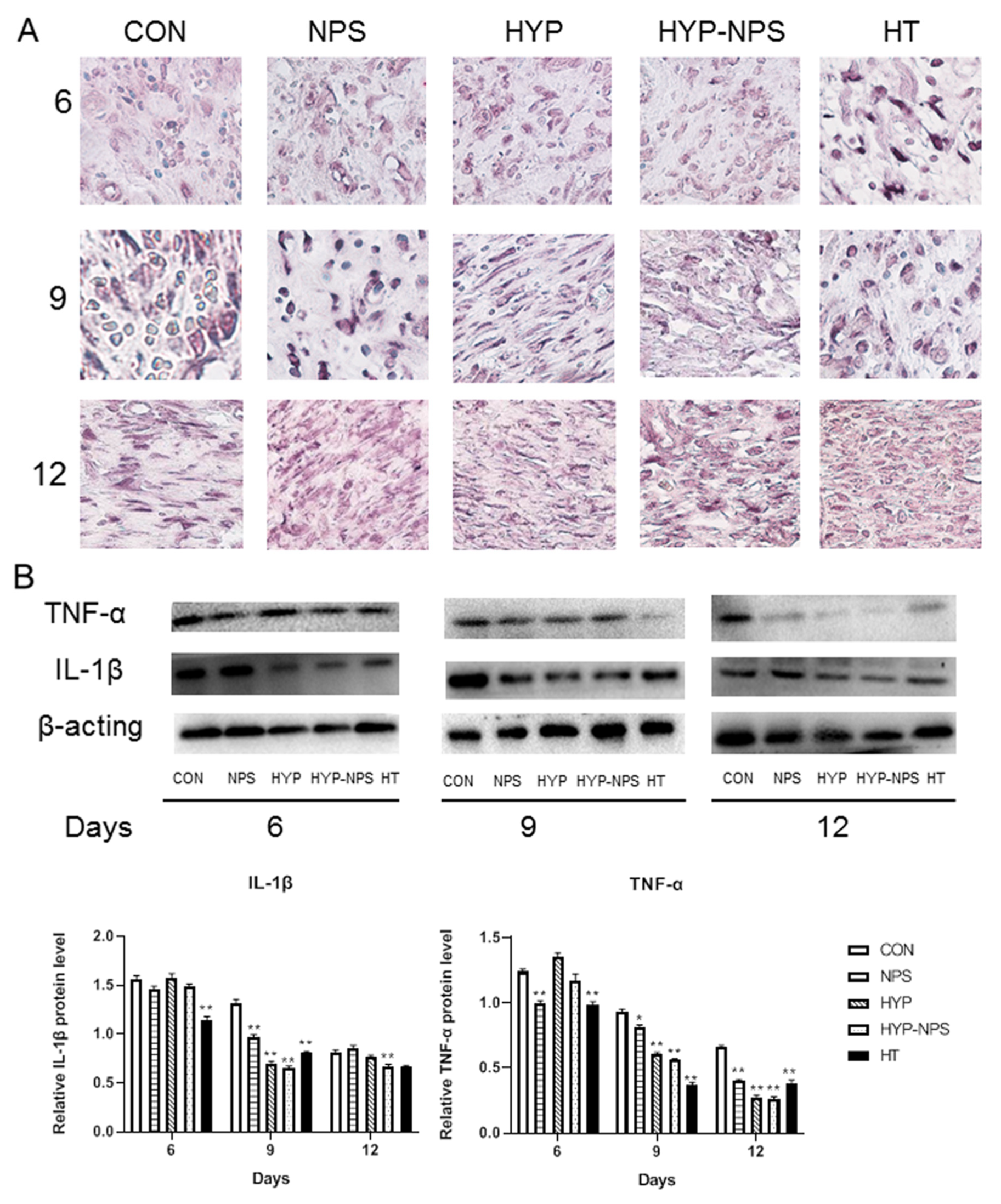
3.8. Expression Analysis of Inflammatory Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapp, S.; Santamaria, N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int. Wound J. 2017, 14, 1108–1119. [Google Scholar] [CrossRef]
- Erfurt-Berge, C.; Renner, R. Quality of life in patients with chronic wounds. Hautarzt 2020, 71, 863–869. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branski, L.K.; Gauglitz, G.G.; Herndon, D.N.; Jeschke, M.G. A review of gene and stem cell therapy in cutaneous wound healing. Burns 2009, 35, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S.; Bruckner-Tuderman, L.; Krieg, T.; Shannon, J.D.; Fox, J.W. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J. Proteome Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef]
- Beidler, S.K.; Douillet, C.D.; Berndt, D.F.; Keagy, B.A.; Rich, P.B.; Marston, W.A. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J. Vasc. Surg. 2009, 49, 1013–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Tarnuzzer, R.W.; Schultz, G.S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996, 4, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [PubMed]
- Mollica, A.; Locatelli, M.; Stefanucci, A.; Pinnen, F. Synthesis and bioactivity of secondary metabolites from marine sponges containing dibrominated indolic systems. Molecules 2012, 17, 6083–6099. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Zhu, X.; Cai, W.; Qiu, L. Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-gamma Dependent on PI3K/Akt/mTOR Signal Pathway. Int. J. Mol. Sci. 2017, 18, 844. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Cai, W.; Wang, X.; Liu, Y.; Hou, B.; Zhu, X.; Qiu, L. Vaccaria hypaphorine alleviates lipopolysaccharide-induced inflammation via inactivation of NFkappaB and ERK pathways in Raw 264.7 cells. BMC Complement. Altern. Med. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wu, J. Recent development in chitosan nanocomposites for surface-based biosensor applications. Electrophoresis 2019, 40, 2084–2097. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104 Pt A, 1003–1011. [Google Scholar] [CrossRef]
- Ye, C.; Zou, H.; Peng, Y.; Liu, X.; Chen, Z. Preparation of chitosan-collagen sponge and its application in wound dressing. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2004, 21, 259–260. [Google Scholar]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tondervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Jayanudin; Fahrurrozi, M.; Wirawan, S.K.; Rochmadi. Preparation of Chitosan Microcapsules Containing Red Ginger Oleoresin Using Emulsion Crosslinking Method. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018809917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, B.; Qi, M.; Sun, J.; Ai, M.; Ma, X.; Cai, W.; Zhou, Y.; Ni, L.; Hu, J.; Xu, F.; et al. Preparation, characterization and wound healing effect of vaccarin-chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 165 Pt B, 3169–3179. [Google Scholar] [CrossRef]
- Su, F.Y.; Lin, K.J.; Sonaje, K.; Wey, S.P.; Yen, T.C.; Ho, Y.C.; Panda, N.; Chuang, E.Y.; Maiti, B.; Sung, H.W. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials 2012, 33, 2801–2811. [Google Scholar] [CrossRef]
- Mohtasham, A.Z.; Tanideh, N.; Seddighi, A.; Mokhtari, M.; Amini, M.; Shakouri, P.A.; Manafi, A.; Hashemi, S.S.; Mehrabani, D. The Effect of Lithospermum officinale, Silver Sulfadiazine and Alpha Ointments in Healing of Burn Wound Injuries in Rat. World J. Plast Surg. 2017, 6, 313–318. [Google Scholar]
- Tate, G.; Mandell, B.F.; Laposata, M.; Ohliger, D.; Baker, D.G.; Schumacher, H.R.; Zurier, R.B. Suppression of acute and chronic inflammation by dietary gamma linolenic acid. J. Rheumatol. 1989, 16, 729–734. [Google Scholar]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Singh, N.; van der Merwe, S.M.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Engineering and Development of Chitosan-Based Nanocoatings for Ocular Contact Lenses. J. Pharm. Sci. 2019, 108, 1540–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef] [Green Version]
- Woo, K.; Ayello, E.A.; Sibbald, R.G. The edge effect: Current therapeutic options to advance the wound edge. Adv. Skin Wound Care 2007, 20, 99–117. [Google Scholar] [CrossRef]
- Edmonds, M. Body of knowledge around the diabetic foot and limb salvage. J. Cardiovasc. Surg. 2012, 53, 605–616. [Google Scholar]
- Wall, I.B.; Moseley, R.; Baird, D.M.; Kipling, D.; Giles, P.; Laffafian, I.; Price, P.E.; Thomas, D.W.; Stephens, P. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J. Investig. Dermatol. 2008, 128, 2526–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Shestakova, V.G.; Nikityuk, D.B.; Bazhenov, D.V.; Banin, V.V. Relationship of Structural and Tissue Components of Full-Layer Skin Wound and Mathematical Modeling of the Healing Process. Bull. Exp. Biol. Med. 2020, 169, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, T.; Wang, S.; He, W.; Qian, W.; Luo, G. Effects of IL-1beta and TNF-alpha on the Expression of P311 in Vascular Endothelial Cells and Wound Healing in Mice. Front. Physiol. 2020, 11, 545008. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Dong, M.; Li, Y.; Zhou, Q.; Yang, L. The proinflammatory cytokines IL-1beta and TNF-alpha modulate corneal epithelial wound healing through p16(Ink4a) suppressing STAT3 activity. J. Cell Physiol. 2020, 235, 10081–10093. [Google Scholar] [CrossRef]
- Xue, X.; Falcon, D.M. The Role of Immune Cells and Cytokines in Intestinal Wound Healing. Int. J. Mol. Sci. 2019, 20, 6097. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loots, M.A.; Lamme, E.N.; Zeegelaar, J.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J. Investig. Dermatol. 1998, 111, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Patel, U.; Rajasingh, S.; Samanta, S.; Cao, T.; Dawn, B.; Rajasingh, J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov. Today 2017, 22, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.; Zhu, X.; Yu, X.; Ai, M.; Cai, W.; Du, B.; Hou, B.; Qiu, L. Preparation of W/O Hypaphorine–Chitosan Nanoparticles and Its Application on Promoting Chronic Wound Healing via Alleviating Inflammation Block. Nanomaterials 2021, 11, 2830. https://doi.org/10.3390/nano11112830
Qi M, Zhu X, Yu X, Ai M, Cai W, Du B, Hou B, Qiu L. Preparation of W/O Hypaphorine–Chitosan Nanoparticles and Its Application on Promoting Chronic Wound Healing via Alleviating Inflammation Block. Nanomaterials. 2021; 11(11):2830. https://doi.org/10.3390/nano11112830
Chicago/Turabian StyleQi, Mengting, Xuerui Zhu, Xiaoyi Yu, Min Ai, Weiwei Cai, Bin Du, Bao Hou, and Liying Qiu. 2021. "Preparation of W/O Hypaphorine–Chitosan Nanoparticles and Its Application on Promoting Chronic Wound Healing via Alleviating Inflammation Block" Nanomaterials 11, no. 11: 2830. https://doi.org/10.3390/nano11112830
APA StyleQi, M., Zhu, X., Yu, X., Ai, M., Cai, W., Du, B., Hou, B., & Qiu, L. (2021). Preparation of W/O Hypaphorine–Chitosan Nanoparticles and Its Application on Promoting Chronic Wound Healing via Alleviating Inflammation Block. Nanomaterials, 11(11), 2830. https://doi.org/10.3390/nano11112830





