Sensing Performance of Thermal Electronic Noses: A Comparison between ZnO and SnO2 Nanowires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SnO2 and ZnO Nanowires
2.2. Material Characterization
2.3. Fabrication of the Sensor
2.4. Gas Sensor Measurements
2.5. Machine Learning
3. Results and Discussion
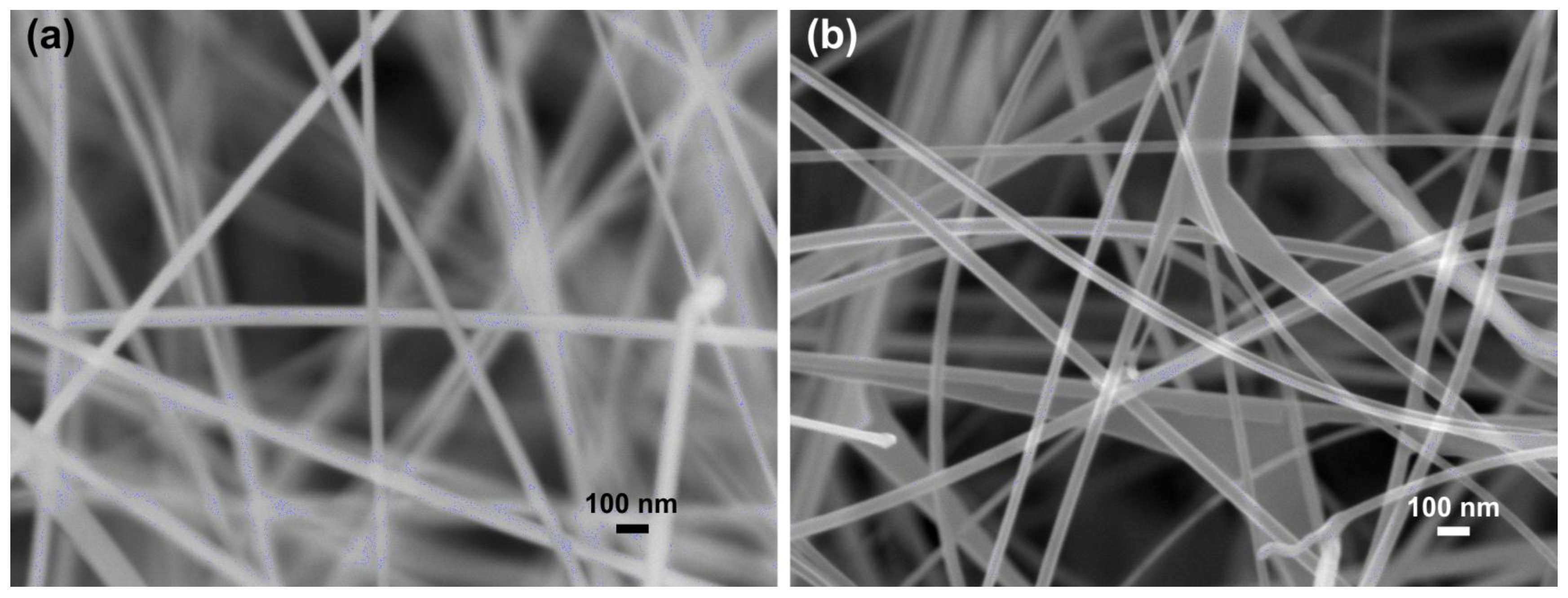
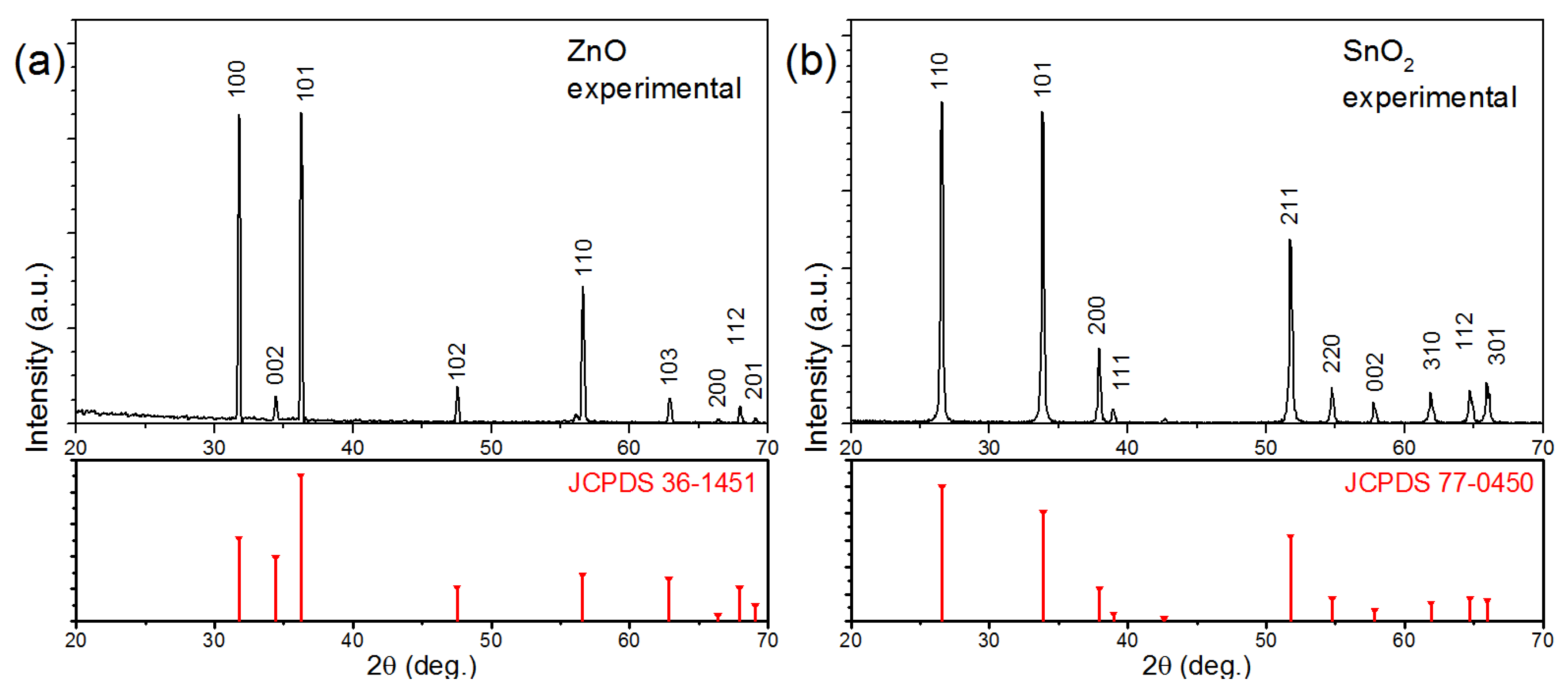
3.1. Nanowire Characterization
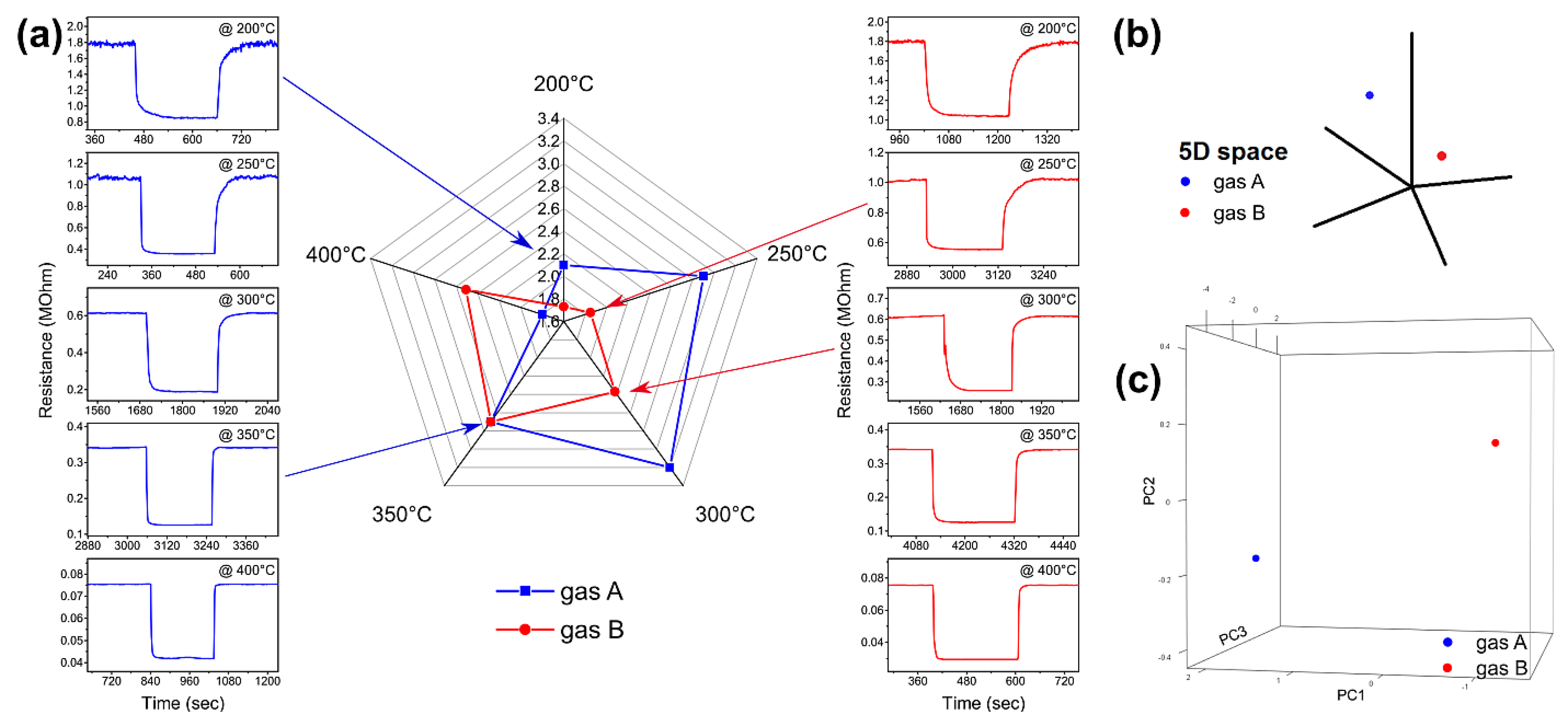
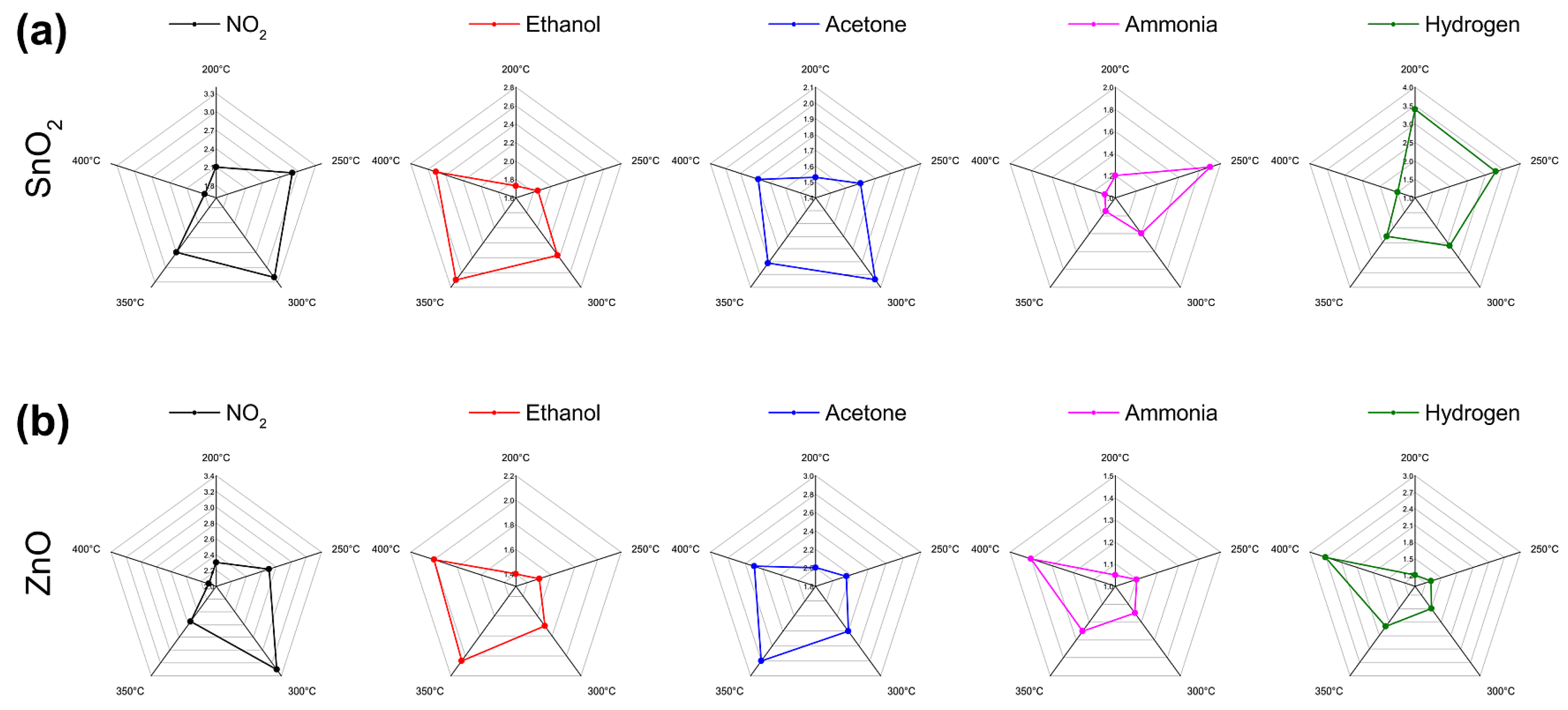
3.2. Gas Sensing Measurements
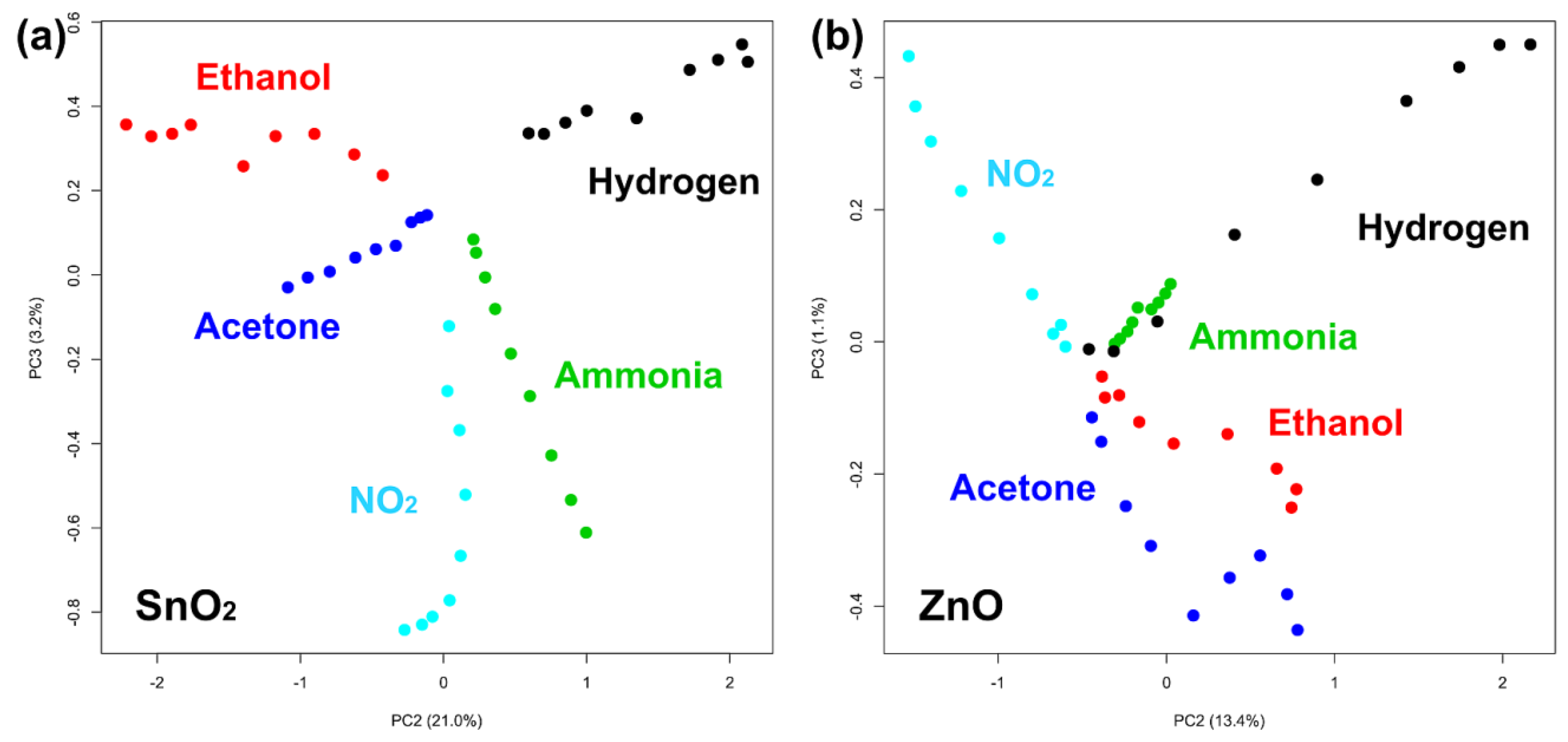
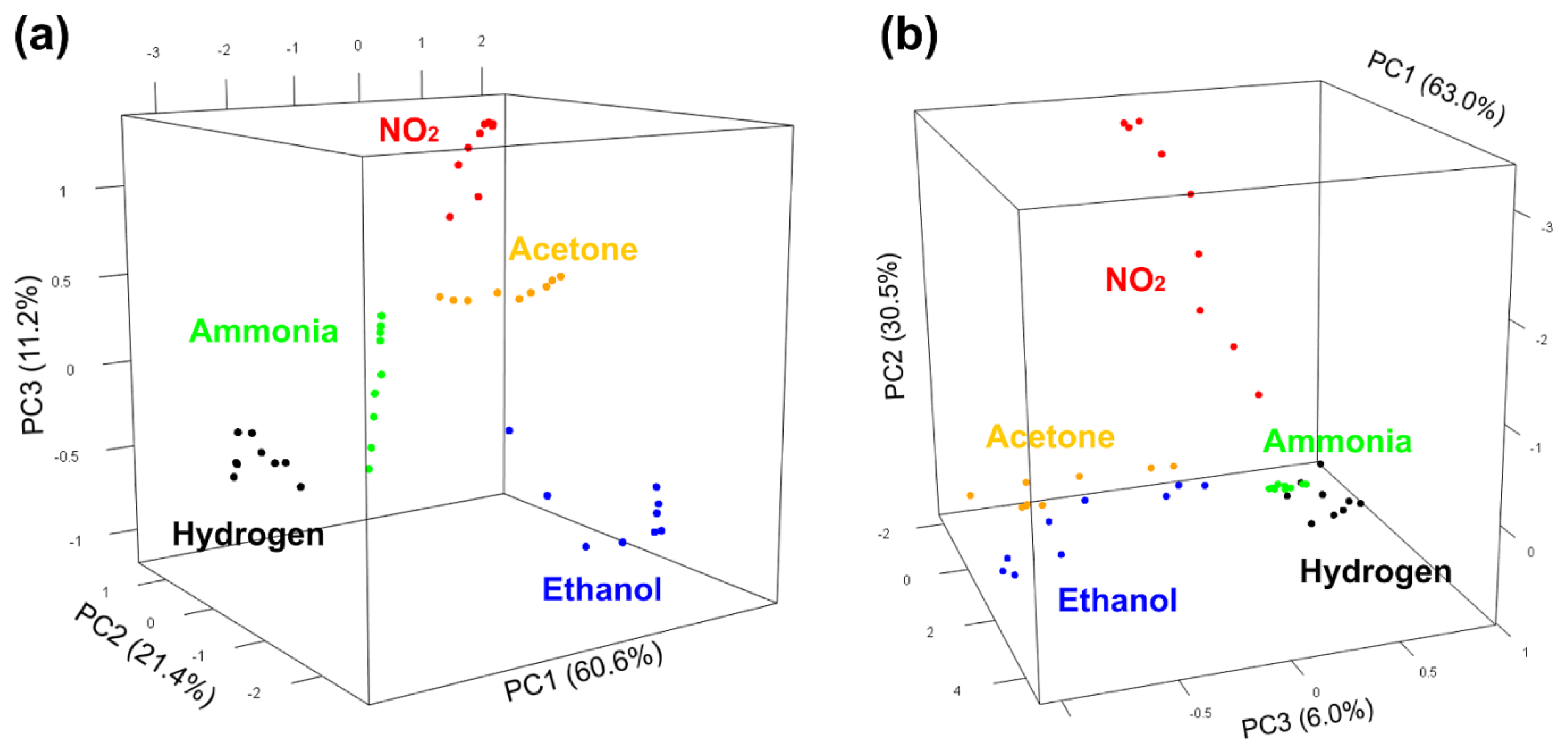
3.3. Qualitative Dinstinction
3.4. Classification
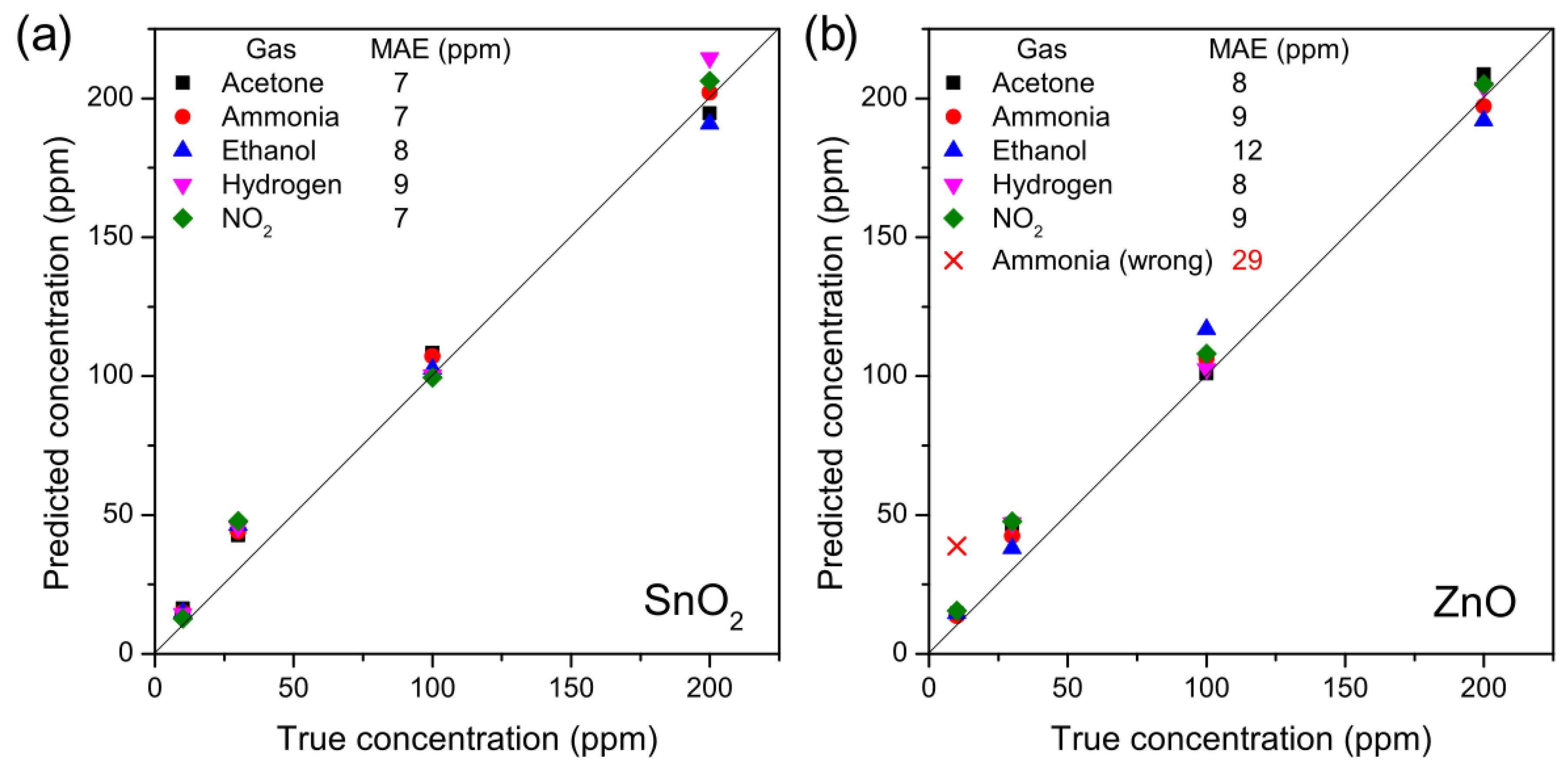
3.5. Quantification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Xing, J.; Wang, S.; Chang, X.; Liu, S.; Shi, A.; Liu, B.; Sahu, S.K. Understand the local and regional contributions on air pollution from the view of human health impacts. Front. Environ. Sci. Eng. 2021, 15, 88. [Google Scholar] [CrossRef]
- Zhou, X.; Strezov, V.; Jiang, Y.; Kan, T.; Evans, T. Temporal and spatial variations of air pollution across China from 2015 to 2018. J. Environ. Sci. 2022, 112, 161–169. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Noh, Y.; Boor, B.E.; Shannahan, J.H.; Troy, C.D.; Jafvert, C.T.; Whelton, A.J. Emergency responder and public health considerations for plastic sewer lining chemical waste exposures in indoor environments. J. Hazard. Mater. 2022, 422, 126832. [Google Scholar] [CrossRef] [PubMed]
- Ghelli, F.; Bellisario, V.; Squillacioti, G.; Grignani, E.; Garzaro, G.; Buglisi, M.; Bergamaschi, E.; Bono, R. Oxidative stress induction in woodworkers occupationally exposed to wood dust and formaldehyde. J. Occup. Med. Toxicol. 2021, 16, 4. [Google Scholar] [CrossRef]
- Huang, L.; Wei, Y.; Zhang, L.; Ma, Z.; Zhao, W. Estimates of emission strengths of 43 VOCs in wintertime residential indoor environments, Beijing. Sci. Total Environ. 2021, 793, 148623. [Google Scholar] [CrossRef]
- Barreca, D.; Gasparotto, A.; Gri, F.; Comini, E.; Maccato, C. Plasma-Assisted Growth of β-MnO2Nanosystems as Gas Sensors for Safety and Food Industry Applications. Adv. Mater. Interfaces 2018, 5, 1800792. [Google Scholar] [CrossRef]
- Tonezzer, M.; Thai, N.; Gasperi, F.; Van Duy, N.; Biasioli, F. Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient. Nanomaterials 2021, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Al Makky, A.; Alaswad, A.; Gibson, D.; Olabi, A.G. Renewable energy scenario and environmental aspects of soil emission measurements. Renew. Sustain. Energy Rev. 2017, 68, 1157–1173. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Wei, Z.; Li, Y.; Qu, J.; Zu, B.; Dou, X. Highly sensitive and rapid chemiresistive sensor towards trace nitro-explosive vapors based on oxygen vacancy-rich and defective crystallized In-doped ZnO. Sens. Actuators B Chem. 2017, 244, 983–991. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, J.-H.; Majhi, S.M.; Weber, M.; Bechelany, M.; Kim, H.W.; Kim, S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102. [Google Scholar] [CrossRef] [Green Version]
- Tonezzer, M. Detection of Mackerel Fish Spoilage with a Gas Sensor Based on One Single SnO2 Nanowire. Chemosensors 2021, 9, 2. [Google Scholar] [CrossRef]
- Bigiani, L.; Zappa, D.; Maccato, C.; Gasparotto, A.; Sada, C.; Comini, E.; Barreca, D. Hydrogen Gas Sensing Performances of p-Type Mn3O4 Nanosystems: The Role of Built-in Mn3O4/Ag and Mn3O4/SnO2 Junctions. Nanomaterials 2020, 10, 511. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.-Y.; Lim, D.-S.; Oh, Y.-J. CO Gas Detection of Al-Doped ZnO Nanostructures with Various Shapes. Jpn. J. Appl. Phys. 2013, 52, 101103. [Google Scholar] [CrossRef]
- Tonezzer, M.; Van Hieu, N. Size-dependent response of single-nanowire gas sensors. Sens. Actuators B Chem. 2012, 163, 146–152. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Simion, C.E.; Tomescu-Stănoiu, A. Differences in the gas sensing properties readout with n and p-type MOX materials. In Proceedings of the International Semiconductor Conference (CAS 2010), Sinaia, Romania, 11–13 October 2010; pp. 201–204. [Google Scholar] [CrossRef]
- Marzorati, D.; Mainardi, L.; Sedda, G.; Gasparri, R.; Spaggiari, L.; Cerveri, P. MOS Sensors Array for the Discrimination of Lung Cancer and At-Risk Subjects with Exhaled Breath Analysis. Chemosensors 2021, 9, 209. [Google Scholar] [CrossRef]
- Moon, H.G.; Jung, Y.; Han, S.D.; Shim, Y.-S.; Shin, B.; Lee, T.; Kim, J.-S.; Lee, S.; Jun, S.C.; Park, H.-H.; et al. Chemiresistive Electronic Nose toward Detection of Biomarkers in Exhaled Breath. ACS Appl. Mater. Interfaces 2016, 8, 20969–20976. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; de León-Martínez, L.D.; Gorocica-Rosete, P.; Pérez-Padilla, R.; Domínguez-Reyes, C.A.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Mehta, G.; Zamora-Mendoza, B.N.; Flores-Ramírez, R. Application of chemoresistive gas sensors and chemometric analysis to differentiate the fingerprints of global volatile organic compounds from diseases. Preliminary results of COPD, lung cancer and breast cancer. Clin. Chim. Acta 2021, 518, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, V.V.; Kiselev, I.; Frietsch, M.; Goschnick, J. Temperature Gradient Effect on Gas Discrimination Power of a Metal-Oxide Thin-Film Sensor Microarray. Sensors 2004, 4, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Sysoev, V.V.; Goschnick, J.; Schneider, T.; Strelcov, A.E.; Kolmakov, A. A Gradient Microarray Electronic Nose Based on Percolating SnO2Nanowire Sensing Elements. Nano Lett. 2007, 7, 3182–3188. [Google Scholar] [CrossRef]
- Thai, N.X.; Tonezzer, M.; Masera, L.; Nguyen, H.; Van Duy, N.; Hoa, N.D. Multi gas sensors using one nanomaterial, temperature gradient, and machine learning algorithms for discrimination of gases and their concentration. Anal. Chim. Acta 2020, 1124, 85–93. [Google Scholar] [CrossRef]
- Thai, N.X.; Van Duy, N.; Hung, C.M.; Nguyen, H.; Tonezzer, M.; Van Hieu, N.; Hoa, N.D. Prototype edge-grown nanowire sensor array for the real-time monitoring and classification of multiple gases. J. Sci. Adv. Mater. Devices 2020, 5, 409–416. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.S.; Tonezzer, M. Selective gas detection and quantification using a resistive sensor based on Pd-decorated soda-lime glass. Sens. Actuators B Chem. 2021, 335, 129714. [Google Scholar] [CrossRef]
- Tischner, A.; Maier, T.; Stepper, C.; Köck, A. Ultrathin SnO2 gas sensors fabricated by spray pyrolysis for the detection of humidity and carbon monoxide. Sens. Actuators B Chem. 2008, 134, 796–802. [Google Scholar] [CrossRef]
- Acetone. Available online: https://www.osha.gov/chemicaldata/476 (accessed on 21 September 2021).
- Ammonia. Available online: https://www.osha.gov/chemicaldata/623 (accessed on 21 September 2021).
- Ethanol. Available online: https://www.osha.gov/chemicaldata/1034 (accessed on 21 September 2021).
- Hydrogen. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.103 (accessed on 21 September 2021).
- Nitrogen Dioxide. Available online: https://www.osha.gov/chemicaldata/21 (accessed on 21 September 2021).
- Khan, A.H.; Thomson, B.; Debnath, R.; Motayed, A.; Rao, M.V. Nanowire-Based Sensor Array for Detection of Cross-Sensitive Gases Using PCA and Machine Learning Algorithms. IEEE Sens. J. 2020, 20, 6020–6028. [Google Scholar] [CrossRef]
- Huang, Y.; Doh, I.-J.; Bae, E. Design and Validation of a Portable Machine Learning-Based Electronic Nose. Sensors 2021, 21, 3923. [Google Scholar] [CrossRef]
| Estimated | ||||||
|---|---|---|---|---|---|---|
| Acetone | Ammonia | Ethanol | Hydrogen | NO2 | ||
| Acetone | 4 | |||||
| Ammonia | 4 | |||||
| True | Ethanol | 4 | ||||
| Hydrogen | 4 | |||||
| NO2 | 4 |
| Estimated | ||||||
|---|---|---|---|---|---|---|
| Acetone | Ammonia | Ethanol | Hydrogen | NO2 | ||
| Acetone | 4 | |||||
| Ammonia | 4 | 1 | ||||
| True | Ethanol | 4 | ||||
| Hydrogen | 3 | |||||
| NO2 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonezzer, M.; Armellini, C.; Toniutti, L. Sensing Performance of Thermal Electronic Noses: A Comparison between ZnO and SnO2 Nanowires. Nanomaterials 2021, 11, 2773. https://doi.org/10.3390/nano11112773
Tonezzer M, Armellini C, Toniutti L. Sensing Performance of Thermal Electronic Noses: A Comparison between ZnO and SnO2 Nanowires. Nanomaterials. 2021; 11(11):2773. https://doi.org/10.3390/nano11112773
Chicago/Turabian StyleTonezzer, Matteo, Cristina Armellini, and Laura Toniutti. 2021. "Sensing Performance of Thermal Electronic Noses: A Comparison between ZnO and SnO2 Nanowires" Nanomaterials 11, no. 11: 2773. https://doi.org/10.3390/nano11112773
APA StyleTonezzer, M., Armellini, C., & Toniutti, L. (2021). Sensing Performance of Thermal Electronic Noses: A Comparison between ZnO and SnO2 Nanowires. Nanomaterials, 11(11), 2773. https://doi.org/10.3390/nano11112773







