Preparation of Barium-Hexaferrite/Gold Janus Nanoplatelets Using the Pickering Emulsion Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
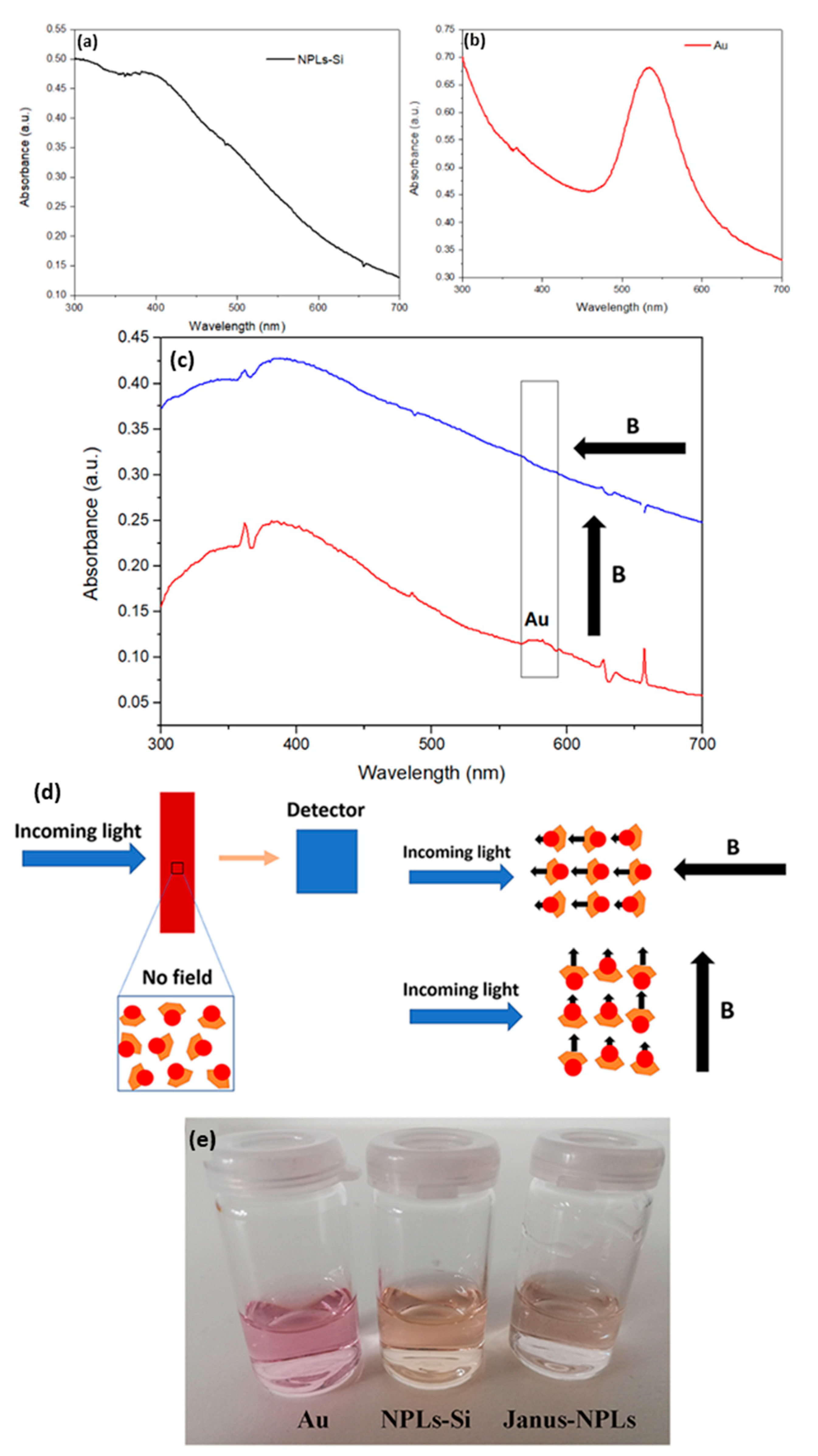
3. Results and Discussion
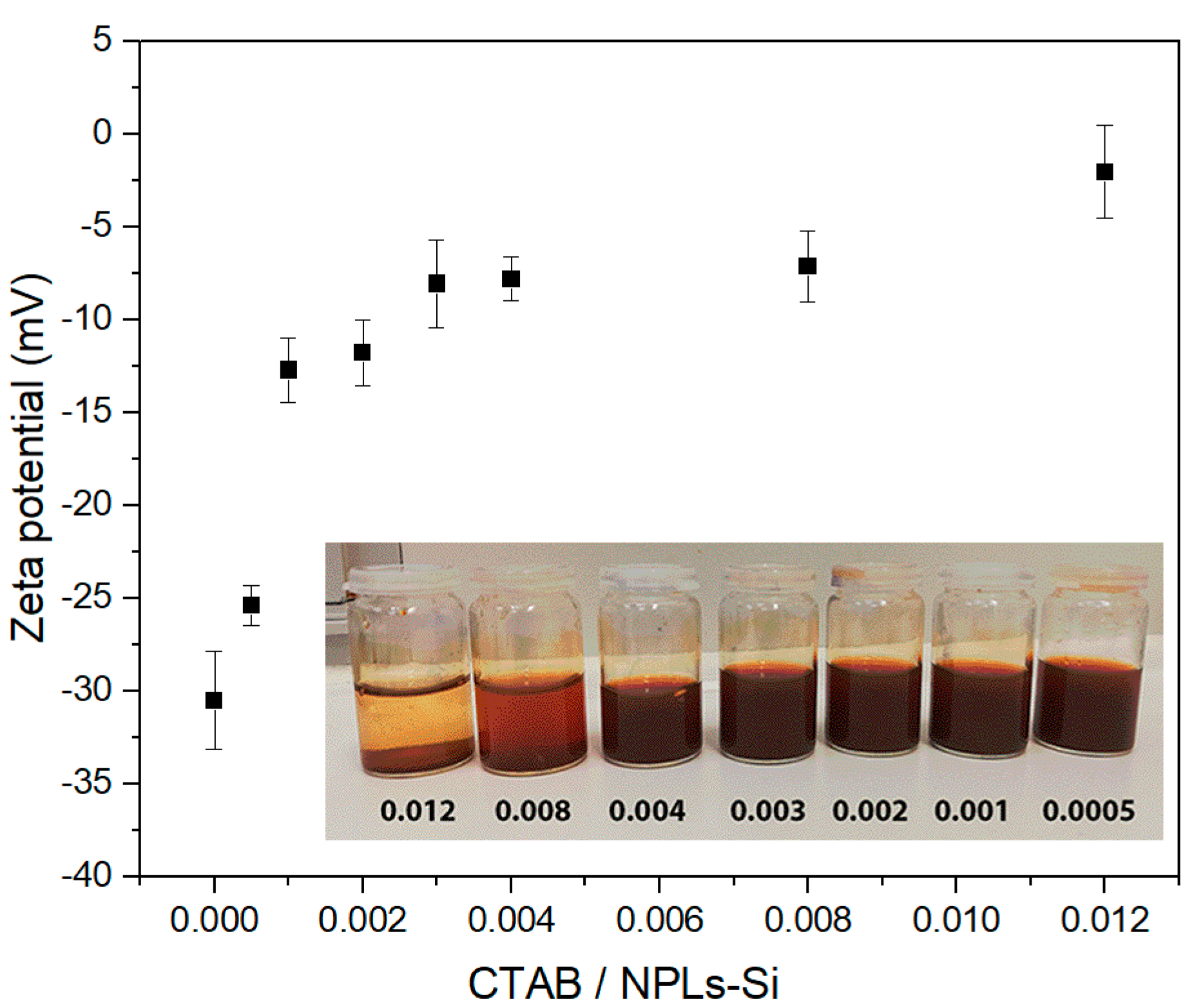
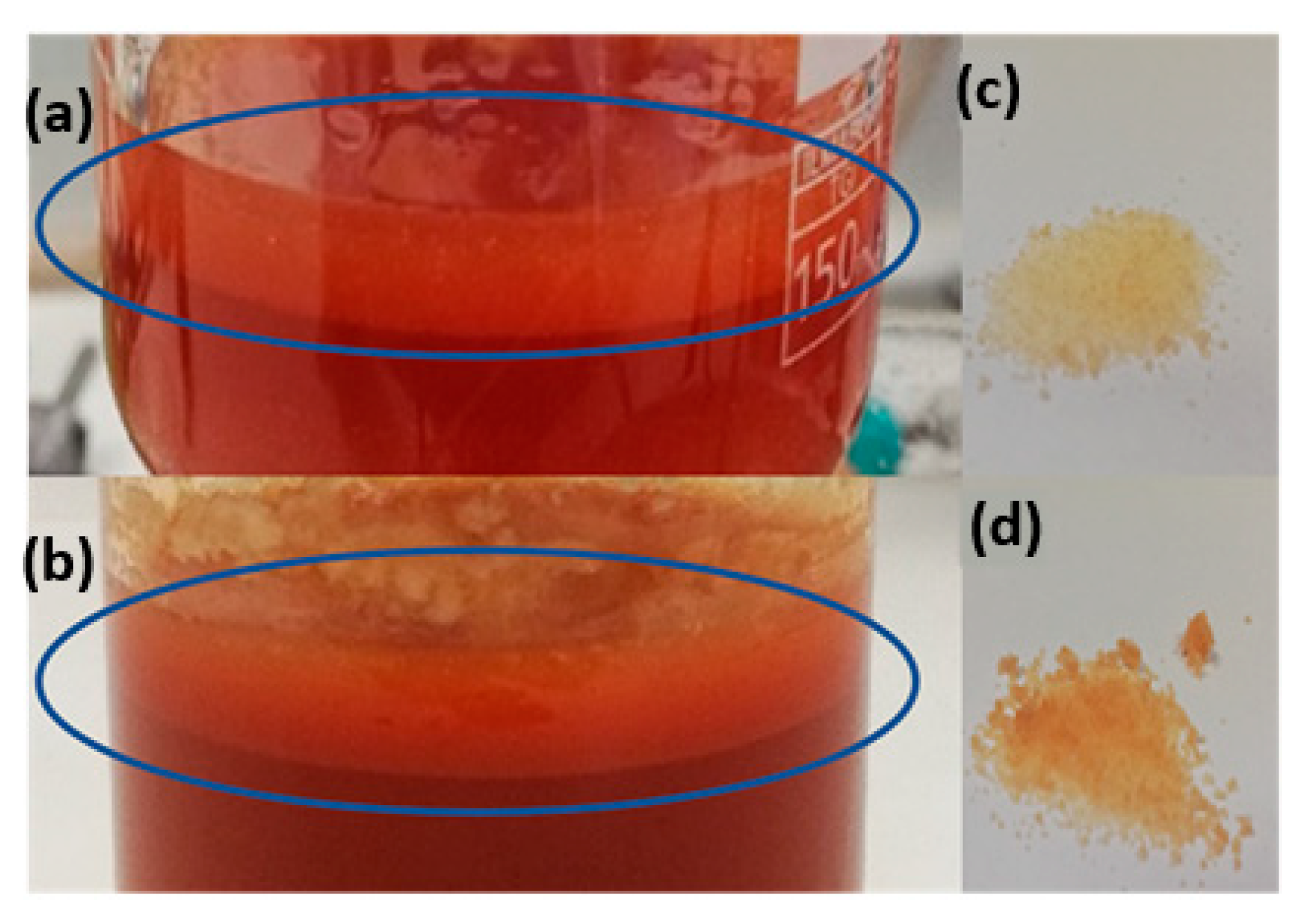
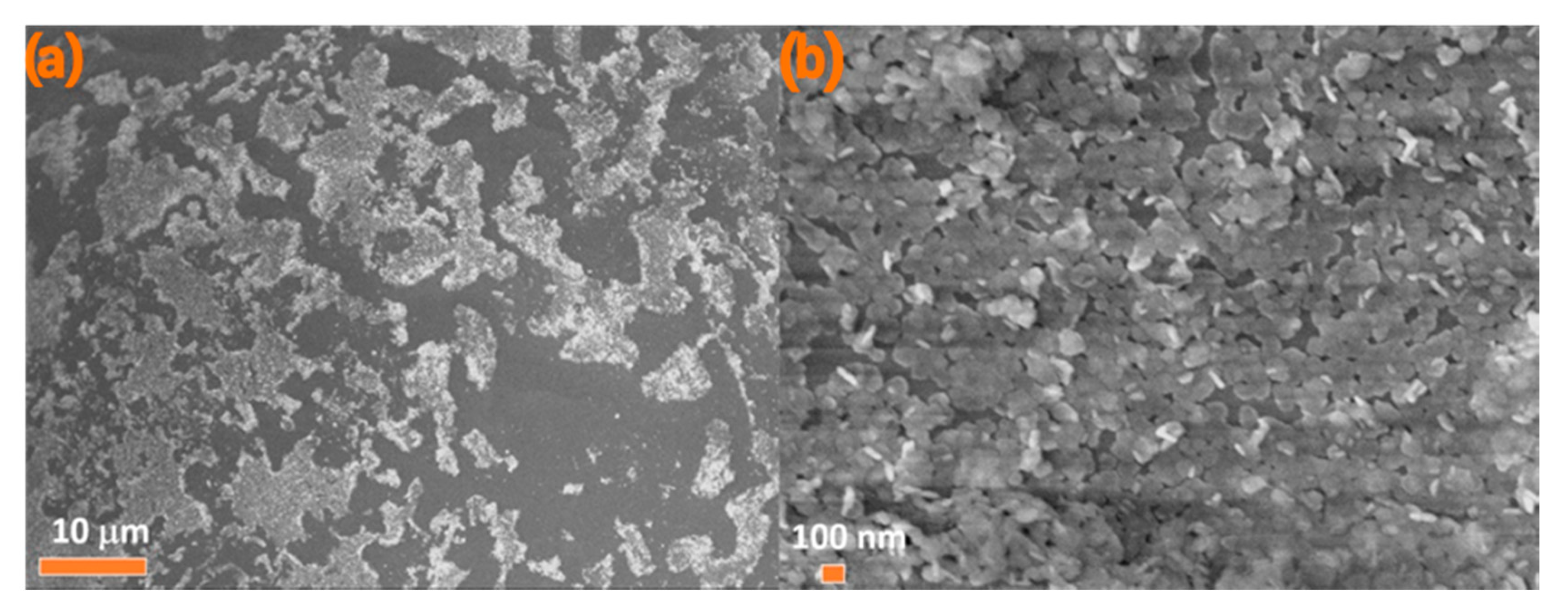
3.1. Optimization of the Emulsification Process
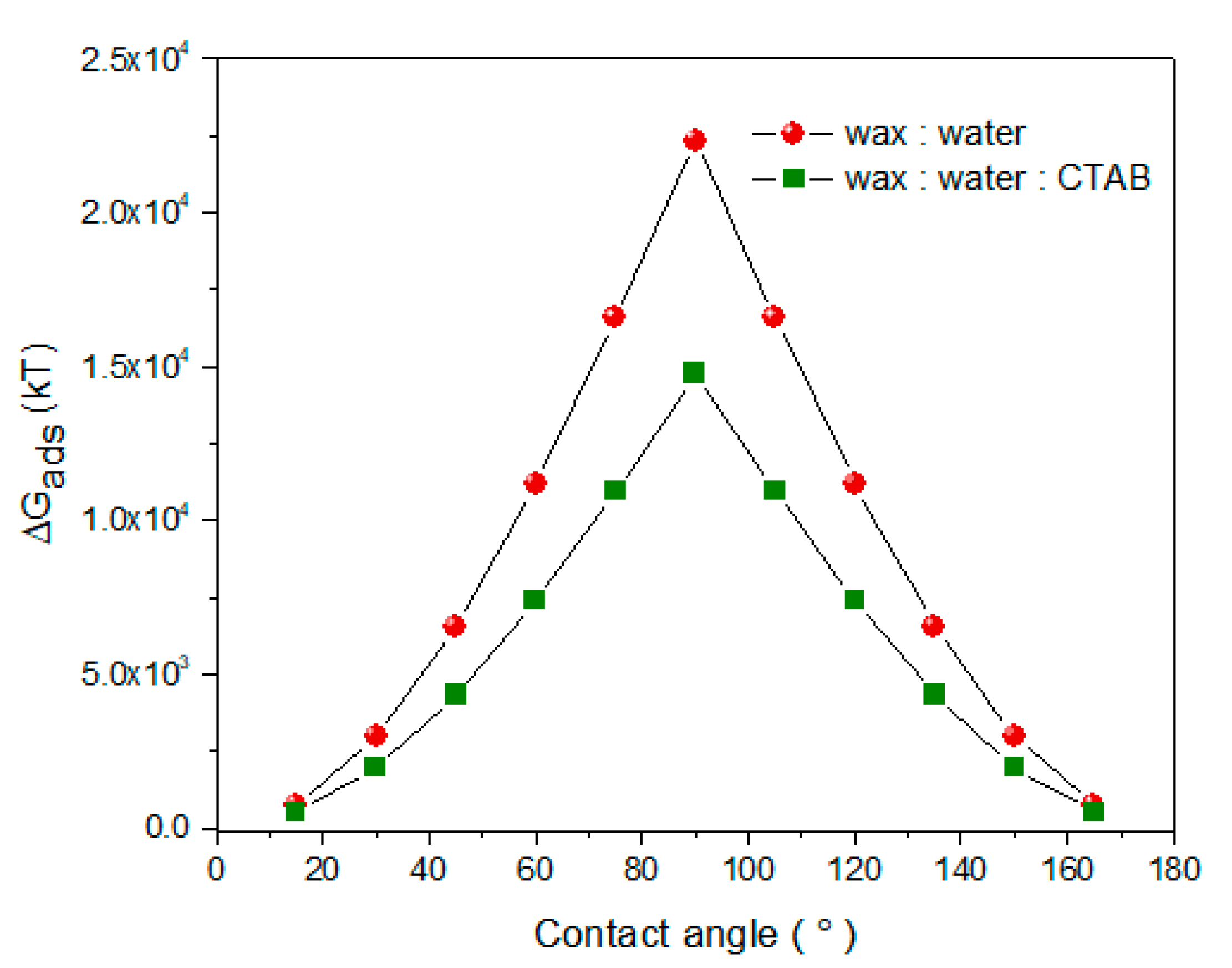
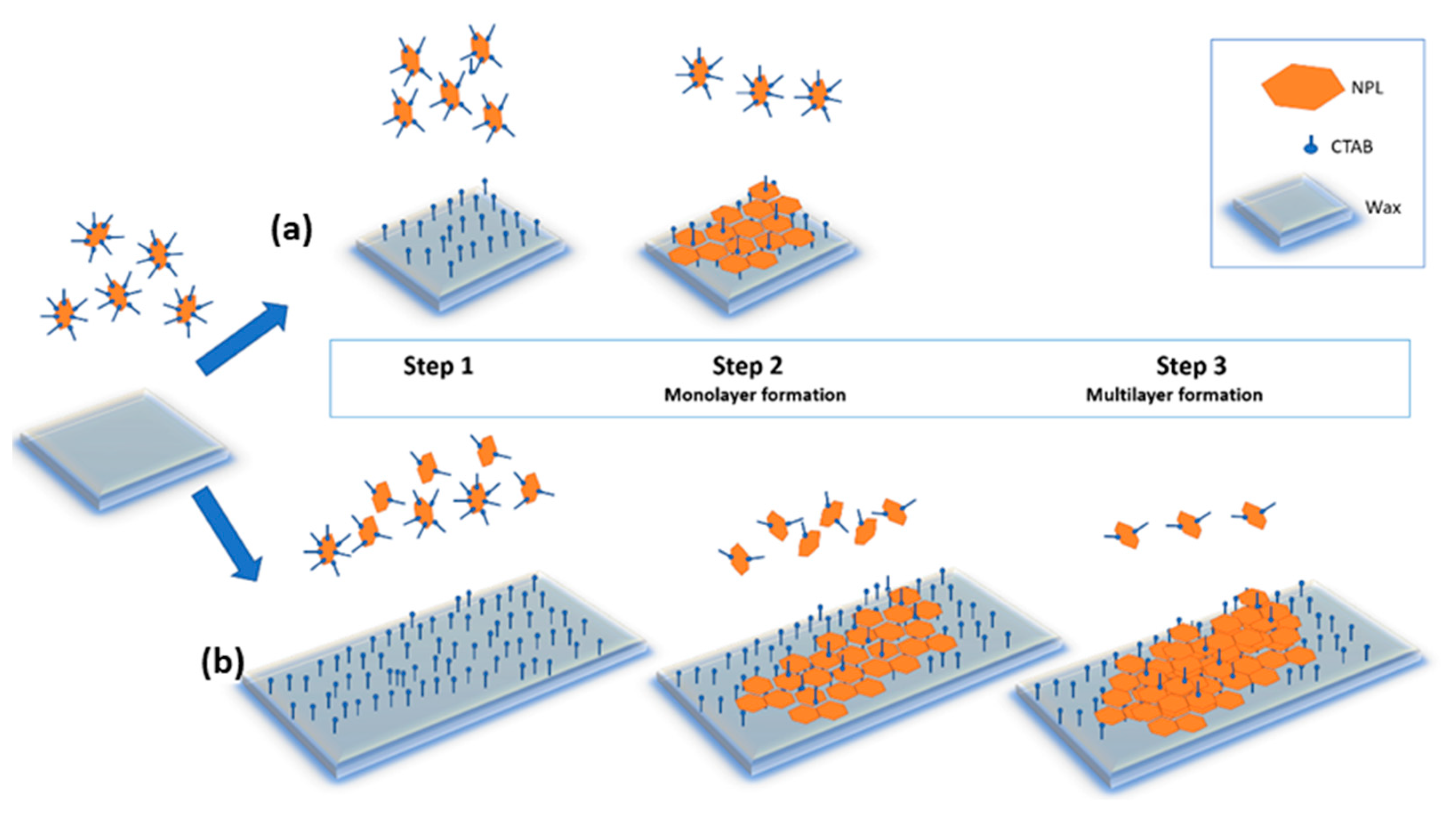
3.2. Mechanism of Adsorption of NPLs-Si onto Wax
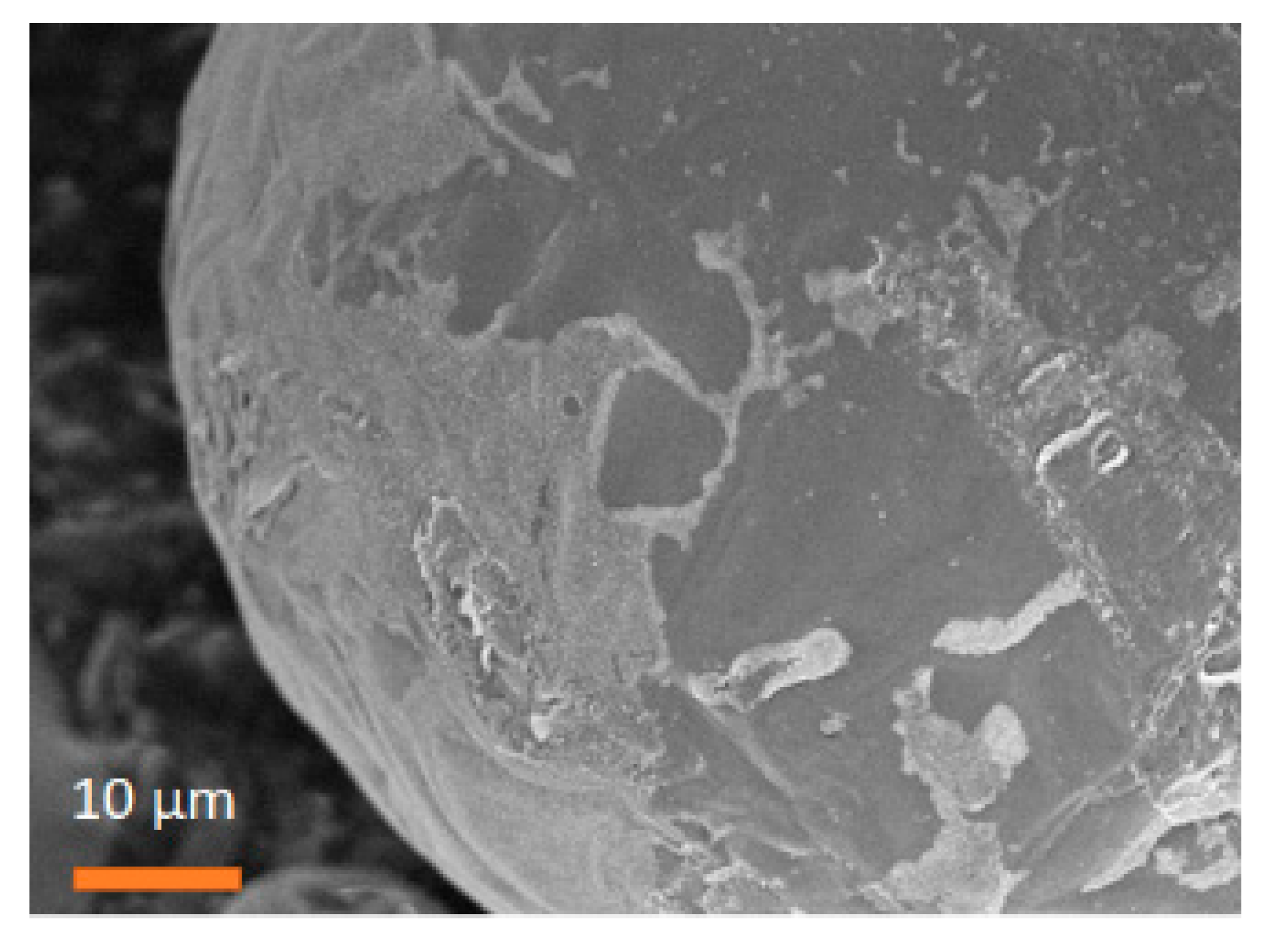
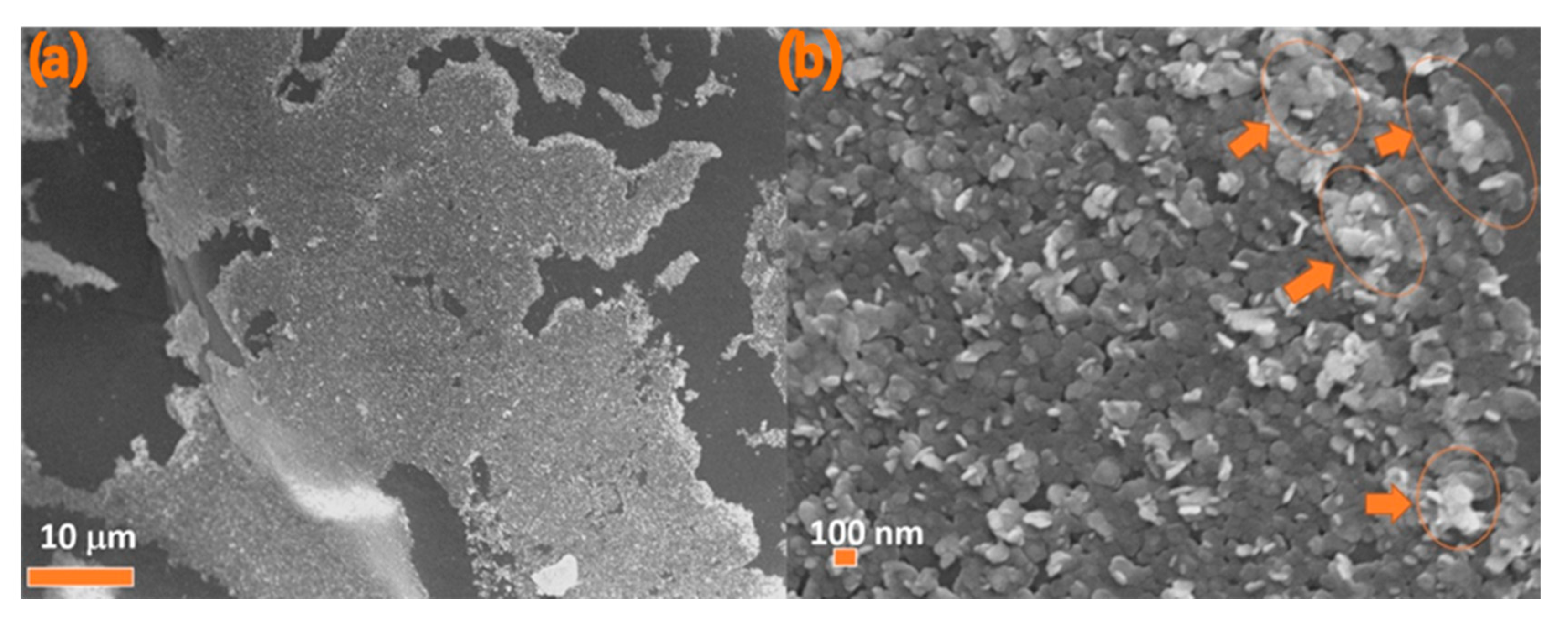
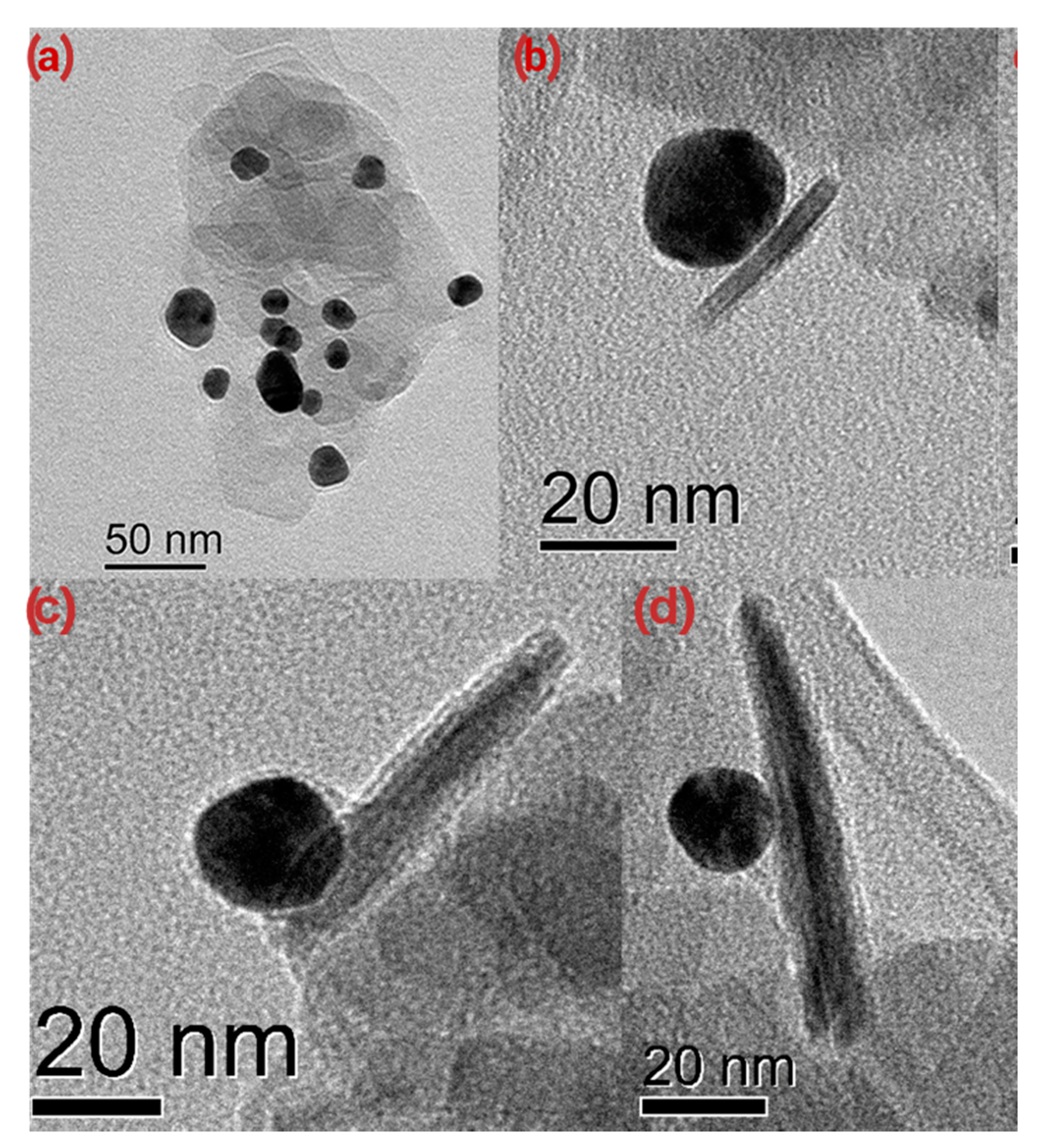
3.3. Janus BHF NPLs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Genes, P.D. Soft matter. Sciences 1992, 256, 495–498. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Janus nanoparticles: Recent advances in their interfacial and biomedical applications. ACS Appl. Nano Mater. 2019, 2, 1738–1757. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Synthesis, properties and applications of Janus nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Song, Y.; Chen, S. Janus nanoparticles: Preparation, characterization, and applications. Chem.–Asian J. 2013, 9, 418–430. [Google Scholar] [CrossRef]
- Marschelke, C.; Fery, A.; Synytska, A. Janus particles: From concepts to environmentally friendly materials and sustainable applications. Colloid Polym. Sci. 2020, 298, 841–865. [Google Scholar] [CrossRef] [Green Version]
- Yánez-Sedeño, P.; Campuzano, S.; Pingarrón, J. Janus particles for (bio)sensing. Appl. Mater. Today 2017, 9, 276–288. [Google Scholar] [CrossRef]
- Campuzano, S.; Gamella, M.; Serafín, V.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Magnetic janus particles for static and dynamic (bio)sensing. Magnetochemistry 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Reguera, J.; Curcio, A.; Muñoz-Noval, Á.; Kuttner, C.; Van De Walle, A.; Liz-Marzán, L.M.; Wilhelm, C. Janus magnetic-plasmonic nanoparticles for magnetically guided and thermally activated cancer therapy. Small 2020, 16, e1904960. [Google Scholar] [CrossRef]
- Reguera, J.; de Aberasturi, D.J.; Winckelmans, N.; Langer, J.; Bals, S.; Liz-Marzán, L.M. Synthesis of Janus plasmonic–magnetic, star–sphere nanoparticles, and their application in SERS detection. Faraday Discuss. 2016, 191, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Mulikova, T.; Abduraimova, A.; Molkenova, A.; Em, S.; Duisenbayeva, B.; Han, D.-W.; Atabaev, T.S. Mesoporous silica decorated with gold nanoparticles as a promising nanoprobe for effective CT X-ray attenuation and potential drug delivery. Nano-Struct. Nano-Objects 2021, 26, 100712. [Google Scholar] [CrossRef]
- Li, Q.; Hu, E.; Yu, K.; Lu, M.; Xie, R.; Lu, F.; Lu, B.; Bao, R.; Lan, G. Magnetic field-mediated Janus particles with sustained driving capability for severe bleeding control in perforating and inflected wounds. Bioact. Mater. 2021, 6, 4625–4639. [Google Scholar] [CrossRef]
- Rahiminezhad, Z.; Tamaddon, A.M.; Borandeh, S.; Abolmaali, S.S. Janus nanoparticles: New generation of multifunctional nanocarriers in drug delivery, bioimaging and theranostics. Appl. Mater. Today 2020, 18, 100513. [Google Scholar] [CrossRef]
- Khoee, S.; Nouri, A. Preparation of Janus nanoparticles and its application in drug delivery. In Design and Development of New Nanocarriers; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 145–180. [Google Scholar]
- Kaewsaneha, C.; Tangboriboonrat, P.; Polpanich, D.; Eissa, M.; Elaissari, A. Preparation of Janus colloidal particles via Pickering emulsion: An overview. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 35–42. [Google Scholar] [CrossRef]
- Thompson, K.L.; Williams, M.; Armes, S.P. Colloidosomes: Synthesis, properties and applications. J. Colloid Interface Sci. 2015, 447, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Jiang, S.; Granick, S. Simple method to produce janus colloidal particles in large quantity. Langmuir 2006, 22, 9495–9499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Granick, S. Controlling the geometry (Janus Balance) of amphiphilic colloidal particles. Langmuir 2008, 24, 2438–2445. [Google Scholar] [CrossRef]
- Paniagua, G.; Villalonga, A.; Eguílaz, M.; Vegas, B.; Parrado, C.; Rivas, G.; Díez, P.; Villalonga, R. Amperometric aptasensor for carcinoembryonic antigen based on the use of bifunctionalized Janus nanoparticles as biorecognition-signaling element. Anal. Chim. Acta 2019, 1061, 84–91. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Zhang, Y.; Zhang, G.; Zhang, C.; He, Y.; Dong, C.; Shuang, S. A novel cell-penetrating Janus nanoprobe for ratiometric fluorescence detection of pH in living cells. Talanta 2019, 209, 120436. [Google Scholar] [CrossRef]
- He, X.; Liu, Q.; Xu, Z. Treatment of oily wastewaters using magnetic Janus nanoparticles of asymmetric surface wettability. J. Colloid Interface Sci. 2020, 568, 207–220. [Google Scholar] [CrossRef]
- Wu, H.; Yi, W.; Chen, Z.; Wang, H.; Du, Q. Janus graphene oxide nanosheets prepared via Pickering emulsion template. Carbon 2015, 93, 473–483. [Google Scholar] [CrossRef]
- Kadam, R.; Maas, M.; Rezwan, K. Selective, agglomerate-free separation of bacteria using biofunctionalized, magnetic janus nanoparticles. ACS Appl. Bio Mater. 2019, 2, 3520–3531. [Google Scholar] [CrossRef]
- Khoee, S.; Keivanshokouh, A. Anisotropic modification of SPIONs surface with thiol and alkyne groups for fabrication of poly (2-hydroxyethyl methacrylate)/polydopamine amphiphilic Janus nanoparticles via double-click reaction. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124777. [Google Scholar] [CrossRef]
- Kadam, R.; Zilli, M.; Maas, M.; Rezwan, K. Nanoscale Janus particles with dual protein functionalization. Part. Part. Syst. Charact. 2018, 35, 1700332. [Google Scholar] [CrossRef]
- Tan, J.S.J.; Wong, S.L.Y.; Chen, Z. Preparation of Janus titanium dioxide particles via ultraviolet irradiation of pickering emulsions. Adv. Mater. Interfaces 2020, 7, 1901961. [Google Scholar] [CrossRef]
- Avossa, J.; Esteves, A.C.C. Influence of experimental parameters on the formation and stability of silica-wax colloidosomes. J. Colloid Interface Sci. 2020, 561, 244–256. [Google Scholar] [CrossRef]
- Synytska, A.; Kirillova, M.S.A.; Isa, L. Synthesis and contact angle measurements of Janus particles. ChemPlusChem 2014, 79, 656. [Google Scholar] [CrossRef]
- Deshmukh, O.S.; van den Ende, D.; Stuart, M.C.; Mugele, F.; Duits, M.H.G. Hard and soft colloids atfluid interfaces: Adsorption, interactions, assembly & rheology. Adv. Colloid Interface Sci. 2015, 222, 215–227. [Google Scholar]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 2000, 16, 8622–8631. [Google Scholar] [CrossRef]
- Schmitt, V.; Destribats, M.; Backov, R. Colloidal particles as liquid dispersion stabilizer: Pickering emulsions and materials thereof. Comptes Rendus Phys. 2014, 15, 761–774. [Google Scholar] [CrossRef]
- Guo, Y.; Khan, A.U.; Cao, K.; Liu, G. Janus plasmonic silver nanoplatelets for interface stabilization. ACS Appl. Nano Mater. 2018, 1, 5377–5381. [Google Scholar] [CrossRef]
- Vis, M.; Opdam, J.; Van’t Oor, I.S.J.; Soligno, G.; van Roij, R.; Tromp, R.H.; Erne, B.H. Water-in-water emulsions stabilized by nanoplates. ACS Macro Lett. 2015, 4, 965. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Du, K.; Glogowski, E.; Emrick, T.; Russell, T.P.; Dinsmore, A.D. Adsorption energy of nano- and microparticles at liquid−liquid interfaces. Langmuir 2010, 26, 12518–12522. [Google Scholar] [CrossRef]
- Ruhland, T.M.; Gröschel, A.H.; Ballard, N.; Skelhon, T.S.; Walther, A.; Müller, A.H.; Bon, S.A. Influence of Janus particle shape on their interfacial behavior at liquid−liquid interfaces. Langmuir 2013, 29, 1388–1394. [Google Scholar] [CrossRef]
- Yan, N.; Masliyah, J.H. Adsorption and desorption of clay particles at the oil-water interface. J. Colloid Interface Sci. 1994, 168, 386–392. [Google Scholar] [CrossRef]
- Brunier, B.; Othman, N.S.; Chevalier, Y.; Bourgeat-Lami, E. Partitioning of laponite clay platelets in pickering emulsion polymerization. Langmuir 2016, 32, 112–124. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, K.H.; Hwang, J.; Sung, M.; Kim, J.E.; Park, B.J.; Kim, J.W. Janus amphiphilic nanoplatelets as smart colloid surfactants with complementary face-to-face interactions. Chem. Comm. 2020, 56, 6031. [Google Scholar] [CrossRef]
- Liang, F.; Shen, K.; Qu, X.; Zhang, C.; Wang, Q.; Li, J.; Liu, J.; Yang, Z. Inorganic Janus nanosheets. Angew. Chem. 2011, 123, 2427–2430. [Google Scholar] [CrossRef]
- Lisjak, D.; Drofenik, M. Chemical substitution—An alternative strategy for controlling the particle size of barium ferrite. Cryst. Growth Des. 2012, 12, 5174–5179. [Google Scholar] [CrossRef]
- Goršak, T.; Makovec, D.; Javornik, U.; Belec, B.; Kralj, S.; Lisjak, D. A functionalization strategy for the dispersion of permanently magnetic barium-hexaferrite nanoplatelets in complex biological media. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 119–127. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; London, E. Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal. Biochem. 1984, 139, 408–412. [Google Scholar] [CrossRef]
- Pallavicini, P.; Cabrini, E.; Casu, A.; Dacarro, G.; Diaz-Fernandez, Y.A.; Falqui, A.; Milanese, C.; Vita, F. Silane-coated magnetic nanoparticles with surface thiol functions for conjugation with gold nanostars. Dalton Trans. 2015, 44, 21088–21098. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Binks, B.P.; Rodrigues, J.A.; Frith, W.J. Synergistic interaction in emulsions stabilized by a mixture of silica nanoparticles and cationic surfactant. Langmuir 2007, 23, 3626–3636. [Google Scholar] [CrossRef]
- Schröder, A.; Sprakel, J.; Schroën, K.; Spaen, J.N.; Berton-Carabin, C.C. Coalescence stability of Pickering emulsions produced with lipid particles: A microfluidic study. J. Food Eng. 2018, 234, 63–72. [Google Scholar] [CrossRef]
- Villalonga, R.; Díez, P.; Sánchez, A.; Aznar, E.; Martínez-Máñez, R.; Pingarrón, J.M. Enzyme-controlled sensing–actuating nanomachine based on Janus au–mesoporous silica nanoparticles. Chem. Eur. J. 2013, 19, 7889. [Google Scholar] [CrossRef]
- Doolaane, A.A.; Ismail, A.F.I.; Mansor, N.; Nor, N.H.M.; Mohamed, F. Effect of surfactants on plasmid DNA stability and release from poly (D, L-lactide-co-glycolide) microspheres. Trop. J. Pharm. Res. 2015, 14, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Proverbio, Z.; Bardavid, S.; Arancibia, E.; Schulz, P. Hydrophile–lipophile balance and solubility parameter of cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 167–171. [Google Scholar] [CrossRef]
- Gadhave, A. Determination of hydrophilic-lipophilic balance value. Int. J. Sci. Res. 2014, 3, 573–575. [Google Scholar]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, G.-H. Recent studies of pickering emulsions: Particles make the difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, F.; Sun, X.; Li, R.; Guo, Y.; Li, C.; Zhang, L.; Xing, F.; Wang, W.; Gao, J. Factors that affect pickering emulsions stabilized by graphene oxide. ACS Appl. Mater. Interfaces 2013, 5, 4843–4855. [Google Scholar] [CrossRef]
- Giermanska-Kahn, J.; Laine, V.; Arditty, S.; Schmitt, V.; Leal-Calderon, F. Particle-stabilized emulsions comprised of solid droplets. Langmuir 2005, 21, 4316–4323. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, H.; Xu, G.; Tan, Y.; Zhang, J.; Lv, X. Influence of CTAB and SDS on the properties of oil-in-water nano-emulsion with paraffin and span 20/Tween 20. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 60–67. [Google Scholar] [CrossRef]
- Lan, Q.; Yang, F.; Zhang, S.; Liu, S.; Xu, J.; Sun, D. Synergistic effect of silica nanoparticle and cetyltrimethyl ammonium bromide on the stabilization of O/W emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 126–135. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Ridel, L.; Bolzinger, M.-A.; Gilon-Delepine, N.; Dugas, P.-Y.; Chevalier, Y. Pickering emulsions stabilized by charged nanoparticles. Soft Matter 2016, 12, 7564–7576. [Google Scholar] [CrossRef]
- Loudet, J.C.; Alsayed, A.M.; Zhang, J.; Yodh, A.G. Capillary interactions between anisotropic colloidal particles. Phys. Rev. Lett. 2005, 94, 018301. [Google Scholar] [CrossRef]
- Bostjančič, P.H.; Tomsič, M.; Jamnik, A.; Lisjak, D.; Mertelj, A. Electrostatic interactions between barium hexaferrite nanoplatelets in alcohol suspensions. J. Phys. Chem. C 2019, 123, 23272–23279. [Google Scholar] [CrossRef] [Green Version]
- Shaghaghi, B.; Sepideh, K.; Shahin, B. Preparation of multifunctional Janus nanoparticles on the basis of SPIONs as targeted drug delivery system. Int. J. Pharm. 2019, 559, 1–12. [Google Scholar] [CrossRef]
- Dewi, M.R.; Laufersky, G.; Nann, T. A highly efficient ligand exchange reaction on gold nanoparticles: Preserving their size, shape and colloidal stability. RSC Adv. 2014, 4, 34217–34220. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential applicationas colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Ferk, G.; Krajnc, P.; Hamler, A.; Mertelj, A.; Cebollada, F.; Drofenik, M.; Lisjak, D. Monolithic magneto-optical nanocomposites of barium hexaferrite platelets in PMMA. Sci. Rep. 2015, 5, 11395. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]

| Core Particles | Diameter (nm) | Shape of Particles | Mass of Particles (mg) | Wax to Water Ratio | Speed of Treatment (rpm) | Duration of Treatment | Reference |
|---|---|---|---|---|---|---|---|
| SiO2 | 70 | Sphere | 200 | 1:10 | 9000 | 80 s | [18] |
| SiO2-NH2 | 172 | Sphere | 250 | 1:5 | 2200 | 2 h | [19] |
| Fe3O4 | 50–100 | Sphere | 100 | 1:60 | 22,000 | 300 s | [20] |
| Graphene oxide | 100–1000 | Nanosheets | 2000 | 1:10 | 12,000 | 12 min | [21] |
| Fe3O4@ SiO2 | 45 | Sphere | 140 | 1:50 | 9500 | 90 s | [22] |
| Fe3O4 | Sphere | 200 | 1:6 | 1650 | 2 h | [23] | |
| SiO2 | 80 | Sphere | 140 | 1:50 | 9500 | 90 | [24] |
| TiO2 | 155–1000 | Sphere | 1–5% ww | 1:10 | 15,000 | 2 min | [25] |
| Sample | Wax to Water Ratio (w/w) | CTAB to NPLs-Si Ratio (w/w) | NPLs-Si (%) | Wax (%) * | CTAB (%) * | Speed Regime | Emulsion |
|---|---|---|---|---|---|---|---|
| 1 | 1:10 | 0.001 | 0.09 | 9.08 | 9 × 10−5 | (1) 9000 rpm, 3 min; (2) 6000 rpm, 3 min | Formed, but all the NPLs are rinsed from wax balls |
| 2 | 1:10 | 0.001 | 0.04 | 9.08 | 4 × 10−5 | (1) 9000 rpm, 3 min; (2) 6000 rpm, 3 min | Formed, but most of the NPLs are rinsed from wax balls |
| 3 | 1:10 | 0.001 | 0.03 | 9.08 | 3 × 10−5 | (1) 9000 rpm, 3 min; (2) 6000 rpm, 3 min | Formed |
| 4 | 1:10 | 0.001 | 0.03 | 9.08 | 3 × 10−5 | 3000 rpm, 35 min | Formed |
| 5 | 1:10 | 0.002 | 0.03 | 9.08 | 7 × 10−5 | 3000 rpm, 35 min | Formed |
| 6 | 1:10 | 0.003 | 0.03 | 9.08 | 10−4 | 3000 rpm, 35 min | Formed |
| 7 | 1:10 | 0.003 | 0.03 | 9.08 | 10−4 | (1) 3000 rpm, 32 min; (2) 9000–12,000 rpm, 3 min | Formed |
| 8 | 1:10.8 | 0.003 | 0.03 | 8.50 | 9 × 10−5 | (1) 3000 rpm, 32 min; (2) 9000–12,000 rpm, 3 min | Formed |
| 9 | 1:12.7 | 0.003 | 0.03 | 7.30 | 9 × 10−5 | (1) 3000 rpm, 32 min; (2) 9000–12,000 rpm, 3 min | Formed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papan, J.; Hribar Boštjančič, P.; Mertelj, A.; Lisjak, D. Preparation of Barium-Hexaferrite/Gold Janus Nanoplatelets Using the Pickering Emulsion Method. Nanomaterials 2021, 11, 2797. https://doi.org/10.3390/nano11112797
Papan J, Hribar Boštjančič P, Mertelj A, Lisjak D. Preparation of Barium-Hexaferrite/Gold Janus Nanoplatelets Using the Pickering Emulsion Method. Nanomaterials. 2021; 11(11):2797. https://doi.org/10.3390/nano11112797
Chicago/Turabian StylePapan, Jelena, Patricija Hribar Boštjančič, Alenka Mertelj, and Darja Lisjak. 2021. "Preparation of Barium-Hexaferrite/Gold Janus Nanoplatelets Using the Pickering Emulsion Method" Nanomaterials 11, no. 11: 2797. https://doi.org/10.3390/nano11112797






