Characteristics, Toxic Effects, and Analytical Methods of Microplastics in the Atmosphere
Abstract
:1. Introduction
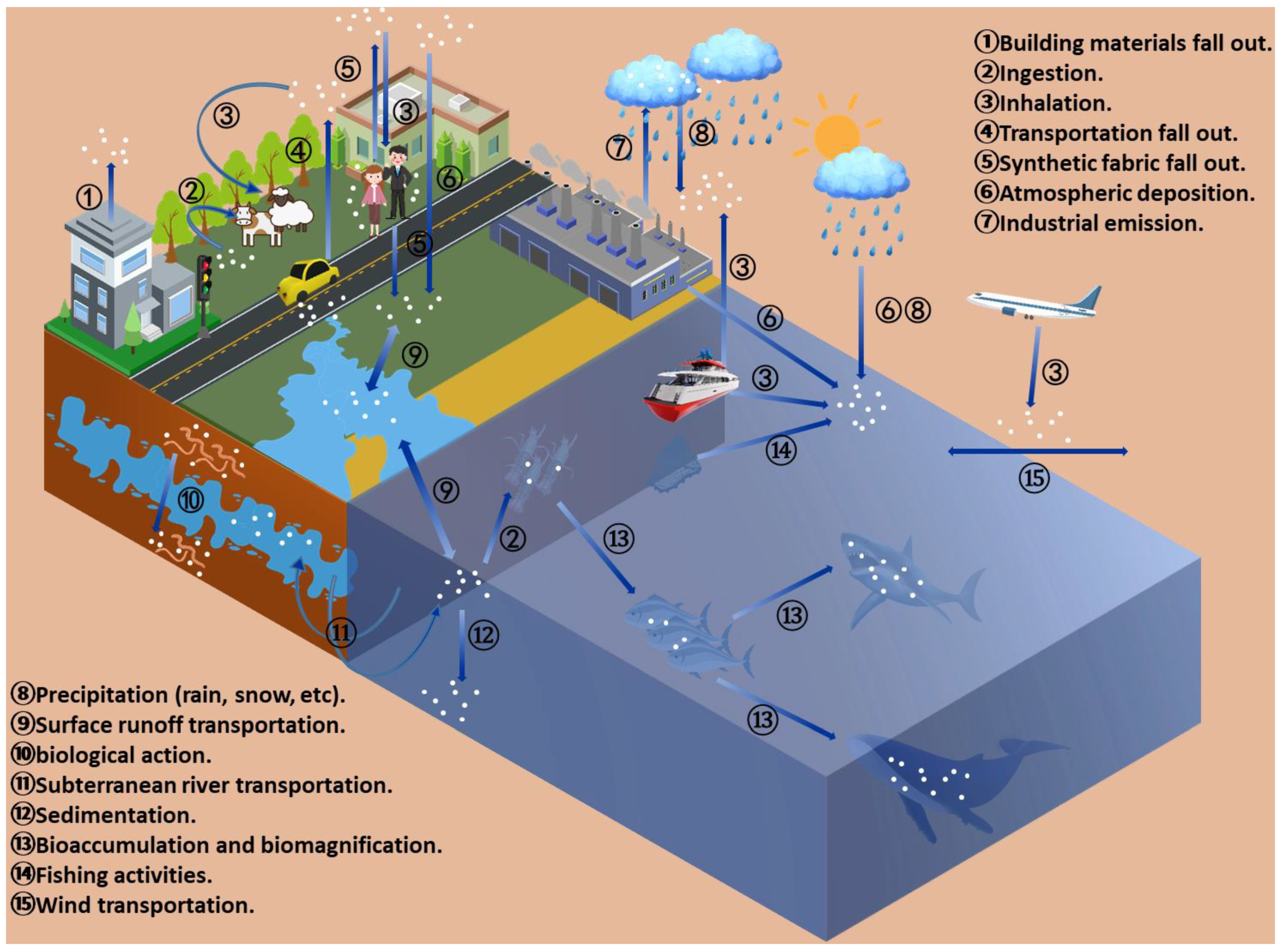
2. The Global Distribution of Atmospheric MPs and Associated Influencing Factors
2.1. Distribution Profile
2.2. Influencing Factors
2.2.1. Vertical Concentration Gradient
2.2.2. Meteorological Conditions
2.2.3. Indoor and Outdoor Atmospheric Settings
2.2.4. Regional Environmental Conditions
2.3. Gaps in and Prospective Research on Distribution Characteristics of Atmospheric MPs
- (1)
- It is difficult to confirm the extent of MP pollution in the atmosphere around the world. It is suggested that systematic spatial and temporal studies be conducted on the distribution of MPs in the atmosphere, to further clarify the concentrations, types, and occurrence of atmospheric MP pollution in different regions and determine the sources, distribution, and fate of atmospheric MPs in different regions.
- (2)
- We found that the experimental methods for studying atmospheric MPs in the past papers were different and no standard methods for collection and characterization of MPs were validated, which greatly reduced the experimental efficiency. In addition, the measurement criteria and units used were so varied that it is hard to intuitively make a comparison with the experimental findings of researchers using different standards (e.g., there is no way to compare the concentrations of MPs in units of m2/day and m3/day). It is suggested that the use of more efficient sampling and analysis methods be unified and the industry standards for measuring MP concentration, type, and occurrence be standardized.
3. Sources of Atmospheric MPs
3.1. Sources
3.1.1. Synthetic Textiles
3.1.2. Transportation
3.1.3. Dust
3.1.4. Other Small Sources
3.1.5. Gaps in and Prospective Research on the Sources of Atmospheric MPs
- (1)
- Continue to optimize the methods and tools for atmospheric MP characterization and component analysis and identification to more clearly identify the sources of MPs and avoid unclear and inaccurate source identification caused by rough differentiation.
- (2)
- Establish a pollution source localization method suitable for atmospheric MPs, which can trace the source more accurately than characterization or component analysis.
3.2. Transportation and Fate of Atmospheric MPs
3.2.1. Migration
3.2.2. Inhalation
3.2.3. Gaps in and Prospective Research on the Destinations of Atmospheric MPs
- (1)
- Further explore the factors affecting the fate of atmospheric MPs and understand the different destinations of MPs in the atmospheric environment under different conditions.
- (2)
- Establish a spatial model and related software suitable for integrating the diffusion and migration trajectories of atmospheric MPs and the pollutants adsorbed by them.
- (3)
- Investigate the difference in the quantities and proportions of MPs absorbed by people in different areas and under different conditions in the same area as well as body burden and associated risks of atmospheric MPs.
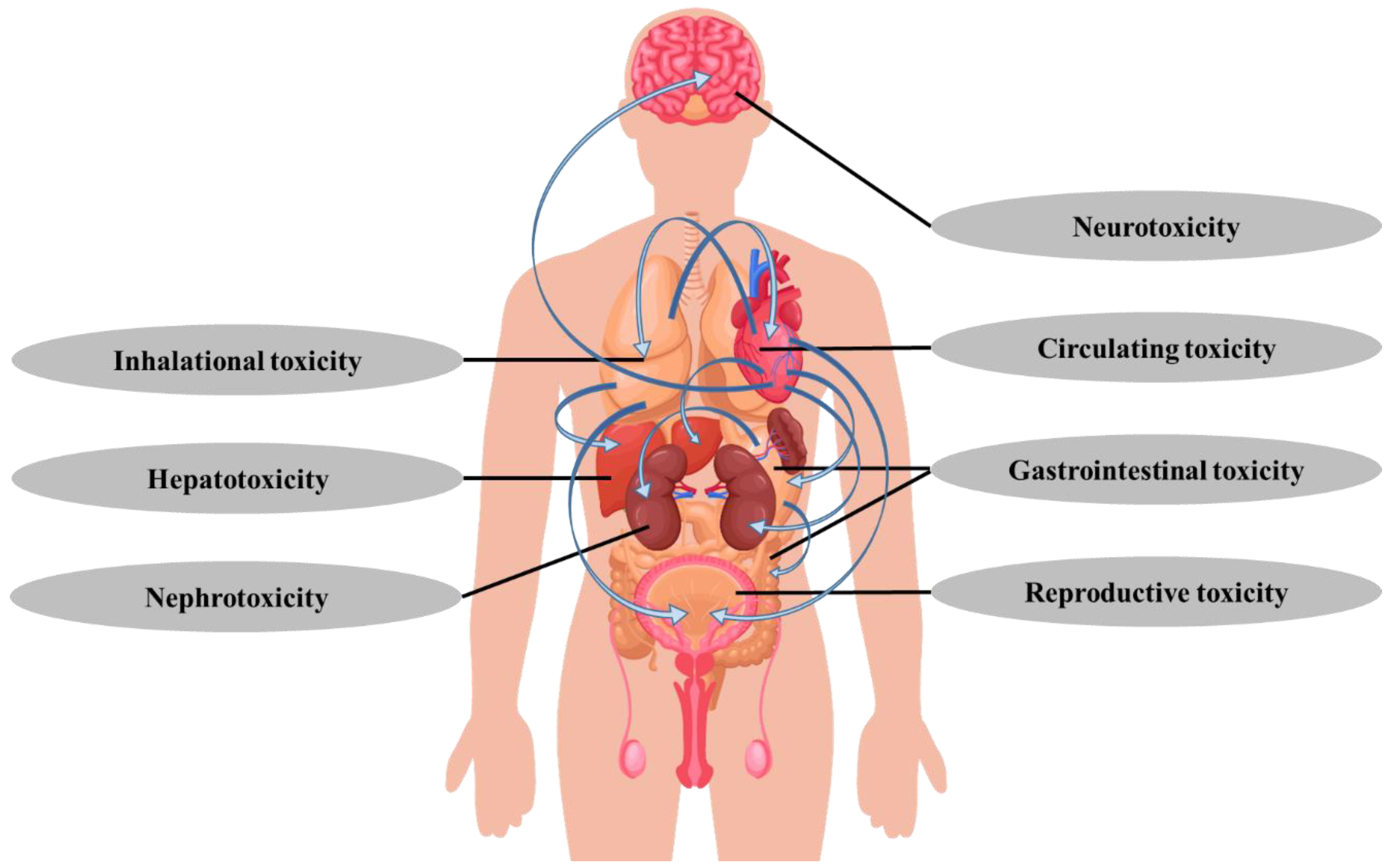
4. Toxic Effects
4.1. Inhalational-Based Toxicity
4.2. Other Toxic Effects
4.3. Joint Toxic Effects
4.4. Gaps in and Prospective Research on the Toxic Effects of Atmospheric MPs on Animals and Humans
- (1)
- In vivo experiments should be conducted to explore the different negative effects of MPs or NPs with different physical or chemical properties (such as different types, sizes, occurrences, crystallinity, and surface charge) on animal and human health after inhalation. An animal model should be established to study the movement trajectory and deposition proportion of and harmful substances released by atmospheric MPs in the body.
- (2)
- To better understand the harmful additive impact of atmospheric MPs adsorbed with other pollutants as a pollutant internalization carrier, more research into the toxic additive effect of the two is required.
5. Existing Analytical Methods and Gaps in Measuring Atmospheric MPs
- (1)
- To further develop and research some efficient methods and instruments. On the one hand, a great number of MPs should be sampled and accurately identified in a short period. On the other hand, we should be able to further identify more types of plastics and plastics of smaller sizes through microfiltration and various pollutants adsorbed on them.
- (2)
- To develop a set of uniform standard methods for sampling and identification. For this, scientific data generated in different regions for atmospheric MPs should be compared.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Capillo, G.; Savoca, S.; Panarello, G.; Mancuso, M.; Branca, C.; Romano, V.; D’Angelo, G.; Bottari, T.; Spano, N. Quali-quantitative analysis of plastics and synthetic microfibers found in demersal species from Southern Tyrrhenian Sea (Central Mediterranean). Mar. Pollut. Bull. 2020, 150, 110596. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Toumi, H.; Abidli, S.; Bejaoui, M. Microplastics in freshwater environment: The first evaluation in sediments from seven water streams surrounding the lagoon of Bizerte (Northern Tunisia). Environ. Sci. Pollut. Res. Int. 2019, 26, 14673–14682. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Perez-Guevara, F.; Elizalde-Martinez, I.; Shruti, V.C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic pollution in commercial salt for human consumption: A review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lu, F. Municipal solid waste (MSW) landfill: A source of microplastics?—Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Gieré, R.; Sommer, F.; Dietze, V.; Baum, A.; Maschowski, C. Tire-wear particles as a major component of microplastics in the environment. In Proceedings of the GSA Annual Meeting, Indianapolis, IN, USA, 4–7 November 2018. [Google Scholar]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Blasing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Coppock, R.; Lindeque, P.K.; Altin, D.; Reed, S.; Pond, D.W.; Sorensen, L.; Galloway, T.S.; Booth, A.M. Effects of Nylon Microplastic on Feeding, Lipid Accumulation, and Moulting in a Coldwater Copepod. Environ. Sci. Technol. 2019, 53, 7075–7082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Cho, H.J.; Kim, E.; Huh, Y.H.; Kim, H.J.; Kim, B.; Kang, T.; Lee, J.S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, R.; Voy, C.; Chen, S.; Di Giulio, R.T. Nanoplastics Decrease the Toxicity of a Complex PAH Mixture but Impair Mitochondrial Energy Production in Developing Zebrafish. Environ. Sci. Technol. 2019, 53, 8405–8415. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus moara) through Disrupting Hepatic Lipid Homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Savoca, S.; Capillo, G.; Mancuso, M.; Bottari, T.; Crupi, R.; Branca, C.; Romano, V.; Faggio, C.; D’Angelo, G.; Spano, N. Microplastics occurrence in the Tyrrhenian waters and in the gastrointestinal tract of two congener species of seabreams. Environ. Toxicol. Pharmacol. 2019, 67, 35–41. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Holloczki, O.; Gehrke, S. Can Nanoplastics Alter Cell Membranes? Chemphyschem 2020, 21, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Diepens, N.J.; Koelmans, A.A. Accumulation of Plastic Debris and Associated Contaminants in Aquatic Food Webs. Environ. Sci. Technol. 2018, 52, 8510–8520. [Google Scholar] [CrossRef]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical Pollutants Sorbed to Ingested Microbeads from Personal Care Products Accumulate in Fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef]
- Ebere, E.C.; Ngozi, V.E. Microplastics, an emerging concern: A review of analytical techniques for detecting and quantifying MPs. Anal. Methods Environ. Chem. J. 2019, 2, 13–30. [Google Scholar]
- González-Pleiter, M.; Edo, C.; Aguilera, Á.; Viúdez-Moreiras, D.; Pulido-Reyes, G.; González-Toril, E.; Osuna, S.; de Diego-Castilla, G.; Leganés, F.; Fernández-Piñas, F.; et al. Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci. Total. Environ. 2021, 761, 143213. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634. [Google Scholar] [CrossRef]
- Rubio, L.; Marcos, R.; Hernandez, A. Potential adverse health effects of ingested micro- and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J. Toxicol. Environ. Health B Crit. Rev. 2020, 23, 51–68. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. Int. 2017, 24, 24928–24935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tian, C.; Luo, Y. Various forms and deposition fluxes of microplastics identified in the coastal urban atmosphere. Chin. Sci. Bull. 2017, 62, 3902–3909. [Google Scholar] [CrossRef] [Green Version]
- Kaya, T.A.; Yurtsever, M.; Çiftçi Bayraktar, S. Ubiquitous exposure to microfiber pollution in the air. Eur. Phys. J. Plus 2018, 133, 488. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total. Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int. 2019, 132, 105127. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Wu, T.; Wang, X.; Song, Z.; Zong, C.; Wei, N.; Li, D. Consistent Transport of Terrestrial Microplastics to the Ocean through Atmosphere. Environ. Sci. Technol. 2019, 53, 10612–10619. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liu, K.; Zhu, L.; Song, Z.; Li, D. Atmospheric microplastic over the South China Sea and East Indian Ocean: Abundance, distribution and source. J. Hazard. Mater. 2020, 389, 121846. [Google Scholar] [CrossRef]
- Li, Y.; Shao, L.; Wang, W.; Zhang, M.; Feng, X.; Li, W.; Zhang, D. Airborne fiber particles: Types, size and concentration observed in Beijing. Sci. Total Environ. 2020, 705, 135967. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful?Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation; IUCN: Gland, Switzerland, 2017; p. 43. [Google Scholar]
- Fernando, H.J.S.; Lee, S.M.; Anderson, J.; Princevac, M.; Pardyjak, E.; Grossman-Clarke, S. Urban Fluid Mechanics: Air Circulation and Contaminant Dispersion in Cities. Environ. Fluid Mech. 2001, 1, 107–164. [Google Scholar] [CrossRef]
- Ambrosini, R.; Azzoni, R.S.; Pittino, F.; Diolaiuti, G.; Franzetti, A.; Parolini, M. First evidence of microplastic contamination in the supraglacial debris of an alpine glacier. Environ. Pollut. 2019, 253, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, F.; Cocca, M.; Avella, M.; Thompson, R.C. Microfiber Release to Water, Via Laundering, and to Air, via Everyday Use: A Comparison between Polyester Clothing with Differing Textile Parameters. Environ. Sci. Technol. 2020, 54, 3288–3296. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, G.; Li, Y.; Huang, J.; Zhao, J. Analysis of the Status Quo of China’s Anti-pandemic Textiles and Suggestions fo Innovative Development. China Text. Lead. 2021, 02, 83–87. [Google Scholar]
- Kole, P.J.; Lohr, A.J.; Van Belleghem, F.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Eisentraut, P.; Dümichen, E.; Ruhl, A.S.; Jekel, M.; Albrecht, M.; Gehde, M.; Braun, U. Two Birds with One Stone—Fast and Simultaneous Analysis of Microplastics: Microparticles Derived from Thermoplastics and Tire Wear. Environ. Sci. Technol. Lett. 2018, 5, 608–613. [Google Scholar] [CrossRef]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment—A review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)—A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total. Environ. 2020, 733, 137823. [Google Scholar] [CrossRef]
- Sommer, F.; Dietze, V.; Baum, A.; Sauer, J.; Gilge, S.; Maschowski, C.; Gieré, R. Tire Abrasion as a Major Source of Microplastics in the Environment. Aerosol Air Qual. Res. 2018, 18, 2014–2028. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124. [Google Scholar] [CrossRef]
- Kausar, A. A review of filled and pristine polycarbonate blends and their applications. J. Plast. Film. Sheeting 2017, 34, 60–97. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Nieuwenhuijsen, M.J.; Colvile, R.N. Fine particulate matter and carbon monoxide exposure concentrations in urban street transport microenvironments. Atmos. Environ. 2007, 41, 4781–4810. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Browne Mark, A.; Galloway Tamara, S.; Thompson Richard, C. Spatial patterns of plastic debris along Estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Alzona, J.; Cohen, B.L.; Rudolph, H.e.a. Indoor-outdoor relationships for airborne particulate matter of outdoor origin. Atmos. Environ. 1979, 13, 55–60. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Gilmour, P.S.; Brown, D.M.; MacNee, W. Ultrafine particles: Mechanisms of lung injury. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2000, 358, 2741–2749. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Carney Almroth, B.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hoet, P.H.M.; Nemery, B. In vitro toxicity assessment of polyvinyl chloride particles and comparison of six cellular systems. J. Toxicol. Environ. Health Part A 2002, 65, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.-M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Chen, Y.-C.; Chen, H.-H.; Lee, J.-S.; Lin, C.-H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Paget, V.; Dekali, S.; Kortulewski, T.; Grall, R.; Gamez, C.; Blazy, K.; Aguerre-Chariol, O.; Chevillard, S.; Braun, A.; Rat, P.; et al. Specific Uptake and Genotoxicity Induced by Polystyrene Nanobeads with Distinct Surface Chemistry on Human Lung Epithelial Cells and Macrophages. PLoS ONE 2015, 10, e0123297. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Chankeshwara, S.V.; Thielbeer, F.; Jeong, J.; Donaldson, K.; Bradley, M.; Cho, W.-S. Surface charge determines the lung inflammogenicity: A study with polystyrene nanoparticles. Nanotoxicology 2016, 10, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.K.; Kaw, J.L.; Srivastava, S.P.; Seth, P.K. Some biochemical and histopathological changes induced by polyvinyl chloride dust in rat lung. Environ. Res. 1978, 16, 333–341. [Google Scholar] [CrossRef]
- Atis, S.; Tutluoglu, B.; Levent, E.; Ozturk, C.; Tunaci, A.; Sahin, K.; Saral, A.; Oktay, I.; Kanik, A.; Nemery, B. The respiratory effects of occupational polypropylene flock exposure. Eur. Respir. J. 2005, 25, 110–117. [Google Scholar] [CrossRef]
- Pimentel, J.C.; Avila, R.; Lourenco, A.G. Respiratory disease caused by synthetic fibres: A new occupational disease. Thorax 1975, 30, 204. [Google Scholar] [CrossRef] [Green Version]
- Porter, D.W.; Castranova, V.; Robinson, V.A.; Hubbs, A.F.; Mercer, R.R.; Scabilloni, J.; Goldsmith, T.; Schwegler-Berry, D.; Battelli, L.; Washko, R.; et al. Acute inflammatory reaction in rats after intratracheal instillation of material collected from a nylon flocking plant. J. Toxicol. Environ. Health A 1999, 57, 25–45. [Google Scholar]
- Xu, H.; Verbeken, E.; Vanhooren, H.M.; Nemery, B.; Hoet, P.H. Pulmonary toxicity of polyvinyl chloride particles after a single intratracheal instillation in rats. Time course and comparison with silica. Toxicol. Appl. Pharmacol. 2004, 194, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Campopiano, A.; Cardinali, G.; Casciardi, S.; De Simone, P.; Kovacs, D.; Perniconi, B.; Spagnoli, G.; Ursini, C.L.; Fanizza, C. Cytotoxic and oxidative effects induced by man-made vitreous fibers (MMVFs) in a human mesothelial cell line. Toxicology 2004, 201, 219–229. [Google Scholar] [CrossRef]
- Jones, A.E.; Watts, J.A.; Debelak, J.P.; Thornton, L.R.; Younger, J.G.; Kline, J.A. Inhibition of prostaglandin synthesis during polystyrene microsphere-induced pulmonary embolism in the rat. AJP Lung Cell. Mol. Physiol. 2003, 284, L1072–L1081. [Google Scholar] [CrossRef] [Green Version]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Canonico, B.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.Y.; Kaplan, G.G.; Madsen, K.L. Air pollution effects on the gut microbiota: A link between exposure and inflammatory disease. Gut Microbes 2014, 5, 215–219. [Google Scholar] [CrossRef]
- Linares, V.; Belles, M.; Domingo, J.L. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol 2015, 89, 335–356. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef]
- Brun, N.R.; Koch, B.E.V.; Varela, M.; Peijnenburg, W.J.G.M.; Spaink, H.P.; Vijver, M.G. Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ. Sci. Nano 2018, 5, 904–916. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Wang, J.; Cai, L. Current understanding of microplastics in the environment: Occurrence, fate, risks, and what we should do. Integr. Environ. Assess. Manag. 2017, 13, 476–482. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Goncalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Dong, S.; Qu, M.; Rui, Q.; Wang, D. Combinational effect of titanium dioxide nanoparticles and nanopolystyrene particles at environmentally relevant concentrations on nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 161, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.; Infantini, V.; Scoponi, M.; Rastelli, E.; Corinaldesi, C.; Mistri, M. Microplastics in the sediments of Terra Nova Bay (Ross Sea, Antarctica). Mar. Pollut. Bull. 2017, 122, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Gallagher, A.; Rees, A.; Rowe, R.; Stevens, J.; Wright, P. Microplastics in the Solent estuarine complex, UK: An initial assessment. Mar. Pollut. Bull. 2016, 102, 243–249. [Google Scholar] [CrossRef]
- Zhao, J.; Ran, W.; Teng, J.; Liu, Y.; Liu, H.; Yin, X.; Cao, R.; Wang, Q. Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 2018, 640-641, 637–645. [Google Scholar] [CrossRef]
- Imhof, H.K.; Laforsch, C.; Wiesheu, A.C.; Schmid, J.; Anger, P.M.; Niessner, R.; Ivleva, N.P. Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res. 2016, 98, 64–74. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.A.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Li, D. Microplastic in three urban estuaries, China. Environ. Pollut. 2015, 206, 597–604. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 mum) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [Green Version]
- Huppertsberg, S.; Knepper, T.P. Instrumental analysis of microplastics-benefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Dumichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Cooper, D.A.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of microplastics in local marine organisms using a multi-technology system. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Weidner, S.M.; Sarah, T. Mass spectrometry of synthetic polymers. Anal. Chem. 2010, 82, 4811–4829. [Google Scholar] [CrossRef]
- Foerch, R.; Beamson, G.; Briggs, D. XPS valence band analysis of plasma-treated polymers. Surf. Interface Anal. 1991, 17, 842–846. [Google Scholar] [CrossRef]
- Magri, D.; Sanchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef]
- Malyuskin, O. Microplastic Detection in Soil and Water Using Resonance Microwave Spectroscopy: A Feasibility Study. IEEE Sens. J. 2020, 20, 14817–14826. [Google Scholar] [CrossRef]
| Location | Year | Sample Type | MP Type | Shape | Concentration (Item/Particle Number) | Size | Reference |
|---|---|---|---|---|---|---|---|
| Paris | 2014 | Urban outdoor air deposition | NA | Fiber, fragment | 29–280/m2/day | 0.1–5 mm | [30] |
| Paris | 2014–2015 | Urban outdoor air deposition | NA | Fiber | 110 ± 96/m2/day | 0.05–5 mm | [32] |
| Paris | 2014–2015 | Suburban outdoor air deposition | NA | Fiber | 53 ± 38/m2/day | 0.05–5 mm | [32] |
| Paris | 2015 | Urban indoor air | PA, PP, PE | Fiber | 0.4–59.4 (5.4)/m3 | 0.05–3.25 mm | [31] |
| Paris | 2015 | Urban outdoor air | PA, PP, PE | Fiber | 0.3–1.5 (0.9)/m3 | 0.05–1.65 mm | [31] |
| Dongguan | 2016 | Urban outdoor air deposition | PE, PP, PS | Fiber, foam, fragment, film | 175–313/m2/day | Minimum: <0.2 mmMaximum: >4.2 mm | [33] |
| Yantai | 2016 | Urban outdoor air deposition | PET, PVC, PE, PS | Fiber, fragment, film, foam | 2.33 × 1013/160 km2/year | 0.05–1 mm | [34] |
| Sakarya | 2016–2017 | Crowded area outdoor air | PA, PUR, PE, PP, PES | Fiber, fragment | 9067–30,793/L | 0.05–0.5 mm | [35] |
| Edinburg | 2017 | Indoor air of houses | NA | Fiber | 5 ± 33/sample | NA | [36] |
| Trent catchment | 2017–2018 | River catchment air deposition | NA | Fiber | 2.9–128.42/m2/day | NA | [37] |
| Shanghai | 2018 | Municipal outdoor air | PET, PE, PES, PAN, PAA, RY, EVA, EP, ALK | Fiber, fragment, granule | 0–4.18 (1.42 ± 1.42)/m3 | 23.07–9554.88 μm | [38] |
| Shanghai | 2019 | Urban outdoor air | PET, EP, PE, ALK, RY, PP, PA, PS | Fiber, fragment, microbead | 0–2 (0.41)/m3 | 12.35–2191.32 μm | [39] |
| Asaluyeh | 2017 | Urban and industrial outdoor air | NA | Fiber, fragment, film | 0.3–1.1/m3 | 2–100 μm | [40] |
| West Pacific Ocean | 2018–2019 | Ocean air | PET, PE, PE-PP, PES, ALK, EP, PA, PAN, PR, PMA, PP, PS, PVA, PVC | Fiber, fragment, granule, microbead | 0–1.37 (0.06 ± 0.16)/m3 | 16.14–2086.69 μm | [41] |
| Pyrenees | 2017–2018 | Remote air deposition | PS, PE, PP, PVC, PET | Fiber, fragment, film | 365 ± 69/m2/day | Minimum: <0.025 mmMaximum: >2.6 mm | [42] |
| Hamburg | 2017–2018 | Urban and rural outdoor air deposition | PE, EVA, PTFE, PVA, PET | Fragment, fiber | 136.5–512/m2/day | Minimum: <0.063 mmMaximum: >0.3 mm | [43] |
| Aarhus | 2017 | Indoor air of apartments | PES, PA, PS, PE, PUR | Fragment, fiber | 1.7–16.2 (9.3 ± 5.8)/m3 | 4–398 μm | [44] |
| London | 2018 | Urban outdoor air deposition | PAN, PES, PA, PP, PVC, PE, PET, PS, PUR, petroleum, resin, acrylic | Fragment, film, granule, foam | 771 ± 167/m2/day | 75–1080 μm | [45] |
| Karimata Strait | 2019 | Strait air | PET | Fiber | 0–0.8/100 m3 | 382.15 | [46] |
| Pearl River Estuary | 2019 | River estuary air | PA, PEP, PET, PP | Fiber | 3–7.7/100 m3 | 288.2–1117.62 μm | [46] |
| South China Sea | 2019 | Ocean air | PET, PEVA, PP | Fiber, fragment | 0–3.1/100 m3 | 286.1–1861.78 μm | [46] |
| East Indian Ocean | 2019 | Ocean air | PAN-AA, PET, PP, PR | Fiber, fragment | 0–0.8/100 m3 | 58.591–988.37 μm | [46] |
| Beijing | NA | Urban outdoor air deposition | NA | Fiber | Surface layer: 5.7 × 10−3/mLRoof: 5.6 × 10−3/mL | 5–200 μm | [47] |
| Alcalá de Henares- Guadalajara, Valladolid | 2020 | Rural and sub-rural PBL air | PET, PA, acrylic | Fiber | Rural area: 1/sampleSub-rural area: 3/sample | 0–9.8 μm | [27] |
| Guadalajara | 2020 | Urban PBL air | PU, PS, PA, acrylic | Fragment, fiber | 6/sample | NA | [27] |
| Madrid | 2020 | Urban PBL air | PA, PU, PET, PB, PE, PP | Fragment, fiber | 12/sample | NA | [27] |
| Classification | Chemicals | Affected Species | Resulting Toxicity | Reference |
|---|---|---|---|---|
| Ingredient | C6H6 | Human | Mutagenic risk | [94] |
| C6H5OH | Human | Mutagenic risk | [94] | |
| BD | Human | Cancer risk | [94] | |
| VCM | Human | Cancer risk | [94] | |
| Adsorption | Au | Danio rerio | Embryo: ① Oxidative stress ② Inflammation | [16] |
| CBz | Mytilus galloprovincialis | Larva: Excessive oxidation of digestive glands | [95] | |
| Cu | Danio rerio | Inflammation | [91] | |
| PAHs | Danio rerio | Metabolic disorders | [18] | |
| PCBs | Human | Neurotoxicity | [92] | |
| Dyestuff | Pyrene | Mytilus galloprovincialis | ① Immune responses ② Lysosomal compartment dysfunction ③ Peroxisome dysfunction ④ Antioxidant system disruption ⑤ Neurotoxic effects | [96] |
| Flame retardants | PBDEs | Human | ① Thyroid homeostasis disruption ② Neurotoxicity ③ Reproductive changes ④ Cancer risk | [89] |
| Paint coat | TiO2 | Caenorhabditis elegan | Oxidative stress | [97] |
| Plasticizer | BPA | Danio rerio | Neurotoxicity | [98] |
| Rat | Estrogen disorder | [99] | ||
| Human | ① Enzyme abnormality and damage of the liver ② Pancreatic cell dysfunction ③ Thyroid hormone disorder ④ Promotion of obesity ⑤ Cardiovascular disease ⑥ Low insulin levels | [99] | ||
| DEHP, MEHP | Rat | Inhibition of estrogen levels | [90] | |
| PAEs | Human | ① Increased risk of cardiovascular disease ② Reproductive system disruption | [90] |
| Methods | Tools | Medium | Plastic Components | Optimal Size | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Spectral analysis | FT-IR | Water, oil, air | RY, PE, PET, PAA | >20 μm | ① It does not destroy the sample. ② Pretreatment is simple. ③ The type of plastic particles can be determined. | It is difficult to identify the types of plastic particles that are aged or have contaminated surfaces. | [100,101,102,103] |
| RM | Water, air | PA, PC, PE, PP, PS, PET, PVC, PMP, PCL, PMMA | 0.5–20 μm | ① It does not destroy the sample. ② It supports nano-sample imaging. ③ It supports low sample amount identification. ④ It is environmentally friendly. | ① The measurement time is long. ② Fluorescence interference is easy to produce. ③ The signal-to-noise ratio is low. ④ The use of laser as the light source leads to background emission and sample degradation. | [104,105,106,107,108] | |
| Thermal analysis | TGA-DSC | NA | PE, PP, etc. | NA | ① The operation is simple. ② Less sample is required (1–20 mg). ③ Accuracy is high. | ① It is difficult to distinguish the polymers with similar transition temperatures. ② It is difficult to identify copolymers. ③ The samples are destroyed. ④ It cannot identify the morphology, size, and quantity of the plastic particles. | [108,109,110,111,112] |
| Py-GC-MS | NA | PA, PC, PE, PS, PP, rubber, PET, PVC, PMMA | NA | ① Less sample is required (5–200 μg). ② The microplastic type and weight and additives can be identified simultaneously without pretreatment. ③ The accuracy is high. ④ It recognizes copolymers. | ① The samples are destroyed. ② It cannot identify the morphology, size, and quantity of the plastic particles. | [110,111,112,113,114,115] | |
| TED-GC-MS | NA | PA, PE, PP, PS, PET | NA | It involves simple pretreatment and operation. | [108,110,112,116] | ||
| Other analytical methods | SEM-EDS | Majority | Majority | ≥1 nm | ① Imaging is at the nanoscale. ② Elements can be identified | ① It is expensive. ② Work efficiency is low. | [108,110,112,117,118] |
| MS | Majority | Majority | ≥1 nm | It can identify the structure, molecular weight, degree of polymerization, functional group, and end group structure of the plastic particles. | Different samples require different ionizing reagents (poor applicability). | [108,119,120] | |
| XPS | Majority | Majority | >10 nm | It can identify elemental composition and content, chemical state, molecular structure, and chemical bonds. | It cannot identify the nanoplastic types definitely. | [121,122] | |
| RMR | Water, oil | Majority | >50 μm | ① The cost is low. ② It is convenient for real-time field detection. | ① It is only used to detect the concentration. ② It requires specific calibration samples. | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; He, Y.; Yan, Y.; Junaid, M.; Wang, J. Characteristics, Toxic Effects, and Analytical Methods of Microplastics in the Atmosphere. Nanomaterials 2021, 11, 2747. https://doi.org/10.3390/nano11102747
Yang H, He Y, Yan Y, Junaid M, Wang J. Characteristics, Toxic Effects, and Analytical Methods of Microplastics in the Atmosphere. Nanomaterials. 2021; 11(10):2747. https://doi.org/10.3390/nano11102747
Chicago/Turabian StyleYang, Huirong, Yinglin He, Yumeng Yan, Muhammad Junaid, and Jun Wang. 2021. "Characteristics, Toxic Effects, and Analytical Methods of Microplastics in the Atmosphere" Nanomaterials 11, no. 10: 2747. https://doi.org/10.3390/nano11102747
APA StyleYang, H., He, Y., Yan, Y., Junaid, M., & Wang, J. (2021). Characteristics, Toxic Effects, and Analytical Methods of Microplastics in the Atmosphere. Nanomaterials, 11(10), 2747. https://doi.org/10.3390/nano11102747






