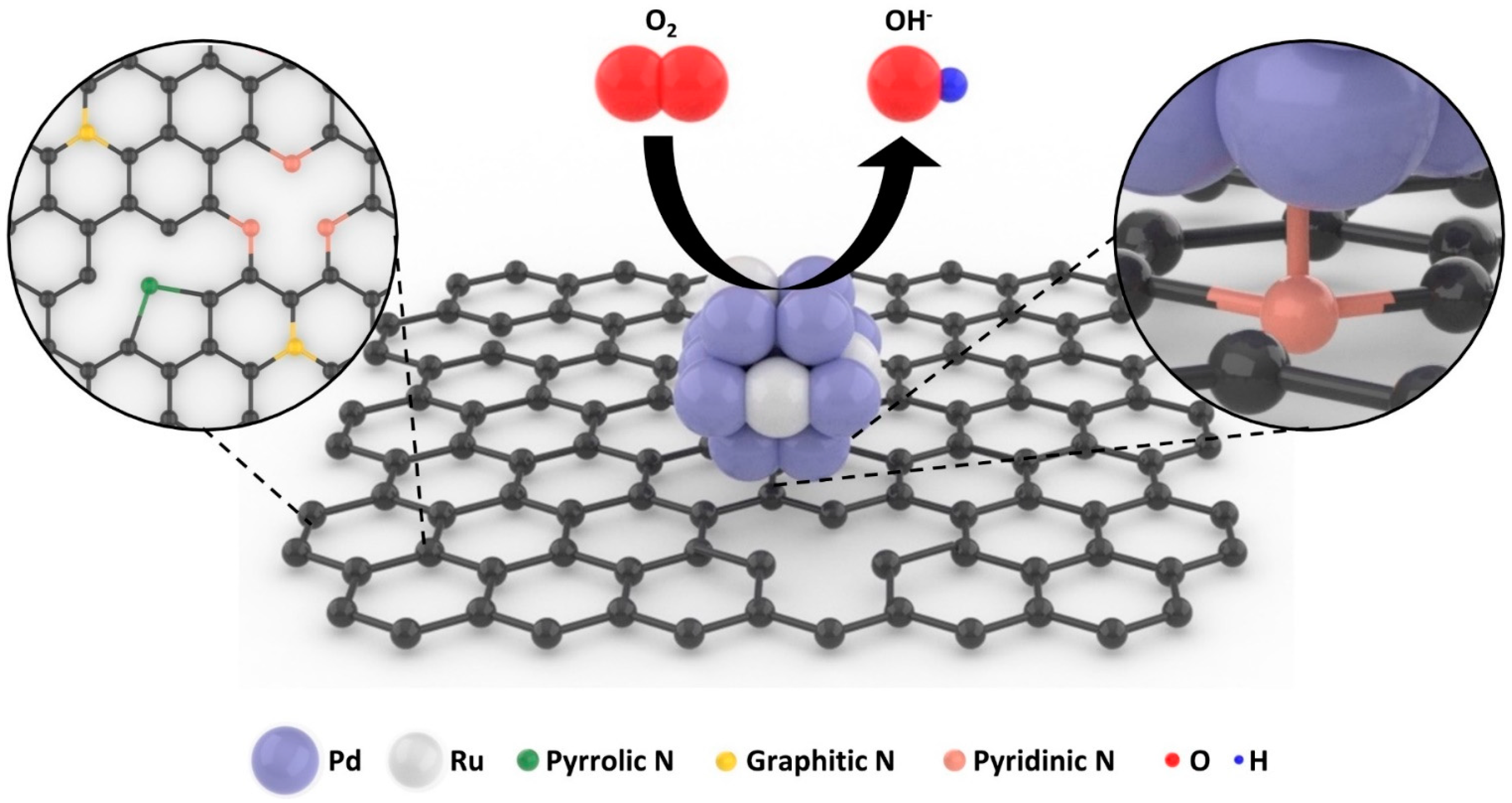
Nitrogen-Doped Reduced Graphene Oxide Supported Pd4.7Ru Nanoparticles Electrocatalyst for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NrGO and rGO
2.2. Synthesis of Pd–Ru NPs on NrGO and rGO
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhowmik, T.; Kundu, M.K.; Barman, S. Highly active and durable Pd nanoparticles-porous graphitic carbon nitride composite for electrocatalytic oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 14768–14777. [Google Scholar] [CrossRef]
- Tian, J.; Wu, W.; Tang, Z.; Wu, Y.; Burns, R.; Tichnell, B.; Liu, Z.; Chen, S. Oxygen reduction reaction and hydrogen evolution reaction catalyzed by Pd–Ru nanoparticles encapsulated in porous carbon nanosheets. Catalysts 2018, 8, 329. [Google Scholar] [CrossRef] [Green Version]
- Hubkowska, K.; Łukaszewski, M.; Czerwiński, A. Pd–Ru electrodeposits with high hydrogen absorption capacity. Electrochem. Commun. 2012, 20, 175–177. [Google Scholar] [CrossRef]
- Xiao, M.; Gao, L.; Wang, Y.; Wang, X.; Zhu, J.; Jin, Z.; Liu, C.; Chen, H.; Li, G.; Ge, J. Engineering energy level of metal center: Ru single-atom site for efficient and durable oxygen reduction catalysis. J. Am. Chem. Soc. 2019, 141, 19800–19806. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Xue, C.; An, H.; Yang, G. Facile construction of MoS2/CdS eutectic clusters anchored on rGO edge with enhanced hydrogen generation performance. Catal. Today 2018, 317, 99–107. [Google Scholar] [CrossRef]
- Cui, X.L.; Long, Y.; Zhou, X.; Yu, G.Q.; Yang, J.; Yuan, M.; Ma, J.T.; Dong, Z.P. Pd-doped Ni nanoparticle-modified N-doped carbon nanocatalyst with high Pd atom utilization for the transfer hydrogenation of nitroarenes. Green Chem. 2018, 20, 1121–1130. [Google Scholar] [CrossRef]
- Warczinski, L.; Hu, B.; Eckhard, T.; Peng, B.X.; Muhler, M.; Hattig, C. Anchoring of palladium nanoparticles on N-doped mesoporous carbon. Phys. Chem. Chem. Phys. 2020, 22, 21317–21325. [Google Scholar] [CrossRef]
- Li, D.; Duan, X.; Sun, H.; Kang, J.; Zhang, H.; Tade, M.O.; Wang, S. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: The effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 2017, 115, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. Acs Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.H.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- He, F.; Li, K.; Yin, C.; Wang, Y.; Tang, H.; Wu, Z.J. Single Pd atoms supported by graphitic carbon nitride, a potential oxygen reduction reaction catalyst from theoretical perspective. Carbon 2017, 114, 619–627. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, Y.; Zou, X.; Hu, B.; Qiang, Y.; Yu, D.; Yin, W.; Chen, C. Hydrothermal synthesis of a new kind of N-doped graphene gel-like hybrid as an enhanced ORR electrocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 10842–10850. [Google Scholar] [CrossRef]
- Skorupska, M.; Ilnicka, A.; Lukaszewicz, J.P. N-doped graphene foam obtained by microwave-assisted exfoliation of graphite. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.I.; Jo, S.G.; Hong, N.E.; Ye, B.; Lee, S.; Dow, H.S.; Lee, D.H.; Lee, J.W. Enhanced activity and durability of Pt nanoparticles supported on reduced graphene oxide for oxygen reduction catalysts of proton exchange membrane fuel cells. Catal. Today 2020, 352, 10–17. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeon, H.J.; Shin, H.-J.; Kang, J.K. Superparamagnetic Fe3O4 nanoparticles–carbon nitride nanotube hybrids for highly efficient peroxidase mimetic catalysts. Chem. Commun. 2012, 48, 422–424. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeong, H.M.; Lee, G.H.; Jung, Y.W.; Jo, S.G.; Kang, J.K. Agglomeration-Free Fe3O4 anchored via nitrogen mediation of carbon nanotubes for high-performance arsenic adsorption. J. Environ. Chem. Eng. 2021, 9, 104772. [Google Scholar] [CrossRef]
- Escudero-Escribano, M.; Malacrida, P.; Hansen, M.H.; Vej-Hansen, U.G.; Velázquez-Palenzuela, A.; Tripkovic, V.; Schiøtz, J.; Rossmeisl, J.; Stephens, I.E.; Chorkendorff, I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Podjaski, F.; Weber, D.; Zhang, S.; Diehl, L.; Eger, R.; Duppel, V.; Alarcón-Lladó, E.; Richter, G.; Haase, F.; Fontcuberta i Morral, A.; et al. Rational strain engineering in delafossite oxides for highly efficient hydrogen evolution catalysis in acidic media. Nat. Catal. 2020, 3, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.H.; Mao, S.J.; Li, M.M.; Wei, Z.Z.; Xu, F.; Li, H.R.; Wang, Y. RuPd Alloy Nanoparticles Supported on N-Doped Carbon as an Efficient and Stable Catalyst for Benzoic Acid Hydrogenation. Acs Catal. 2015, 5, 3100–3107. [Google Scholar] [CrossRef]
- Ion-Ebrașu, D.; Andrei, R.D.; Enache, S.; Căprărescu, S.; Negrilă, C.C.; Jianu, C.; Enache, A.; Boerașu, I.; Carcadea, E.; Varlam, M. Nitrogen Functionalization of CVD Grown Three-Dimensional Graphene Foam for Hydrogen Evolution Reactions in Alkaline Media. Materials 2021, 14, 4952. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, J.; Li, N.; Liu, C.; Zhou, Y.; Yu, X.; Sun, L.; Song, Y.; Zhang, S.; Wang, Z. Nitrogen-doped graphene prepared by thermal annealing of fluorinated graphene oxide as supercapacitor electrode. J. Chem. Technol. Biotechnol. 2019, 94, 3530–3537. [Google Scholar] [CrossRef]
- Mu, X.W.; Yuan, B.H.; Feng, X.M.; Qiu, S.L.; Song, L.; Hu, Y. The effect of doped heteroatoms (nitrogen, boron, phosphorus) on inhibition thermal oxidation of reduced graphene oxide. RSC Adv. 2016, 6, 105021–105029. [Google Scholar] [CrossRef]
- Vinod, K.R.; Saravanan, P.; Kumar, T.R.S.; Radha, R.; Balasubramaniam, M.; Balakumar, S. Enhanced shielding effectiveness in nanohybrids of graphene derivatives with Fe3O4 and epsilon-Fe3N in the X-band microwave region. Nanoscale 2018, 10, 12018–12034. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, V.K.; Mohanty, T. Coexistence of interfacial stress and charge transfer in graphene oxide-based magnetic nanocomposites. J. Mater. Sci. 2017, 52, 7677–7687. [Google Scholar] [CrossRef]
- Ni, Z.H.; Wang, Y.Y.; Yu, T.; Shen, Z.X. Raman Spectroscopy and Imaging of Graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Y.; Gao, Q.M.; Zhang, H.; Tian, W.Q.; Tan, Y.L.; Qian, W.W.; Liu, Z.P. Low content Pt nanoparticles anchored on N-doped reduced graphene oxide with high and stable electrocatalytic activity for oxygen reduction reaction. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, R.K.; Bhattacharjee, H.; Mohan, S.V.; Verma, A. Nitrogen doped graphene supported alpha-MnO2 nanorods for efficient ORR in a microbial fuel cell. RSC Adv. 2016, 6, 110091–110101. [Google Scholar] [CrossRef]
- Ma, J.W.; Habrioux, A.; Luo, Y.; Ramos-Sanchez, G.; Calvillo, L.; Granozzi, G.; Balbuena, P.B.; Alonso-Vante, N. Electronic interaction between platinum nanoparticles and nitrogen-doped reduced graphene oxide: Effect on the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 11891–11904. [Google Scholar] [CrossRef]
- Lee, J.W.; Viswan, R.; Choi, Y.J.; Lee, Y.; Kim, S.Y.; Cho, J.; Jo, Y.; Kang, J.K. Facile Fabrication and Superparamagnetism of Silica-Shielded Magnetite Nanoparticles on Carbon Nitride Nanotubes. Adv. Funct. Mater. 2009, 19, 2213–2218. [Google Scholar] [CrossRef]
- Peng, L.; Shang, Y.; Gao, B.; Xu, X. Co3O4 anchored in N, S heteroatom co-doped porous carbons for degradation of organic contaminant: Role of pyridinic N-Co binding and high tolerance of chloride. Appl. Catal. B Environ. 2021, 282, 119484. [Google Scholar] [CrossRef]
- Lasch, K.; Jorissen, L.; Friedrich, K.A.; Garche, J. The function of ruthenium oxides in Pt-Ru catalysts for methanol electro-oxidation at low temperatures. J. Solid State Electrochem. 2003, 7, 619–625. [Google Scholar] [CrossRef]
- Xiong, H.F.; Lester, K.; Ressler, T.; Schlogl, R.; Allard, L.F.; Datye, A. Metastable Pd <-> PdO Structures During High Temperature Methane Oxidation. Catal. Lett. 2017, 147, 1095–1103. [Google Scholar] [CrossRef]
- Rahul, R.; Singh, R.K.; Bera, B.; Devivaraprasad, R.; Neergat, M. The role of surface oxygenated-species and adsorbed hydrogen in the oxygen reduction reaction (ORR) mechanism and product selectivity on Pd-based catalysts in acid media. Phys. Chem. Chem. Phys. 2015, 17, 15146–15155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejaz, A.; Jeon, S. The individual role of pyrrolic, pyridinic and graphitic nitrogen in the growth kinetics of Pd NPs on N-rGO followed by a comprehensive study on ORR. Int. J. Hydrogen Energy 2018, 43, 5690–5702. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Fundamentals and applications. Electrochem. Methods 2001, 2, 580–632. [Google Scholar]
- Chai, L.L.; Zhang, L.J.; Wang, X.; Xu, L.Q.; Han, C.; Li, T.T.; Hu, Y.; Qian, J.J.; Huang, S.M. Bottom-up synthesis of MOF-derived hollow N-doped carbon materials for enhanced ORR performance. Carbon 2019, 146, 248–256. [Google Scholar] [CrossRef]
- Kang, B.K.; Im, S.Y.; Lee, J.; Kwag, S.H.; Kwon, S.B.; Tiruneh, S.; Kim, M.J.; Kim, J.H.; Yang, W.S.; Lim, B.; et al. In-situ formation of MOF derived mesoporous Co3N/amorphous N-doped carbon nanocubes as an efficient electrocatalytic oxygen evolution reaction. Nano Res. 2019, 12, 1605–1611. [Google Scholar] [CrossRef]
- Bai, L.; Duan, Z.Y.; Wen, X.D.; Si, R.; Zhang, Q.Q.; Guan, J.Q. Highly Dispersed Ruthenium-Based Multifunctional Electrocatalyst. ACS Catal. 2019, 9, 9897–9904. [Google Scholar] [CrossRef]

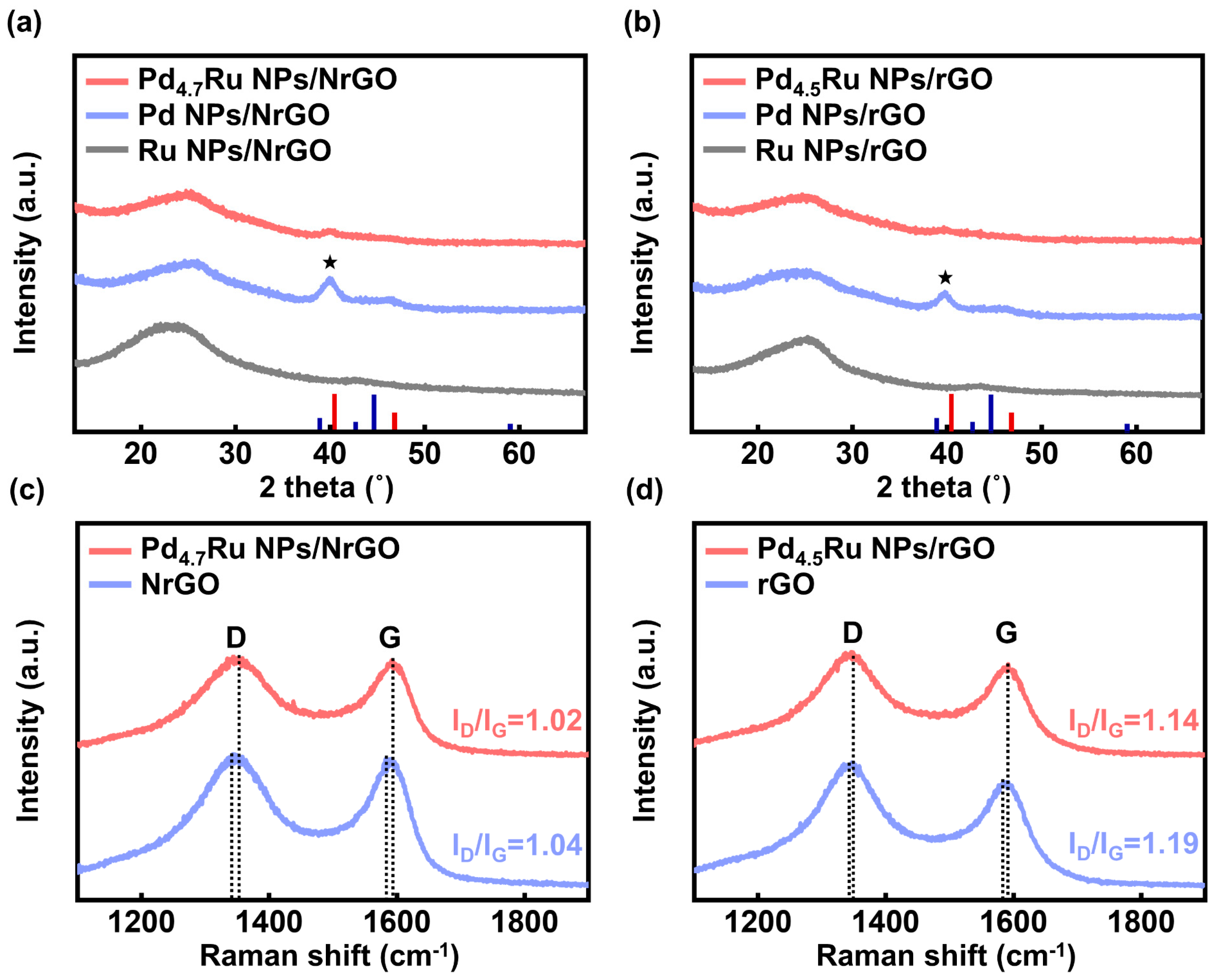
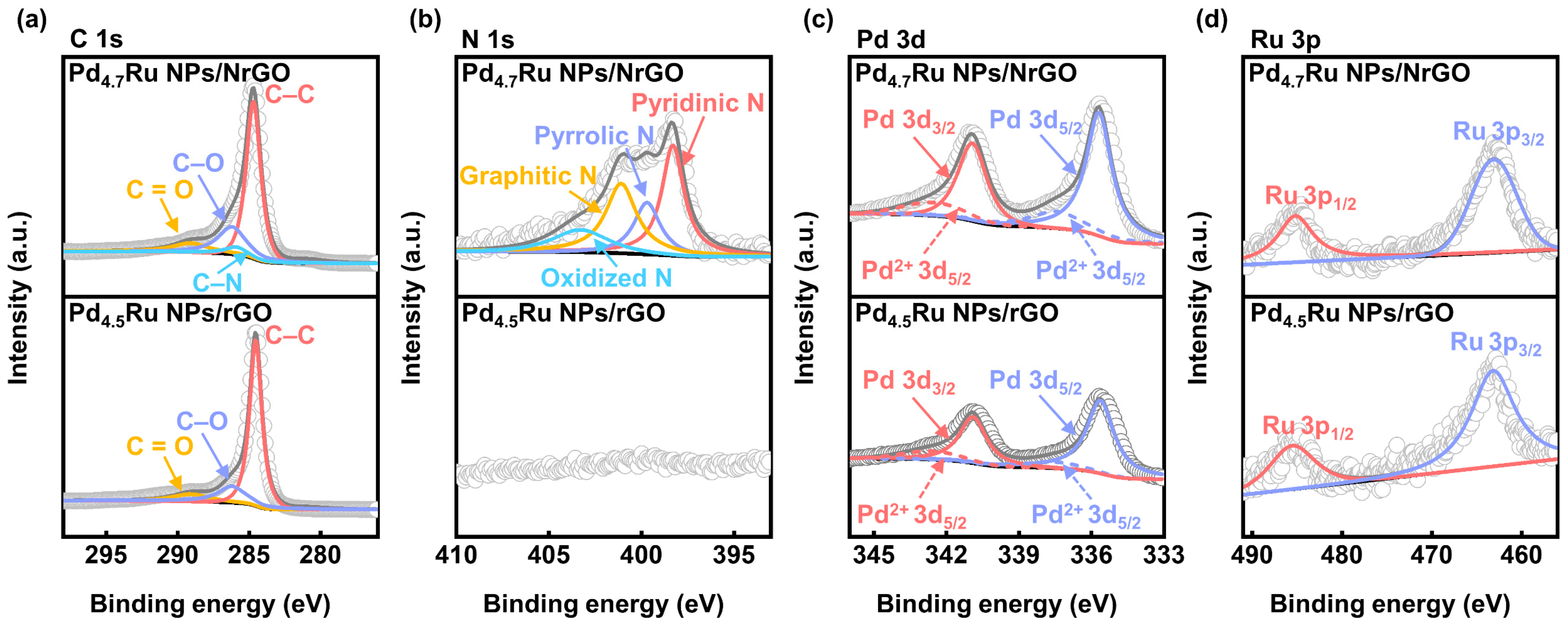
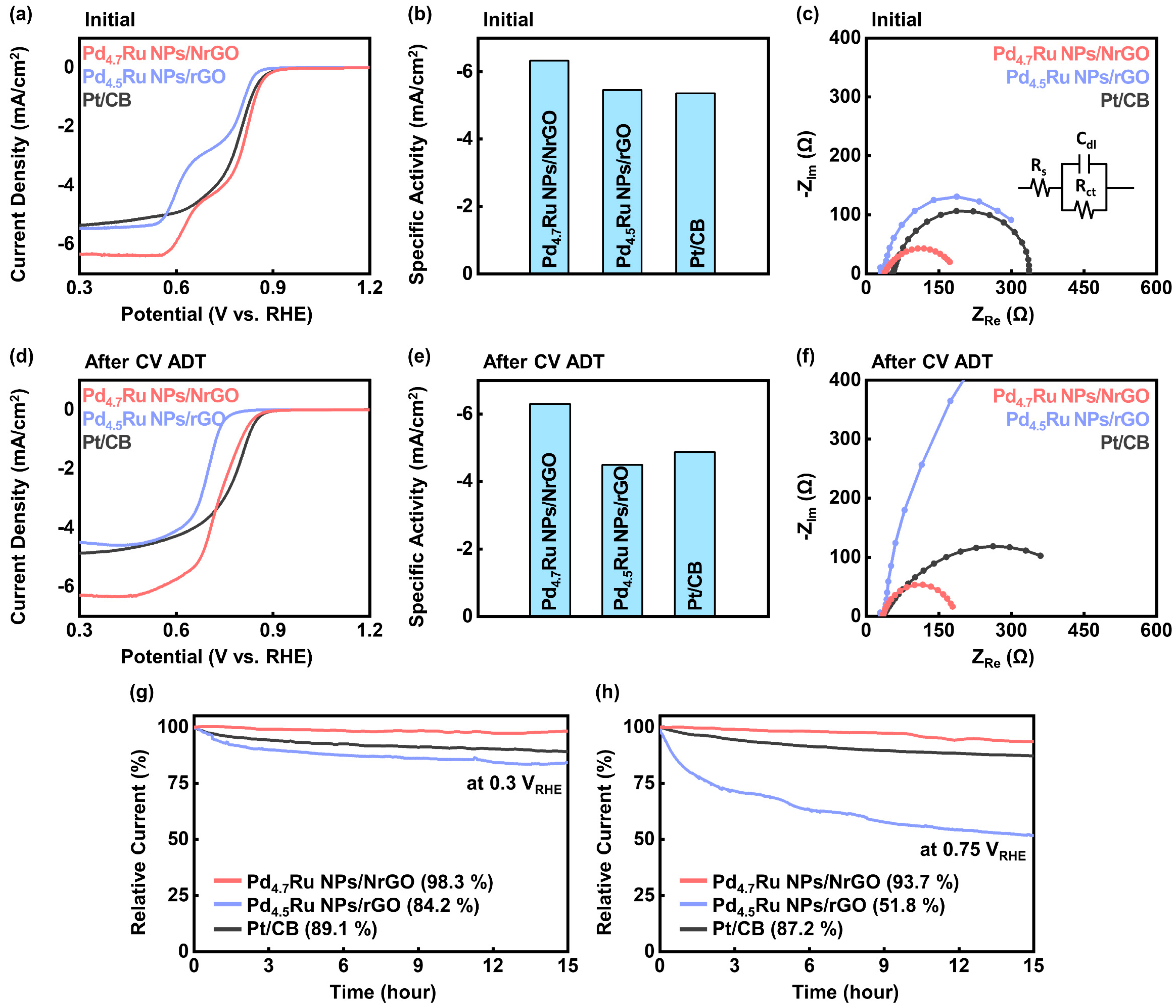
| Sample | Raman Shift (cm−1) | |
|---|---|---|
| D Band | G Band | |
| Pd4.7Ru NPs/NrGO | 1349.7 | 1591.6 |
| Pd4.5Ru NPs/rGO | 1346.2 | 1591.7 |
| NrGO | 1343.6 | 1588.3 |
| rGO | 1343.4 | 1588.7 |
| Sample | Onset Potential at −0.1 mA/cm2 (VRHE) | Half-Wave Potential (VRHE) | Current Density at 0.3 VRHE (mA/cm2) | Rct (Ω) | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | After ADT | Initial | After ADT | Initial | After ADT | Initial | After ADT | |
| Pd4.7Ru NPs/NrGO | 0.913 | 0.870 | 0.792 | 0.760 | −6.33 | −6.30 | 115.2 | 119.2 |
| Pd4.5Ru NPs/rGO | 0.863 | 0.787 | 0.713 | 0.694 | −5.46 | −4.49 | 327.7 | 2063.0 |
| Pt/CB | 0.908 | 0.877 | 0.785 | 0.775 | −5.36 | −4.87 | 283.8 | 428.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.-R.; Jo, S.G.; Varyambath, A.; Kim, J.; Lee, J.W. Nitrogen-Doped Reduced Graphene Oxide Supported Pd4.7Ru Nanoparticles Electrocatalyst for Oxygen Reduction Reaction. Nanomaterials 2021, 11, 2727. https://doi.org/10.3390/nano11102727
Park G-R, Jo SG, Varyambath A, Kim J, Lee JW. Nitrogen-Doped Reduced Graphene Oxide Supported Pd4.7Ru Nanoparticles Electrocatalyst for Oxygen Reduction Reaction. Nanomaterials. 2021; 11(10):2727. https://doi.org/10.3390/nano11102727
Chicago/Turabian StylePark, Gil-Ryeong, Seung Geun Jo, Anuraj Varyambath, Jeonghyun Kim, and Jung Woo Lee. 2021. "Nitrogen-Doped Reduced Graphene Oxide Supported Pd4.7Ru Nanoparticles Electrocatalyst for Oxygen Reduction Reaction" Nanomaterials 11, no. 10: 2727. https://doi.org/10.3390/nano11102727
APA StylePark, G.-R., Jo, S. G., Varyambath, A., Kim, J., & Lee, J. W. (2021). Nitrogen-Doped Reduced Graphene Oxide Supported Pd4.7Ru Nanoparticles Electrocatalyst for Oxygen Reduction Reaction. Nanomaterials, 11(10), 2727. https://doi.org/10.3390/nano11102727







