New Insights into the Reactivity of Detonation Nanodiamonds during the First Stages of Graphitization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanodiamond Powder
2.2. Annealing under Vacuum
2.3. Annealing under Argon
2.4. Homogenization of Annealed DND in Water
2.5. Fourier Transform Infrared (FTIR) Measurements
2.6. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.7. Raman Analysis
2.8. Thermogravimetric Analysis (TGA)
2.9. High-Resolution Transmission Electron Microscopy (HR-TEM) Observations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; García, H. Diamond Nanoparticles in Heterogeneous Catalysis. Chem. Mater. 2020, 32, 4116–4143. [Google Scholar] [CrossRef]
- Ivanov, M.; Shenderova, O. Nanodiamond-based nanolubricants for motor oils. Curr. Opin. Solid State Mater. Sci. 2017, 21, 17–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part. B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Wang, H.; Lee, D.-K.; Chen, K.-Y.; Chen, J.-Y.; Zhang, K.; Silva, A.; Ho, C.-M.; Ho, D. Mechanism-Independent Optimization of Combinatorial Nanodiamond and Unmodified Drug Delivery Using a Phenotypically Driven Platform Technology. ACS Nano 2015, 9, 3332–3344. [Google Scholar] [CrossRef]
- Van der Laan, K.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds for In Vivo Applications. Small 2018, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Claveau, S.; Nehlig, É.; Garcia-Argote, S.; Feuillastre, S.; Pieters, G.; Girard, H.A.; Arnault, J.-C.; Treussart, F.; Bertrand, J.-R. Delivery of siRNA to Ewing Sarcoma Tumor Xenografted on Mice, Using Hydrogenated Detonation Nanodiamonds: Treatment Efficacy and Tissue Distribution. Nanomaterials 2020, 10, 553. [Google Scholar] [CrossRef] [Green Version]
- Shvidchenko, A.V.; Eidelman, E.D.; Vul’, A.Y.; Kuznetsov, N.M.; Stolyarova, D.Y.; Belousov, S.I.; Chvalun, S.N. Colloids of detonation nanodiamond particles for advanced applications. Adv. Colloid Interface Sci. 2019, 268, 64–81. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Ermilova, M.M.; Efimov, M.N.; Zemtsov, L.M.; Orekhova, N.V.; Karpacheva, G.P.; Bondarenko, G.N.; Zhilyaeva, N.A.; Muraviev, D.N.; Yaroslavtsev, A.B. Detonation nanodiamonds as catalysts of steam reforming of ethanol. Russ. Chem. Bull. 2013, 62, 2317–2321. [Google Scholar] [CrossRef]
- Zhang, L.; Hamers, R.J. Photocatalytic reduction of CO2 to CO by diamond nanoparticles. Diam. Relat. Mater. 2017, 78, 24–30. [Google Scholar] [CrossRef]
- Mi, Z.; Chen, C.-B.; Tan, H.Q.; Dou, Y.; Yang, C.; Turaga, S.P.; Ren, M.; Vajandar, S.K.; Yuen, G.H.; Osipowicz, T.; et al. Quantifying nanodiamonds biodistribution in whole cells with correlative iono-nanoscopy. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Grall, R.; Girard, H.; Saad, L.; Petit, T.; Gesset, C.; Combis-Schlumberger, M.; Paget, V.; Delic, J.; Arnault, J.-C.; Chevillard, S. Impairing the radioresistance of cancer cells by hydrogenated nanodiamonds. Biomaterials 2015, 61, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Brun, E.; Girard, H.A.; Arnault, J.; Mermoux, M.; Sicard-Roselli, C. Hydrogen plasma treated nanodiamonds lead to an overproduction of hydroxyl radicals and solvated electrons in solution under ionizing radiation. Carbon N. Y. 2020, 162, 510–518. [Google Scholar] [CrossRef]
- Wehling, J.; Dringen, R.; Zare, R.N.; Maas, M.; Rezwan, K. Bactericidal activity of partially oxidized nanodiamonds. ACS Nano 2014, 8, 6475–6483. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Su, D.S.; Centi, G.; Perathoner, S. Catalysis by hybrid sp2/sp3 nanodiamonds and their role in the design of advanced nanocarbon materials. Chem. Soc. Rev. 2018, 47, 8438–8473. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Meinhardt, T.; Jarre, G.; Ozawa, M.; Vrdoljak, P.; Schöll, A.; Reinert, F.; Krueger, A. Deagglomeration and surface modification of thermally annealed nanoscale diamond. J. Colloid Interface Sci. 2011, 354, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Maksimova, N.I.; Mestl, G.; Kuznetsov, V.L.; Keller, V.; Schlögl, R.; Keller, N. Oxidative dehydrogenation of ethylbenzene to styrene over ultra-dispersed diamond and onion-like carbon. Carbon N. Y. 2007, 45, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.L.; Butenko, Y.V. Diamond phase transitions at nanoscale. In Ultananocrystalline Diamond, 2nd ed.; Shenderova, O.A., Gruen, D.M., Eds.; Elsevier: Oxford, UK, 2012; ISBN 978-1-4377-3465-2. [Google Scholar]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef] [Green Version]
- Mykhaylyk, O.O.; Solonin, Y.M.; Batchelder, D.N.; Brydson, R. Transformation of nanodiamond into carbon onions: A comparative study by high-resolution transmission electron microscopy, electron energy-loss spectroscopy, X-ray diffraction, small-angle X-ray scattering, and ultraviolet Raman spectroscopy. J. Appl. Phys. 2005, 97, 074302. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Aslan, M.; Weingarth, D.; Presser, V. Understanding structure and porosity of nanodiamond-derived carbon onions. Carbon N. Y. 2015, 84, 584–598. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E.; Molina-Ontoria, A.; Echegoyen, L. Post-modification by low-temperature annealing of carbon nano-onions in the presence of carbohydrates. Carbon N. Y. 2014, 67, 304–317. [Google Scholar] [CrossRef]
- Xie, F.Y.; Xie, W.G.; Gong, L.; Zhang, W.H.; Chen, S.H.; Zhang, Q.Z.; Chen, J. Surface characterization on graphitization of nanodiamond powder annealed in nitrogen ambient. Surf. Interface Anal. 2010, 42, 1514–1518. [Google Scholar] [CrossRef]
- Aleksenskii, A.E.; Baidakova, M.V.; Vul, A.Y.; Dideikin, A.T.; Siklitskii, V.I.; Vul’, S.P. Effect of hydrogen on the structure of ultradisperse diamond. Phys. Solid State 2000, 42, 1575–1578. [Google Scholar] [CrossRef]
- Fugaciu, F.; Hermann, H.; Seifert, G. Concentric-shell fullerenes and diamond particles: A molecular-dynamics study. Phys. Rev. B 1999, 60, 10711–10714. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Zilberberg, I.L.; Butenko, Y.V.; Chuvilin, A.L.; Segall, B. Theoretical study of the formation of closed curved graphite-like structures during annealing of diamond surface. J. Appl. Phys. 1999, 86, 863. [Google Scholar] [CrossRef]
- Raty, J.-Y.; Galli, G.; Bostedt, C.; van Buuren, T.; Terminello, L. Quantum Confinement and Fullerenelike Surface Reconstructions in Nanodiamonds. Phys. Rev. Lett. 2003, 90, 037401. [Google Scholar] [CrossRef]
- Ray, M.A.; Shenderova, O.; Hook, W.; Martin, A.; Grishko, V.; Tyler, T.; Cunningham, G.B.; McGuire, G. Cold plasma functionalization of nanodiamond particles. Diam. Relat. Mater. 2006, 15, 1809–1812. [Google Scholar] [CrossRef]
- Petit, T.; Arnault, J.C.; Girard, H.A.; Sennour, M.; Bergonzo, P. Early stages of surface graphitization on nanodiamond probed by X-ray photoelectron spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 84, 233407. [Google Scholar] [CrossRef]
- Petit, T.; Arnault, J.-C.; Girard, H.A.; Sennour, M.; Kang, T.-Y.; Cheng, C.-L.; Bergonzo, P. Oxygen hole doping of nanodiamond. Nanoscale 2012, 4, 6792. [Google Scholar] [CrossRef] [PubMed]
- Cebik, J.; McDonough, J.K.; Peerally, F.; Medrano, R.; Neitzel, I.; Gogotsi, Y.; Osswald, S. Raman spectroscopy study of the nanodiamond-to-carbon onion transformation. Nanotechnology 2013, 24, 205703. [Google Scholar] [CrossRef]
- Panich, A.M.; Shames, A.I.; Sergeev, N.A.; Olszewski, M.; McDonough, J.K.; Mochalin, V.N.; Gogotsi, Y. Nanodiamond graphitization: A magnetic resonance study. J. Phys. Condens. Matter 2013, 25, 245303. [Google Scholar] [CrossRef] [PubMed]
- Koshcheev, A.P. Thermodesorption mass spectrometry in the light of solution of the problem of certification and unification of the surface properties of detonation nano-diamonds. Russ. J. Gen. Chem. 2009, 79, 2033–2044. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR spectroscopy of nanodiamonds: Methods and interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Ackermann, J.; Krueger, A. Efficient surface functionalization of detonation nanodiamond using ozone under ambient conditions. Nanoscale 2019, 11, 8012–8019. [Google Scholar] [CrossRef]
- Stehlik, S.; Glatzel, T.; Pichot, V.; Pawlak, R.; Meyer, E.; Spitzer, D.; Rezek, B. Water interaction with hydrogenated and oxidized detonation nanodiamonds—Microscopic and spectroscopic analyses. Diam. Relat. Mater. 2016, 63, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Petit, T.; Puskar, L.; Dolenko, T.; Choudhury, S.; Ritter, E.; Burikov, S.; Laptinskiy, K.; Brzustowski, Q.; Schade, U.; Yuzawa, H.; et al. Unusual Water Hydrogen Bond Network around Hydrogenated Nanodiamonds. J. Phys. Chem. C 2017, 121, 5185–5194. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-L.; Chen, C.-F.; Shaio, W.-C.; Tsai, D.-S.; Chen, K.-H. The CH stretching features on diamonds of different origins. Diam. Relat. Mater. 2005, 14, 1455–1462. [Google Scholar] [CrossRef]
- Aleksenskiǐ, A.E.; Baǐdakova, M.V.; Vul, A.Y.; Davydov, V.Y.; Pevtsova, Y.A. Diamond-graphite phase transition in ultradisperse-diamond clusters. Phys. Solid State 1997, 39, 1007–1015. [Google Scholar] [CrossRef]
- Mermoux, M.; Crisci, A.; Petit, T.; Girard, H.A.; Arnault, J.-C. Surface Modifications of Detonation Nanodiamonds Probed by Multiwavelength Raman Spectroscopy. J. Phys. Chem. C 2014, 118, 23415–23425. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]
- Brenier, R.; Mugnier, J.; Mirica, E. XPS study of amorphous zirconium oxide films prepared by sol-gel. Appl. Surf. Sci. 1999, 143, 85–91. [Google Scholar] [CrossRef]
- Ozawa, M.; Inaguma, M.; Takahashi, M.; Kataoka, F.; Krüger, A.; Ōsawa, E. Preparation and Behavior of Brownish, Clear Nanodiamond Colloids. Adv. Mater. 2007, 19, 1201–1206. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. On elemental composition and crystal-chemical parameters of detonation nanodiamonds. J. Superhard Mater. 2009, 31, 158–164. [Google Scholar] [CrossRef]
- Arnault, J.C. X-ray Photoemission Spectroscopy applied to nanodiamonds: From surface chemistry to in situ reactivity. Diam. Relat. Mater. 2018, 84, 157–168. [Google Scholar] [CrossRef]
- Stehlik, S.; Mermoux, M.; Schummer, B.; Vanek, O.; Kolarova, K.; Stenclova, P.; Vlk, A.; Ledinsky, M.; Pfeifer, R.; Romanyuk, O.; et al. Size effects on surface chemistry and Raman spectra of Sub-5 nm oxidized high-pressure high-temperature and detonation nanodiamonds. J. Phys. Chem. C 2021, 125, 5647–5669. [Google Scholar] [CrossRef]
- Girard, H.; Simon, N.; Ballutaud, D.; Herlem, M.; Etcheberry, A. Effect of anodic and cathodic treatments on the charge transfer of boron doped diamond electrodes. Diam. Relat. Mater. 2007, 16, 316–325. [Google Scholar] [CrossRef]
- Smith, J.M.; Meynell, S.A.; Bleszynski Jayich, A.C.; Meijer, J. Colour centre generation in diamond for quantum technologies. Nanophotonics 2019, 8, 1889–1906. [Google Scholar] [CrossRef]
- Turner, S.; Lebedev, O.I.; Shenderova, O.; Vlasov, I.I.; Verbeeck, J.; Van Tendeloo, G. Determination of Size, Morphology, and Nitrogen Impurity Location in Treated Detonation Nanodiamond by Transmission Electron Microscopy. Adv. Funct. Mater. 2009, 19, 2116–2124. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Laikhtman, A.; Lafosse, A.; Le Coat, Y.; Azria, R.; Hoffman, A. Interaction of water vapor with bare and hydrogenated diamond film surfaces. Surf. Sci. 2004, 551, 99–105. [Google Scholar] [CrossRef]
- Petit, T.; Pflüger, M.; Tolksdorf, D.; Xiao, J.; Aziz, E.F. Valence holes observed in nanodiamonds dispersed in water. Nanoscale 2015, 7, 2987–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehlig, E.; Garcia-Argote, S.; Feuillastre, S.; Moskura, M.; Charpentier, T.; Schleguel, M.; Girard, H.A.; Arnault, J.-C.; Pieters, G. Using hydrogen isotope incorporation as a tool to unravel the surfaces of hydrogen-treated nanodiamonds. Nanoscale 2019, 11, 8027–8036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitev, D.P.; Townsend, A.T.; Paull, B.; Nesterenko, P.N. Microwave-assisted purification of detonation nanodiamond. Diam. Relat. Mater. 2014, 48, 37–46. [Google Scholar] [CrossRef]
- Arnault, J.C.; Petit, T.; Girard, H.A.; Gesset, C.; Combis-Schlumberger, M.; Sennour, M.; Koscheev, A.; Khomich, A.A.; Vlasov, I.; Shenderova, O. Surface graphitization of ozone treated detonation nanodiamonds. Phys. Status Solidi Appl. Mater. Sci. 2014, 211, 2739–2743. [Google Scholar] [CrossRef]
- Ahmed, A.-I.; Mandal, S.; Gines, L.; Williams, O.A.; Cheng, C.-L. Low temperature catalytic reactivity of nanodiamond in molecular hydrogen. Carbon N. Y. 2016, 110, 438–442. [Google Scholar] [CrossRef]
- Thalassinos, G.; Stacey, A.; Dontschuk, N.; Murdoch, B.J.; Mayes, E.; Girard, H.A.; Abdullahi, I.M.; Thomsen, L.; Tadich, A.; Arnault, J.; et al. Fluorescence and Physico-Chemical Properties of Hydrogenated Detonation Nanodiamonds. C J. Carbon Res. 2020, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Shenderova, O.; Panich, A.M.; Moseenkov, S.; Hens, S.C.; Kuznetsov, V.; Vieth, H.-M. Hydroxylated Detonation Nanodiamond: FTIR, XPS, and NMR Studies. J. Phys. Chem. C 2011, 115, 19005–19011. [Google Scholar] [CrossRef]
- Williams, O.A.; Hees, J.; Dieker, C.; Jäger, W.; Kirste, L.; Nebel, C.E. Size-Dependent Reactivity of Diamond Nanoparticles. ACS Nano 2010, 4, 4824–4830. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, K.; Ji, S.; Jib, S. FTIR studies on the spectral changes of the surface functional groups of ultradispersed diamond powder synthesized by explosive detonation after treatment in hydrogen, nitrogen, methane and air at different temperatures. J. Chem. Soc. Faraday Trans. 1996, 92, 3401–3406. [Google Scholar] [CrossRef]
- Stehlik, S.; Henych, J.; Stenclova, P.; Kral, R.; Zemenova, P.; Pangrac, J.; Vanek, O.; Kromka, A.; Rezek, B. Size and nitrogen inhomogeneity in detonation and laser synthesized primary nanodiamond particles revealed via salt-assisted deaggregation. Carbon 2021, 171, 230–239. [Google Scholar] [CrossRef]
- Scholz, J.; McQuillan, A.J.; Holt, K.B. Redox transformations at nanodiamond surfaces revealed by in situ infrared spectroscopy. Chem. Commun. 2011, 47, 12140–12142. [Google Scholar] [CrossRef]
- Liang, Y.; Ozawa, M.; Krueger, A. A General Procedure to Functionalize Agglomerating Nanoparticles Demonstrated on Nanodiamond. ACS Nano 2009, 3, 2288–2296. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. IX. Data for 41 elemental solids over the 50 eV to 30 keV range. Surf. Interface Anal. 2011, 43, 689–713. [Google Scholar] [CrossRef]
- Baer, D.; Engelhard, M.; Gaspar, D. Challenges in Applying Surface Analysis Methods to Nanoparticles and Nanostructured Materials. J. Surf. Anal. 2005, 12, 101–108. [Google Scholar]
- Baer, D.R.; Engelhard, M.H. XPS analysis of nanostructured materials and biological surfaces. J. Electron. Spectros. Relat. Phenom. 2010, 178–179, 415–432. [Google Scholar] [CrossRef]
- Shenderova, O.A.; McGuire, G.E. Science and engineering of nanodiamond particle surfaces for biological applications (Review). Biointerphases 2015, 10, 030802. [Google Scholar] [CrossRef]

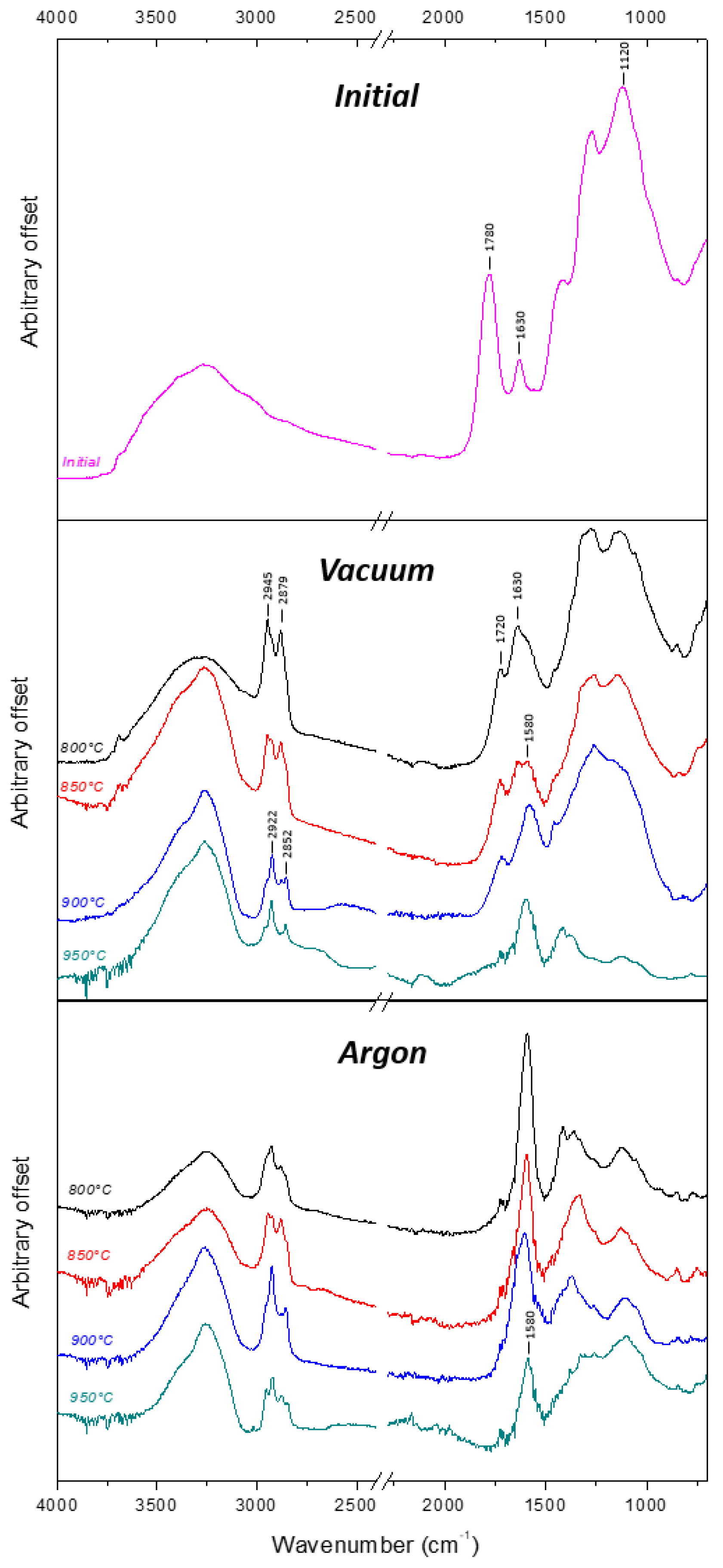
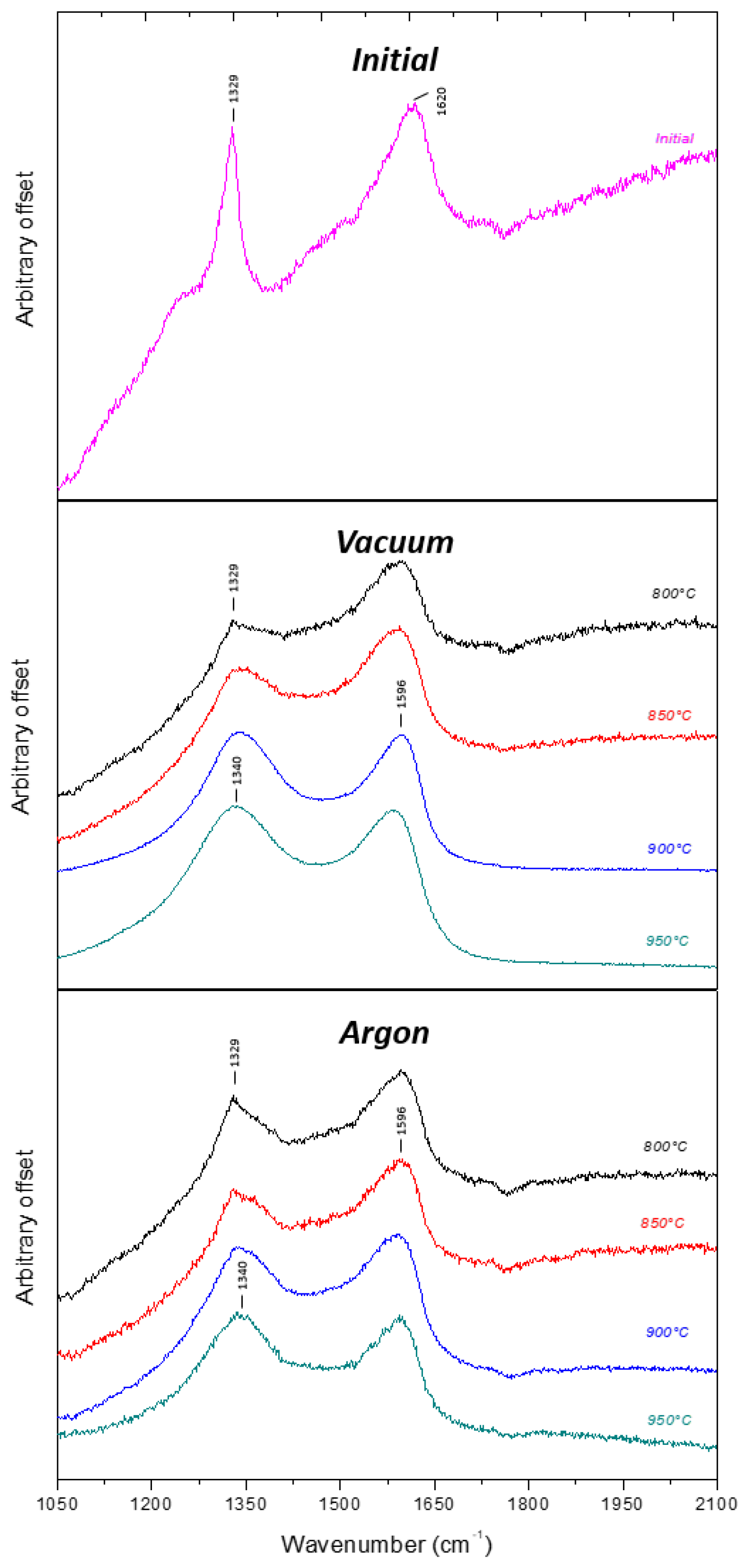
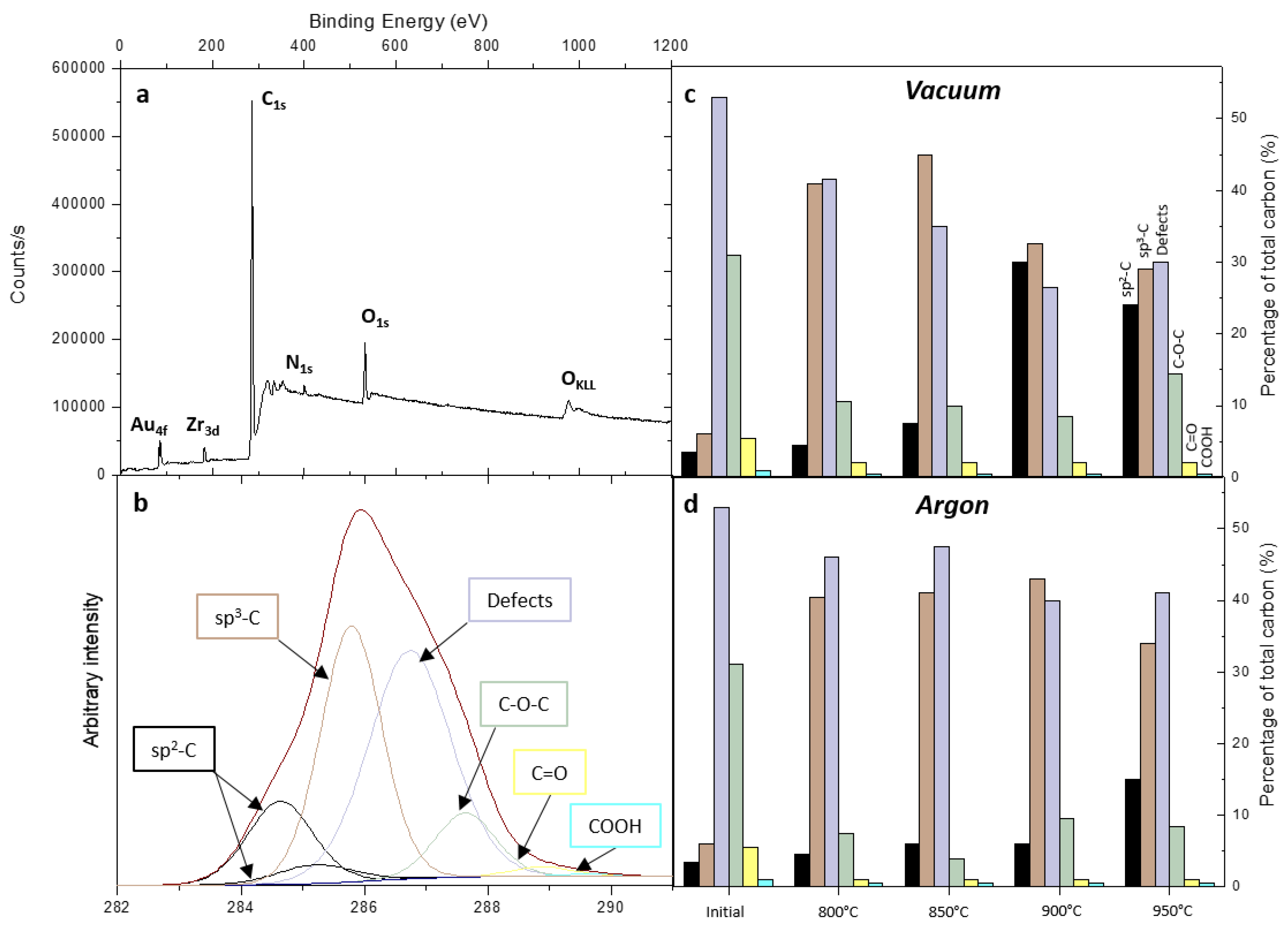

| Temperature °C | Oxygen at. % | Nitrogen at. % | |
|---|---|---|---|
| Initial | 9.3 | 1.9 | |
| Vacuum | 800 | 5.7 | 1.6 |
| 850 | 5.3 | 1.3 | |
| 900 | 6.1 | 1.2 | |
| 950 | 5.2 | 1.3 | |
| Argon | 800 | 5.1 | 1.5 |
| 850 | 3.6 | 1.5 | |
| 900 | 5.0 | 1.4 | |
| 950 | 4.0 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducrozet, F.; Girard, H.A.; Leroy, J.; Larquet, E.; Florea, I.; Brun, E.; Sicard-Roselli, C.; Arnault, J.-C. New Insights into the Reactivity of Detonation Nanodiamonds during the First Stages of Graphitization. Nanomaterials 2021, 11, 2671. https://doi.org/10.3390/nano11102671
Ducrozet F, Girard HA, Leroy J, Larquet E, Florea I, Brun E, Sicard-Roselli C, Arnault J-C. New Insights into the Reactivity of Detonation Nanodiamonds during the First Stages of Graphitization. Nanomaterials. 2021; 11(10):2671. https://doi.org/10.3390/nano11102671
Chicago/Turabian StyleDucrozet, Florent, Hugues A. Girard, Jocelyne Leroy, Eric Larquet, Ileana Florea, Emilie Brun, Cécile Sicard-Roselli, and Jean-Charles Arnault. 2021. "New Insights into the Reactivity of Detonation Nanodiamonds during the First Stages of Graphitization" Nanomaterials 11, no. 10: 2671. https://doi.org/10.3390/nano11102671
APA StyleDucrozet, F., Girard, H. A., Leroy, J., Larquet, E., Florea, I., Brun, E., Sicard-Roselli, C., & Arnault, J.-C. (2021). New Insights into the Reactivity of Detonation Nanodiamonds during the First Stages of Graphitization. Nanomaterials, 11(10), 2671. https://doi.org/10.3390/nano11102671







