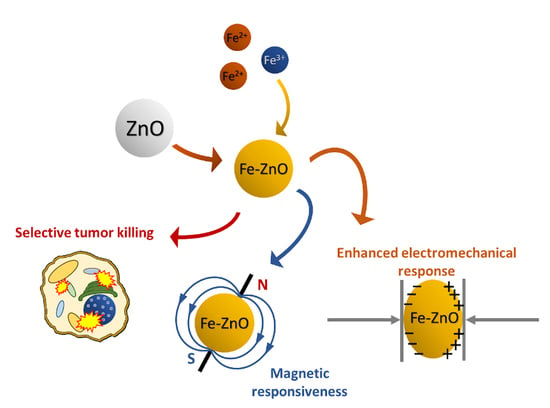
Iron-Doped ZnO Nanoparticles as Multifunctional Nanoplatforms for Theranostics
Abstract
1. Introduction
2. Materials and Methods
2.1. ZnO and Fe:ZnO Nanoparticle Synthesis Procedure
2.2. ZnO and Fe:ZnO Nanoparticle Functionalization Procedure
2.3. Physicochemical Characterization
2.4. Biological Characterization
2.4.1. Suspension Cell Lines
2.4.2. Adherent Cell Line
2.4.3. Cytotoxicity
2.4.4. Uptake
3. Results and Discussion
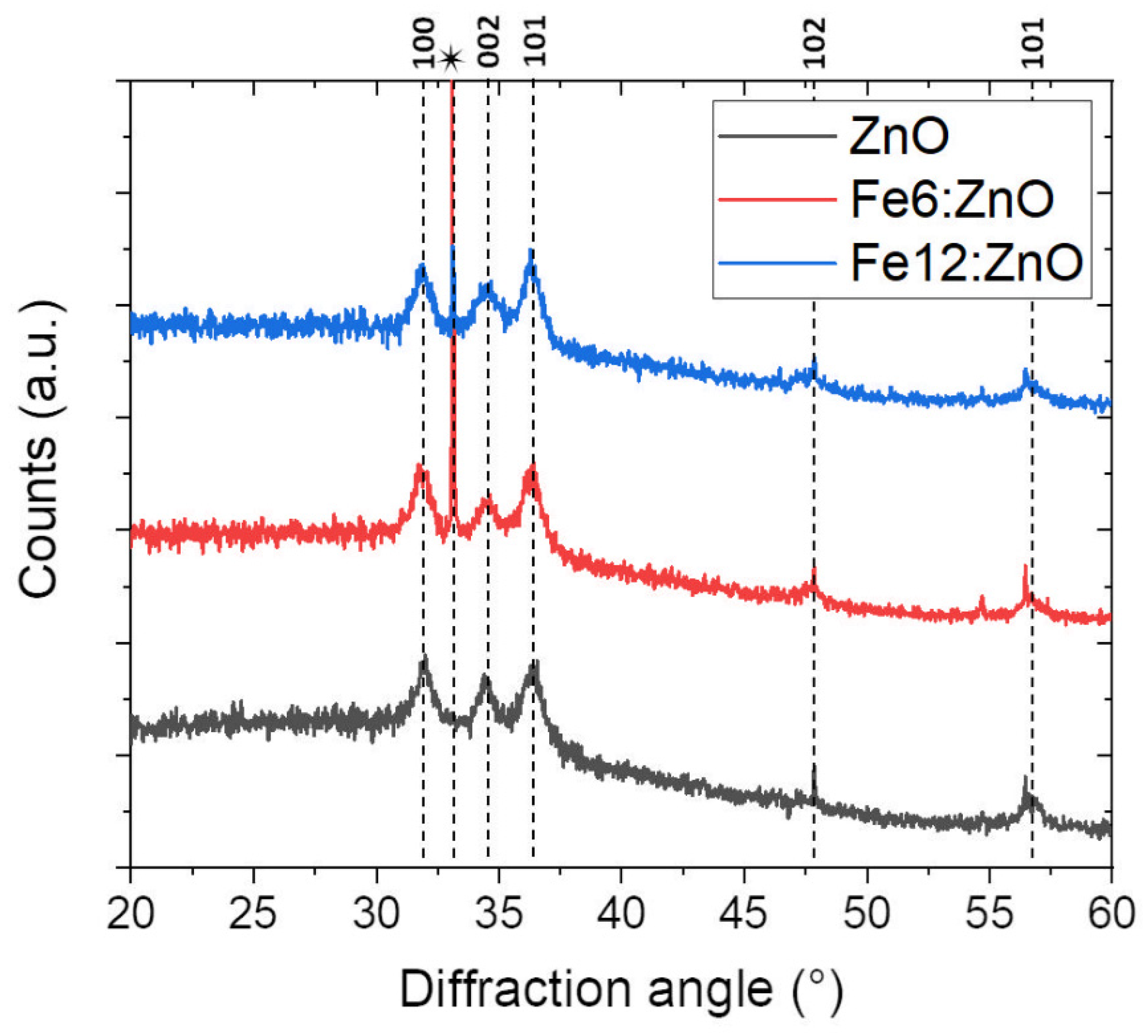
3.1. Structural and Morphological Characterization
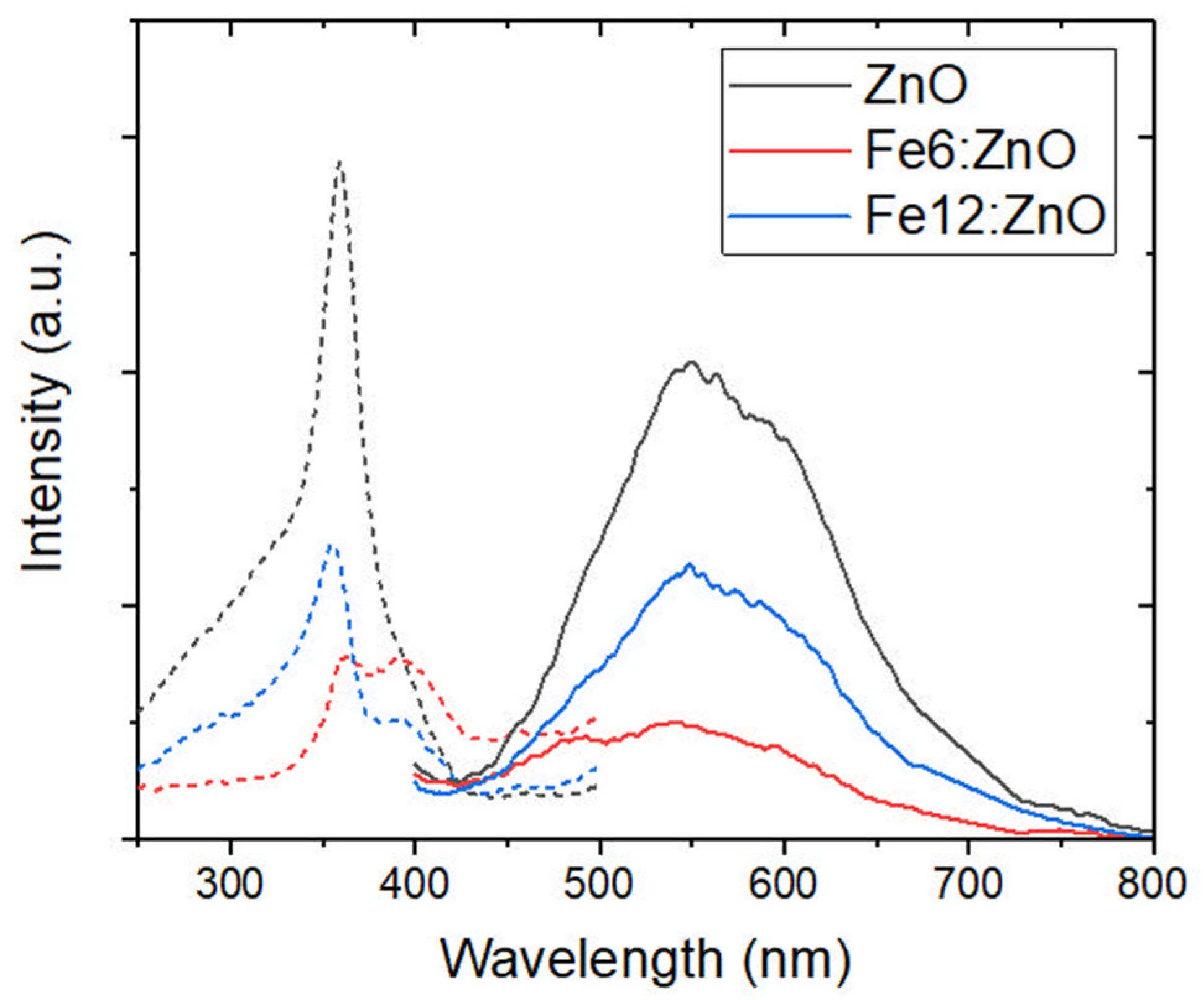
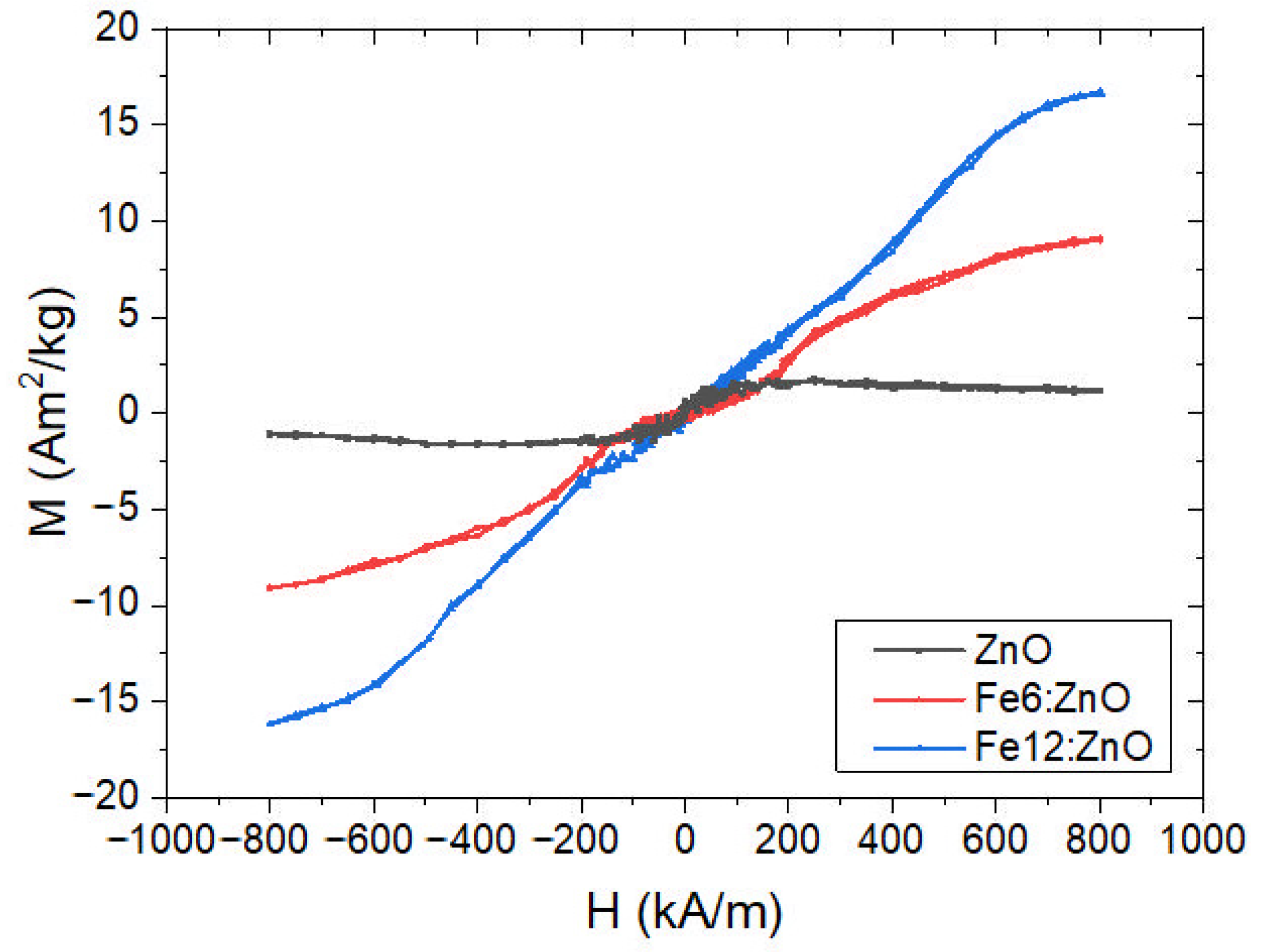
3.2. Optical, Magnetic and Piezoelectric Characterization
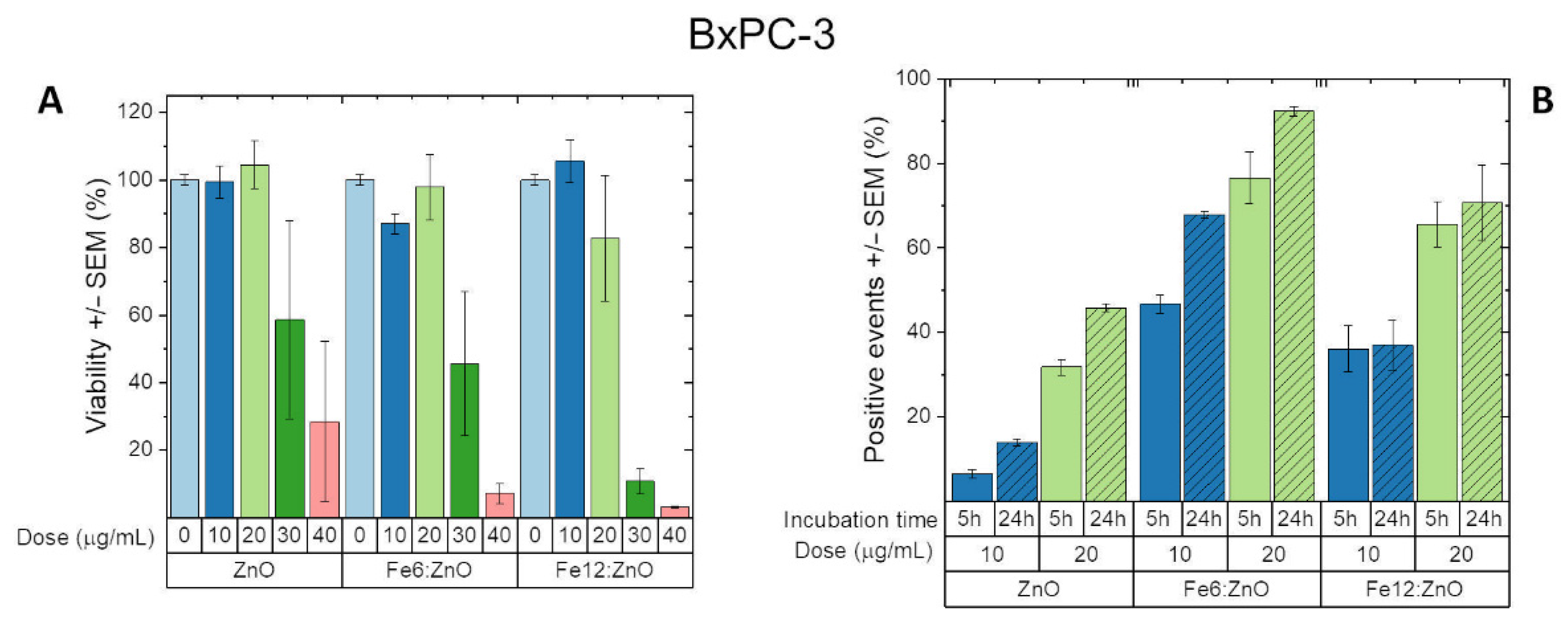
3.3. Biological Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and Drug Delivery Using Theranostic Nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-Based Theranostic Agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles as Controlled Release Drug Delivery and Gene Transfection Carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in Cancer Therapy and Diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Ancona, A.; Dumontel, B.; Garino, N.; Demarco, B.; Chatzitheodoridou, D.; Fazzini, W.; Engelke, H.; Cauda, V. Lipid-Coated Zinc Oxide Nanoparticles as Innovative ROS-Generators for Photodynamic Therapy in Cancer Cells. Nanomaterials 2018, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Racca, L.; Limongi, T.; Vighetto, V.; Dumontel, B.; Ancona, A.; Canta, M.; Canavese, G.; Garino, N.; Cauda, V. Zinc Oxide Nanocrystals and High-Energy Shock Waves: A New Synergy for the Treatment of Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-Assisted Ultrasound: A Special Focus on Sonodynamic Therapy against Cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Brazzale, C.; Canaparo, R.; Racca, L.; Foglietta, F.; Durando, G.; Fantozzi, R.; Caliceti, P.; Salmaso, S.; Serpe, L. Enhanced Selective Sonosensitizing Efficacy of Ultrasound-Based Anticancer Treatment by Targeted Gold Nanoparticles. Nanomedicine 2016, 11, 3053–3070. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Cauda, V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Dumontel, B.; Susa, F.; Limongi, T.; Canta, M.; Racca, L.; Chiodoni, A.; Garino, N.; Chiabotto, G.; Centomo, M.L.; Pignochino, Y.; et al. ZnO Nanocrystals Shuttled by Extracellular Vesicles as Effective Trojan Nano-Horses against Cancer Cells. Nanomedicine 2019, 14, 2815–2833. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential Killing of Cancer Cells and Activated Human T Cells Using ZnO Nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and Applications of ZnO Nanostructures (ZONSs): A Review. Crit. Rev. Solid State Mater. Sci. 2021, 1–43. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Marino, A.; Arai, S.; Hou, Y.; Sinibaldi, E.; Pellegrino, M.; Chang, Y.-T.; Mazzolai, B.; Mattoli, V.; Suzuki, M.; Ciofani, G. Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation. ACS Nano 2015, 9, 7678–7689. [Google Scholar] [CrossRef]
- Genchi, G.G.; Ceseracciu, L.; Marino, A.; Labardi, M.; Marras, S.; Pignatelli, F.; Bruschini, L.; Mattoli, V.; Ciofani, G. P(VDF-TrFE)/BaTiO3 Nanoparticle Composite Films Mediate Piezoelectric Stimulation and Promote Differentiation of SH-SY5Y Neuroblastoma Cells. Adv. Healthc. Mater. 2016, 5, 1808–1820. [Google Scholar] [CrossRef]
- Marino, A.; Almici, E.; Migliorin, S.; Tapeinos, C.; Battaglini, M.; Cappello, V.; Marchetti, M.; de Vito, G.; Cicchi, R.; Pavone, F.S.; et al. Piezoelectric Barium Titanate Nanostimulators for the Treatment of Glioblastoma Multiforme. J. Colloid Interface Sci. 2019, 538, 449–461. [Google Scholar] [CrossRef]
- Yoon, J.-K.; Misra, M.; Yu, S.J.; Kim, H.Y.; Bhang, S.H.; Song, S.Y.; Lee, J.-R.; Ryu, S.; Choo, Y.W.; Jeong, G.-J.; et al. Thermosensitive, Stretchable, and Piezoelectric Substrate for Generation of Myogenic Cell Sheet Fragments from Human Mesenchymal Stem Cells for Skeletal Muscle Regeneration. Adv. Funct. Mater. 2017, 27, 1703853. [Google Scholar] [CrossRef]
- Vighetto, V.; Ancona, A.; Racca, L.; Limongi, T.; Troia, A.; Canavese, G.; Cauda, V. The Synergistic Effect of Nanocrystals Combined With Ultrasound in the Generation of Reactive Oxygen Species for Biomedical Applications. Front. Bioeng. Biotechnol. 2019, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications. Nanomaterials 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef] [PubMed]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Xiong, H.-M. Photoluminescent ZnO Nanoparticles and Their Biological Applications. Materials 2015, 8, 3101–3127. [Google Scholar] [CrossRef]
- Laurenti, M.; Castellino, M.; Perrone, D.; Asvarov, A.; Canavese, G.; Chiolerio, A. Lead-Free Piezoelectrics: V 3+ to V 5+ Ion Conversion Promoting the Performances of V-Doped Zinc Oxide. Sci. Rep. 2017, 7, 41957. [Google Scholar] [CrossRef]
- Laurenti, M.; Canavese, G.; Sacco, A.; Fontana, M.; Bejtka, K.; Castellino, M.; Pirri, C.F.; Cauda, V. Nanobranched ZnO Structure: P-Type Doping Induces Piezoelectric Voltage Generation and Ferroelectric–Photovoltaic Effect. Adv. Mater. 2015, 27, 4218–4223. [Google Scholar] [CrossRef]
- Laurenti, M.; Garino, N.; Canavese, G.; Hernandéz, S.; Cauda, V. Piezo- and Photocatalytic Activity of Ferroelectric ZnO:Sb Thin Films for the Efficient Degradation of Rhodamine-β Dye Pollutant. ACS Appl. Mater. Interfaces 2020, 12, 25798–25808. [Google Scholar] [CrossRef]
- Ravichandran, K.; Karthika, K.; Sakthivel, B.; Jabena Begum, N.; Snega, S.; Swaminathan, K.; Senthamilselvi, V. Tuning the Combined Magnetic and Antibacterial Properties of ZnO Nanopowders through Mn Doping for Biomedical Applications. J. Magn. Magn. Mater. 2014, 358–359, 50–55. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, S.; Schmidt, H. Magnetic Properties of ZnO Nanopowders. J. Alloys Compd. 2009, 487, 665–667. [Google Scholar] [CrossRef]
- El-Hilo, M.; Dakhel, A.A. Structural and Magnetic Properties of Mn-Doped ZnO Powders. J. Magn. Magn. Mater. 2011, 323, 2202–2205. [Google Scholar] [CrossRef]
- Barui, S.; Gerbaldo, R.; Garino, N.; Brescia, R.; Laviano, F.; Cauda, V. Facile Chemical Synthesis of Doped ZnO Nanocrystals Exploiting Oleic Acid. Nanomaterials 2020, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.M.; Jappor, H.R.; Al-Marzoki, K.; Al-Hydary, I.A.; Edrees, S.J.; Shukur, M.M. Unraveling the Effect of Gd Doping on the Structural, Optical, and Magnetic Properties of ZnO Based Diluted Magnetic Semiconductor Nanorods. RSC Adv. 2019, 9, 33207–33221. [Google Scholar] [CrossRef]
- Santos, D.A.A.; Macêdo, M.A. Study of the Magnetic and Structural Properties of Mn-, Fe-, and Co-Doped ZnO Powder. Phys. B Condens. Matter 2012, 407, 3229–3232. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Yun, F.; Morkoc, H. Ferromagnetism of ZnO and GaN: A Review. J. Mater. Sci. Mater. Electron. 2015, 16, 555–597. [Google Scholar] [CrossRef]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased Dissolution of ZnO by Iron Doping Yields Nanoparticles with Reduced Toxicity in the Rodent Lung and Zebrafish Embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef]
- Dumontel, B.; Canta, M.; Engelke, H.; Chiodoni, A.; Racca, L.; Ancona, A.; Limongi, T.; Canavese, G.; Cauda, V. Enhanced Biostability and Cellular Uptake of Zinc Oxide Nanocrystals Shielded with a Phospholipid Bilayer. J. Mater. Chem. B 2017, 5, 8799–8813. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; repr. as paperback; Wiley: Chichester, UK, 2010; ISBN 978-0-470-09307-8. [Google Scholar]
- Biesinger, M.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, L.M.; Liao, J.-W.; Peng, Z.-A.; Lai, J.-H. Doping Effects on the Characteristics of Fe:ZnO Films: Valence Transition and Hopping Transport. J. Electrochem. Soc. 2008, 156, H138. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Sharma, S.K.; Vargas, J.M.; Knobel, M.; Gautam, S.; Chae, K.H.; Kim, D.K.; Kim, Y.K.; et al. Structural and Magnetic Properties of Chemically Synthesized Fe Doped ZnO. J. Appl. Phys. 2009, 105, 07C520. [Google Scholar] [CrossRef]
- Luo, J.T.; Yang, Y.C.; Zhu, X.Y.; Chen, G.; Zeng, F.; Pan, F. Enhanced Electromechanical Response of Fe-Doped ZnO Films by Modulating the Chemical State and Ionic Size of the Fe Dopant. Phys. Rev. B 2010, 82, 014116. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Jiang, H.; Wang, C.; Wang, H.; Wang, X. A Strategy for ZnO Nanorod Mediated Multi-Mode Cancer Treatment. Biomaterials 2011, 32, 1906–1914. [Google Scholar] [CrossRef]
- Li, J.; Guo, D.; Wang, X.; Wang, H.; Jiang, H.; Chen, B. The Photodynamic Effect of Different Size ZnO Nanoparticles on Cancer Cell Proliferation In Vitro. Nanoscale Res Lett 2010, 5, 1063–1071. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative Eco-Toxicity of Nanoscale TiO2, SiO2, and ZnO Water Suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Gamage, J.; Zhang, Z. Applications of Photocatalytic Disinfection. Int. J. Photoenergy 2010, 2010, e764870. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-Gap Determination from Diffuse Reflectance Measurements of Semiconductor Films, and Application to Photoelectrochemical Water-Splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Gaur, L.K.; Gairola, P.; Gairola, S.P.; Mathpal, M.C.; Kumar, P.; Kumar, S.; Kushavah, D.; Agrahari, V.; Aragon, F.F.H.; Soler, M.A.G.; et al. Cobalt Doping Induced Shape Transformation and Its Effect on Luminescence in Zinc Oxide Rod-like Nanostructures. J. Alloys Compd. 2021, 868, 159189. [Google Scholar] [CrossRef]
- Fan, H.J.; Scholz, R.; Kolb, F.M.; Zacharias, M.; Gösele, U.; Heyroth, F.; Eisenschmidt, C.; Hempel, T.; Christen, J. On the Growth Mechanism and Optical Properties of ZnO Multi-Layer Nanosheets. Appl. Phys. A 2004, 79, 1895–1900. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Cao, H.; Chang, R.P.H. Growth Mechanism and Properties of ZnO Nanorods Synthesized by Plasma-Enhanced Chemical Vapor Deposition. J. Appl. Phys. 2004, 95, 3141–3147. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical Properties of ZnO Nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- Srinivasulu, T.; Saritha, K.; Reddy, K.T.R. Synthesis and Characterization of Fe-Doped ZnO Thin Films Deposited by Chemical Spray Pyrolysis. Mod. Electron. Mater. 2017, 3, 76–85. [Google Scholar] [CrossRef]
- Beltrán, J.J.; Barrero, C.A.; Punnoose, A. Understanding the Role of Iron in the Magnetism of Fe Doped ZnO Nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 15284–15296. [Google Scholar] [CrossRef]
- Garcia, M.A.; Merino, J.M.; Fernández Pinel, E.; Quesada, A.; de la Venta, J.; Ruíz González, M.L.; Castro, G.R.; Crespo, P.; Llopis, J.; González-Calbet, J.M.; et al. Magnetic Properties of ZnO Nanoparticles. Nano Lett. 2007, 7, 1489–1494. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.Q.; Wang, D.D.; Gong, J.; Cao, C.Y.; Tang, Z.; Huang, L.R. Effect of Thermal Annealing on the Structure and Magnetism of Fe-Doped ZnO Nanocrystals Synthesized by Solid State Reaction. J. Magn. Magn. Mater. 2010, 322, 3642–3647. [Google Scholar] [CrossRef]
- Chen, Z.C.; Zhuge, L.J.; Wu, X.M.; Meng, Y.D. Initial Study on the Structure and Optical Properties of Zn1−xFexO Films. Thin Solid Film. 2007, 515, 5462–5465. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, D.; Lin, F.; Shi, W.; Ma, X. Fe-Doped ZnO Magnetic Semiconductor by Mechanical Alloying. J. Alloys Compd. 2007, 436, 30–33. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; He, Y.; Li, L.; Zhang, D. Structure, Morphology and Properties of Fe-Doped ZnO Films Prepared by Facing-Target Magnetron Sputtering System. Appl. Surf. Sci. 2009, 255, 6881–6887. [Google Scholar] [CrossRef]
- Kumar, K.; Chitkara, M.; Sandhu, I.S.; Mehta, D.; Kumar, S. Photocatalytic, Optical and Magnetic Properties of Fe-Doped ZnO Nanoparticles Prepared by Chemical Route. J. Alloys Compd. 2014, 588, 681–689. [Google Scholar] [CrossRef]
- Cheng, W.; Ma, X. Structural, Optical and Magnetic Properties of Fe-Doped ZnO. J. Phys. Conf. Ser. 2009, 152, 012039. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Das Sarma, S.; Millis, A.J. Transition Temperature of Ferromagnetic Semiconductors: A Dynamical Mean Field Study. Phys. Rev. Lett. 2001, 87, 227202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Zhou, X.L.; Miao, H.C.; Cai, H.R.; Li, F.X. Mechanism of Crystal-Symmetry Dependent Deformation in Ferroelectric Ceramics: Experiments versus Model. J. Appl. Phys. 2013, 113, 214111. [Google Scholar] [CrossRef]
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Rossetti, G.A.; Rödel, J. BaTiO3-Based Piezoelectrics: Fundamentals, Current Status, and Perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef]
- Pan, F.; Luo, J.; Yang, Y.; Wang, X.; Zeng, F. Giant Piezoresponse and Promising Application of Environmental Friendly Small-Ion-Doped ZnO. Sci. China Technol. Sci. 2012, 55, 421–436. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Lorenzoni, M.; Fontana, M.; Canavese, G.; Cauda, V.; Pirri, C.F. Evaluation of the Piezoelectric Properties and Voltage Generation of Flexible Zinc Oxide Thin Films. Nanotechnology 2015, 26, 215704. [Google Scholar] [CrossRef]
- Laurenti, M.; Verna, A.; Chiolerio, A. Evidence of Negative Capacitance in Piezoelectric ZnO Thin Films Sputtered on Interdigital Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 24470–24479. [Google Scholar] [CrossRef]
- Manshian, B.B.; Pokhrel, S.; Himmelreich, U.; Tämm, K.; Sikk, L.; Fernández, A.; Rallo, R.; Tamm, T.; Mädler, L.; Soenen, S.J. In Silico Design of Optimal Dissolution Kinetics of Fe-Doped ZnO Nanoparticles Results in Cancer-Specific Toxicity in a Preclinical Rodent Model. Adv. Healthc. Mater. 2017, 6, 1601379. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective Toxicity of ZnO Nanoparticles toward Gram-Positive Bacteria and Cancer Cells by Apoptosis through Lipid Peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]

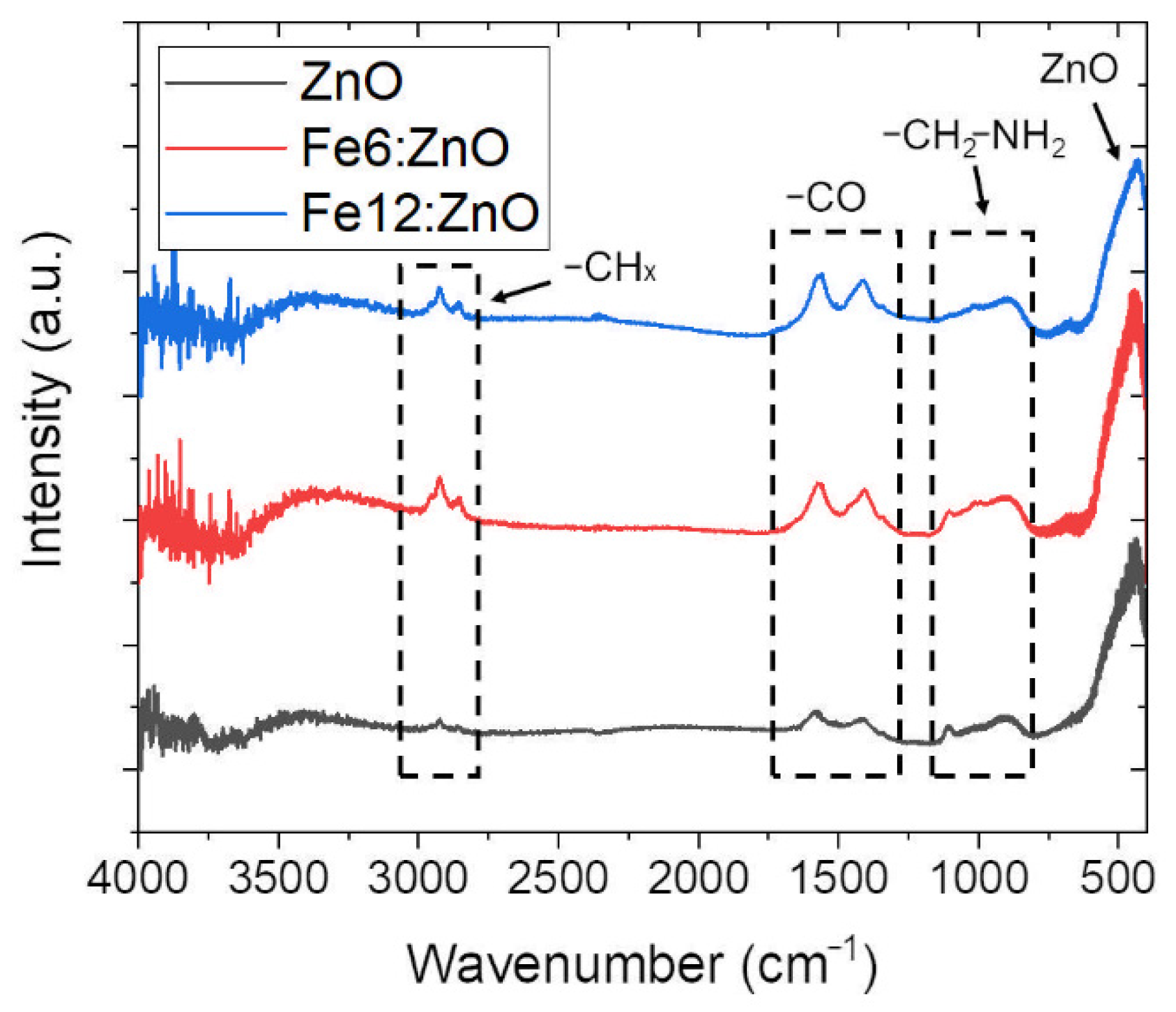
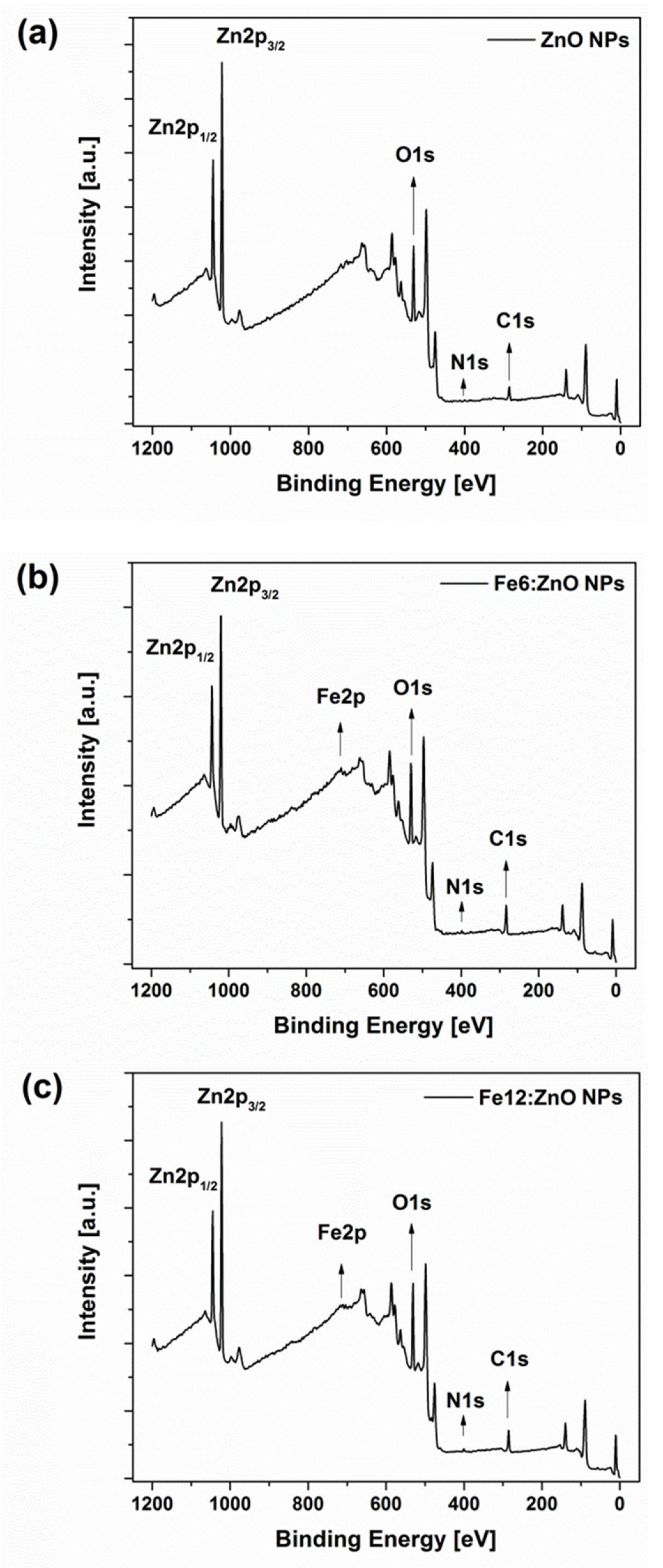

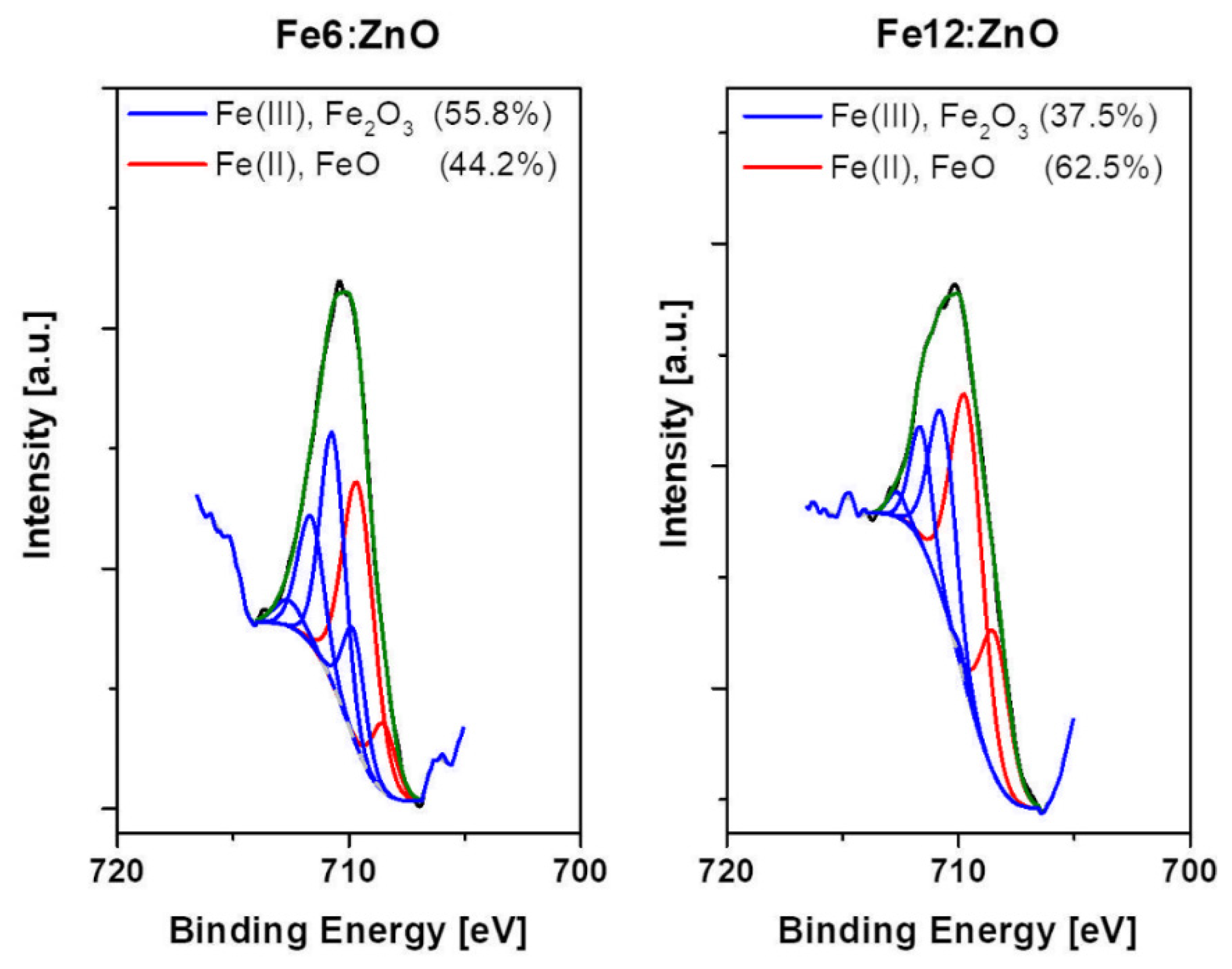



| Sample | Zn (at.%) | Fe (at.%) | O (at.%) |
|---|---|---|---|
| ZnO | 18.65 | - | 81.35 |
| Fe6:ZnO | 23.68 | 1.20 | 74.82 |
| Fe12:ZnO | 22.70 | 1.90 | 75.4 |
| Sample | C (at.%) | Zn (at.%) | O (at.%) | N (at.%) | Fe (at.%) |
|---|---|---|---|---|---|
| ZnO | 14.8 ± 0.5 | 39.3 ± 0.4 | 43.7 ± 0.4 | 2.2 ± 0.4 | - |
| Fe6:ZnO | 24.3 ± 0.5 | 29.8 ± 0.3 | 41.8 ± 0.4 | 2.3 ± 0.3 | 1.8 ± 0.3 |
| Fe12:ZnO | 28.1 ± 0.6 | 27.8 ± 0.3 | 38.8 ± 0.4 | 2.8 ± 0.4 | 2.5 ± 0.4 |
| Sample | (100) |
|---|---|
| ZnO | 6.0 nm |
| Fe6:ZnO | 8.3 nm |
| Fe12:ZnO | 12 nm |
| Sample | ΔΘ (100) | ΔΘ (002) | ΔΘ (101) |
|---|---|---|---|
| Fe6:ZnO | −0.121° | +0.061° | +0.020° |
| Fe12:ZnO | −0.088° | +0.114° | −0.032° |
| Sample | DH in Ethanol | PDI in Ethanol | DH in Water | PDI In Water |
|---|---|---|---|---|
| ZnO | 98.5 ± 0.2 nm | 0.154 ± 0.019 | 116.2 ± 0.4 nm | 0.148 ± 0.011 |
| Fe6:ZnO | 120.4 ± 0.2 nm | 0.133 ± 0.010 | 139.0 ± 1.8 nm | 0.138 ± 0.025 |
| Fe12:ZnO | 124.4 ± 0.4 nm | 0.144 ± 0.021 | 167.6 ± 1.7 nm | 0.121 ± 0.003 |
| Sample | Z-Potential after Functionalization |
|---|---|
| 0 at.% | 22.7 ± 0.9 mV |
| 6 at.% | 26.4 ± 0.4 mV |
| 12 at.% | 25.5 ± 0.5 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carofiglio, M.; Laurenti, M.; Vighetto, V.; Racca, L.; Barui, S.; Garino, N.; Gerbaldo, R.; Laviano, F.; Cauda, V. Iron-Doped ZnO Nanoparticles as Multifunctional Nanoplatforms for Theranostics. Nanomaterials 2021, 11, 2628. https://doi.org/10.3390/nano11102628
Carofiglio M, Laurenti M, Vighetto V, Racca L, Barui S, Garino N, Gerbaldo R, Laviano F, Cauda V. Iron-Doped ZnO Nanoparticles as Multifunctional Nanoplatforms for Theranostics. Nanomaterials. 2021; 11(10):2628. https://doi.org/10.3390/nano11102628
Chicago/Turabian StyleCarofiglio, Marco, Marco Laurenti, Veronica Vighetto, Luisa Racca, Sugata Barui, Nadia Garino, Roberto Gerbaldo, Francesco Laviano, and Valentina Cauda. 2021. "Iron-Doped ZnO Nanoparticles as Multifunctional Nanoplatforms for Theranostics" Nanomaterials 11, no. 10: 2628. https://doi.org/10.3390/nano11102628
APA StyleCarofiglio, M., Laurenti, M., Vighetto, V., Racca, L., Barui, S., Garino, N., Gerbaldo, R., Laviano, F., & Cauda, V. (2021). Iron-Doped ZnO Nanoparticles as Multifunctional Nanoplatforms for Theranostics. Nanomaterials, 11(10), 2628. https://doi.org/10.3390/nano11102628