Silver Nanowire Networks: Ways to Enhance Their Physical Properties and Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Details about AgNWs and Deposition Technique
2.2. Deposition of Oxide Coating by AP-SALD
2.3. In Situ Resistance Measurement
2.4. Characterization of AgNWs and AgNW Networks
3. Results and Discussion
3.1. Brief Overview of Main Properties of AgNW Networks
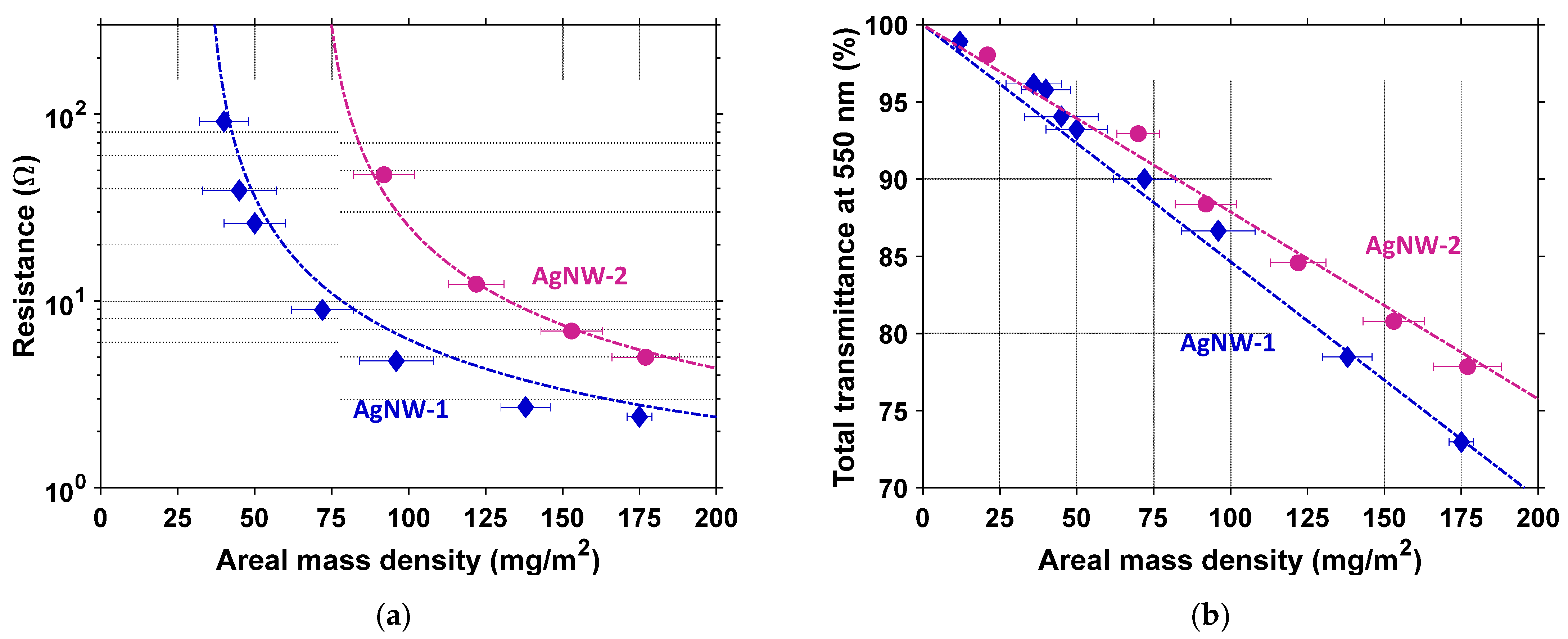
3.2. Comparison of Optical and Electrical Properties of AgNWs with Different Synthesis Conditions
3.2.1. Effects on Morphology and Optical Properties
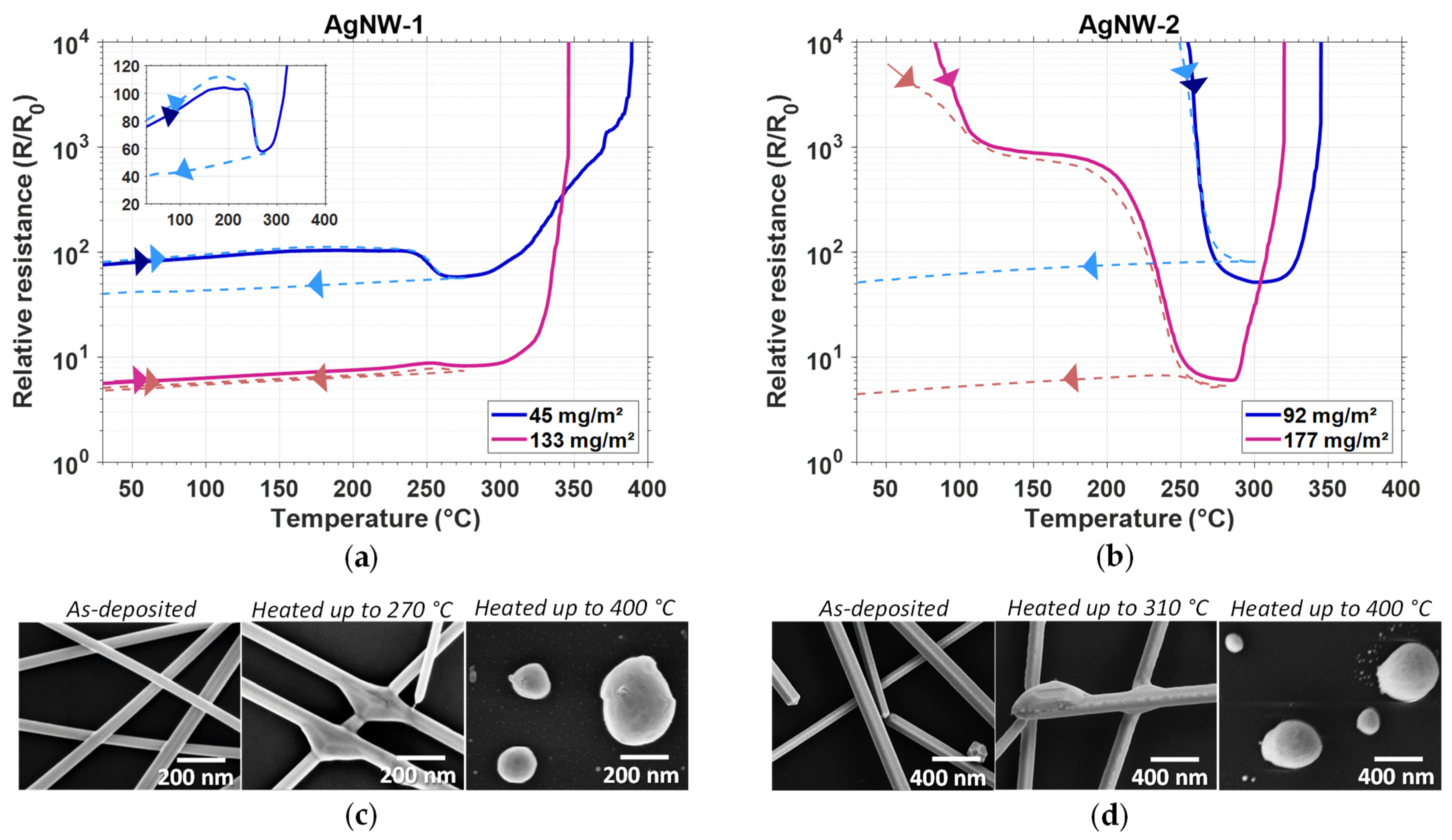
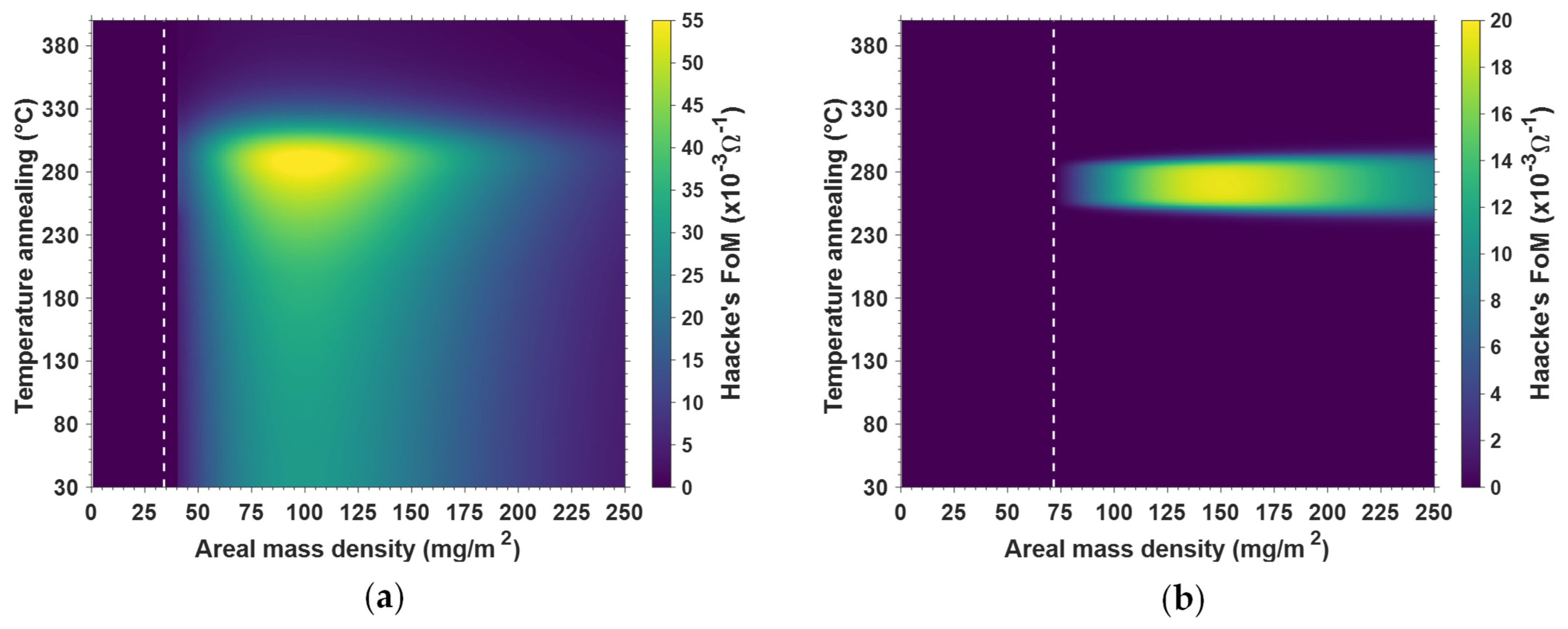
3.2.2. Effects of Thermal Treatment on Electrical Conductivity
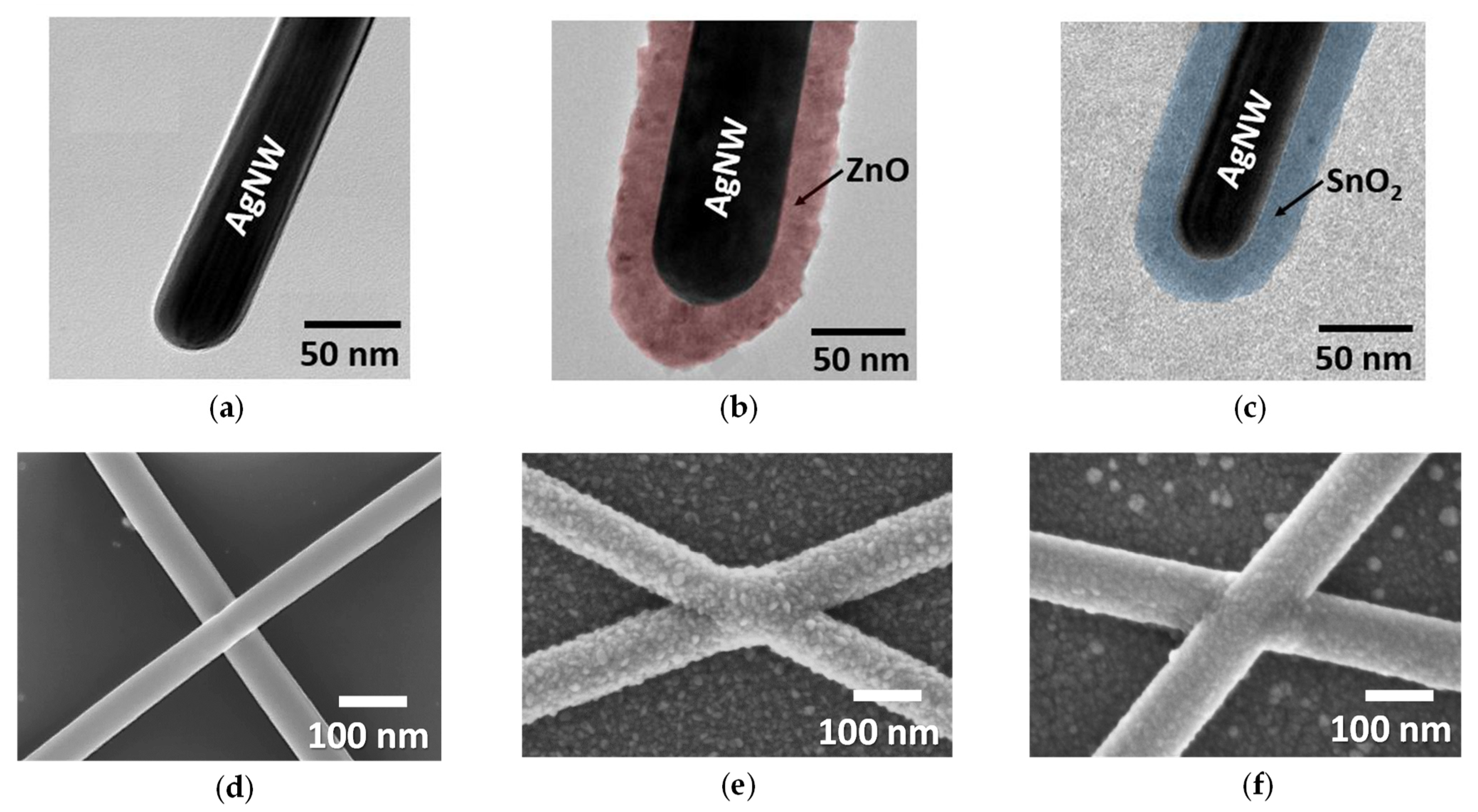
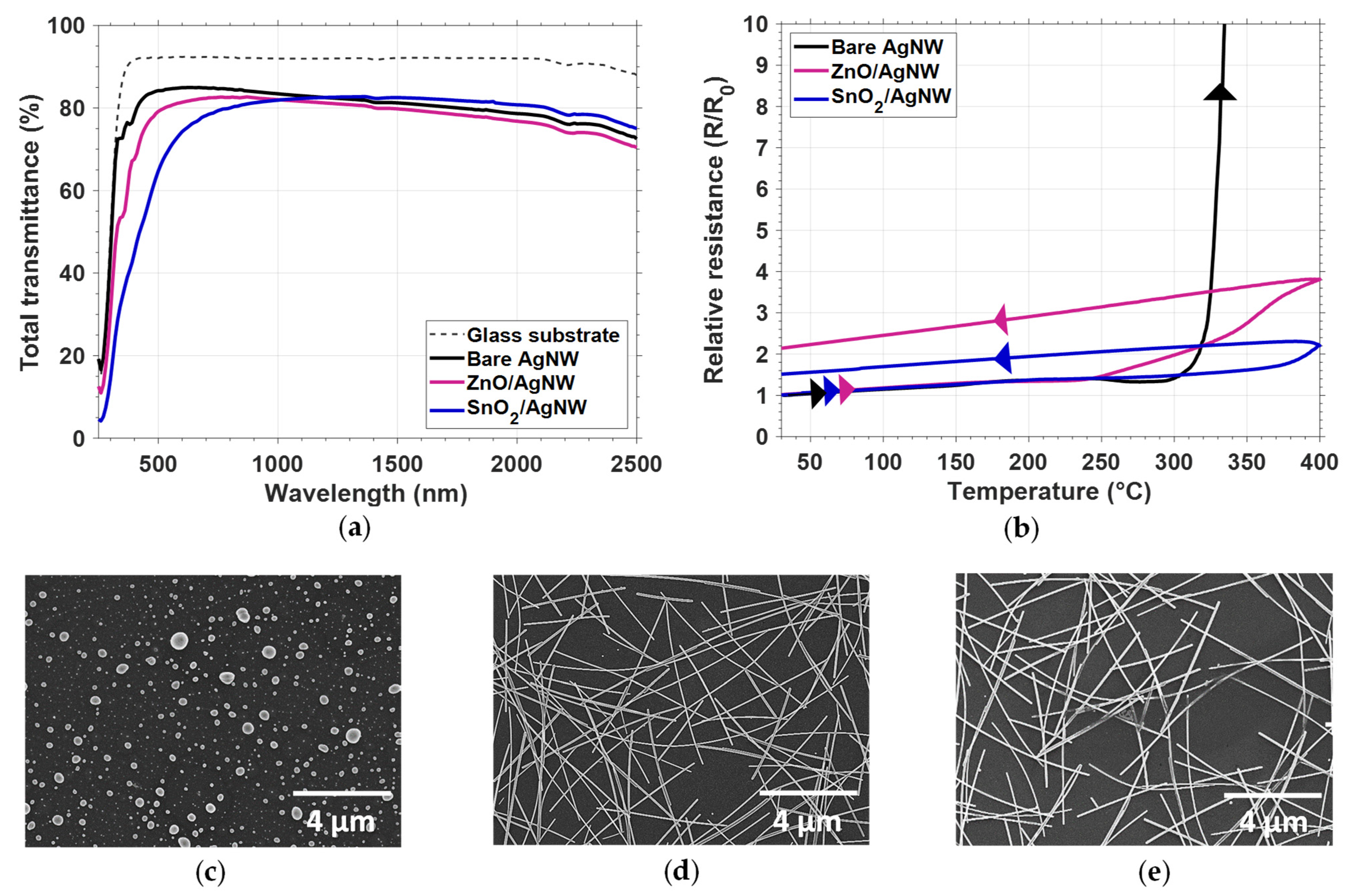
3.3. Influence of Thin Oxide Coating on AgNW Network Stability
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and poly (vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D.; et al. Copper nanowires as fully transparent conductive electrodes. Sci. Rep. 2013, 3, srep02323. [Google Scholar] [CrossRef] [Green Version]
- Rathmell, A.R.; Nguyen, M.; Chi, M.; Wiley, B.J. Synthesis of oxidation-resistant cupronickel nanowires for transparent conducting nanowire networks. Nano Lett. 2012, 12, 3193–3199. [Google Scholar] [CrossRef]
- Kim, K.; Kwon, H.-C.; Ma, S.; Lee, E.; Yun, S.-C.; Jang, G.; Yang, H.; Moon, J. All-solution-processed thermally and chemically stable copper–nickel core–shell nanowire-based composite window electrodes for perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 30337–30347. [Google Scholar] [CrossRef] [PubMed]
- Sannicolo, T.; Lagrange, M.; Cabos, A.; Celle, C.; Simonato, J.-P.; Bellet, D. Metallic nanowire-based transparent electrodes for next generation flexible devices: A review. Small 2016, 12, 6052–6075. [Google Scholar] [CrossRef] [PubMed]
- Bellet, D.; Lagrange, M.; Sannicolo, T.; Aghazadehchors, S.; Nguyen, V.H.; Langley, D.P.; Muñoz-Rojas, D.; Jiménez, C.; Bréchet, Y.; Nguyen, N.D. Transparent electrodes based on silver nanowire networks: From physical considerations towards device integration. Materials 2017, 10, 570. [Google Scholar] [CrossRef]
- Sohn, H.; Park, C.; Oh, J.-M.; Kang, S.W.; Kim, M.-J. Silver nanowire networks: Mechano-electric properties and applications. Materials 2019, 12, 2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Zhang, Y.; Zheng, Z. Metal-based flexible transparent electrodes: Challenges and recent advances. Adv. Electron. Mater. 2021, 7, 2001121. [Google Scholar] [CrossRef]
- Singh, M.; Rana, S. Silver and copper nanowire films as cost-effective and robust transparent electrode in energy harvesting through photovoltaic: A review. Mater. Today Commun. 2020, 24, 101317. [Google Scholar] [CrossRef]
- Sannicolo, T.; Chae, W.H.; Mwaura, J.; Bulović, V.; Grossman, J.C. Silver nanowire back electrode stabilized with graphene oxide encapsulation for inverted semitransparent organic solar cells with longer lifetime. ACS Appl. Energy Mater. 2021, 4, 1431–1441. [Google Scholar] [CrossRef]
- Celle, C.; Mayousse, C.; Moreau, E.; Basti, H.; Carella, A.; Simonato, J.-P. Highly flexible transparent film heaters based on random networks of silver nanowires. Nano Res. 2012, 5, 427–433. [Google Scholar] [CrossRef]
- Sorel, S.; Bellet, D.; Coleman, J.N. Relationship between material properties and transparent heater performance for both bulk-like and percolative nanostructured networks. ACS Nano 2014, 8, 4805–4814. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, D.T.; Schultheiss, A.; Muñoz-Rojas, D.; Celle, C.; Carella, A.; Simonato, J.; Bellet, D. Transparent heaters: A review. Adv. Funct. Mater. 2020, 30, 1910225. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Ahn, Y.; Lee, E.-W.; Park, L.S.; Lee, Y. Highly efficient and low voltage silver nanowire-based OLEDs employing a n-type hole injection layer. Nanoscale 2014, 6, 8565–8570. [Google Scholar] [CrossRef]
- Wang, J.-L.; Lu, Y.-R.; Li, H.-H.; Liu, J.-W.; Yu, S.-H. Large area co-assembly of nanowires for flexible transparent smart windows. J. Am. Chem. Soc. 2017, 139, 9921–9926. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Byun, J.; Seong, N.; Ha, J.; Kim, H.; Kim, S.; Kim, T.; Im, H.; Kim, D.; Hong, Y. Silver nanowire-embedded PDMS with a multiscale structure for a highly sensitive and robust flexible pressure sensor. Nanoscale 2015, 7, 6208–6215. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, R.; Coskun, S.; Unalan, H.E. Coaxial silver nanowire network core molybdenum oxide shell supercapacitor electrodes. Electrochim. Acta 2016, 193, 39–44. [Google Scholar] [CrossRef]
- Cho, S.; Kang, S.; Pandya, A.; Shanker, R.; Khan, Z.; Lee, Y.; Park, J.; Craig, S.L.; Ko, H. Large-area cross-aligned silver nanowire electrodes for flexible, transparent, and force-sensitive mechanochromic touch screens. ACS Nano 2017, 11, 4346–4357. [Google Scholar] [CrossRef]
- Jiang, S.; Teng, C.P. Fabrication of silver nanowires-loaded polydimethylsiloxane film with antimicrobial activities and cell compatibility. Mater. Sci. Eng. C 2017, 70, 1011–1017. [Google Scholar] [CrossRef]
- Bahcelioglu, E.; Doganay, D.; Coskun, S.; Unalan, H.E.; Erguder, T.H. A point-of-use (POU) water disinfection: Silver nanowire decorated glass fiber filters. J. Water Process. Eng. 2020, 38, 101616. [Google Scholar] [CrossRef]
- Resende, J.; Sekkat, A.; Nguyen, V.H.; Chatin, T.; Jiménez, C.; Burriel, M.; Bellet, D.; Muñoz-Rojas, D. Planar and transparent memristive devices based on titanium oxide coated silver nanowire networks with tunable switching voltage. Small 2021, 17, 2007344. [Google Scholar] [CrossRef]
- Hanauer, S.; Celle, C.; Crivello, C.; Szambolics, H.; Muñoz-Rojas, D.; Bellet, D.; Simonato, J.-P. Transparent and mechanically resistant silver-nanowire-based low-emissivity coatings. ACS Appl. Mater. Interfaces 2021, 13, 21971–21978. [Google Scholar] [CrossRef] [PubMed]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver nanowire networks as flexible, transparent, conducting films: Extremely high DC to optical conductivity ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef]
- Atkinson, J.; Goldthorpe, I.A. Near-infrared properties of silver nanowire networks. Nanotechnology 2020, 31, 365201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, S.; Stewart, I.E.; Wiley, B.J. Copper nanowire networks with transparent oxide shells that prevent oxidation without reducing transmittance. ACS Nano 2014, 8, 9673–9679. [Google Scholar] [CrossRef]
- Celle, C.; Cabos, A.; Fontecave, T.; Laguitton, B.; Benayad, A.; Guettaz, L.; Pélissier, N.; Nguyen, V.H.; Bellet, D.; Muñoz-Rojas, D.; et al. Oxidation of copper nanowire based transparent electrodes in ambient conditions and their stabilization by encapsulation: Application to transparent film heaters. Nanotechnology 2018, 29, 085701. [Google Scholar] [CrossRef]
- Karst, N.; Rey, G.; Doisneau, B.; Roussel, H.; Deshayes, R.; Consonni, V.; Ternon, C.; Bellet, D. Fabrication and characterization of a composite ZnO semiconductor as electron transporting layer in dye-sensitized solar cells. Mater. Sci. Eng. B 2011, 176, 653–659. [Google Scholar] [CrossRef]
- Giusti, G.; Consonni, V.; Puyoo, E.; Bellet, D. High performance ZnO-SnO2: F nanocomposite transparent electrodes for energy applications. ACS Appl. Mater. Interfaces 2014, 6, 14096–14107. [Google Scholar] [CrossRef]
- Mayousse, C.; Celle, C.; Moreau, E.; Mainguet, J.-F.; Carella, A.; Simonato, J.-P. Improvements in purification of silver nanowires by decantation and fabrication of flexible transparent electrodes. Application to capacitive touch sensors. Nanotechnology 2013, 24, 215501. [Google Scholar] [CrossRef]
- Available online: https://www.rdnano.fr/ (accessed on 10 September 2021).
- Papanastasiou, D.T.; Charvin, N.; Resende, J.; Nguyen, V.H.; Sekkat, A.; Muñoz-Rojas, D.; Jiménez, C.; Flandin, L.; Bellet, D. Effects of non-homogeneity and oxide coating on silver nanowire networks under electrical stress: Comparison between experiment and modeling. Nanotechnology 2021, 32, 445702. [Google Scholar] [CrossRef]
- Huerta, C.A.M.; Nguyen, V.H.; Sekkat, A.; Crivello, C.; Toldra-Reig, F.; Veiga, P.B.; Quessada, S.; Jimenez, C.; Muñoz-Rojas, D. Gas-phase 3D printing of functional materials. Adv. Mater. Technol. 2020, 5, 2000657. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Sekkat, A.; Jiménez, C.; Muñoz, D.; Bellet, D.; Muñoz-Rojas, D. Impact of precursor exposure on process efficiency and film properties in spatial atomic layer deposition. Chem. Eng. J. 2021, 403, 126234. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Resende, J.; Jiménez, C.; Deschanvres, J.-L.; Carroy, P.; Muñoz, D.; Bellet, D.; Muñoz-Rojas, D. Deposition of ZnO based thin films by atmospheric pressure spatial atomic layer deposition for application in solar cells. J. Renew. Sustain. Energy 2017, 9, 021203. [Google Scholar] [CrossRef]
- Araki, T.; Jiu, J.; Nogi, M.; Koga, H.; Nagao, S.; Sugahara, T.; Suganuma, K. Low haze transparent electrodes and highly conducting air dried films with ultra-long silver nanowires synthesized by one-step polyol method. Nano Res. 2014, 7, 236–245. [Google Scholar] [CrossRef]
- Lagrange, M.; Langley, D.P.; Giusti, G.; Jiménez, C.; Bréchet, Y.; Bellet, D. Optimization of silver nanowire-based transparent electrodes: Effects of density, size and thermal annealing. Nanoscale 2015, 7, 17410–17423. [Google Scholar] [CrossRef] [PubMed]
- Sannicolo, T.; Charvin, N.; Flandin, L.; Kraus, S.; Papanastasiou, D.T.; Celle, C.; Simonato, J.-P.; Muñoz-Rojas, D.; Jiménez, C.; Bellet, D. Electrical mapping of silver nanowire networks: A versatile tool for imaging network homogeneity and degradation dynamics during failure. ACS Nano 2018, 12, 4648–4659. [Google Scholar] [CrossRef] [PubMed]
- Weiß, N.; Müller-Meskamp, L.; Selzer, F.; Bormann, L.; Eychmüller, A.; Leo, K.; Gaponik, N. Humidity assisted annealing technique for transparent conductive silver nanowire networks. RSC Adv. 2015, 5, 19659–19665. [Google Scholar] [CrossRef] [Green Version]
- Langley, D.P.; Lagrange, M.; Giusti, G.; Jiménez, C.; Bréchet, Y.; Nguyen, N.D.; Bellet, D. Metallic nanowire networks: Effects of thermal annealing on electrical resistance. Nanoscale 2014, 6, 13535–13543. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.H.; Hwang, I.; Um, H.-D.; Lee, S.; Lee, K.; Park, J.; Shin, H.; Kwon, T.-H.; Kang, S.J.; Seo, K. Cold isostatic-pressured silver nanowire electrodes for flexible organic solar cells via room-temperature processes. Adv. Mater. 2017, 29, 1701479. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Gao, H.; Wang, Y.; Liu, Q.; Huang, S.; Guo, C.F.; Ren, Z. Capillary-force-induced cold welding in silver-nanowire-based flexible transparent electrodes. Nano Lett. 2017, 17, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Deignan, G.; Goldthorpe, I.A. The dependence of silver nanowire stability on network composition and processing parameters. RSC Adv. 2017, 7, 35590–35597. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Canlier, A.; Cho, C.; Rozyyev, V.; Lee, J.-Y.; Han, S.M. Highly transparent Au-coated Ag nanowire transparent electrode with reduction in haze. ACS Appl. Mater. Interfaces 2014, 6, 13527–13534. [Google Scholar] [CrossRef]
- Pham, A.-T.; Nguyen, X.-Q.; Tran, D.-H.; Ngoc Phan, V.; Duong, T.-T.; Nguyen, D.-C. Enhancement of the electrical properties of silver nanowire transparent conductive electrodes by atomic layer deposition coating with zinc oxide. Nanotechnology 2016, 27, 335202. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Nguyen, V.H.; Muñoz-Rojas, D.; Aghazadehchors, S.; Jiménez, C.; Nguyen, N.D.; Bellet, D. Stability enhancement of silver nanowire networks with conformal ZnO coatings deposited by atmospheric pressure spatial atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 19208–19217. [Google Scholar] [CrossRef]
- Mao, Y.; Yang, H.; Hu, C.; Guo, J.; Meng, X.; Yang, Y. Large-scale synthesis of AgNWs with ultra-high aspect ratio above 4000 and their application in conductive thin film. J. Mater. Sci. Mater. Electron. 2017, 28, 5308–5314. [Google Scholar] [CrossRef]
- Hauger, T.C.; Al-Rafia, S.M.I.; Buriak, J.M. Rolling silver nanowire electrodes: Simultaneously addressing adhesion, roughness, and conductivity. ACS Appl. Mater. Interfaces 2013, 5, 12663–12671. [Google Scholar] [CrossRef]
- Tang, H.; Feng, H.; Wang, H.; Wan, X.; Liang, J.; Chen, Y. Highly Conducting MXene–silver nanowire transparent electrodes for flexible organic solar cells. ACS Appl. Mater. Interfaces 2019, 11, 25330–25337. [Google Scholar] [CrossRef]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Wang, G.; Bi, L.; Wei, W.; Zhang, X.; Gu, Y.; Huang, L.; Yin, H.; Li, Y.; Chen, G.; Wu, Z.; et al. Strongly adhesive silver nanowire ink makes delamination-free transparent conductive films possible. ACS Appl. Nano Mater. 2019, 2, 6707–6714. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Krosnicki, G.; Bras, J. Positive impact of cellulose nanofibrils on silver nanowire coatings for transparent conductive films. J. Mater. Chem. C 2016, 4, 10945–10954. [Google Scholar] [CrossRef]
- Aharony, A.; Stauffer, D. Introduction to Percolation Theory; Taylor & Francis: Oxford, UK, 1991; ISBN 9781135747831. [Google Scholar]
- Bid, A.; Bora, A.; Raychaudhuri, A.K. Temperature dependence of the resistance of metallic nanowires of diameter greater than 15 Nm: Applicability of Bloch-Gruneisen theorem. Phys. Rev. B 2006, 74, 035426. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Geng, Y.; Cao, Y.; Yan, Y. Uniform high-reflectivity silver film deposited by planar magnetron sputtering. Vacuum 2021, 185, 109999. [Google Scholar] [CrossRef]
- Bergin, S.M.; Chen, Y.-H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.-Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Jang, J.; Park, T.; Park, Y.M.; Park, J.S.; Kim, Y.-K.; Lee, H.-K.; Jeon, E.-C.; Lee, D.-K.; Ahn, B.; et al. Electrodeposited silver nanowire transparent conducting electrodes for thin-film solar cells. ACS Appl. Mater. Interfaces 2020, 12, 6169–6175. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol synthesis of silver nanowires: An extensive parametric study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- Hwang, J.; Shim, Y.; Yoon, S.-M.; Lee, S.H.; Park, S.-H. Influence of polyvinylpyrrolidone (PVP) capping layer on silver nanowire networks: Theoretical and experimental studies. RSC Adv. 2016, 6, 30972–30977. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, Y.; Liu, X.; Liu, G.; Shan, F. Welded silver nanowire networks as high-performance transparent conductive electrodes: Welding techniques and device applications. Appl. Mater. Today 2020, 20, 100634. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, P.; Lee, D.; Lee, S.S.; Ko, S.H. Large-scale synthesis and characterization of very long silver nanowires via successive multistep growth. Cryst. Growth Des. 2012, 12, 5598–5605. [Google Scholar] [CrossRef]
- Vigonski, S.; Jansson, V.; Vlassov, S.; Polyakov, B.; Baibuz, E.; Oras, S.; Aabloo, A.; Djurabekova, F.; Zadin, V. Au nanowire junction breakup through surface atom diffusion. Nanotechnology 2018, 29, 015704. [Google Scholar] [CrossRef] [Green Version]
- Langley, D.P.; Lagrange, M.; Nguyen, N.D.; Bellet, D. Percolation in networks of 1-dimensional objects: Comparison between Monte Carlo simulations and experimental observations. Nanoscale Horiz. 2018, 3, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Papanastasiou, D.T.; Toupance, T.; Servant, L.; Tréguer-Delapierre, M.; Bellet, D.; Goldthorpe, I.A. Rapid synthesis of ultra-long silver nanowires for high performance transparent electrodes. Nanoscale Adv. 2020, 2, 3804–3808. [Google Scholar] [CrossRef]
- Patil, J.J.; Chae, W.H.; Trebach, A.; Carter, K.; Lee, E.; Sannicolo, T.; Grossman, J.C. Failing forward: Stability of transparent electrodes based on metal nanowire networks. Adv. Mater. 2020, 33, 2004356. [Google Scholar] [CrossRef] [PubMed]
- Aghazadehchors, S.; Nguyen, V.H.; Muñoz-Rojas, D.; Jiménez, C.; Rapenne, L.; Nguyen, N.D.; Bellet, D. Versatility of bilayer metal oxide coatings on silver nanowire networks for enhanced stability with minimal transparency loss. Nanoscale 2019, 11, 19969–19979. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Resende, J.; Papanastasiou, D.T.; Fontanals, N.; Jiménez, C.; Muñoz-Rojas, D.; Bellet, D. Low-cost fabrication of flexible transparent electrodes based on Al doped ZnO and silver nanowire nanocomposites: Impact of the network density. Nanoscale 2019, 11, 12097–12107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Yang, S.; Kuttner, E.; Taylor, A.A.; Salemmilani, R.; Liu, X.; Moskovits, M.; Wu, B.; Dehestani, A.; et al. Protecting the nanoscale properties of Ag nanowires with a solution-grown SnO2 monolayer as corrosion inhibitor. J. Am. Chem. Soc. 2019, 141, 13977–13986. [Google Scholar] [CrossRef]
- Yu, S.; Liu, X.; Dong, H.; Wang, X.; Li, L. Flexible high-performance SnO2/AgNWs bilayer transparent conductors for flexible transparent heater applications. Ceram. Int. 2021, 47, 20379–20386. [Google Scholar] [CrossRef]
- Rey, G.; Ternon, C.; Modreanu, M.; Mescot, X.; Consonni, V.; Bellet, D. Electron scattering mechanisms in fluorine-doped SnO2 thin films. J. Appl. Phys. 2013, 114, 183713. [Google Scholar] [CrossRef] [Green Version]
- PVlighthouse. Available online: https://www.pvlighthouse.com.au/refractive-index-library (accessed on 10 September 2021).
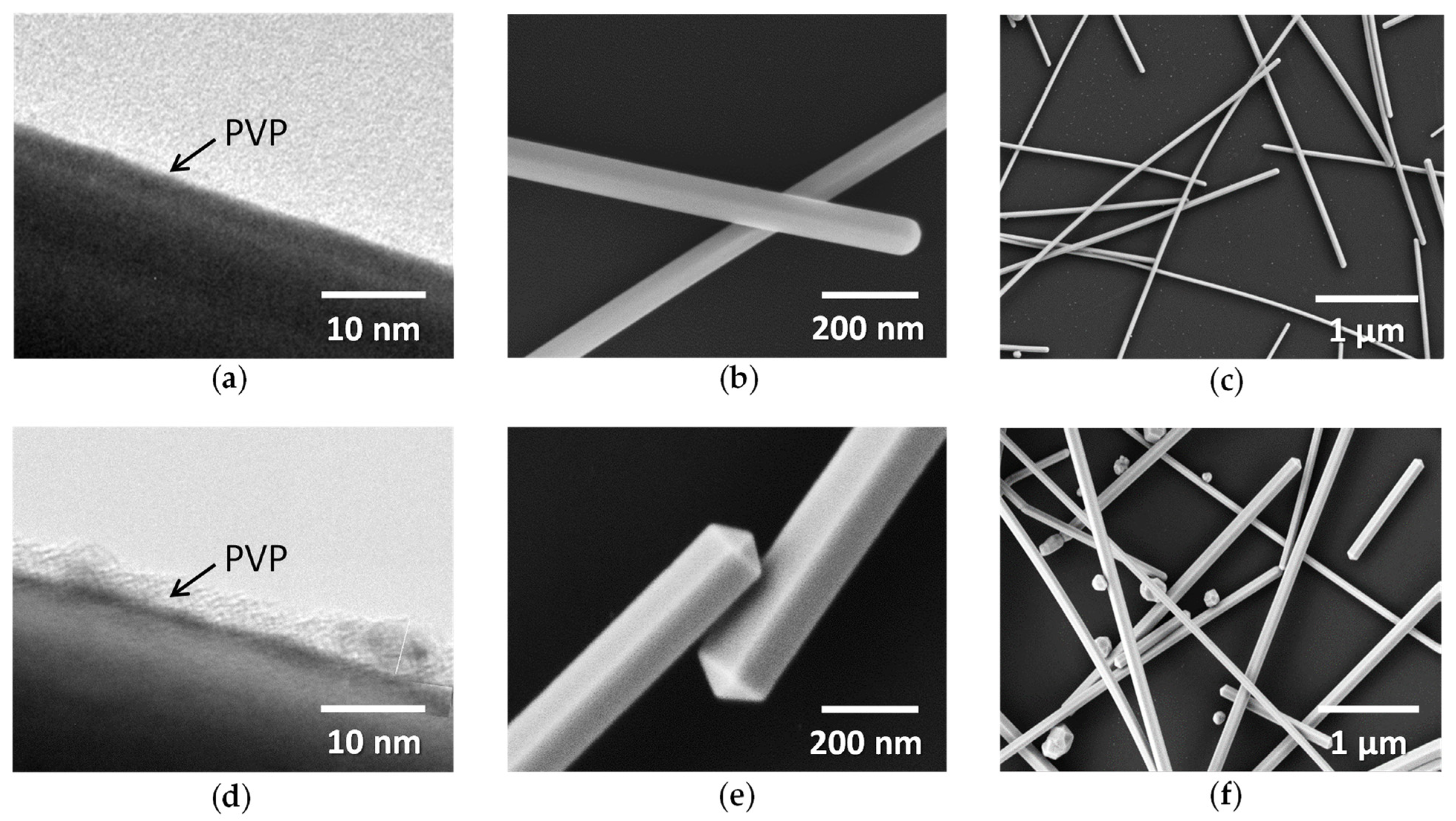
| Name | Suppliers | Details of the Synthesis | Dimensions of AgNWs |
|---|---|---|---|
| AgNW-1 | CEA-LITEN (France) | (1) Stirring of a 160 mL ethyleneglycol (EG) solution with NaCl (1 mM) and PVP (3.54 g, Mw = 40,000 g/mol) at 120 °C and cooling down to room temperature (2) Addition of this solution into 80 mL of an EG solution of AgNO3 (1.36 g, 0.1 M) at 120 °C and further heated at 160 °C, refluxed for 80 min and cooled down to room temperature (3) Washing of the remaining grey residue containing nanowires after two settlement days with acetone and dispersion of the AgNWs in methanol | L = 8 ± 3 µm D = 79 ± 10 nm |
| AgNW-2 | R&Dnano (France) | (1) Stirring of an EG solution with NaCl (5.5 µM), PVP (72 mM) and AgNO3 (59 mM) at room temperature (2) Heating of the solution at 130 °C for 5 h (3) Washing steps (three steps with water and one with isopropanol), followed by a centrifugation step | L = 11 ± 5.8 µm D = 119 ± 23 nm |
| Associated Network Properties | Ways to Optimize These Properties | References |
|---|---|---|
| Optical transparency and electrical resistance (evaluated by ) |
| [36,37] |
| [38] | |
| [39,40,41,42] | |
| Present work | |
| Morphological stability |
| [37,43] |
| [44] | |
| [45,46] Present work | |
| Haziness |
| [45,47] |
| Surface roughness |
| [42,48] |
| [49,50] | |
| Adhesion to substrate |
| [22,51,52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardet, L.; Papanastasiou, D.T.; Crivello, C.; Akbari, M.; Resende, J.; Sekkat, A.; Sanchez-Velasquez, C.; Rapenne, L.; Jiménez, C.; Muñoz-Rojas, D.; et al. Silver Nanowire Networks: Ways to Enhance Their Physical Properties and Stability. Nanomaterials 2021, 11, 2785. https://doi.org/10.3390/nano11112785
Bardet L, Papanastasiou DT, Crivello C, Akbari M, Resende J, Sekkat A, Sanchez-Velasquez C, Rapenne L, Jiménez C, Muñoz-Rojas D, et al. Silver Nanowire Networks: Ways to Enhance Their Physical Properties and Stability. Nanomaterials. 2021; 11(11):2785. https://doi.org/10.3390/nano11112785
Chicago/Turabian StyleBardet, Laetitia, Dorina T. Papanastasiou, Chiara Crivello, Masoud Akbari, João Resende, Abderrahime Sekkat, Camilo Sanchez-Velasquez, Laetitia Rapenne, Carmen Jiménez, David Muñoz-Rojas, and et al. 2021. "Silver Nanowire Networks: Ways to Enhance Their Physical Properties and Stability" Nanomaterials 11, no. 11: 2785. https://doi.org/10.3390/nano11112785








