Metal Cluster Size-Dependent Activation Energies of Growth of Single-Chirality Single-Walled Carbon Nanotubes inside Metallocene-Filled Single-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Filled SWCNTs
2.2. Preparation of SWCNT Films
2.3. Opening of SWCNT Ends
2.4. Filling of SWCNT Channels
2.5. Formation of DWCNTs
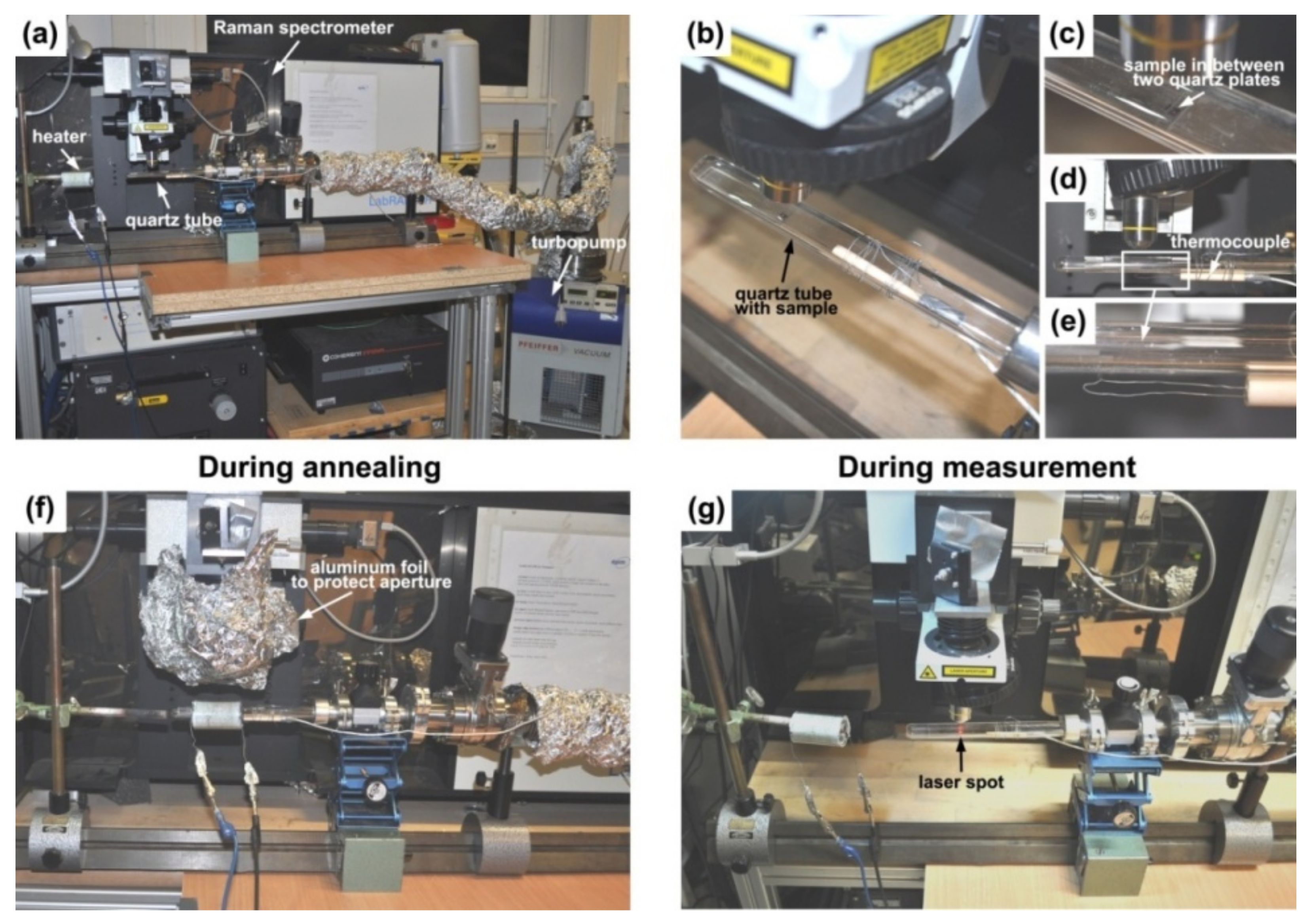
2.5.1. In Situ Annealing
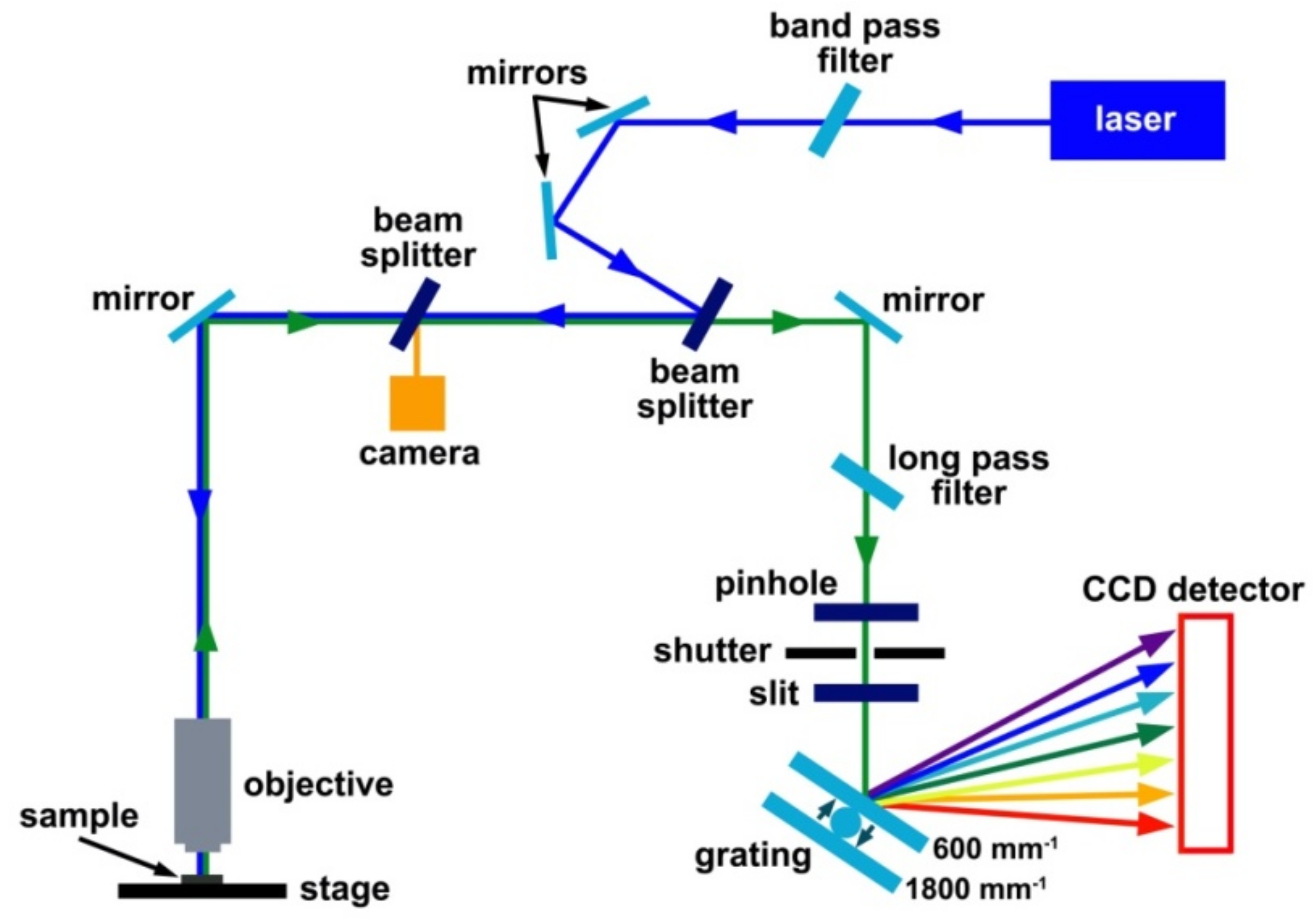
2.5.2. Instrumentation for Raman Spectroscopy
3. Results
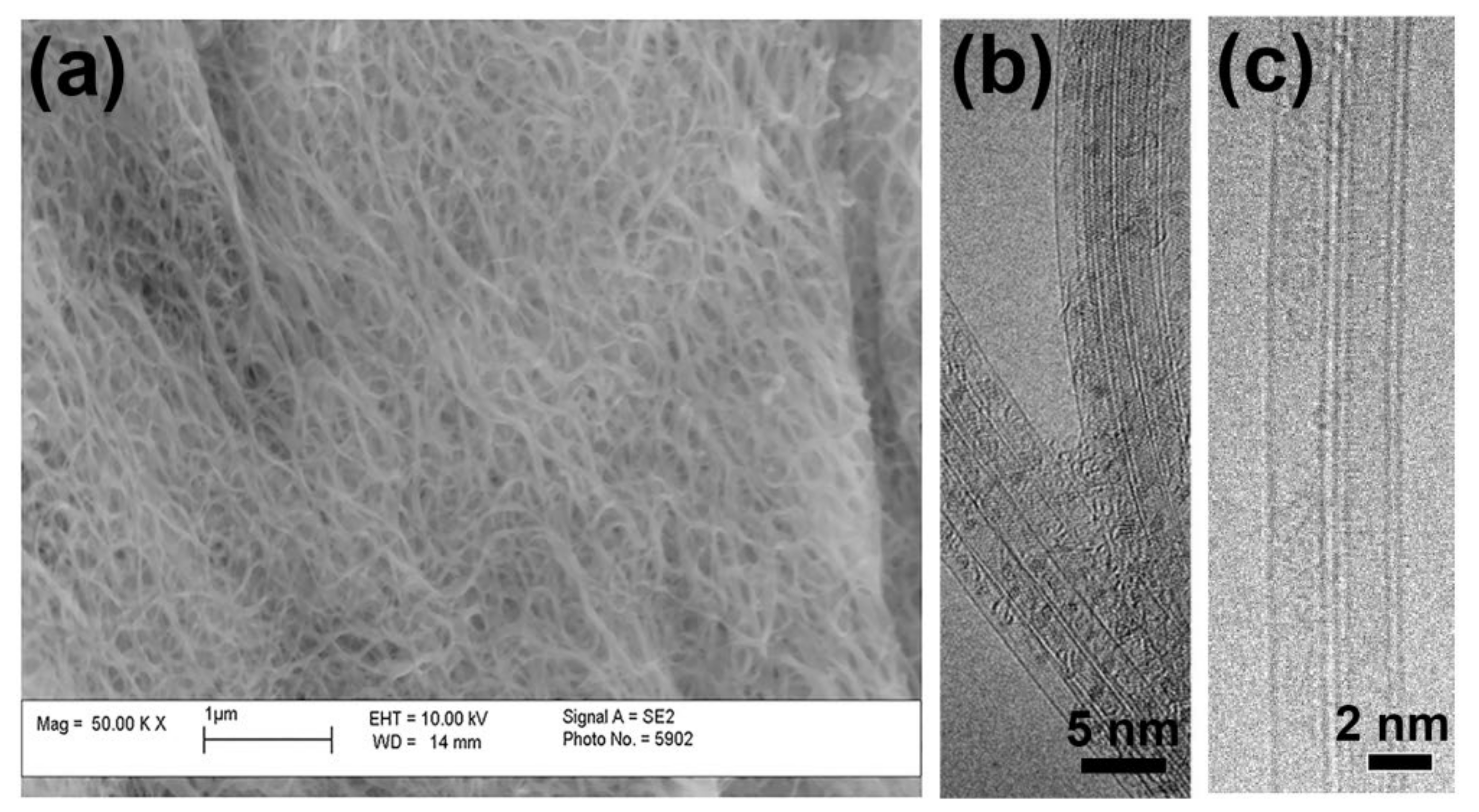
3.1. Scanning and Transmission Electron Microscopy
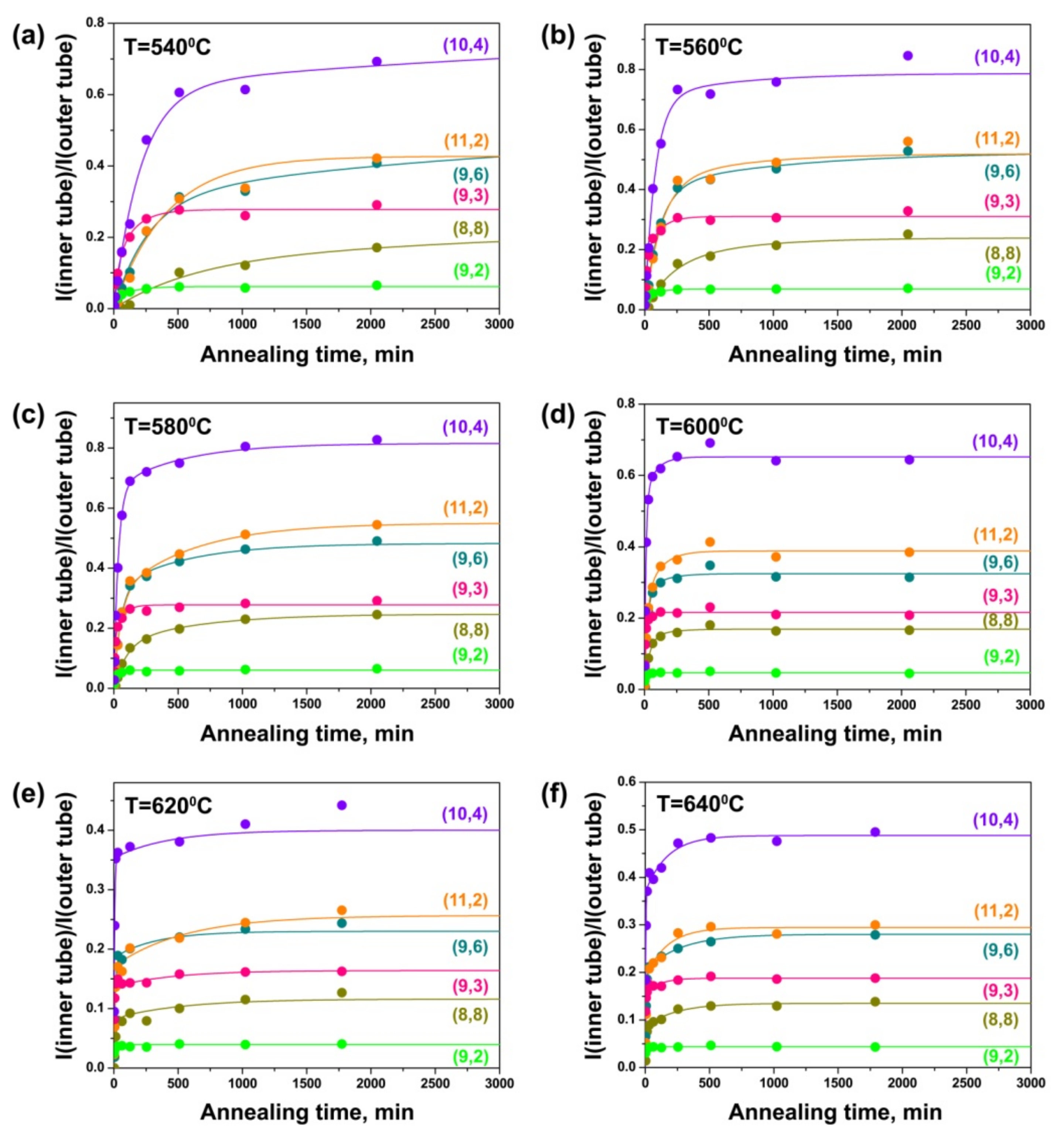
3.2. In Situ Raman Spectroscopy at Laser Wavelengths of 633 and 568 nm
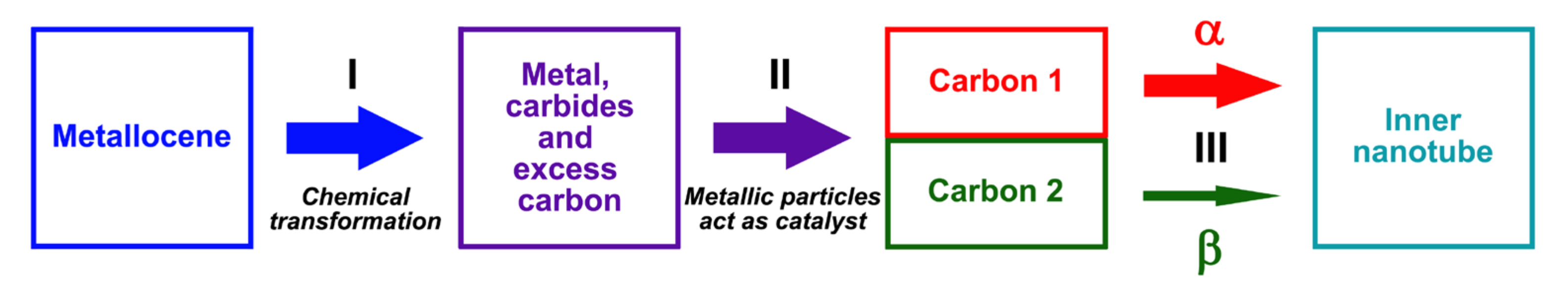
3.3. Growth Model and Calculation of Growth Rates of Inner Tubes
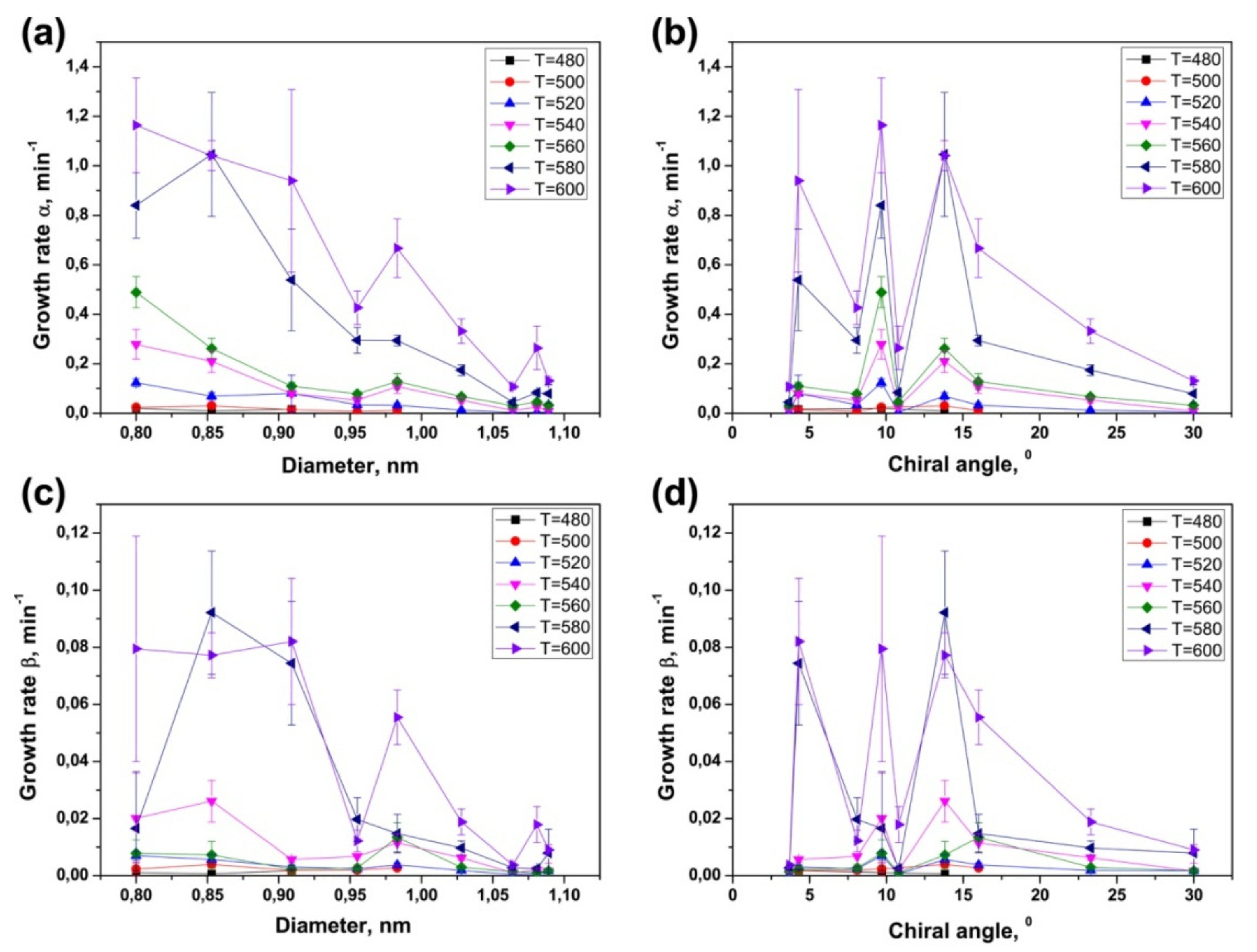
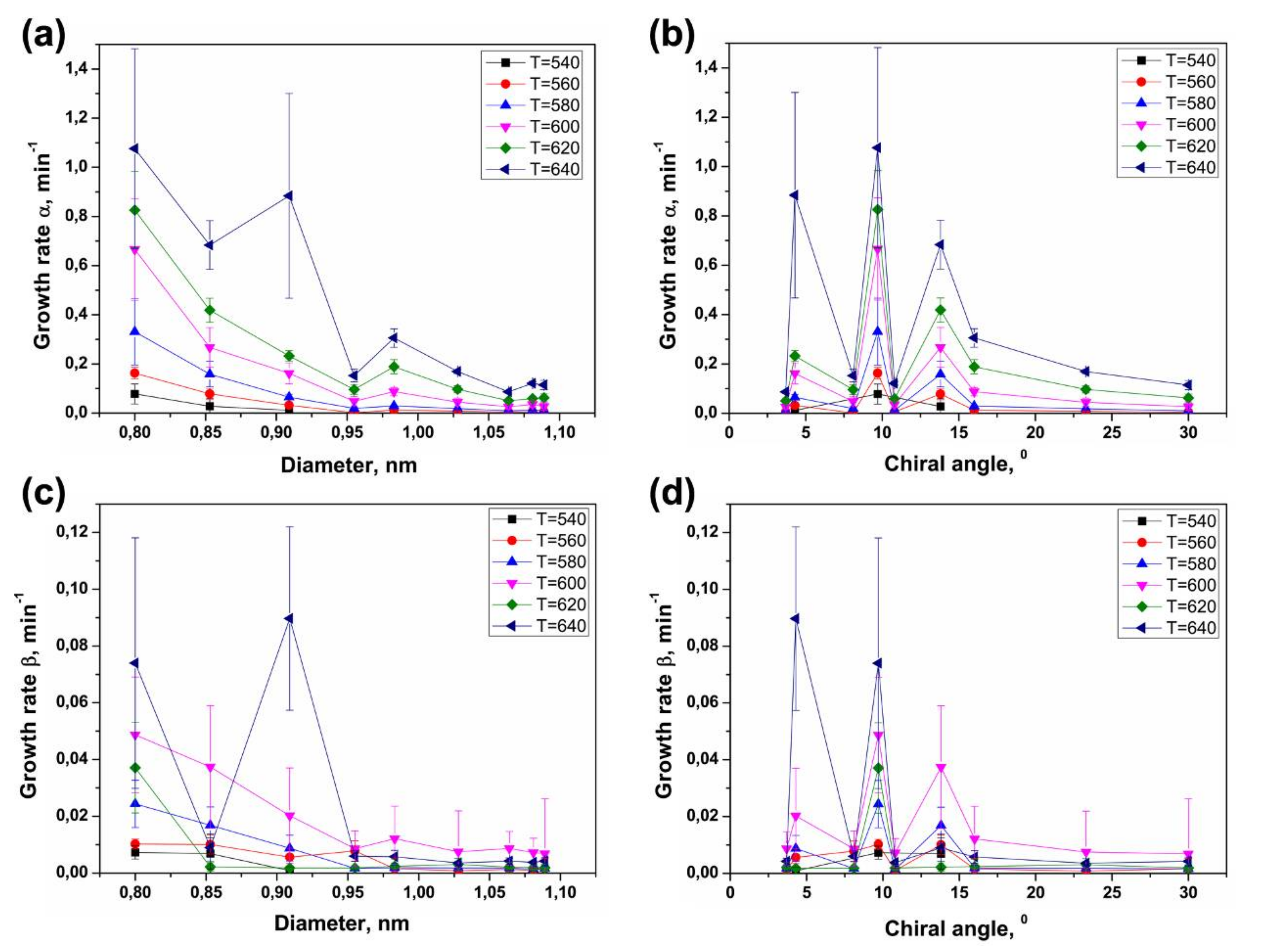
3.4. Dependence of Growth Rates of Inner Tubes on Their Diameter and Annealing Temperature
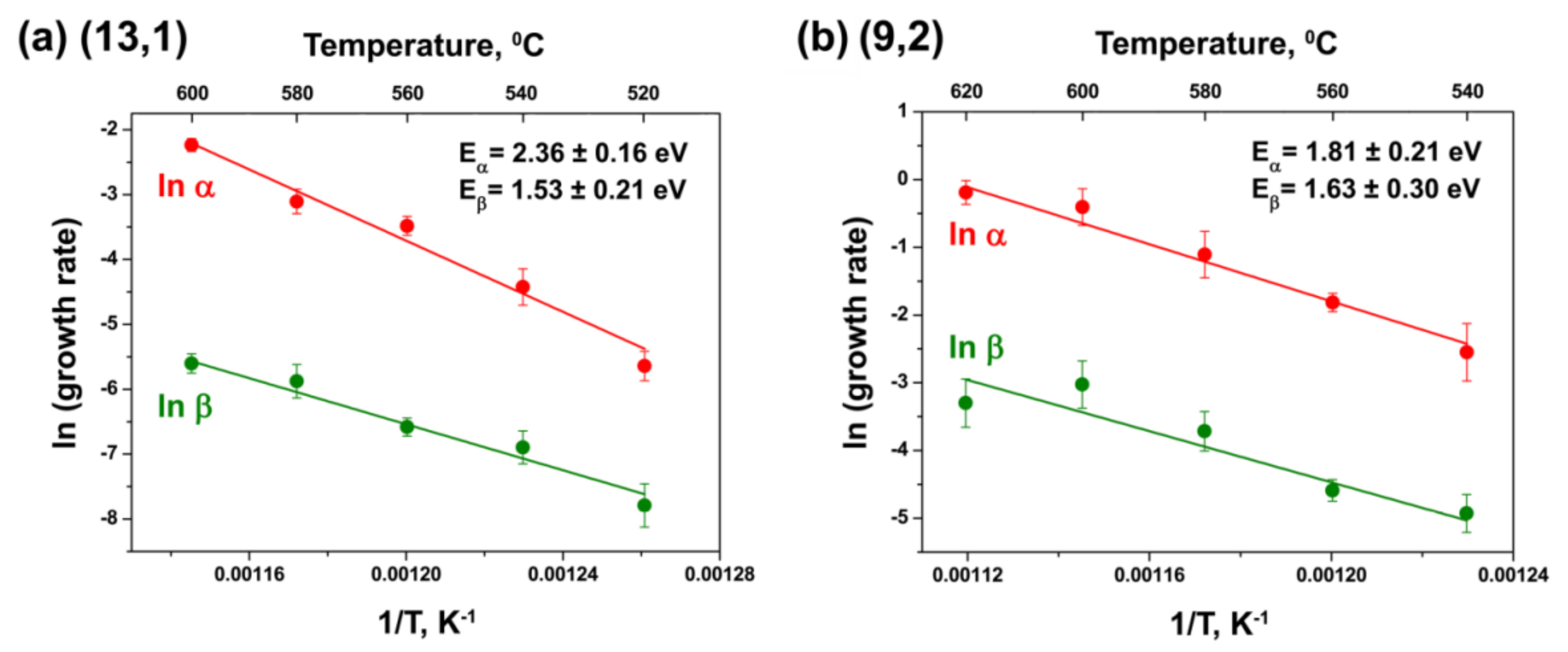
3.5. Calculation of Activation Energies of the Inner Tube Growth
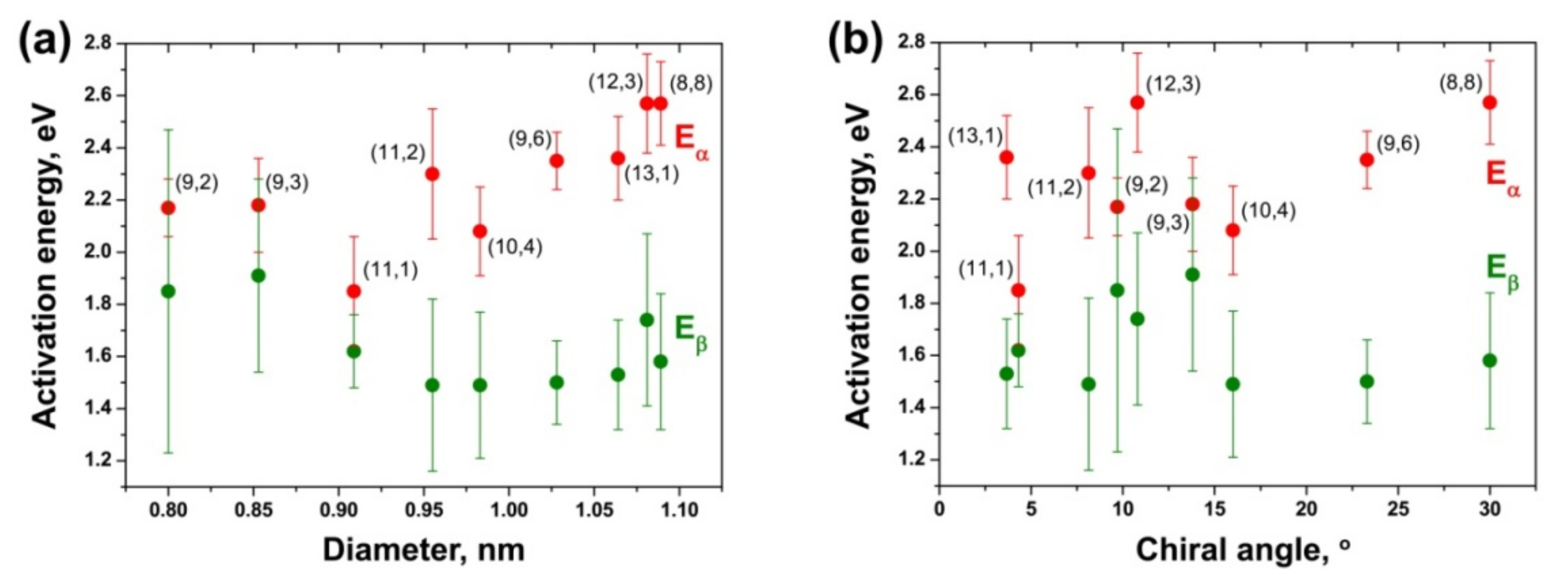
3.6. Dependence of Activation Energies of the Inner Tube Growth on Their Diameter and Chirality
3.7. Discussion of the Growth Process of Inner Tubes
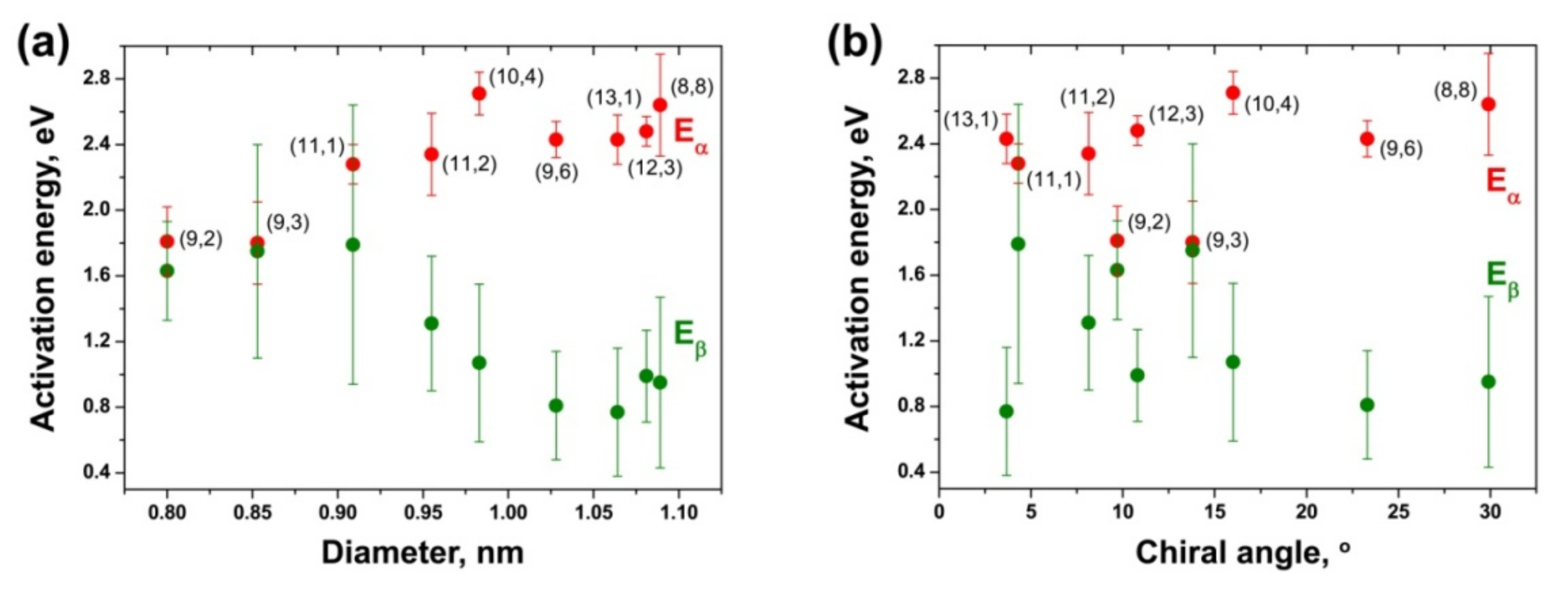
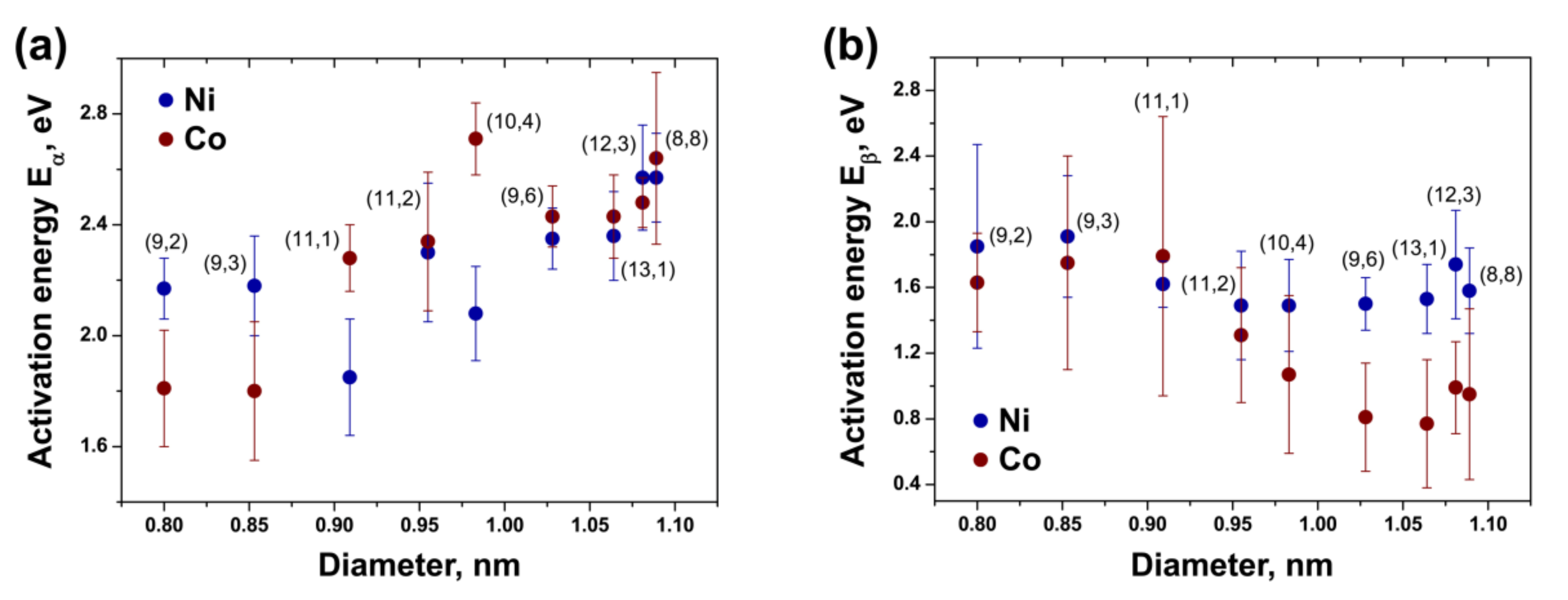
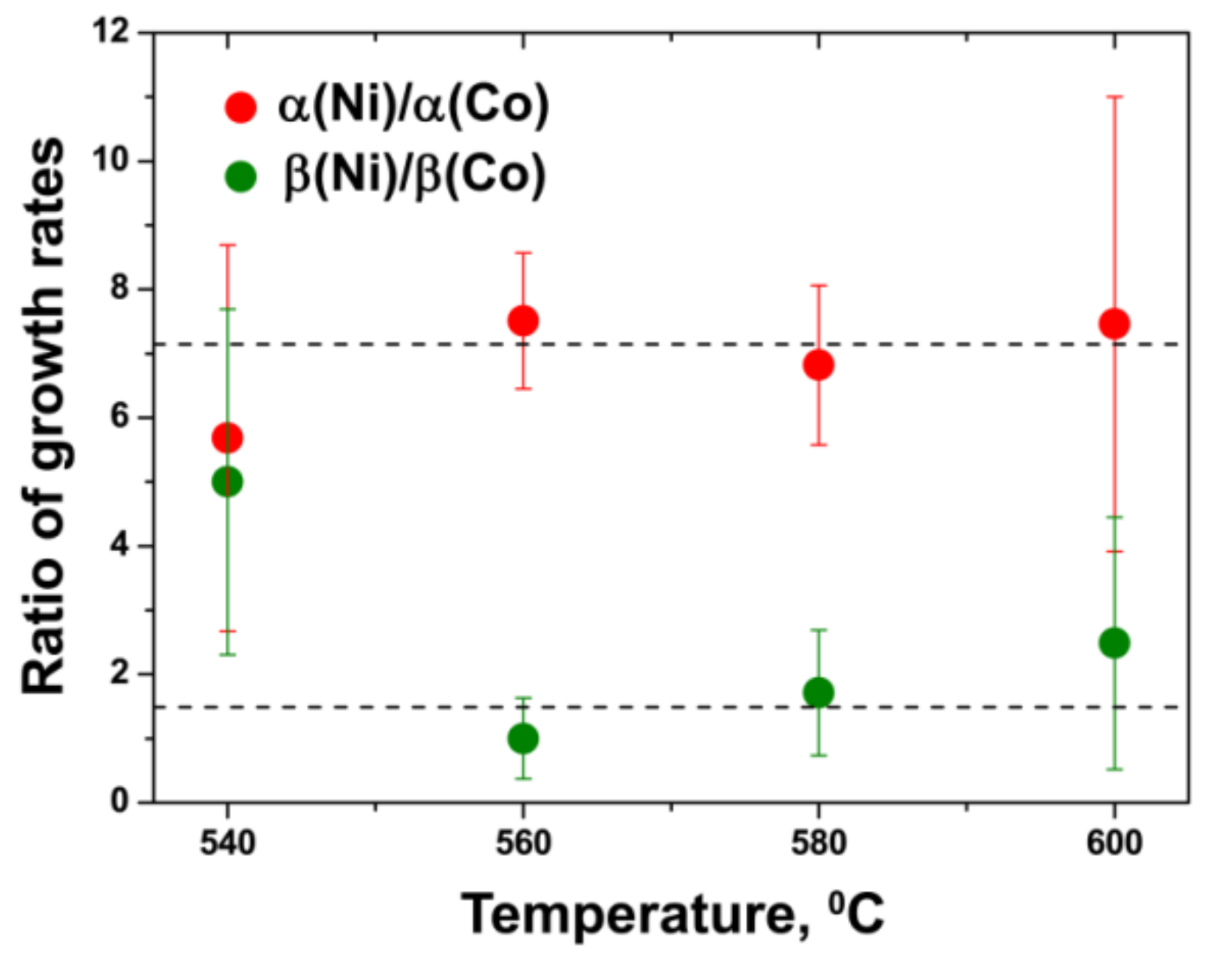
3.8. Comparison of Activation Energies of the Inner Tube Growth inside the NiCp2- and CoCp2-Filled SWCNTs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Klang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Charlier, J.C.; Blase, X.; Roche, S. Electronic and transport properties of nanotubes. Rev. Mod. Phys. 2007, 79, 677–732. [Google Scholar] [CrossRef]
- Dai, H.J. Nanotube growth and characterization. In Carbon Nanotubes: Topics in Applied Physics; Dresselhaus, M.S., Dresselhaus, G., Avouris, A., Eds.; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2001; Volume 80, pp. 29–53. [Google Scholar]
- Joselevich, E.; Dai, H.J.; Liu, J.; Hata, K.; Windle, A.H. Carbon nanotube synthesis and organization. In Carbon Nanotubes; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2008; Volume 111, pp. 101–164. [Google Scholar]
- Li, Y.M.; Mann, D.; Rolandi, M.; Kim, W.; Ural, A.; Hung, S.; Javey, A.; Cao, J.; Wang, D.W.; Yenilmez, E.; et al. Preferential growth of semiconducting single-walled carbon nanotubes by a plasma enhanced CVD method. Nano Lett. 2004, 4, 317–321. [Google Scholar] [CrossRef]
- Ding, L.; Tselev, A.; Wang, J.Y.; Yuan, D.N.; Chu, H.B.; McNicholas, T.P.; Li, Y.; Liu, J. Selective Growth of Well-Aligned Semiconducting Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V. Advances in tailoring the electronic properties of single-walled carbon nanotubes. Prog. Mater. Sci. 2016, 77, 125–211. [Google Scholar] [CrossRef]
- Tans, S.J.; Verschueren, A.R.M.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49–52. [Google Scholar] [CrossRef]
- Bachtold, A.; Hadley, P.; Nakanishi, T.; Dekker, C. Logic circuits with carbon nanotube transistors. Science 2001, 294, 1317–1320. [Google Scholar] [CrossRef]
- Chen, Z.H.; Appenzeller, J.; Lin, Y.M.; Sippel-Oakley, J.; Rinzler, A.G.; Tang, J.Y.; Wind, S.J.; Solomon, P.M.; Avouris, P. An integrated logic circuit assembled on a single carbon nanotube. Science 2006, 311, 1735. [Google Scholar] [CrossRef]
- Avouris, P.; Chen, Z.H.; Perebeinos, V. Carbon-based electronics. Nat. Nanotechnol. 2007, 2, 605–615. [Google Scholar] [CrossRef]
- Artukovic, E.; Kaempgen, M.; Hecht, D.S.; Roth, S.; Gruner, G. Transparent and flexible carbon nanotube transistors. Nano Lett. 2005, 5, 757–760. [Google Scholar] [CrossRef]
- Cao, Q.; Kim, H.S.; Pimparkar, N.; Kulkarni, J.P.; Wang, C.J.; Shim, M.; Roy, K.; Alam, M.A.; Rogers, J.A. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature 2008, 454, 495–500. [Google Scholar] [CrossRef]
- Huang, Y.X.; Palkar, P.V.; Li, L.J.; Zhang, H.; Chen, P. Integrating carbon nanotubes and lipid bilayer for biosensing. Biosens. Bioelectron. 2010, 25, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, E.; Murakami, Y.; Kadowaki, M.; Maruyama, S. Growth dynamics of vertically aligned single-walled carbon nanotubes from insitu measurements. Carbon 2008, 46, 923–930. [Google Scholar] [CrossRef]
- Picher, M.; Anglaret, E.; Arenal, R.; Jourdain, V. Self-Deactivation of Single-Walled Carbon Nanotube Growth Studied by insitu Raman Measurements. Nano Lett. 2009, 9, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Vinten, P.; Lefebvre, J.; Finnie, P. Kinetic critical temperature and optimized chemical vapor deposition growth of carbon nanotubes. Chem. Phys. Lett. 2009, 469, 293–297. [Google Scholar] [CrossRef]
- Yasuda, S.; Futaba, D.N.; Yamada, T.; Yumura, M.; Hata, K. Gas Dwell Time Control for Rapid and Long Lifetime Growth of Single-Walled Carbon Nanotube Forests. Nano Lett. 2011, 11, 3617–3623. [Google Scholar] [CrossRef]
- Rao, R.; Liptak, D.; Cherukuri, T.; Yakobson, B.I.; Maruyama, B. Insitu evidence for chirality-dependent growth rates of individual carbon nanotubes. Nat. Mater. 2012, 11, 213–216. [Google Scholar] [CrossRef]
- Liu, B.L.; Liu, J.; Tu, X.M.; Zhang, J.L.; Zheng, M.; Zhou, C.W. Chirality-Dependent Vapor-Phase Epitaxial Growth and Termination of Single-Wall Carbon Nanotubes. Nano Lett. 2013, 13, 4416–4421. [Google Scholar] [CrossRef]
- Chen, G.H.; Davis, R.C.; Kimura, H.; Sakurai, S.; Yumura, M.; Futaba, D.N.; Hata, K. The relationship between the growth rate and the lifetime in carbon nanotube synthesis. Nanoscale 2015, 7, 8873–8878. [Google Scholar] [CrossRef]
- Shiozawa, H.; Pichler, T.; Gruneis, A.; Pfeiffer, R.; Kuzmany, H.; Liu, Z.; Suenaga, K.; Kataura, H. A catalytic reaction inside a single-walled carbon nanotube. Adv. Mater. 2008, 20, 1443–1449. [Google Scholar] [CrossRef]
- Shiozawa, H.; Pichler, T.; Kramberger, C.; Rummeli, M.; Batchelor, D.; Liu, Z.; Suenaga, K.; Kataura, H.; Silva, S.R.P. Screening the Missing Electron: Nanochemistry in Action. Phys. Rev. Lett. 2009, 102, 046804. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, H.; Pichler, T.; Kramberger, C.; Gruneis, A.; Knupfer, M.; Buchner, B.; Zolyomi, V.; Koltai, J.; Kurti, J.; Batchelor, D.; et al. Fine tuning the charge transfer in carbon nanotubes via the interconversion of encapsulated molecules. Phys. Rev. B 2008, 77, 153402. [Google Scholar] [CrossRef]
- Saito, T.; Ohshima, S.; Okazaki, T.; Ohmori, S.; Yumura, M.; Iijima, S. Selective Diameter Control of Single-Walled Carbon Nanotubes in the Gas-Phase Synthesis. J. Nanosci. Nanotechnol. 2008, 8, 6153–6157. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ohmori, S.; Shukla, B.; Yumura, M.; Iijima, S. A Novel Method for Characterizing the Diameter of Single-Wall Carbon Nanotubes by Optical Absorption Spectra. Appl. Phys. Express 2009, 2, 095006. [Google Scholar] [CrossRef]
- Fabian, G.; Kramberger, C.; Friedrich, A.; Simon, F.; Pichler, T. A broadband and high throughput single-monochromator Raman spectrometer: Application for single-wall carbon nanotubes. Rev. Sci. Instrum. 2011, 82, 023905. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.T.; Maciel, I.O.; Pesce, P.B.C.; Pimenta, M.A.; Doorn, S.K.; Qian, H.; Hartschuh, A.; Steiner, M.; Grigorian, L.; Hata, K.; et al. Nature of the constant factor in the relation between radial breathing mode frequency and tube diameter for single-wall carbon nanotubes. Phys. Rev. B 2008, 77, 241403. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Plank, W.; Pfeiffer, R.; Schaman, C.; Kuzmany, H.; Calvaresi, M.; Zerbetto, F.; Meyer, J. Electronic Structure of Carbon Nanotubes with Ultrahigh Curvature. ACS Nano 2010, 4, 4515–4522. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.; Simon, F.; Kuzmany, H.; Popov, V.N. Fine structure of the radial breathing mode of double-wall carbon nanotubes. Phys. Rev. B 2005, 72, 161404. [Google Scholar] [CrossRef]
- Dubay, O.; Kresse, G. Density functional calculations for C60 peapods. Phys. Rev. B 2004, 70, 165424. [Google Scholar] [CrossRef]
- Pfab, W.; Fischer, E.O. ZurKristallstruktur Der Di-cyclopentadienyl-verbindungen Des ZweiwertigenEisens, Kobalts Und Nickels. Z. Anorg. Allg. Chem. 1953, 274, 316–322. [Google Scholar] [CrossRef]
- Hedberg, L.; Hedberg, K. Molecular Structure of Dicyclopentadienylnickel (C5H5)2Ni. J. Chem. Phys. 1970, 53, 1228. [Google Scholar] [CrossRef]
- Hedberg, A.K.; Hedberg, L.; Hedberg, K. Molecular-structure of Di picyclopentadienylcobalt, (C5H5)2Co, By Gaseous Electron-diffraction. J. Chem. Phys. 1975, 63, 1262–1266. [Google Scholar] [CrossRef]
- Kealy, T.J.; Pauson, P.L. A New Type of Organo-iron Compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Miller, S.A.; Tebboth, J.A.; Tremaine, J.F. Dicyclopentadienyliron. J. Chem. Soc. 1952, 632–635. [Google Scholar] [CrossRef]
- Wilkinson, G.; Rosenblum, M.; Whiting, M.C.; Woodward, R.B. The Structure of Iron Bis-cyclopentadienyl. J. Am. Chem. Soc. 1952, 74, 2125–2126. [Google Scholar] [CrossRef]
- Fischer, E.O.; Pfab, W. Cyclopentadien-metallkomplexe, Ein NeuerTypMetallorganischerVerbindungen. Z. Naturforsch. B 1952, 7, 377–379. [Google Scholar] [CrossRef]
- Eiland, P.F.; Pepinsky, R. X-ray Examination of Iron Biscyclopentadienyl. J. Am. Chem. Soc. 1952, 74, 4971. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Orgel, L.E. Bis-cyclopentadienyl Iron—A Molecular Sandwich. Nature 1953, 171, 121–122. [Google Scholar] [CrossRef]
- Seibold, E.A.; Sutton, L.E. Structure of Ferrocene. J. Chem. Phys. 1955, 23, 1967. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Orgel, L.E.; Rich, A. The Crystal Structure of Ferrocene. Acta Crystallogr. 1956, 9, 373–375. [Google Scholar] [CrossRef]
- Bohn, R.K.; Haaland, A. On Molecular Structure of Ferrocene Fe(C5H5)2. J. Organomet. Chem. 1966, 5, 470. [Google Scholar] [CrossRef]
- Haaland, A.; Nilsson, J.E. Determination of Barriers to Internal Rotation by Means of Electron Diffraction. Ferrocene and Ruthenocene. Acta Chem. Scand. 1968, 22, 2653. [Google Scholar] [CrossRef]
- Seiler, P.; Dunitz, J.D. New Interpretation of the Disordered Crystal-structure of Ferrocene. Acta Crystallogr. B 1979, 35, 1068–1074. [Google Scholar] [CrossRef]
- Swart, M. Metal-ligand bonding in metallocenes: Differentiation between spin state, electrostatic and covalent bonding. Inorg. Chim. Acta 2007, 360, 179–189. [Google Scholar] [CrossRef]
- Bunder, W.; Weiss, E. Reigning of Crystal-Structure of Dicyclopentadienylcobalt. J. Organomet. Chem. 1975, 92, 65–68. [Google Scholar]
- Almenningen, A.; Gard, E.; Haaland, A.; Brunvoll, J. Dynamic Jahn-Teller Effect and Average Structure of Dicyclopentadienylcobalt, (C5H5)2Co, Studied by Gas-phase Electron-diffraction. J. Organomet. Chem. 1976, 107, 273–279. [Google Scholar] [CrossRef]
- Antipin, M.Y.; Boese, R.; Augart, N.; Schmid, G. Redetermination of the Cobaltocene Crystal-structure at 100 K and 297 K—Comparison with Ferrocene and Nickelocene. Struct. Chem. 1993, 4, 91–101. [Google Scholar] [CrossRef]
- Ketkov, S.Y.; Selzle, H.L. Threshold Ionization of Cobaltocene: The Metallocene Molecule Revealing Zero Kinetic Energy States. Angew. Chem.-Int. Ed. 2012, 51, 11527–11530. [Google Scholar] [CrossRef]
- Ronova, I.A.; Bochvar, D.A.; Chistjak, A.L.; Struchko, Y.T.; Alekseev, N.V. Electron Diffraction Study in Vapour Phase of Molecular Structure of Dicyclopentadienylnickel. J. Organomet. Chem. 1969, 18, 337. [Google Scholar] [CrossRef]
- Seiler, P.; Dunitz, J.D. The Structure of Nickelocene at Room-temperature and at 101 K. Acta Crystallogr. B 1980, 36, 2255–2260. [Google Scholar] [CrossRef]
- Coates, G.E.; Green, M.L.H.; Wade, K. Organometallic Compounds, Volume 2, The Transition Elements; Methuen: London, UK, 1968. [Google Scholar]
- Kharlamova, M.V.; Sauer, M.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Doping of single-walled carbon nanotubes controlled via chemical transformation of encapsulated nickelocene. Nanoscale 2015, 7, 1383–1391. [Google Scholar] [CrossRef]
- Greenwood, N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 5th ed.; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Endregard, M.; Nicholson, D.G.; Stocker, M.; Lamble, G.M. Adsorption and Thermal Decomposition of Cobalticenium Ions on AlPO4-5 studied by X-Ray Absorption Spectroscopy, 13C Solid-state NMR and FTIR. J. Mater. Chem. 1995, 5, 785–791. [Google Scholar] [CrossRef]
- Bower, C.; Zhou, O.; Zhu, W.; Werder, D.J.; Jin, S.H. Nucleation and growth of carbon nanotubes by microwave plasma chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 2767–2769. [Google Scholar] [CrossRef]
- Choi, Y.C.; Shin, Y.M.; Lee, Y.H.; Lee, B.S.; Park, G.S.; Choi, W.B.; Lee, N.S.; Kim, J.M. Controlling the diameter, growth rate, and density of vertically aligned carbon nanotubes synthesized by microwave plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2000, 76, 2367–2369. [Google Scholar] [CrossRef]
- Chhowalla, M.; Teo, K.B.K.; Ducati, C.; Rupesinghe, N.L.; Amaratunga, G.A.J.; Ferrari, A.C.; Roy, D.; Robertson, J.; Milne, W.I. Growth process conditions of vertically aligned carbon nanotubes using plasma enhanced chemical vapor deposition. J. Appl. Phys. 2001, 90, 5308–5317. [Google Scholar] [CrossRef]
- Huang, Z.P.; Wang, D.Z.; Wen, J.G.; Sennett, M.; Gibson, H.; Ren, Z.F. Effect of nickel, iron and cobalt on growth of aligned carbon nanotubes. Appl. Phys. A 2002, 74, 387–391. [Google Scholar] [CrossRef]
- Ducati, C.; Alexandrou, I.; Chhowalla, M.; Amaratunga, G.A.J.; Robertson, J. Temperature selective growth of carbon nanotubes by chemical vapor deposition. J. Appl. Phys. 2002, 92, 3299–3303. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.M.; Shi, S.C.; Chen, C.F. Low-temperature synthesis multiwalled carbon nanotubes by microwave plasma chemical vapor deposition using CH4-CO2 gas mixture. Jpn. J. Appl. Phys. 2003, 42, 614–619. [Google Scholar] [CrossRef]
- Lin, M.; Tan, J.P.Y.; Boothroyd, C.; Loh, K.P.; Tok, E.S.; Foo, Y.L. Direct observation of single-walled carbon nanotube growth at the atomistic scale. Nano Lett. 2006, 6, 449–452. [Google Scholar] [CrossRef]
- Baker, R.T.K. Catalytic Growth of Carbon Filaments. Carbon 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Chiang, W.H.; Sankaran, R.M. Relating carbon nanotube growth parameters to the size and composition of nanocatalysts. Diam. Relat. Mater. 2009, 18, 946–952. [Google Scholar] [CrossRef]
- Patole, S.P.; Kim, H.; Choi, J.; Kim, Y.; Baik, S.; Yoo, J.B. Kinetics of catalyst size dependent carbon nanotube growth by growth interruption studies. Appl. Phys. Lett. 2010, 96, 094101. [Google Scholar] [CrossRef]
- Cervantes-Sodi, F.; McNicholas, T.P.; Simmons, J.G.; Liu, J.; Csanyi, G.; Ferrari, A.C.; Curtarolo, S. Viscous State Effect on the Activity of Fe Nanocatalysts. ACS Nano 2010, 4, 6950–6956. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Somorjai, G.A. Introduction to Surface Chemistry and Catalysis; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Baker, R.T.K.; Harris, P.S.; Thomas, R.B.; Waite, R.J. Formation of Filamentous Carbon from Iron, Cobalt and Chromium Catalyzed Decomposition of Acetylene. J. Catal. 1973, 30, 86–95. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Chludzinski, J.J.; Dudash, N.S.; Simoens, A.J. The Formation of Filamentous Carbon from Decomposition of Acetylene Over Vanadium and Molybdenum. Carbon 1983, 21, 463–468. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Chludzinski, J.J.; Lund, C.R.F. Further-Studies of the Formation of Filamentous Carbon from the Interaction of Supported Iron Particles with Acetylene. Carbon 1987, 25, 295–303. [Google Scholar] [CrossRef]
- Gorbunov, A.; Jost, O.; Pompe, W.; Graff, A. Solid-liquid-solid growth mechanism of single-wall carbon nanotubes. Carbon 2002, 40, 113–118. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, Y.T.; Park, J.H.; Ryu, H.; Lee, H.J.; Choi, S.Y.; Choo, J.B. Dependence of the vertically aligned growth of carbon nanotubes on the catalysts. J. Phys. Chem. B 2002, 106, 9286–9290. [Google Scholar] [CrossRef]
- Lee, Y.T.; Park, J.; Choi, Y.S.; Ryu, H.; Lee, H.J. Temperature-dependent growth of vertically aligned carbon nanotubes in the range 800–1100 °C. J. Phys. Chem. B 2002, 106, 7614–7618. [Google Scholar] [CrossRef]
- Hofmann, S.; Ducati, C.; Robertson, J.; Kleinsorge, B. Low-temperature growth of carbon nanotubes by plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2003, 83, 135–137. [Google Scholar] [CrossRef]
- Lee, Y.T.; Kim, N.S.; Park, J.; Han, J.B.; Choi, Y.S.; Ryu, H.; Lee, H.J. Temperature-dependent growth of carbon nanotubes by pyrolysis of ferrocene and acetylene in the range between 700 and 1000 °C. Chem. Phys. Lett. 2003, 372, 853–859. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, Y.T.; Park, J.; Han, J.B.; Choi, Y.S.; Choi, S.Y.; Choo, J.; Lee, G.H. Vertically aligned carbon nanotubes grown by pyrolysis of iron, cobalt, and nickel phthalocyanines. J. Phys. Chem. B 2003, 107, 9249–9255. [Google Scholar] [CrossRef]
- Lee, Y.T.; Kim, N.S.; Bae, S.Y.; Park, J.; Yu, S.C.; Ryu, H.; Lee, H.J. Growth of vertically aligned nitrogen-doped carbon nanotubes: Control of the nitrogen content over the temperature range 900–1100 °C. J. Phys. Chem. B 2003, 107, 12958–12963. [Google Scholar] [CrossRef]
- Perez-Cabero, M.; Romeo, E.; Royo, C.; Monzon, A.; Guerrero-Ruiz, A.; Rodriguez-Ramos, I. Growing mechanism of CNTs: A kinetic approach. J. Catal. 2004, 224, 197–205. [Google Scholar] [CrossRef]
- Bartsch, K.; Biedermann, K.; Gemming, T.; Leonhardt, A. On the diffusion-controlled growth of multiwalled carbon nanotubes. J. Appl. Phys. 2005, 97, 114301. [Google Scholar] [CrossRef]
- Kim, K.E.; Kim, K.J.; Jung, W.S.; Bae, S.Y.; Park, J.; Choi, J.; Choo, J. Investigation on the temperature-dependent growth rate of carbon nanotubes using chemical vapor deposition of ferrocene and acetylene. Chem. Phys. Lett. 2005, 401, 459–464. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, K.L.; Feng, C.; Chen, Z.; Fan, S.S. A growth mark method for studying growth mechanism of carbon nanotube arrays. Carbon 2005, 43, 2850–2856. [Google Scholar] [CrossRef]
- Hofmann, S.; Csanyi, G.; Ferrari, A.C.; Payne, M.C.; Robertson, J. Surface diffusion: The low activation energy path for nanotube growth. Phys. Rev. Lett. 2005, 95, 036101. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Geohegan, D.B.; Jesse, S.; Ivanov, I.N.; Eres, G. In situ measurements and modeling of carbon nanotube array growth kinetics during chemical vapor deposition. Appl. Phys. A 2005, 81, 223–240. [Google Scholar] [CrossRef]
- Kim, S.H.; Zachariah, M.R. In-flight kinetic measurements of the aerosol growth of carbon nanotubes by electrical mobility classification. J. Phys. Chem. B 2006, 110, 4555–4562. [Google Scholar] [CrossRef]
- Ni, L.; Kuroda, K.; Zhou, L.P.; Kizuka, T.; Ohta, K.; Matsuishi, K.; Nakamura, J. Kinetic study of carbon nanotube synthesis over Mo/Co/MgO catalysts. Carbon 2006, 44, 2265–2272. [Google Scholar] [CrossRef]
- Zhu, L.B.; Xu, J.W.; Xiao, F.; Jiang, H.J.; Hess, D.W.; Wong, C.P. The growth of carbon nanotube stacks in the kinetics-controlled regime. Carbon 2007, 45, 344–348. [Google Scholar] [CrossRef]
- Bronikowski, M.J. Longer nanotubes at lower temperatures: The influence of effective activation energies on carbon nanotube growth by thermal chemical vapor deposition. J. Phys. Chem. C 2007, 111, 17705–17712. [Google Scholar] [CrossRef]
- Chiang, W.H.; Sankaran, R.M. Microplasma synthesis of metal nanoparticles for gas-phase studies of catalyzed carbon nanotube growth. Appl. Phys. Lett. 2007, 91, 121503. [Google Scholar] [CrossRef]
- Pal, S.K.; Talapatra, S.; Kar, S.; Ci, L.; Vajtai, R.; Borca-Tasciuc, T.; Schadler, L.S.; Ajayan, P.M. Time and temperature dependence of multi-walled carbon nanotube growth on Inconel 600. Nanotechnology 2008, 19, 045610. [Google Scholar] [CrossRef]
- Wirth, C.T.; Zhang, C.; Zhong, G.F.; Hofmann, S.; Robertson, J. Diffusion- and Reaction-Limited Growth of Carbon Nanotube Forests. ACS Nano 2009, 3, 3560–3566. [Google Scholar] [CrossRef] [PubMed]
- Meshot, E.R.; Plata, D.L.; Tawfick, S.; Zhang, Y.Y.; Verploegen, E.A.; Hart, A.J. Engineering Vertically Aligned Carbon Nanotube Growth by Decoupled Thermal Treatment of Precursor and Catalyst. ACS Nano 2009, 3, 2477–2486. [Google Scholar] [CrossRef]
- Nessim, G.D.; Seita, M.; O’Brien, K.P.; Hart, A.J.; Bonaparte, R.K.; Mitchell, R.R.; Thompson, C.V. Low Temperature Synthesis of Vertically Aligned Carbon Nanotubes with Electrical Contact to Metallic Substrates Enabled by Thermal Decomposition of the Carbon Feedstock. Nano Lett. 2009, 9, 3398–3405. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, Y.T.; Chen, W.Y.; Wei, J.L. Parameter setting on growth of carbon nanotubes over transition metal/alumina catalysts in a fluidized bed reactor. Powder Technol. 2009, 192, 16–22. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, Y.T.; Lin, J.Y.; Wei, J.L. Synthesis of carbon nanotubes over Niand Co-supported CaCO3 catalysts using catalytic chemical vapor deposition. Mater. Chem. Phys. 2009, 114, 702–708. [Google Scholar] [CrossRef]
- In, J.B.; Grigoropoulos, C.P.; Chernov, A.A.; Noy, A. Growth Kinetics of Vertically Aligned Carbon Nanotube Arrays in Clean Oxygen-free Conditions. ACS Nano 2011, 5, 9602–9610. [Google Scholar] [CrossRef] [PubMed]
- Van de Burgt, Y.; Bellouard, Y.; Mandamparambil, R. Kinetics of laser-assisted carbon nanotube growth. Phys. Chem. Chem. Phys. 2014, 16, 5162–5173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voelskow, K.; Becker, M.J.; Xia, W.; Muhler, M.; Turek, T. The influence of kinetics, mass transfer and catalyst deactivation on the growth rate of multiwalled carbon nanotubes from ethene on a cobalt-based catalyst. Chem. Eng. J. 2014, 244, 68–74. [Google Scholar] [CrossRef]
- Le Claire, A.D. Diffusion in solid metals and alloys. In Landolt-Börnstein: Numerical Data and Functional Rerelationships in Science and Technology; Mehrer, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 26, p. 471. [Google Scholar]
- Sharma, R.; Chee, S.W.; Herzing, A.; Miranda, R.; Rez, P. Evaluation of the Role of Au in Improving Catalytic Activity of Ni Nanoparticles for the Formation of One-Dimensional Carbon Nanostructures. Nano Lett. 2011, 11, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Saeys, M. First principles study of the stability and the formation kinetics of subsurface and bulk carbon on a Ni catalyst. J. Phys. Chem. C 2008, 112, 9679–9685. [Google Scholar] [CrossRef]
- Lander, J.J.; Kern, H.E.; Beach, A.L. Solubility and Diffusion Coefficient of Carbon in Nickel—Reaction Rates of Nickel-Carbon Alloys with Barium Oxide. J. Appl. Phys. 1952, 23, 1305–1309. [Google Scholar] [CrossRef]
- Diamond, S.; Wert, C. Diffusion of Carbon in Nickel above and below Curie Temperature. Trans. Met. Soc. AIME 1967, 239, 705. [Google Scholar]
- Kovenskiy, I.I. Phys. Metals Metallogr.1693, 16, 107.
- Cermak, J.; Rollert, F.; Mehrer, H. Diffusion of C-14 in Fcc Cobalt. Z. Metallk. 1990, 81, 81–83. [Google Scholar]
- Andrievsky, R.; Gurov, K. Self-diffusion in interstitial phases. Fiz. Met. Metalloved. 1968, 26, 818–822. [Google Scholar]
- Leng, Y.G.; Shao, H.Y.; Wang, Y.T.; Suzuki, M.; Li, X.G. A new method to synthesize nickel carbide (Ni3C) nanoparticles in solution. J. Nanosci. Nanotechnol. 2006, 6, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, S.; Levenson, L.L. Formation and Decomposition of Nickel Carbide in Evaporated Nickel Films on Graphite. Thin Solid Films 1978, 53, 31–36. [Google Scholar] [CrossRef]
- Kovacs, G.J.; Bertoti, I.; Radnoczi, G. X-ray photoelectron spectroscopic study of magnetron sputtered carbon-nickel composite films. Thin Solid Films 2008, 516, 7942–7946. [Google Scholar] [CrossRef]
- Kitaura, R.; Imazu, N.; Kobayashi, K.; Shinohara, H. Fabrication of metal nanowires in carbon nanotubes via versatile nano-template reaction. Nano Lett. 2008, 8, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, R.; Nakanishi, R.; Saito, T.; Yoshikawa, H.; Awaga, K.; Shinohara, H. High-Yield Synthesis of Ultrathin Metal Nanowires in Carbon Nanotubes. Angew. Chem.-Int. Ed. 2009, 48, 8298–8302. [Google Scholar] [CrossRef]
- Meunier, V.; Muramatsu, H.; Hayashi, T.; Kim, Y.A.; Shimamoto, D.; Terrones, H.; Dresselhaus, M.S.; Terrones, M.; Endo, M.; Sumpter, B.G. Properties of One-Dimensional Molybdenum Nanowires in a Confined Environment. Nano Lett. 2009, 9, 1487–1492. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, P.J.; Pan, X.L.; Liu, H.Y.; Li, M.R.; Bao, X.H. Self-Assembly of Atomically Thin and Unusual Face-Centered Cubic Re Nanowires within Carbon Nanotubes. Chem. Mater. 2015, 27, 1569–1573. [Google Scholar] [CrossRef]
- Sloan, J.; Wright, D.M.; Woo, H.G.; Bailey, S.; Brown, G.; York, A.P.E.; Coleman, K.S.; Hutchison, J.L.; Green, M.L.H. Capillarity and silver nanowire formation observed in single walled carbon nanotubes. Chem. Commun. 1999, 699–700. [Google Scholar] [CrossRef]
- Grobert, N.; Mayne, M.; Terrones, M.; Sloan, J.; Dunin-Borkowski, R.E.; Kamalakaran, R.; Seeger, T.; Terrones, H.; Ruhle, M.; Walton, D.R.M.; et al. Alloy nanowires: Invar inside carbon nanotubes. Chem. Commun. 2001, 471–472. [Google Scholar] [CrossRef]
- Bao, J.C.; Tie, C.; Xu, Z.; Suo, Z.Y.; Zhou, Q.F.; Hong, J.M. A facile method for creating an array of metal-filled carbon nanotubes. Adv. Mater. 2002, 14, 1483–1486. [Google Scholar] [CrossRef]
- Satishkumar, B.C.; Govindaraj, A.; Vanitha, P.V.; Raychaudhuri, A.K.; Rao, C.N.R. Barkhausen jumps and related magnetic properties of iron nanowires encapsulated in aligned carbon nanotube bundles. Chem. Phys. Lett. 2002, 362, 301–306. [Google Scholar] [CrossRef]
- Elias, A.L.; Rodriguez-Manzo, J.A.; McCartney, M.R.; Golberg, D.; Zamudio, A.; Baltazar, S.E.; Lopez-Urias, F.; Munoz-Sandoval, E.; Gu, L.; Tang, C.C.; et al. Production and characterization of single-crystal FeCo nanowires inside carbon nanotubes. Nano Lett. 2005, 5, 467–472. [Google Scholar] [CrossRef]
- Guan, L.; Suenaga, K.; Okubo, S.; Okazaki, T.; Lijima, S. Metallic wires of lanthanum atoms inside carbon nanotubes. J. Am. Chem. Soc. 2008, 130, 2162–2163. [Google Scholar] [CrossRef]
- Muramatsu, H.; Hayashi, T.; Kim, Y.A.; Shimamoto, D.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Synthesis and isolation of molybdenum atomic wires. Nano Lett. 2008, 8, 237–240. [Google Scholar] [CrossRef]
- Page, A.J.; Minami, S.; Ohta, Y.; Irle, S.; Morokuma, K. Comparison of single-walled carbon nanotube growth from Fe and Ni nanoparticles using quantum chemical molecular dynamics methods. Carbon 2010, 48, 3014–3026. [Google Scholar] [CrossRef]
- Sen, R.; Govindaraj, A.; Rao, C.N.R. Carbon nanotubes by the metallocene route. Chem. Phys. Lett. 1997, 267, 276–280. [Google Scholar] [CrossRef]
- Karaeva, A.R.; Urvanov, S.A.; Kazennov, N.V.; Mitberg, E.B.; Mordkovich, V.Z. Synthesis, Structure and Electrical Resistivity of Carbon Nanotubes Synthesized over Group VIII Metallocenes. Nanomaterials 2020, 10, 2279. [Google Scholar] [CrossRef]
- Dong, L.; Park, J.G.; Leonhardt, B.E.; Zhang, S.; Liang, R. Continuous Synthesis of Double-Walled Carbon Nanotubes withWater-Assisted Floating Catalyst Chemical Vapor Deposition. Nanomaterials 2020, 10, 365. [Google Scholar] [CrossRef]
- Abdullah, H.B.; Ramli, I.; Ismail, I.; Yusof, N.A. Synthesis and mechanism perspectives of a carbon nanotube aerogel via a floating catalyst chemical vapour deposition method. Bull. Mater. Sci. 2019, 42, 241. [Google Scholar] [CrossRef]
- Mordkovich, V.Z.; Kazennov, N.V.; Ermolaev, V.S.; Zhukova, E.A.; Karaeva, A.R. Scaled-up process for producing longer carbon nanotubes and carbon cotton bymacro-spools. Diam. Relat. Mater. 2018, 83, 15–20. [Google Scholar] [CrossRef]
- Shandakov, S.D.; Kosobutsky, A.V.; Rybakov, M.S.; Sevostyanov, O.G.; Russakov, D.M.; Lomakin, M.V.; Vershinina, A.I.; Chirkova, I.M. Effect of gaseous and condensate products of ethanol decomposition on aerosol CVD synthesis of single-walled carbon nanotubes. Carbon 2018, 126, 522–531. [Google Scholar] [CrossRef]
- Ambriz-Torres, J.M.; Granados-Martínez, F.G.; Contreras-Navarrete, J.D.J.; Gutiérrez-García, C.J.; García-Ruiz, D.L.; Mondragón-Sánchez, M.D.L.; Hernández-Cristóbal, O.; Arredondo-León, Y.; García, L.; Zamora-Peredo, L.; et al. Carbon nanotubes and carbon nanobeads synthesis by one-pot chemical vapor deposition method: Morphology and crystallinity. Mater. Res. Express 2018, 5, 085008. [Google Scholar] [CrossRef]
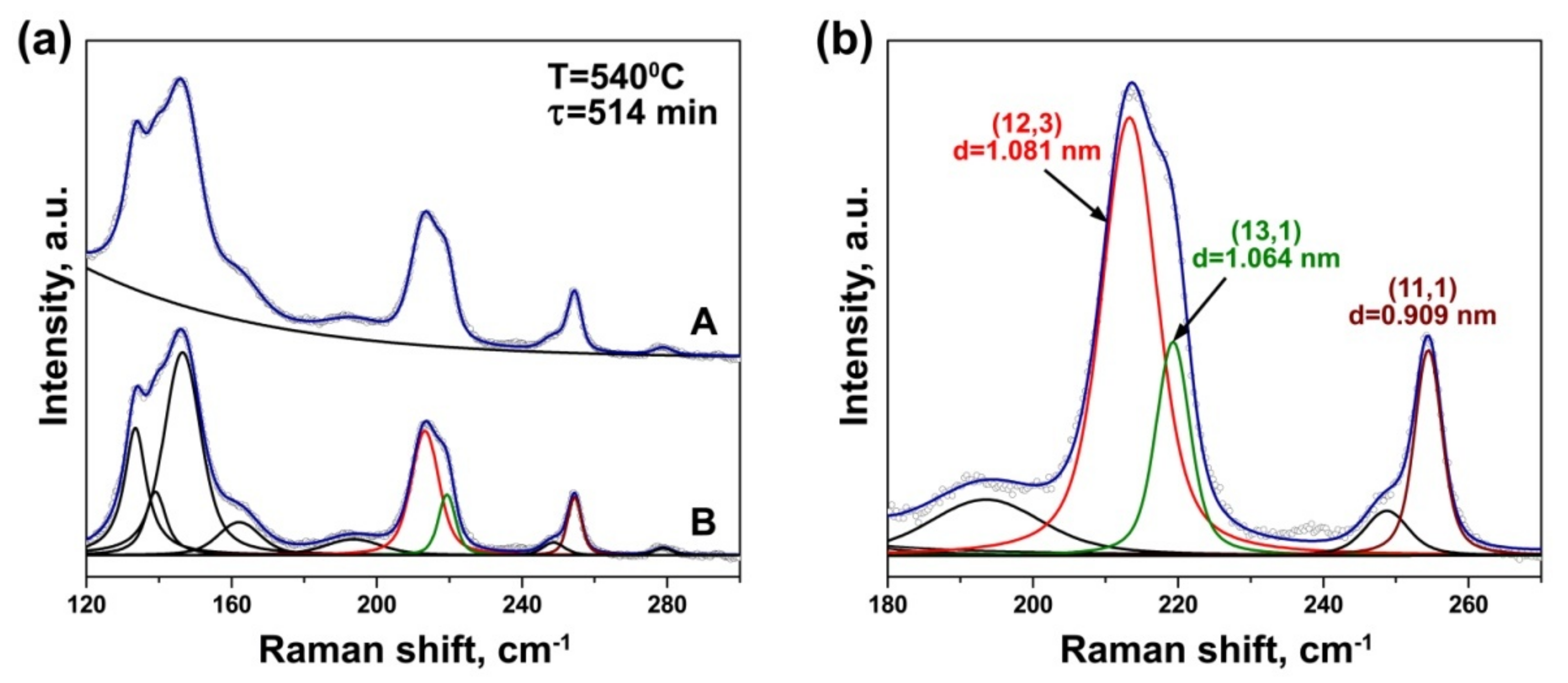
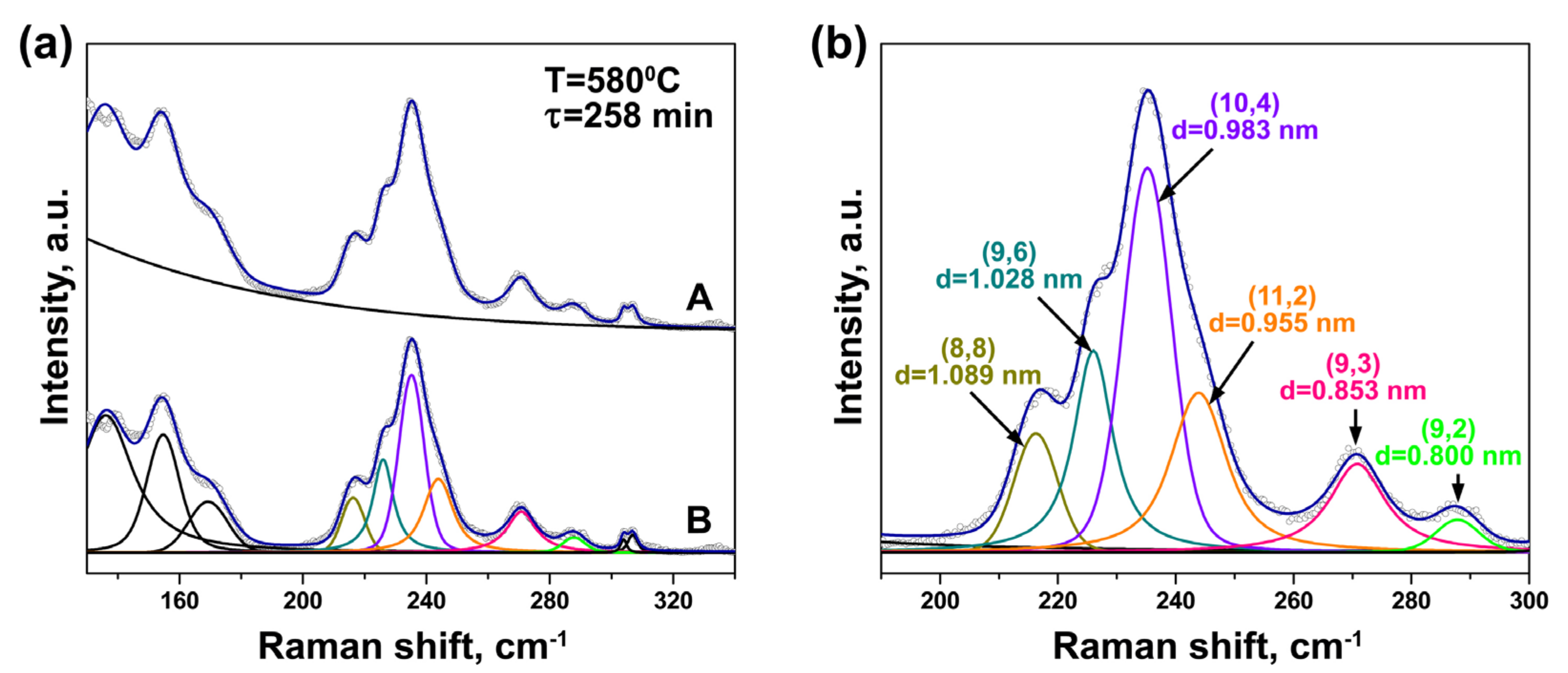

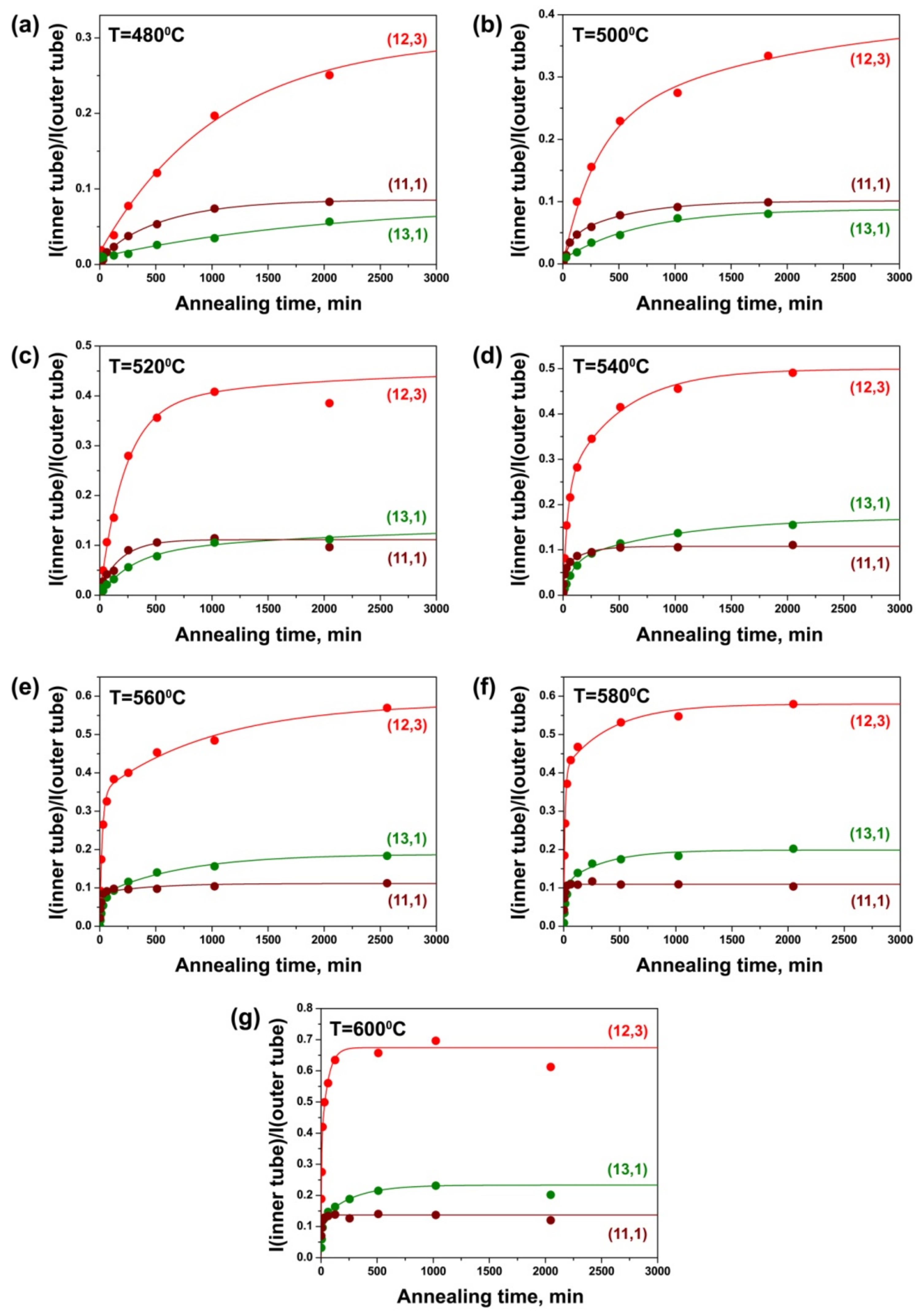
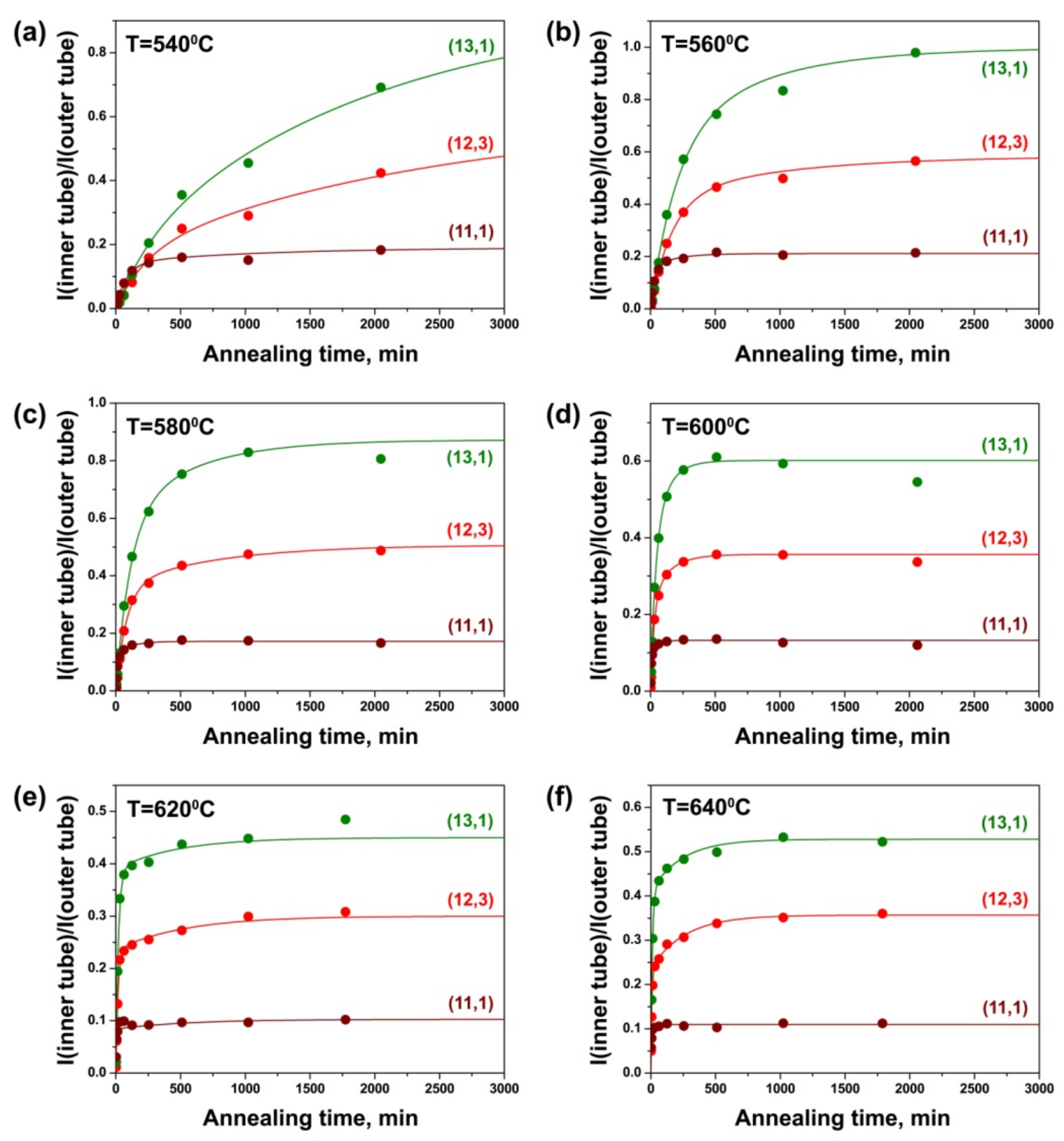
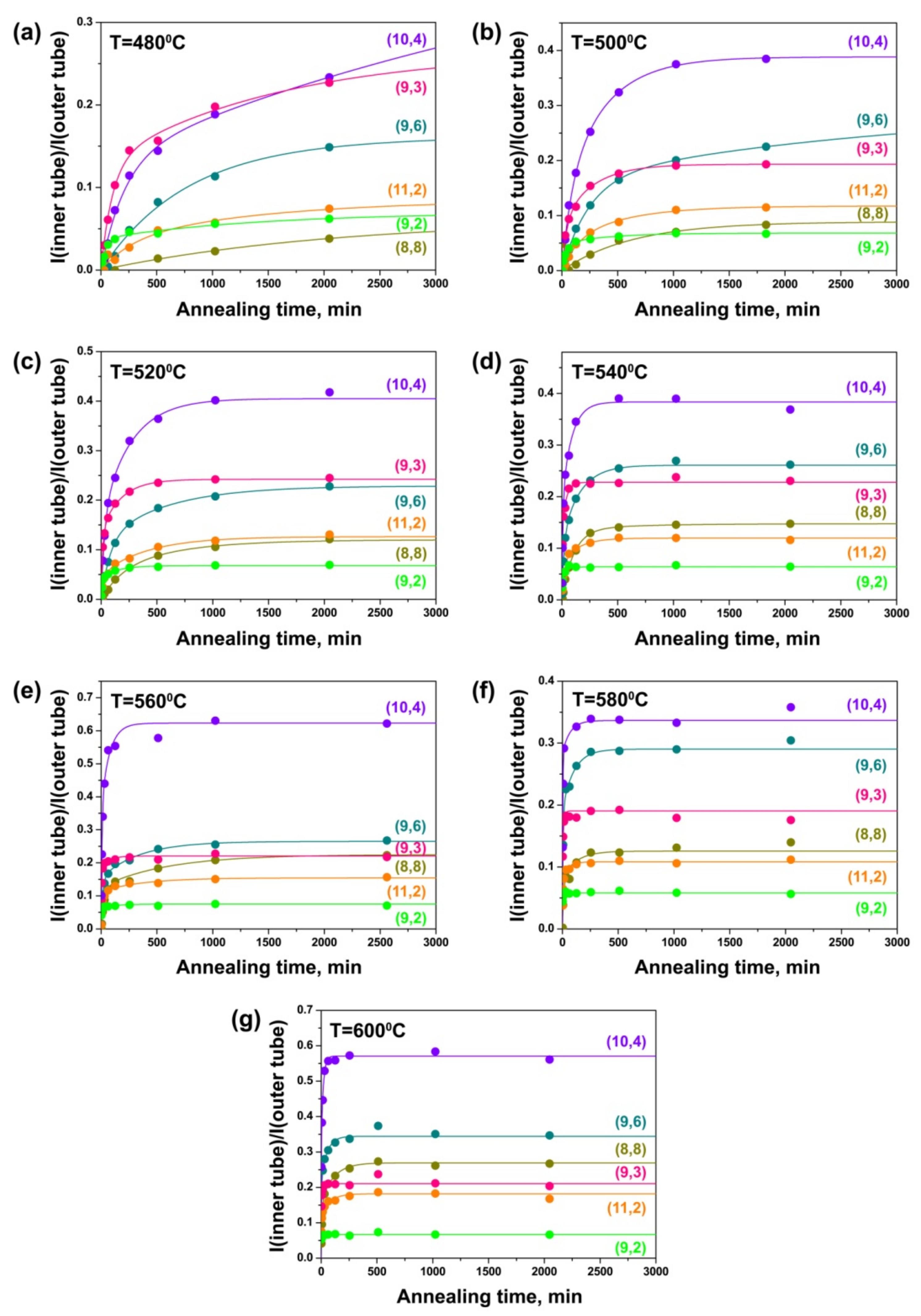

| Laser Wavelength | RBM Peak Position (cm−1) | Inner Nanotube Chirality | Inner Nanotube Diameter (nm) | Inner Nanotube Chiral Angle (°) | Excited Electronic Transition |
|---|---|---|---|---|---|
| 633 nm | 214 | (12,3) | 1.081 | 10.8 | |
| 219 | (13,1) | 1.064 | 3.7 | ||
| 254 | (11,1) | 0.909 | 4.3 | ||
| 568 nm | 215 | (8,8) | 1.089 | 30.0 | |
| 226 | (9,6) | 1.028 | 23.3 | ||
| 235 | (10,4) | 0.983 | 16.0 | ||
| 240 | (11,2) | 0.955 | 8.1 | ||
| 269 | (9,3) | 0.853 | 13.8 | ||
| 285 | (9,2) | 0.800 | 9.7 |
| Inner Nanotube Chirality | Activation Energy (eV) | |||
|---|---|---|---|---|
| Precursor | ||||
| NiCp2 | CoCp2 | |||
| Eα | Eβ | Eα | Eβ | |
| (8,8) | 2.57 ± 0.16 | 1.58 ± 0.26 | 2.64 ± 0.31 | 0.95 ± 0.52 |
| (12,3) | 2.57 ± 0.19 | 1.74 ± 0.33 | 2.48 ± 0.09 | 0.99 ± 0.28 |
| (13,1) | 2.36 ± 0.16 | 1.53 ± 0.21 | 2.43 ± 0.15 | 0.77 ± 0.39 |
| (9,6) | 2.35 ± 0.11 | 1.50 ± 0.16 | 2.43 ± 0.11 | 0.81 ± 0.33 |
| (10,4) | 2.08 ± 0.17 | 1.49 ± 0.28 | 2.71 ± 0.13 | 1.07 ± 0.48 |
| (11,2) | 2.30 ± 0.25 | 1.49 ± 0.33 | 2.34 ± 0.25 | 1.31 ± 0.41 |
| (11,1) | 1.85 ± 0.21 | 1.62 ± 0.14 | 2.28 ± 0.12 | 1.79 ± 0.85 |
| (9,3) | 2.18 ± 0.18 | 1.91 ± 0.37 | 1.80 ± 0.25 | 1.75 ± 0.65 |
| (9,2) | 2.17 ± 0.11 | 1.85 ± 0.62 | 1.81 ± 0.21 | 1.63 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharlamova, M.V.; Kramberger, C. Metal Cluster Size-Dependent Activation Energies of Growth of Single-Chirality Single-Walled Carbon Nanotubes inside Metallocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 2649. https://doi.org/10.3390/nano11102649
Kharlamova MV, Kramberger C. Metal Cluster Size-Dependent Activation Energies of Growth of Single-Chirality Single-Walled Carbon Nanotubes inside Metallocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials. 2021; 11(10):2649. https://doi.org/10.3390/nano11102649
Chicago/Turabian StyleKharlamova, Marianna V., and Christian Kramberger. 2021. "Metal Cluster Size-Dependent Activation Energies of Growth of Single-Chirality Single-Walled Carbon Nanotubes inside Metallocene-Filled Single-Walled Carbon Nanotubes" Nanomaterials 11, no. 10: 2649. https://doi.org/10.3390/nano11102649
APA StyleKharlamova, M. V., & Kramberger, C. (2021). Metal Cluster Size-Dependent Activation Energies of Growth of Single-Chirality Single-Walled Carbon Nanotubes inside Metallocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials, 11(10), 2649. https://doi.org/10.3390/nano11102649







