1. Introduction
Silver nanoparticles (AgNPs) are broadly used in the biomedical and industrial fields and are being incorporated into many daily life products, such as paints and textiles, due to their highly effective antimicrobial properties [
1,
2]. NPs’ physicochemical characteristics, such as small size and large surface area, provide numerous advantages in hundreds of nano-based products; however, negative effects also arise from these unique characteristics, demanding proper toxicological assessment of the possible negative outcomes linked to their release into the environment, in conjunction with an in-depth understanding of their biological behaviour and effects following cellular exposure and internalisation [
3,
4].
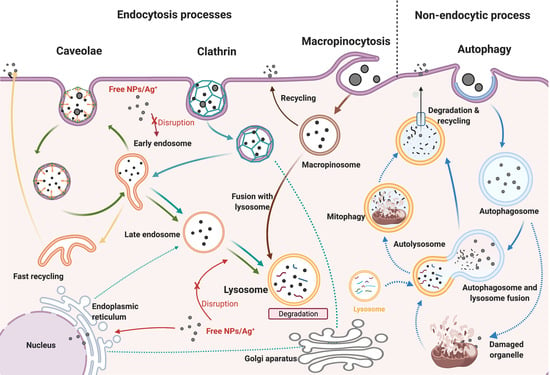
Cellular uptake is one of the most important processes regulating the biological activity of cells, involving the use a range of transport mechanisms to move macromolecules and particles from the surrounding medium across the cell membrane by a process called receptor-mediated endocytosis [
5,
6]. Endocytosis can be broadly divided into phagocytosis and pinocytosis. During phagocytosis, cells (mainly macrophages or dendritic cells) engulf particulate matter to digest it, using receptors on the cell surface for recognition and activation [
5,
7]. On the other hand, pinocytosis uses vesicles to internalise fluids and other molecules, involving different processes, depending on the molecular mechanism activated. Clathrin or caveolin-dependent endocytosis trigger the production of endocytic vesicles called early endosomes (EE), which accept the incoming cargo internalised from the cell membrane [
5]. Caveolae-dependent endocytosis internalises external matter in flask-shaped vesicles, through the binding between receptors on the cell membrane and the external matter [
6,
8]. Finally, macropinocytosis involves the formation of large vesicles, internalising nonspecific cargo and large amounts of fluid [
6,
9]. Autophagy, while not fully recognised as an endocytic pathway, plays an important role in cellular internalisation due to its physiological role in engulfing, transporting, and degrading internalised cargo [
10].
The cellular uptake of NPs involves highly regulated mechanisms that are interrelated with other complex biomolecular interactions [
11]. The use of pharmaceutical inhibitors to assess the endocytic pathways involved in the uptake of NPs has been widely accepted in the scientific community and applied in a variety of cellular models [
8,
12,
13,
14]. The effectiveness of pharmacological inhibitors is determined through assessment of the reduction of uptake of a chemical known to be internalised through that specific endocytic pathway, demonstrating the effectiveness of the inhibitor to block the desired pathway and the assay performance [
8]. The interactions between NPs and the environment imposes challenges for their characterisation as a result of physical and chemical transformations and the required determination of outcomes at the cellular and subcellular levels in relevant aquatic models and under relevant exposure conditions [
11,
15,
16].
Zebrafish have been used for many years as a regulatory test species and has been validated by the Organisation for Economic Cooperation and Development (OECD); however, their use requires an animal handling license and ethical approval. Due to these difficulties, the use of zebrafish embryos is considered a more viable option for assessing acute toxicity without requiring ethical approval (up to 5 days post fertilisation) [
17]. Despite the numerous benefits embryos present in the nanotoxicological field, some of their characteristics, such as the protective chorion, may compromise the assessment of NP-induced biological responses, as the chorion may represent a barrier between NPs and their internalisation. On the other hand, chorion removal involves complex processes with non-environmental relevance, resulting in inhibition of the hatching process, low survival rate, and sensitivity [
18,
19,
20].
The use of fish cells represents a feasible option for fast toxicological screening, including assessment of the mechanisms implicated in the internalisation, distribution, and (adverse) effects of NPs. As an aquatic model, it also has advantages in terms of avoiding the ethical challenges associated with sentient organisms, supporting the transition to in vitro testing. In fact, zebrafish cells, such as embryonic zebrafish cells (ZF4) and zebrafish liver (ZFL) cells, have gained popularity in past years as a potential early-stage model, mainly for the assessment of toxicological and biological responses, including translational gene expression [
21], bacterial invasion [
22], assessment of pharmacological chemicals [
23], and impacts from polystyrene [
24,
25] and metallic NPs [
26,
27,
28]. To date, only a few studies have explored the trafficking and uptake mechanisms of nanomaterials in zebrafish cells, highlighting a key knowledge gap that needs to be urgently explored [
24,
25,
26,
28].
In the present study, we investigated endocytic and non-endocytic (autophagy) mechanisms involved in the internalisation of cargo by ZF4 cell aiming to determine which pathways were utilised for the uptake of different sizes (10, 30, and 100 nm) and concentrations of AgNPs, through the use of pharmacological inhibitors. Our ultimate aim was to demonstrate the utility of the ZF4 adherent continuous cell line for assessment of nanotoxicity, in support of the replacement, reduction, and refinement of animals in experimentation through the 3Rs framework [
29,
30]. Furthermore, ZF4 cells can bridge the gap between chemical analysis and biological responses by assisting in the development of adverse outcome pathways (AOP), which focus on gaining information about the initial interaction of xenobiotics within a biological systems (the molecular initiating event), assessing the series of intermediate events that will eventually lead to an adverse outcome [
30].
2. Materials and Methods
2.1. Characterization of the AgNPs
Polyvinylpyrrolidone (PVP) coated silver nanoparticles (AgNPs) with sizes of 10, 30, and 100 nm were purchased from Nanocomposix, USA. The characterisation of AgNPs was performed in different testing media, such as the simple water matrix Ultrapure Water (UPW) and Complete Culture Media (CCM), prepared with the cell medium (DMEM/F12,11330, Gibco, Amarillo, TX, USA) supplemented with 10% Foetal Bovine Serum (FBS; 10270, Gibco, Amarillo, TX, USA) and 1% penicillin and streptomycin (15070, Gibco, Amarillo, TX, USA). Dynamic light scattering (DLS) (Zetasizer Nano series, Malvern Panalytical, Great Malvern, UK) was used to assess the hydrodynamic size, polydispersity index (PDI), and zeta potential. Ultraviolet visible light (UV–Vis) spectrophotometry was used to determine the optical spectra of the AgNPs (Absmax) and to provide a complementary measure of particle size.
Both techniques were applied in UPW and CCM, with AgNP dispersions prepared at a final concentration of 10 μg/mL and incubated at 28 °C for 0 and 24 h. For size and PDI measurements, the suspensions were placed in disposable polystyrene cuvettes (Sarstedt, Newton, NC, USA, 67.742) at a total volume of 1 mL; for zeta potential measurements, the sample was prepared in a total volume of 700 μL and placed in a folded capillary cell (Malvern, DTS1070); and finally, for UV–Vis measurements, 1 mL of the sample was placed in 3 mL disposable cuvettes (Sarstedt, Newton, NC, USA, 67742). All the samples were prepared fresh and immediately evaluated. Transmission electron microscopy (TEM) samples were prepared in UPW to assess the core size of the NPs. The TEM sample preparation is described in a previous publication [
27]. Briefly, samples with a concentration of 100 μg/mL were prepared fresh, then 15 μL of the AgNPs suspension was loaded onto a copper grid, and after 2 h the sample was gently washed with UPW and left to dry for 24 h.
2.2. Culturing of ZF4 Cells
Embryonic zebrafish (ZF4) cells were cultured as described on the manufacturer’s website (
https://www.atcc.org/products/all/CRL-2050.aspx, accessed on 15 October 2021) and in previous publications [
26,
27]. Briefly, cells were cultured in T75 flasks with a vented cap (Corning, Corning, NY, USA, 430641U) and in CCM. The CCM was prepared with DMEM/F12, supplemented with 10% FBS, 1% penicillin, and streptomycin, and incubated in a humidified atmosphere of 5% CO
2 at 28 °C. Cells were passaged upon reaching 80% confluence (Passage 28) by discarding the cell medium and gently washing with 5 mL of warm phosphate-buffered saline (PBS) (Thermofisher, Waltham, MA, USA, D5837). Then, cells were detached using 1.5 mL of 0.25% trypsin (Gibco, Amarillo, TX, USA, 15090) for 3 min at 28 °C. To maintain the cell line in T75 flasks, 2 mL of the diluted cell suspension was transferred into a new flask, diluted with 8 mL of CCM, and left to grow for one week with 5% CO
2 at 28 °C. For large experiments, 3 mL of the detached cells were transferred to T175 vented cap flasks (Corning, Corning, NY, USA, 431080), diluted with 17 mL of CCM, and left to grow for one week before seeding in well plates for experiments. To maintain the cell line in T175 flasks, cells were washed with 10 mL of PBS and detached with 3 mL of trypsin for 3 min, then 2 mL of the cells were re-seeded in T175 flasks, diluted with 18 mL of CCM, and left to grow for one week.
2.3. Imaging of Intracellular AgNPs by TEM
To confirm the presence of the particulate form (AgNPs) and to provide evidence of the endocytosis pathways activated in ZF4 cells exposed to AgNPs, TEM analysis of the highest AgNPs concentration (10 μg/mL) was performed for each AgNP size. The protocol described by Ellis et al. (2020) was modified and adapted to ZF4 cells [
31]. Briefly, ZF4 cells were seeded in six-well flat bottom plates (Corning, Corning, NY, USA, CLS3736) at a density of 5 × 10
5 cells in a total volume of 2 mL per well, 24 h prior to the study. After 24 h, cells were treated with 10 μg/mL of the 10, 30, and 100 nm AgNPs for 24 h at 28 °C. After the incubation period, the cell medium was removed, and cells were washed with 1 mL of warm PBS and then detached using 0.25% trypsin for 3 min at 28 °C in a total volume of 2 mL. The detached cells were centrifuged for 10 min at 270×
g at 20 °C (Eppendorf, Hamburg, Germany, 5430R). Then, the supernatant was carefully removed, and the cell pellet was diluted with 2.5% glutaraldehyde in 0.1 M PBS provided by the Centre for Electron Microscopy at the University of Birmingham (UK), followed by 10 min of centrifugation at 270×
g at 20 °C. Finally, cells were dehydrated in ethanol and embedded in epoxy resin before sectioning using an ultramicrotome to cut 0.1 μm sections with a diamond knife. Sections were collected onto 300 mesh copper (Cu) grids on carbon film (Agar Scientific, Stansted, UK, AGS160-3) and images were visualised using JEOL 1200EX 80 kV and JEOL 1400EX 80 kV microscopes (JEOL, Tokyo, Japan).
2.4. Visualisation of the Inhibition of Cellular Uptake Pathways by Confocal Microscopy
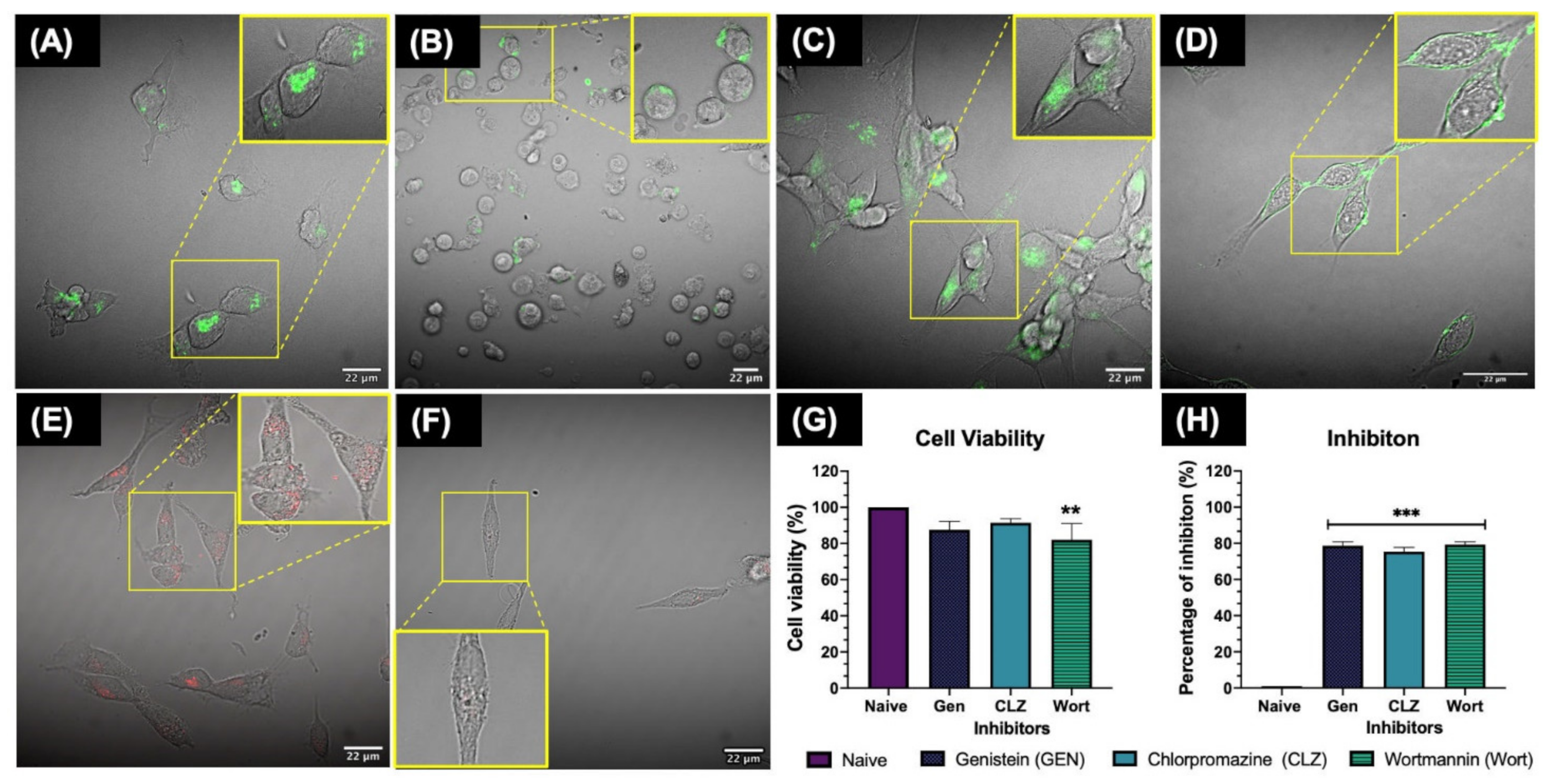
When using inhibitors for endocytosis pathways, it is essential to confirm that the desired uptake pathway was effectively inhibited by the respective chemical inhibitor in order to obtain reliable and representative results. To test the inhibition of the desired cellular uptake pathways, ZF4 cells were seeded in 24 well MatTek 13 mm glass-bottom dishes (MatTek, Ashland, MA, USA, P24G013F) at a density of 100,000 cells/mL in a total volume of 1 mL per well, with DMEM/F12 supplemented with 10% FBS, and 1% of penicillin and streptomycin at 28 °C and 5% CO
2. Twenty-four hours post-seeding, cells were pre-incubated with different concentrations of chemical inhibitors determined based on literature review [
6,
8,
14], selecting the following as the final concentrations which successfully inhibited the desired uptake pathway: 100 μg/mL of genistein (G6649, Sigma, St. Louis, MO, USA) for 20 min at 28 °C to inhibit the caveolae pathway, 10 μg/mL of chlorpromazine (C8138, Sigma, St. Louis, MO, USA) for 30 min at 28 °C to inhibit the clathrin- mediated pathway, and 10 μg/mL of wortmannin (W1628, Sigma, St. Louis, MO, USA) for 10 min at room temperature for the macropinocytosis pathway.
All the inhibitors were prepared at the desired concentration in CCM in a total volume of 500 μL per well. After the incubation period with the chemical inhibitors, the cell medium was removed and the cells were carefully washed with 500 μL of warm PBS, then the respective markers (known to selectively enter cells via a specific pathway) were added for 2 h at 28 °C (in a total volume of 500 μL) as positive controls to confirm that the inhibition was successful. The final concentrations for the transport molecules (controls) were as follows: 1 μg/mL of cholera toxin b (C1655, Sigma, St. Louis, MO, USA) for genistein treatments, 500 μg/mL of Transferrin Conjugate Alexa FluorTM 488 (T13342, ThermoFisher, Waltham, MA, USA) for chlorpromazine treatments, and 500 μg/mL of dextran-Rhodamine B (D1824, ThermoFisher, Waltham, MA, USA) for wortmannin treatments. After the incubation time, the cell medium was removed, and cells were washed twice with warm PBS. Then, cells were fixed for 20 min with 500 μL of 4% Paraformaldehyde (16% Thermofisher, Waltham, MA, USA, 43368) diluted in PBS and then examined by confocal microscopy using a NIKON A1R 808 series microscope (Nikon, Tokyo, Japan). Images were recorded with a 60× objective lens; the red filter was used for dextran-rhodamine B, which has an Excitation/Emission of 647⁄668 nm; the green filter was used for cholera toxin b and transferrin conjugate Alexa Fluor both with an Ext/Em of 495/519 nm. Images were processed using the FIJI open-source image processing tool (V.2.00-RC69, National Institutes of Health, Bethesda, Maryland, MD, USA).
2.5. Quantification of Ag+ Uptake by ZF4 Cells by ICP–MS
To assess the uptake of AgNPs in ZF4 cells by Inductively Coupled Plasma Mass Spectrometry (ICP–MS), different methodologies were tested to standardise the protocol to ensure the maximum detection of ionic silver [
32,
33,
34]. The final protocol was established as follows. First, ZF4 cells were seeded in six-well flat bottom plates (Corning, Corning, NY, USA, CLS3736) at a density of 5 × 10
5 cells per well in a total volume of 2 mL, using DMEM/F12 supplemented with 10% FBS and 1% of penicillin and streptomycin at 28 °C and 5% CO
2. At 24 h post-seeding, ZF4 cells were treated to a low, medium, and high concentration (2.5, 5, and 10 μg/mL as determined in our previous studies using the lactate dehydrogenase (LDH) cytotoxicity assay [
23]) of the three representative AgNPs sizes (10, 30, and 100 nm); then, cells were incubated for 24 h at 5% CO
2 and 28 °C.
After the 24 h incubation period, the cell medium was removed, and 3 mL of aqua regia was added. The aqua regia was prepared with 37% hydrochloric acid (H/1200/PB17, Fisher Scientific, Hampton, NH, USA) and 70% nitric acid (A509-P500, TraceMetalTM, Fisher Scientific, Hampton, NH, USA) in a ratio of 3:1. Cells attached to the six well plates were carefully washed with 500 μL of warm PBS twice to ensure the removal of non-intracellular AgNPs. Cells were detached using 0.25% trypsin for 3 min at 28 °C. Then, 3 mL of aqua regia (prepared as previously described) were added, then cells were resuspended and transferred to a clean glass vial. The glass vials were properly closed and placed in an oven overnight at 70 °C. The next day, the acid digested samples were diluted with ultra-pure water to reach a 2% HNO3 concentration. Then, the diluted samples were filtered with 22 μm syringe filters (E4780-1226, StarLab, Brussels, Belgium) to ensure the full extraction of only ionic silver. Finally, the filtered sample was analysed by ICP–MS (NexION 300×, Perkin Elmer, Waltham, MA, USA). A calibration curve of silver plasma emission standard (Cat. No. 456892C) prepared in 2% HNO3 was used to setup the ICP–MS. Three individual replicates were included for each AgNP size and concentration.
2.6. Quantification of Ag+ by ICP–MS after Inhibition of the Cellular Uptake Pathways
Having confirmed the relevant concentrations of the pharmacological inhibitors by confocal microscopy (see above), ZF4 cells were seeded in six-well flat bottom plates (Corning, Corning, NY, USA, CLS3736) at a density of 5 × 10
5 cells per well in volume of 2 mL, using DMEM/F12 supplemented with 10% FBS and 1% of penicillin and streptomycin at 28 °C and 5% CO
2. After 24 hrs, cells were incubated with 100 μg/mL of genistein for 20 min at 28 °C, 20 μg/mL of chlorpromazine for 30 min at 28 °C, and 10 μg/mL of wortmannin for 10 min at room temperature. Inhibitors were prepared in CCM for a total volume of 2 mL per well. After incubation with the chemical inhibitors, the cell medium was discarded, and cells were treated with 2.5, 5 and 10 μg/mL of AgNPs for 10, 30, and 100 nm sizes for 2 h at 28 °C in a final volume of 2 mL per well. Finally, the cell culture medium was removed; then, cells attached to the six well plates were washed, acid digested, diluted, and analysed by ICP–MS, as described in
Section 2.5. Three individual replicates were analysed for each NP size, concentration, and chemical inhibitor used.
2.7. Detection of Early Endosome (EE) Formation by Fluorescence Measurements
To further understand the endocytosis process, the induction of EE was evaluated by labelling early endosomes with red fluorescent protein (RFP). Cells were transduced with cellLightTM Early Endosomes-RFP BacMam 2.0 (Cat. No. C10587; Thermofisher, Waltham, MA, USA)) in accordance with the manufacturer’s instructions. Briefly, ZF4 cells were seeded in 96-well flat bottom plates (Corning, Corning, NY, USA, Cat. No. 3917) at 8000 cells per well and in a final volume of 200 μL. Cells were left to attach overnight at 28 °C with 5% CO
2. The next day, AgNP treatments of 2.5, 5, and 10 μg/mL for different AgNP sizes (10, 30, and 100) were mixed with 2 μL of BacMam 2.0 reagent (Thermofisher, Waltham, MA, USA) in a final volume of 200 μL per well. Then, the cell medium was gently aspirated, and cells were treated with the AgNPs mixed with the cell light dye and incubated for 24 h at 28 °C with 5% CO
2. After the incubation time, the intensity of the red fluorescent protein of three individual replicates was measured by fluorescent microplate reader (Spark by Tecan, Männedorf, Switzerland) using an excitation and emission of 555/584 nm, respectively. Fluorescence intensity results were normalised to percentage (%) against untreated cells (naive), indicated as
N%.
2.8. Autophagy Response
To evaluate the autophagy response involved in the uptake of AgNPs by ZF4, a modified protocol for autophagy evaluation by flow cytometry was adapted for confocal microscopy [
27]. Briefly, ZF4 cells were seeded in uncoated 24 well MatTek 13 mm glass- bottom dishes (MatTek, Ashland, MA, USA, Cat. No. P24G013F) at a density of 100,000 cells per well in a final volume of 1 mL with CCM, and incubated overnight at 28 °C and 5% CO
2. 24h post-seeding, cells were treated with 2.5, 5, and 10 μg/mL of 10, 30, and 100 nm AgNPs for 24 h. After the incubation period, the cell medium was removed, and 500 μL of CCM mixed with nucleus and lysosome staining was added to the cells and incubated at 28 °C for 30 min. The organelle labelling solution was prepared in warm CCM containing 1 μL/mL of Hoechst 33342 (Thermofisher, Waltham, MA, USA, Cat. No. 62249) to stain the nuclei and 1 μL/mL of LysoTracker
TM Deep Red (Thermofisher, Waltham, MA, USA, Cat. No. L12492). After the incubation period, the medium containing the first dyes was removed, and cells were washed twice with warm PBS. Then, cells were stained using a Cell Meter
TM Autophagy Assay Kit (Cat. No. 23002; Sunnyvale, CA, USA). First, a stock solution was prepared with 20 μL of the Autophagy Green
TM diluted in 10 mL of Stain Buffer. Then, 500 μL of the staining solution was added to cells (per well) and incubated at room temperature for 30 min in the dark. Then, the autophagy staining medium was removed, and cells were washed with warm PBS once. Immediately a fixation was performed with 500 μL of 4% Paraformaldehyde (16% Thermofisher, Waltham, MA, USA, Cat. No. 43368) diluted in PBS. Finally, cells were examined by confocal microscopy using a NIKON A1R 808 series microscope (Nikon, Tokyo, Japan). Images were recorded with a 60× objective lens for all channels. For nucleus identification, the blue laser was used, with an excitation and emission of 350⁄461 nm; the red filter was used for the lysosomes (647⁄668 nm); and for autophagy response, the green filter (495/519 nm) was used. The intensity of three individual cells per replicate (n = 3) were analysed by the FIJI open-source image processing tool (V.2.00-RC69, National Institutes of Health, Bethesda, Maryland, MD, USA) as described in Quevedo et al., 2021.
2.9. Protein Corona Isolation and Analysis of Proteins by Polyacrylamide Gel Electrophoresis (PAGE)
To further understand the implications of the NP–protein interactions, the protein coronas acquired by the incubation of the AgNPs in CCM alone and CCM conditioned with cells attached to the flask were isolated and analysed by mass spectrometry, following the protocol described by Monopoli et al., (2013) [
35]. The full methodology for the incubation and extraction approaches, and the PAGE analysis, are described in
Section S7 in the
Supplementary Materials. Briefly, after exposure to the NPs, the cell medium containing the coronas was removed, then a series of centrifugation and washing steps were performed to remove unbound proteins by centrifugation in PBS at 20,073×
g (Eppendorf, Hamburg, Germany, 5430R) for 20 min. Then, the sample pellet was re-suspended and incubated at 95 °C for 5 min and centrifugated again at 20,073×
g for 30 min. The pellet was diluted with sodium dodecyl sulphate (SDS) and stored at −20 °C for further analysis. The isolated proteins were run on a 12.5% polyacrylamide gel electrophoresis (PAGE) and stained with 25% Coomassie blue. Bands confirming the presence of proteins were sent to the Advanced Mass Spectrometry Facility in the School of Biosciences at the University of Birmingham for analysis. The proteins secreted by the ZF4 cells during the exposure to AgNPs were also evaluated by quantification of the total protein concentrations in the media using a BCA protein assay kit (ThermoFisher, Waltham, MA, USA, 23225).
2.10. Statistical Analysis
The results were normalised against the control for each experiment and then plotted and statistically analysed using GraphPad V8.1 software (GraphPad Software, San Diego, CA, USA) by one or two-way ANOVA, followed by a Bonferroni post-hoc multiple comparison for all the AgNPs treatments against the untreated control (naïve), unless otherwise stated in the figure legend.
4. Discussion
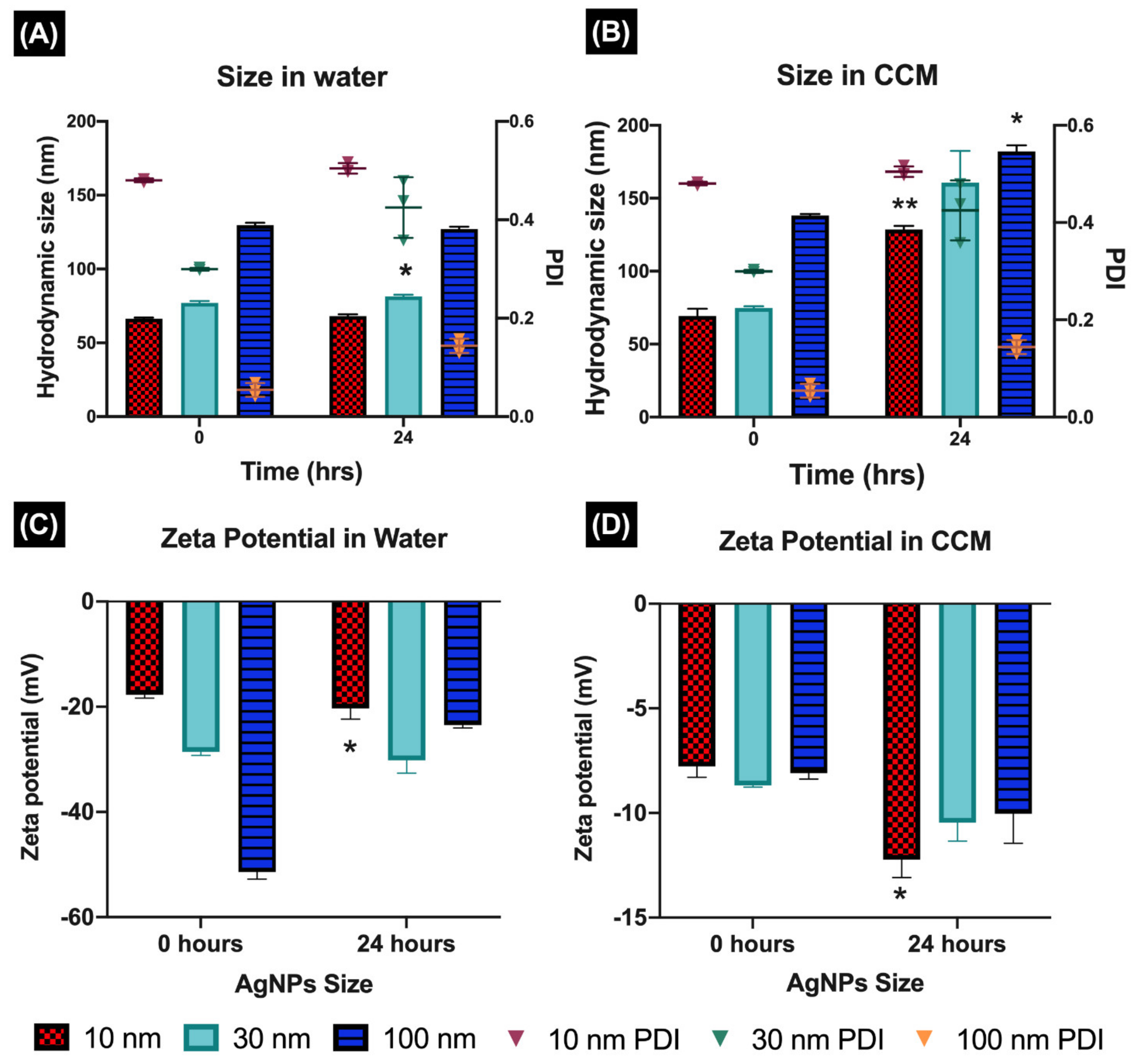
The physicochemical properties of NPs can strongly influence their toxicity in biological systems. However, these characteristics may substantially change upon introduction of the NPs into a complex biological environment, leading to different outcomes [
11,
15,
16]. In this study, we demonstrated that the characterisation in ultra-pure water (UPW) and complete culture media (CCM) displayed different results. The addition of proteins strongly affected the NPs’ characteristics, creating a new biological identity (protein corona), that was reflected in the hydrodynamic size and polydispersity index (PDI), in CCM that was more than double those in water, influencing the NPs’ interactions with the biological system [
11,
38,
39]. Similarly, the protein components in the biological media also affected the surface charge, as seen in the zeta potential results in CCM, which became lower than in water, around −9 mV (for all NPs), as consequence of the serum protein adsorption onto the NPs [
8,
40]. The absorption spectra measured by UV–Vis were also strongly influenced by the biological medium; in the complex media, the samples exhibited significant scattering increase (compared to in UPW) and a second peak, revealing the presence of proteins in the sample. These results can be related to the fact that a considerable fraction of transmitted light does not reach the UV–Vis detector due to the complex nature of the cell medium, recording a higher absorption for these samples [
41].
The intracellular uptake and fate of the NPs can also be influenced by their physicochemical properties, cell type, and constituents in the complex medium, such as proteins, that can modulate the cellular uptake, for example, by enabling the NPs to engage cellular receptors following formation of the protein corona [
42,
43,
44]. In the present study, we found a strong dependence of the AgNP protein corona composition on the time of exposure and the size of the NPs. Isolation of the protein corona by SDS-PAGE illustrated a clear difference in the protein corona identity and evolution over time for the two biological incubation periods. After 2 h, the gel bands showed a single line between 72 and 57 kDa (
Supplementary Materials Figure S3), which may be related to common proteins found in FBS, such as serum albumin (66 kDa), as well as complement proteins such as immunoglobulin, and apolipoproteins [
45,
46]. Similarly, other serum proteins such as α
1-antitrypsin (52 kDa) and keratin type ii cytoskeletal (57–70 kDa) have also been found as part of the protein corona of PVP-AgNPs in CCM (10% FBS) in a different study [
45]. The SDS-PAGE gel also revealed that after 24 h (
Supplementary Materials Figure S3), the smaller AgNPs (10 nm) presented a number of highly visible protein lines along the length of the band for the medium and high AgNP concentrations, showing a clear separation of the protein sizes, ranging between 250 and 258 kDa. This can be related to other serum- related proteins such as Apolipoprotein A-I (28 kDa) and Apolipoprotein A-II (17.4 kDa) [
45], as well as specific ZF4 cellular proteins secreted by the cells in response to damage induced by the AgNPs, including p65 transcription factor (51.1 kDa), retinoic acid receptor RXR-gamma-A, and max-interacting protein 1 (27.6 kDa), proteins that can be found in the nucleus and are related to DNA regulation and binding transcription activity; and the swelling-dependent chloride channel protein (27.4 kDa), which is located in the cytoplasm, nucleus, and plasma membrane of ZF4 cells; and ataxin-3 Fragment (34.5 kDa), involved in protein de-ubiquitination and located in the nucleus [
47]. A full list of the identified proteins can be found in
Supplementary Materials Table S7 in the Supplementary Materials.
The differences between the NPs sizes and their protein composition can perhaps be related to the curvature of the NPs, and to the fact that the 10 nm solution contains a much larger number of particles (NPs/mL) and a much larger surface area for protein adsorption compared to the larger AgNPs at constant mass [
27]. In this regard, small NPs have a higher total Surface Area (SA), which may enable more protein binding compared to the larger sizes, as demonstrated in a previous publication [
27]. Calculations were performed to obtain the total SA for the NPs based on their hydrodynamic size, demonstrating that the smaller NPs have a higher SA (6.22 × 10
−5 m
2/g), compared to the 30 nm (5.78 × 10
−5 m
2/g) and the 100 nm size (3.67 × 10
−5 m
2/g), which was 1.69× smaller than the 10 nm AgNPs (results for highest AgNP concentration). This confirms that that smaller NPs may have a higher likelihood of binding proteins due to their large SA, as suggested by other authors [
3,
45,
48]. The SA results for other concentrations can be found in Quevedo et al., 2021 [
27].
Another aspect to consider is the type of proteins in the complex environment; for example, albumin and fibrinogen are proteins that will bind and dominate the particle surface due to higher abundance, especially at short exposure times. These proteins may eventually be displaced by proteins with higher affinity and slower kinetics, increasing the protein diversity of the protein corona [
44]. For example, certain proteins secreted by ZF4 cells could have a higher affinity for the AgNPs compared to the serum proteins, potentially increasing the competition of proteins and displacement of weakly bound proteins; however, further studies are necessary to fully confirm the role of the secreted proteins in driving AgNP uptake by ZF4 cells [
39,
44].
On the other hand, the uptake of AgNPs may also be strongly related to the size of the NPs and the time of exposure, as suggested by other authors [
49,
50]. Our results for the total Ag in cells demonstrated that after 2 h, the 10 nm AgNPs displayed an overall average uptake of 10% (calculated as the average of the three concentrations) of the available dose, whereas the larger AgNP sizes (30 and 100 nm) displayed an uptake efficiency per cell between 0.3% and 3% of the exposure dose for all concentrations. After 24 h, the uptake for all the sizes greatly increased, with the 10 nm AgNPs showing the highest percentages of uptake, displaying almost 90% uptake for the highest concentration (10 µg/mL), followed by the 100 nm size, with an overall average of 40%, and lastly the 30 nm, with an average uptake of 19%. Interestingly, the larger AgNP size (100 nm) displayed higher percentages of uptake compared to the medium size (30 nm). It has been suggested that there might be an optimal particle size for active uptake; for example, in human cell lines exposed to different AgNPs sizes, the 50 and 100 nm had higher uptake efficiencies compared to the smaller size (20 nm) [
49,
50]. Similarly, Qiang et al., 2020 suggested the smaller sizes up to 20 nm are more likely to be internalised than larger sizes, providing evidence for enhanced uptake and toxicity for smaller sizes (4 nm) in zebrafish embryos [
51]. Another study demonstrated that the uptake efficiency of 5 and 100 nm AgNPs after 24 h was higher (58 and 63% respectively) compared to other sizes (20 nm and 50 nm) [
49], supporting our findings. It is important to mention that the detected total Ag
+ intracellular concentrations can also be linked to the dissolution of the NPs; for example, in a previous study, the 10 and 100 nm presented higher dissolution and uptake values compared to the 30 nm AgNPs in CCM [
27]. This suggests that the high total Ag
+ intracellular concentrations detected by the 10 nm can be linked to a major abundance of dissolved ions in the media due to the high rates of dissolution, whereas the 100 nm results can be a combination of dissolution and sedimentation, which may increase their contact with the cells, and therefore their uptake amount [
27]. Thus, a mixture of NPs and ionic uptake is likely to occur in ZF4 cells.
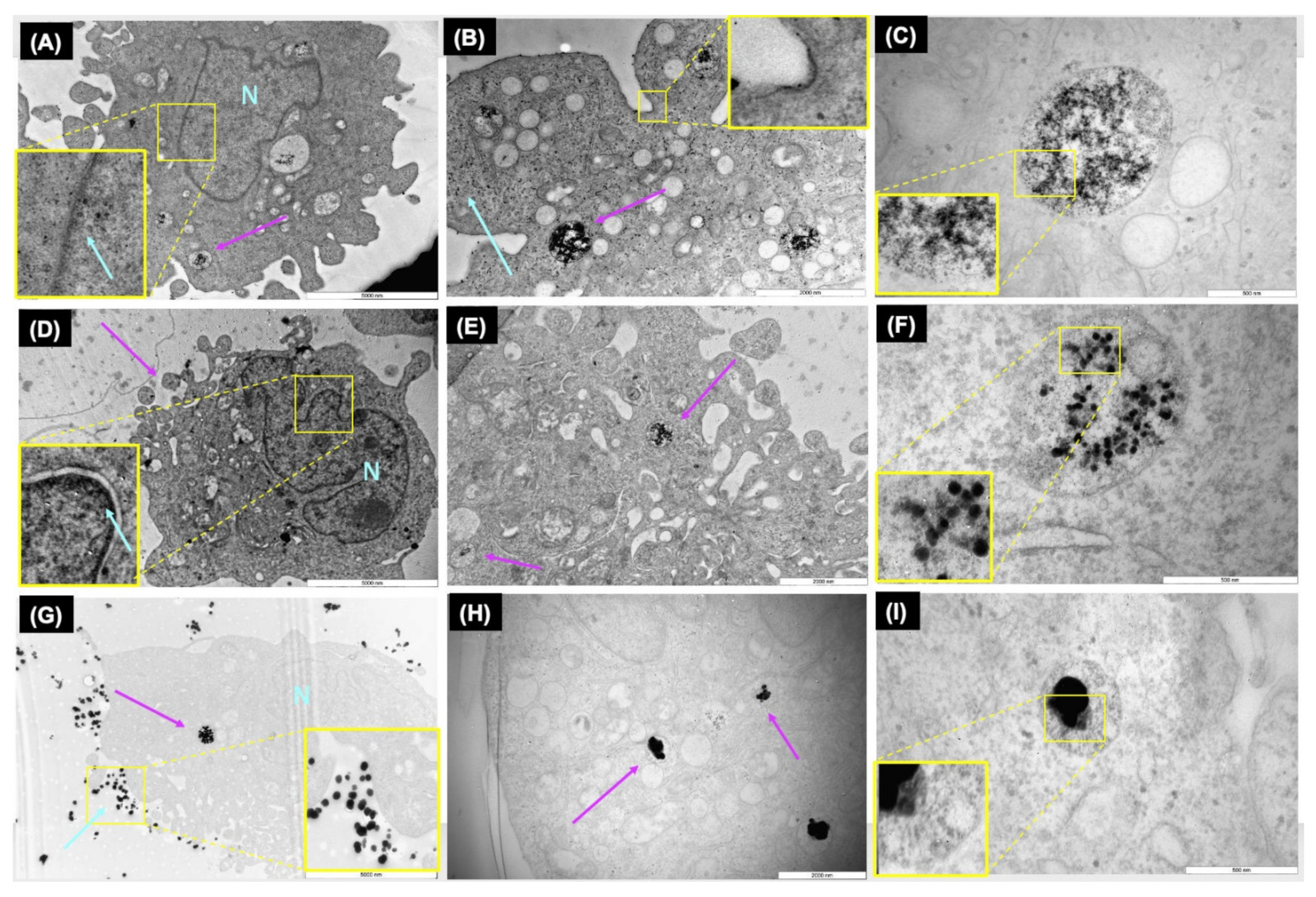
Analysis of the TEM images demonstrated the internalisation of AgNPs as well as the formation of vesicles as part of the endocytic process is activated by ZF4 cells, to deal with exposure to the NPs. Analysis of the internalised NP size indicated a reduction in their initial size, as well as visible loss of density or fragmentation once inside the vesicles for all the NPs, most evident for the 10 nm AgNPs (see
Figure 2C). The TEM images showed that the small and medium AgNPs (10 and 30 nm) were located close to the nucleus (see
Figure 2A,D), as magnification of the images revealed NPs in the nucleus area. This can be related to the easier cellular internalisation of the NPs due to their small size, as well as to the fact that NPs may be able to escape from the vesicles [
52,
53]. This process has been described as the enhanced Trojan horse effect, which refers to the cellular internalisation of metal NPs via an active processes, resulting in an enhanced release of toxic ions such as Ag
+ as a result of cation-induced lysosomal damage or dysfunction [
54].
Other studies agree with these findings; for example, Berry et al., (2007) demonstrated that small gold nanoparticles (5 nm) were visible in the nucleus of human fibroblasts, whereas larger particles (>30 nm) were observed mainly in the cytoplasm, suggesting that the fate of the internalised NPs can also be controlled by the dimensions of the organelle membranes, such as the nuclear pores in the nucleus [
55]. Interestingly, the TEM images for 100 nm showed a larger number of NPs surrounding the cell membrane, with no visible signs of agglomeration. A study by Greulich et al., (2011) demonstrated that large AgNPs (80 nm) were visible in the cytoplasm, and were able to be internalised in hMSC cells as nanoparticulate material and agglomerate once inside the perinuclear region, which is connected to the endo-lysosomal cell compartment [
56]. The NPs’ degradation and their reduction in diameter can also be related to their dissolution (as previously discussed), as well as the lysosomal degradation by hydrolases, which can digest proteins, nucleic acids, lipids, and extracellular agents, as described by other studies [
54,
57].
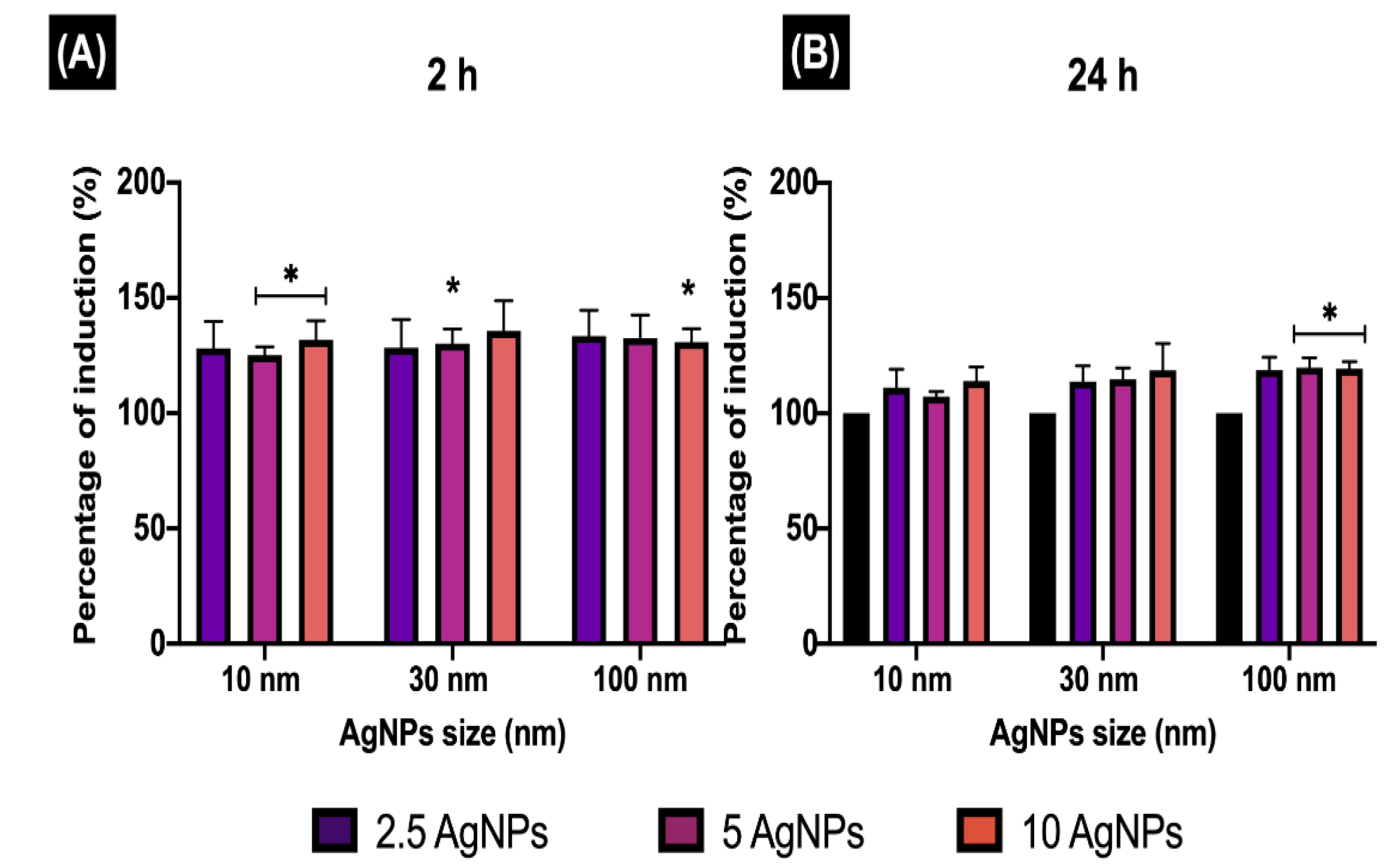
Once the NPs are engulfed by the cell membrane during any endocytic process, the formation of EE is rapidly activated to sort the internalised cargo to different intracellular destinations [
37]. The internalised cargo remains for only a few minutes in EE (between 5 and 10 min); then, the EE progressively acidify and mature to late endosomes [
37]. Interestingly, the results for the EE induction (EEI) revealed higher NP trafficking during the first 2 h for all NP concentrations and sizes, compared to longer exposure times (24 h). The internalisation and detection of AgNPs was confirmed in ZF4, demonstrating the ZF4 cells activate endocytosis processes to deal with the NP exposure. The results for the uptake pathways induced by the AgNPs demonstrated that different endocytotic pathways may take place simultaneously, involving complex NP-cell interactions in their uptake by ZF4 cells, as suggested by other authors [
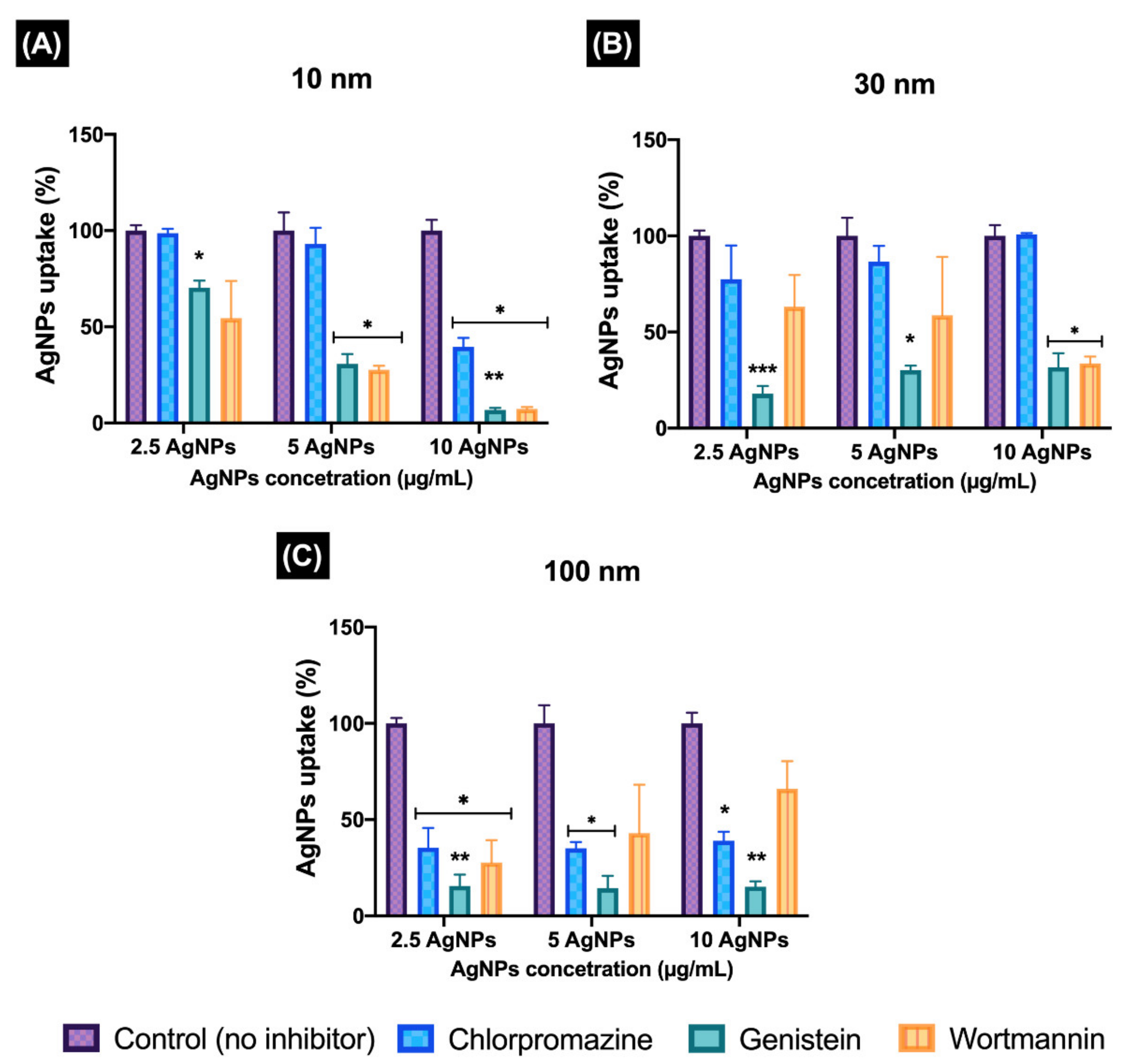
24,
58]. Size and mass concentration also proved to affect the uptake pathway during the assessment with pharmaceutical inhibitors. The results for the inhibition of the endocytosis pathways showed that treatment with chlorpromazine has no effect on the uptake of 10 and 30 nm AgNPs; therefore, the clathrin pathway was not activated for most of the concentrations, except at the 10 µg/mL for the 10 nm size. Here, the caveolae-mediated and macropinocytosis pathways were mainly responsible for the uptake of the 10 nm AgNPs, showing similar percentages of uptake by both pathways. These results agree with a study by Gunduz et al., (2017), who demonstrated that 12 nm AuNPs predominant entered HUVECs via macropinocytosis [
59]. Similarly, NPs could also be internalised due to their small size during the engulfment of large volumes of the extracellular medium, which allows the internalisation of fluid-phase nutrients such as proteins and ATP, and sampling of the environment for foreign agents [
9]. In addition, it has been suggested that cells may internalise secreted vesicles, called exosomes, through macropinocytosis, which can be related to the fact that small NPs are easily internalised (as seen in the uptake data); so perhaps cells are coping with the exposure by encapsulating the NPs inside vesicles, recycling these vesicles to the cell membrane, and then internalising these vesicles again via macropinocytosis [
9,
60].
On the other hand, the 30 nm AgNPs displayed inhibition of the caveolae pathway at all AgNPs concentrations tested; similar results were displayed by the 100 nm size for this pathway, which also showed inhibition of the other evaluated pathways. These results suggest that the 30 and 100 nm uptake are linked to the strong participation of lipid raft-associated receptors during their internalisation [
8]. These results are in agreement with other authors who demonstrated a clear inhibitory effect of genistein treatments on the uptake by human cells of large NPs such as 200 nm PS-COOH NPs and 500 nm latex microspheres, suggesting that caveolae flask-shaped invaginations can internalise larger particles compared to clathrin-coated pits, due to the restricted triskeletal structure of clathrin [
8,
61]. It is important to mention that the NP uptake was measured only at short exposure times (2 h), as it has been reported that the inhibition of one uptake pathway can result in the activation of other endocytic uptake pathways [
8,
14]. When selecting a specific inhibitor for the desired endocytosis pathway, it is important to take into account the effective inhibition as well as cytotoxicity of the pharmacological inhibitors, before their co-incubation with the NPs, as severe disruption (in terms of toxicity) may inactivate or activate other molecular processes as part of the cell’s defence mechanism, which may lead to different outcomes [
8].
Autophagy as endocytosis is involved in the formation of membrane vesicles for the degradation and recycling of intracellular and extracellular components; thus, it could be considered another (non-endocytotic) pathway by which cells could internalise NPs [
62]. It has been suggested that both pathways are interconnected at different stages during the formation, fusion, and trafficking of the vesicles, having a common endpoint at the lysosomes, where the internalised cargo is degraded, and finally eliminated by exocytosis [
62]. The results for the induction of autophagy showed higher induction (%) for the small NPs, whereas the medium and large NPs showed less autophagy levels (<1%). This suggests that autophagy could be directly related to the size of the NPs and the level of stress the cells are under [
10]. In addition, autophagy could potentially be activated during macropinocytosis and caveolae-mediated endocytosis, which were the pathways that showed the highest inhibition in this study. For example, a study by Zhang et al., (2017) has demonstrated that polymeric NPs (PLGA) were also internalised by the autophagy pathway, as NPs were observed in autophagosomes in human cells, suggesting a close link between autophagy and endocytosis [
63]. The increased autophagy response in our study could also be linked to the cytotoxicity of the AgNPs in ZF4 cells, which has been previously demonstrated [
27]. Autophagy is primarily described as a self-protective mechanism; thus, excessive levels of autophagy could be linked to undergoing stress caused by damaged organelles, which result in an alkalising effect on the endosomal system, reducing the functionality of the formed endosomes, causing induction of autophagy to remove the extracellular components and damaged organelles to cope with the NPs [
53].
The cellular internalisation methods assessed in this study provide further insights about the uptake and fate of NPs in cellular models. Besides, fish cell lines have been further described as potential in vitro models for chemical and water testing, including ZFL and ZF4 cell lines [
23,
64]. In this regard, the assessed toxicological responses in ZF4 cells can support the transition towards the replacement, reduction, and refinement of animals in experimentation, by providing evidence to encourage the application of the 3Rs in regulatory ecotoxicity testing [
29,
64]. Similarly, ZF4 cells can work well as a potential model to support the transition to more sustainable and alternative toxicity testing, and for facilitating the acquisition of data for the AOP framework for a 21st century toxicological assessment.
5. Conclusions
The present study demonstrated the internalisation mechanisms of three representative AgNP sizes (10, 30, and 100 nm) in embryonic zebrafish cells (ZF4). The inhibition of the caveolae, clathrin, and macropinocytosis endocytic pathways by pharmaceutical inhibitors (genistein, chlorpromazine, and wortmannin, respectively) confirmed that ZF4 cells’ uptake occurred via different endocytosis mechanisms, depending on the particle size. For example, the results revealed that uptake of the 10 nm size was mainly via macropinocytosis, whereas the uptake of the 30 and 100 nm sizes was mediated via the caveolae-mediated pathway.
In addition, we demonstrated that the AgNP mass concentration, size, and time of exposure of the three different AgNP sizes were closely linked to their intracellular fate, as displayed in the TEM images indicating the formation and disruption of early endosomes, and the activation of the endocytic uptake pathways, such as clathrin, caveolae macropinocytosis, and autophagy as a non-endocytic process. The presence of NPs inside the cells, including in the cytoplasm and close to the nucleus, was confirmed by TEM and correlated with the total Ag content as determined by ICP–MS, although the presence of intact 10 nm AgNPs was challenging to confirm, as the particles underwent some dissolution following internalisation.
Finally, the results presented in this study provide further insights about the cellular uptake mechanisms in ZF4 cells exposed to NPs, highlighting the complexity and interplay between induced intracellular mechanisms and NPs. The confirmation of existence and availability of the well-known endocytotic pathways in the ZF4 cells is an important step towards widespread adoption of this alternative vertebrate model for high-throughput in vitro toxicity testing, supporting the 3Rs initiative for the refinement, reduction, and replacement of animals in experimentation, for safer and sustainable nanotoxicological assessment.