1. Introduction
Ionic liquids (ILs) are considered a novel type of green working fluid used in various fields, such as absorption refrigeration, solar applications, chemistry (gas capture, storage), and electrochemistry (batteries, sensors). In recent years, ionic liquids have been considered a promising alternative to the conventional working fluids (NH
3/H
2O and H
2O/LiBr) used as absorbents in absorption refrigeration systems due to their good thermal stability, high absorption capacity, and very low vapor pressure [
1,
2,
3].
The thermophysical properties of solutions containing ionic liquids may influence their application in absorption refrigeration systems. Refrigerant/ionic liquid solutions are studied in the literature by way of thermodynamic models, mainly equations of state or activity models [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. In addition, two studies on the use of ionic liquids as absorbents have been published by Shiflett and Yokozeki [
16,
17].
In one paper, Yokozeki and Shiflett [
7] carried out a study on the performance of an absorption refrigeration system using NH
3 as the refrigerant and various ionic liquids as absorbents ([Bmim][PF
6], [Hmim]Cl, [Bmim][BF
4], [Emim][SCN], [DMEA][Ac]). The results indicated that the COPs of all the studied solutions were lower than those of the NH
3/H
2O solution.
The thermophysical properties (vapor pressures and heat capacities) of the H
2O + ([Dmim]dmp) system were investigated by Dong et al. [
8]. The results revealed that the coefficient of the performance of the H
2O + [Dmim]dmp system is close to that of the conventional working pair H
2O + LiBr system.
Kim et al. [
9] theoretically investigated the thermodynamic performance of a miniature absorption system using various refrigerant mixtures (R125, R152a, R32, R134a, R143a) /ILs ([Emim][Tf
2N], [Emim][BF
4], [Bmim][BF
4], [Bmim][PF
6], [Hmim][Tf
2N], [Hmim][BF
4], [Hmim][PF
6]) as the working fluids. They found that refrigerant/IL solutions were promising materials for absorption refrigeration systems that utilize low-grade waste heat, such as those of electronic systems.
Kim and Kohl [
10] carried out an analysis of the performance of R134a/[Bmim][PF
6] and R134a/[Hmim][Tf
2N] using the Redlich–Kwong equation of state and a two-phase pressure drop model. They noticed that R134a/[Hmim][Tf
2N] exhibited a higher system efficiency compared to R134a [Hmim][PF
6], except in the case where the solubility difference between the absorber and desorber converged to zero.
In another paper, Kim and Kohl [
11] investigated the cooling capability of the R134/[Bmim][PF
6] used in an absorption refrigeration system. They compared the performance of R134/[Bmim][PF
6] with R134a/[Bmim][PF
6] and found that R134/[Bmim][PF
6] had a 1.9 times higher cooling capability than R134a/[Bmim][PF
6] at a desorber temperature as low as 63 °C. In addition, R134/[Bmim][PF
6] had a coefficient of performance up to three times higher than that of R134a/[Bmim][PF
6]. Chen and Bay [
18] investigated the thermal performance of an absorption refrigeration system using [Emim]Cu
2Cl
5/NH
3 and found that the thermal performance of the [Emim]Cu
2Cl
5/NH
3 solution was better than that of a NH
3/H
2O solution, but slightly lower than that of a LiBr/H
2O solution. In another study, Chen et al. [
19] numerically investigated the thermodynamic performance of an absorption system using [Bmim]Zn
2Cl
5/NH
3. The results revealed that that the [Bmim]Zn
2Cl
5/NH
3 absorption system exhibited higher thermal performance compared to a NaSCN/NH
3 absorption system.
The thermodynamic performance of an absorption chiller using [Emim][dmp]/H
2O was simulated by Zhang and Hu [
20]. Their results showed that the coefficient of performance was lower than that of a H
2O/LiBr solution, concluding that [Emim][dmp] may be a good absorbent for refrigeration systems. Martin et al. [
21] carried out a study on the use of ILs with supercritical CO
2 using a group contribution equation of state and found that the coefficient of performance was lower compared to a conventional NH
3/H
2O system.
Table 1 presents the values of the coefficients of performance for absorption refrigeration systems using ammonia/ionic liquids as working fluids.
Investigations into the application of ammonia/ionic liquids as working fluids in absorption refrigeration systems are limited in the open literature. Moreover, studies on absorption refrigeration systems using ammonia/ionic liquid+nanomaterials as working fluids are not reported in the literature. In order to improve the performance of absorption systems, new working fluids are herein proposed. The thermophysical properties of working fluids are the main data in this evaluation of the performance of absorption refrigeration systems. In this regard, the thermophysical properties of ammonia with graphene (GE) and single-wall carbon nanotubes (SWCNTs), respectively, dispersed into [Bmim]BF4 ionic liquid, are analyzed and discussed. Correlations for the studied properties, required for the modeling and simulation of the performance of various absorption refrigeration systems, are also proposed.
3. Results and Discussions
In this study, the thermo-properties of the NH3/[Bmim]BF4 and NH3/[Bmim]BF4+ CNMs solutions were evaluated for the mass fractions of NH3 in a range of 0.018–0.404 and at temperatures from 293 K to 388 K. Two types of carbon nanomaterials with two weight fractions (0.005 and 0.01), dispersed into an ionic liquid, were considered: graphene (GE) and single-wall carbon nanotubes (SWCNTs). No data for the thermal properties of the [Bmim]BF4 +CNMs mixed with NH3 solutions have been reported in the literature.
3.1. Thermal Conductivity
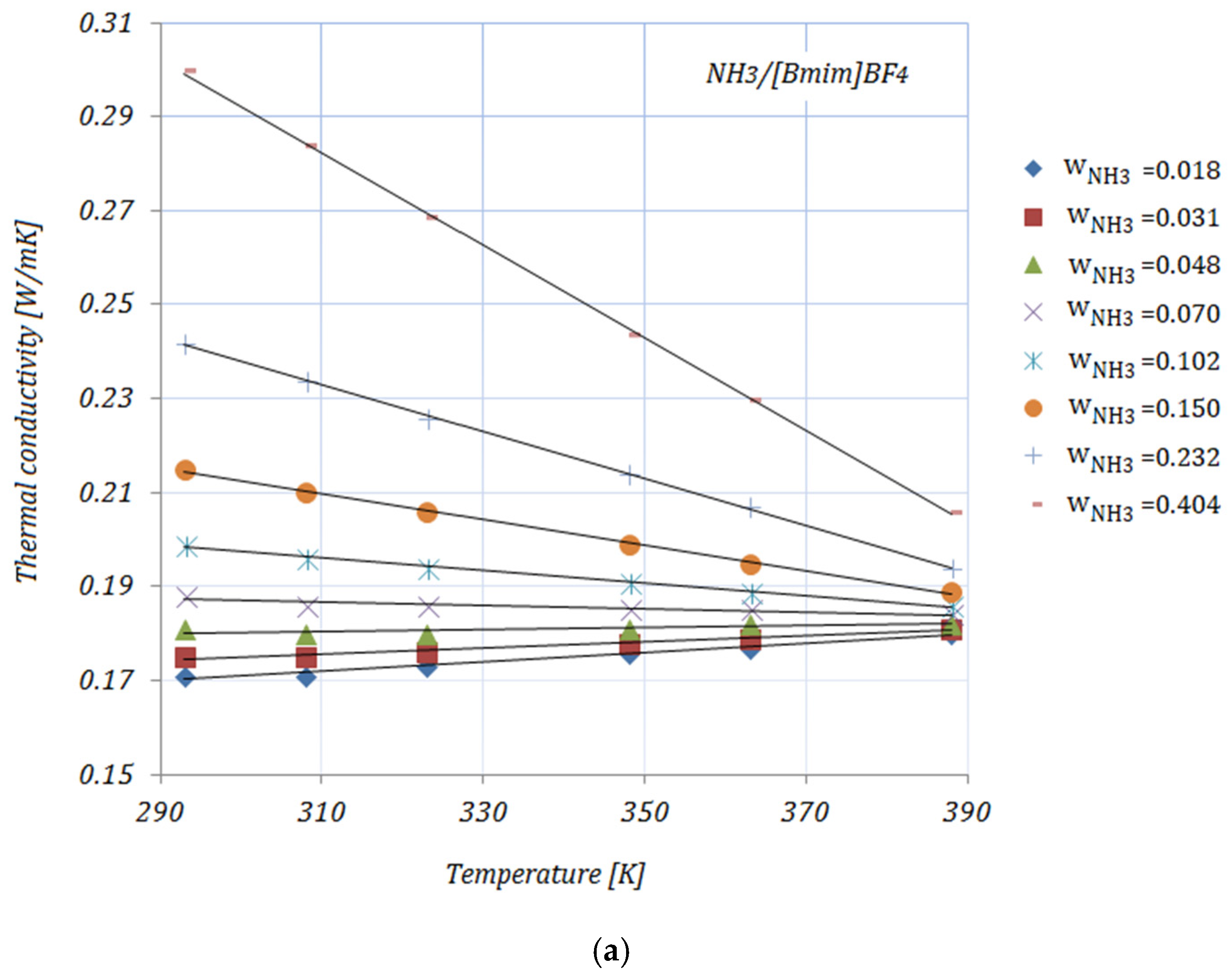
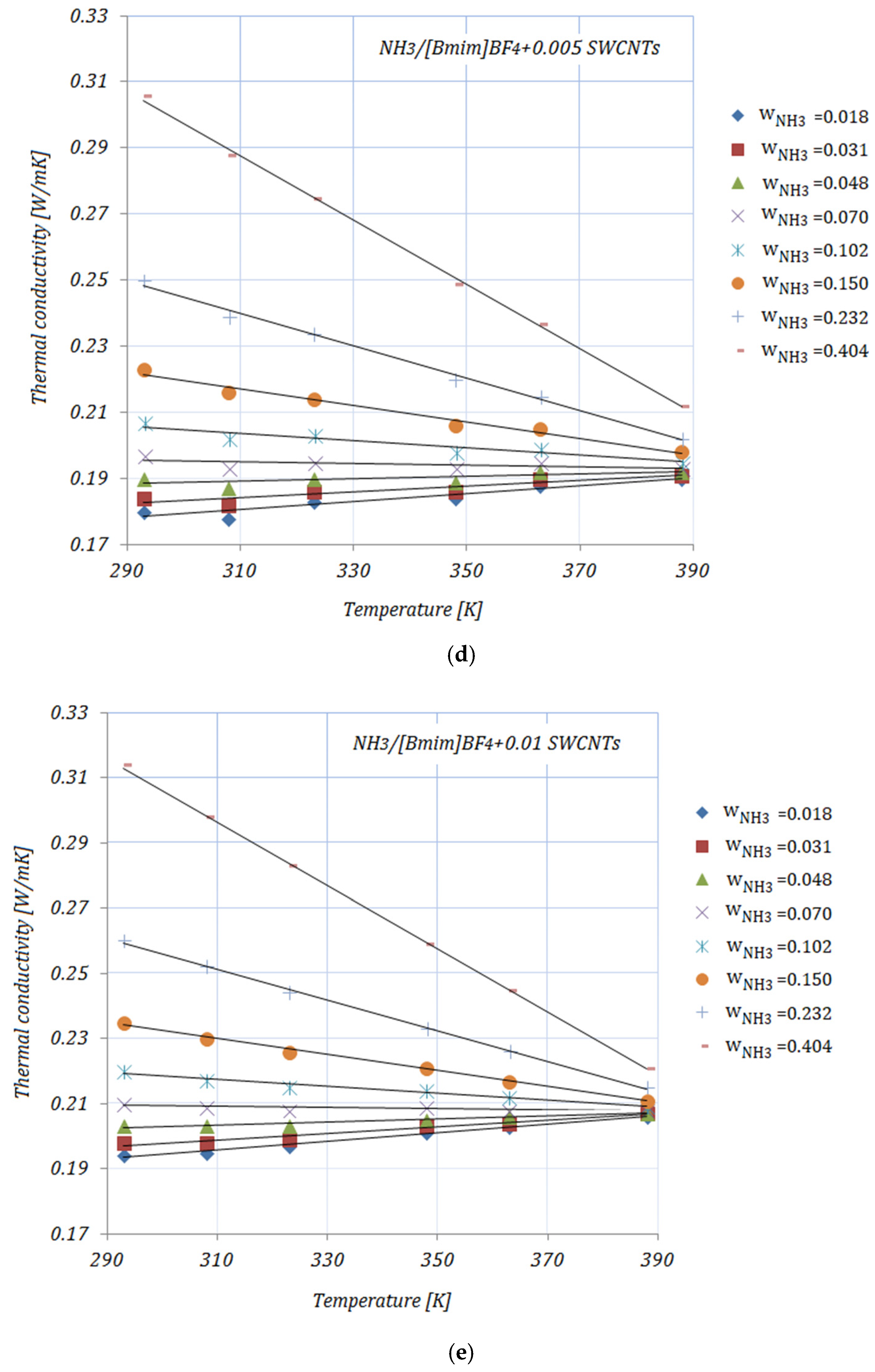
Figure 1a–e shows the variation of the thermal conductivity of NH
3/[B
mim]BF
4, NH
3/[B
mim]BF
4 + GE and NH
3/[B
mim]BF
4 + SWCNTs with the temperature at various NH
3 fractions. With increasing temperatures can be seen that the thermal conductivities of all solutions have an upward trend up to w_NH
3 = 0.048, then with increasing NH
3 fractions (≥0.102), the thermal conductivities decrease with increasing temperatures, but increasing with increasing NH
3 fractions. The addition of carbon nanomaterials to the ionic liquid leads to an enhancement in the solution’s thermal conductivity compared to the base solution. The enhancements in the thermal conductivity of the studied solutions—calculated as
—at minimum and maximum NH
3 fractions—
and
, respectively—are shown in
Table 2:
It can be seen from
Table 2 that the maximum enhancement in thermal conductivity was achieved by
, while the minimum enhancement in thermal conductivity was recorded for
for both NH
3 fractions. In addition, a descending trend in the thermal conductivity of solutions with higher NH
3 fractions may be seen. These results may be explained by the thermal conductivities of carbon nanomaterials dispersed into the ionic liquid. Graphene (GE) exhibits a thermal conductivity of
[
28,
29,
30], while the thermal conductivity of SWCNTs is usually reported to be in the range of
at a standard temperature (25 °C) [
31]. Yu et al. [
32] measured the thermal conductivity of SWCNTs using a chemical vapor deposition method and found a value higher than
. The experimental results related to the thermal conductivity of ionic liquids revealed the increase in thermal conductivity achieved by adding nanoparticles into the ionic liquid and the minor influence of temperature on several ionic liquids containing nanoparticles. The main arguments for these trends are thermal boundary resistance, layering phenomena, and clustering [
33].
The thermal conductivity values are correlated by means of a linear equation as a function of temperature:
The coefficient values
,
, and
are given in
Table 3.
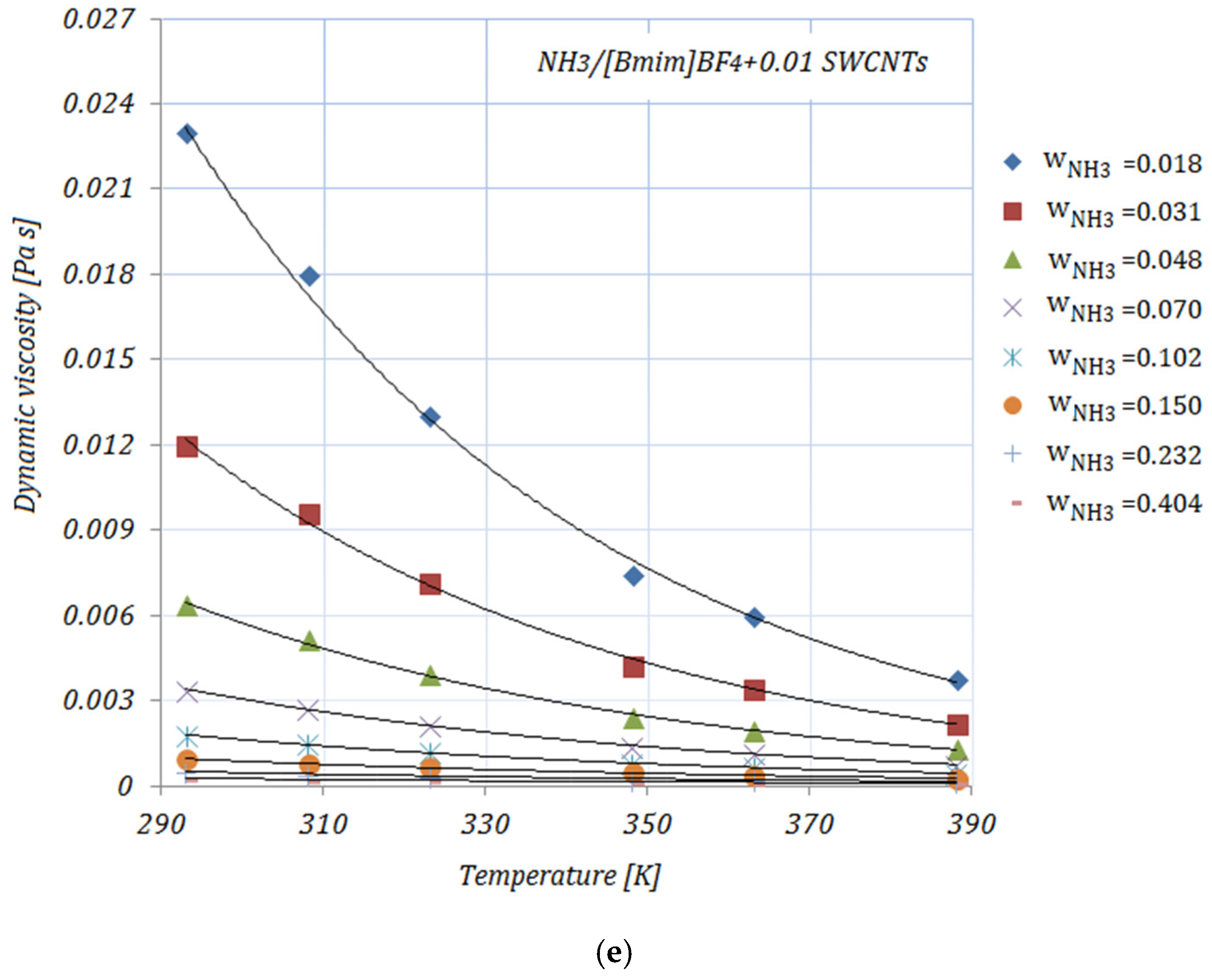
3.2. Dynamic Viscosity
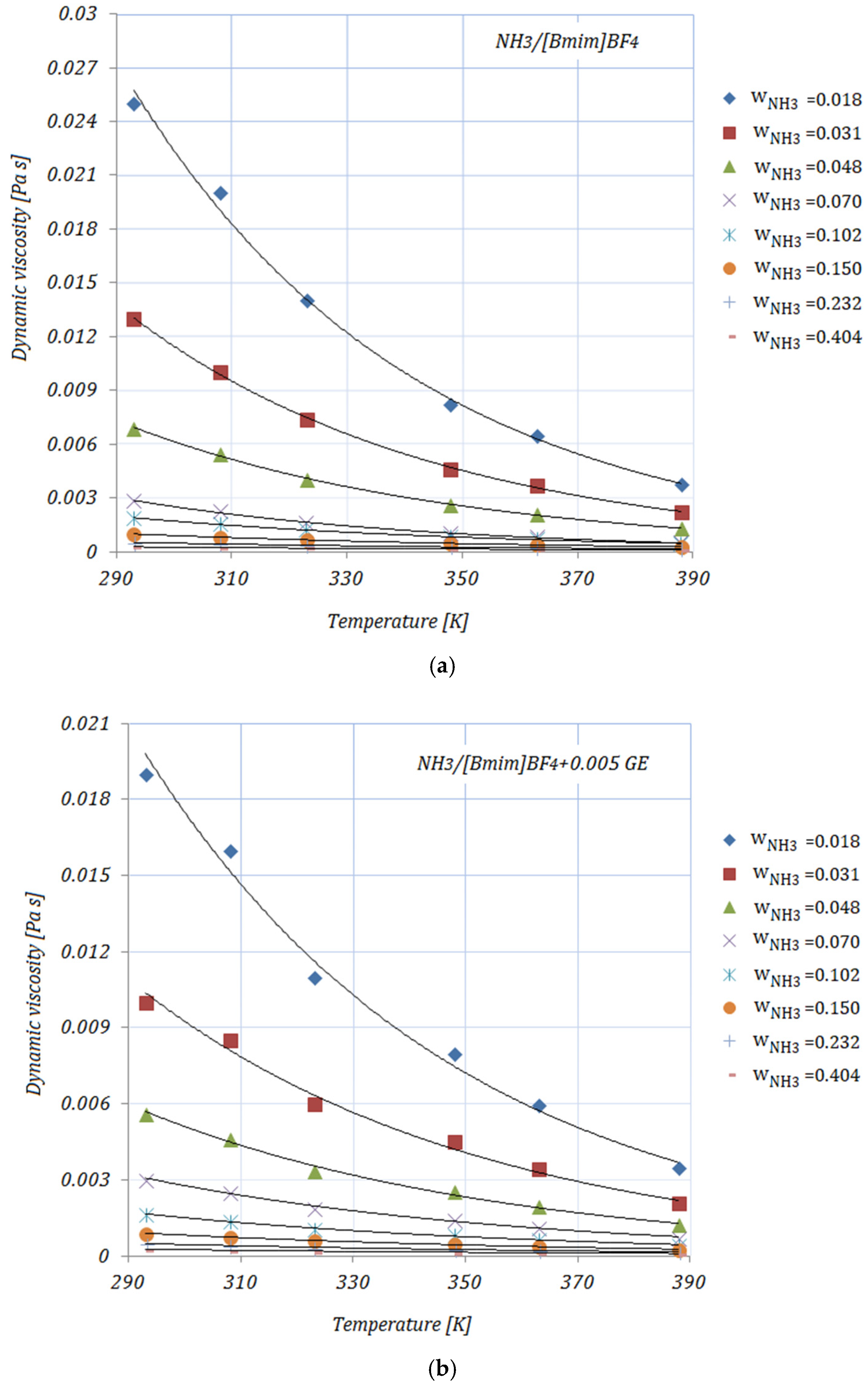
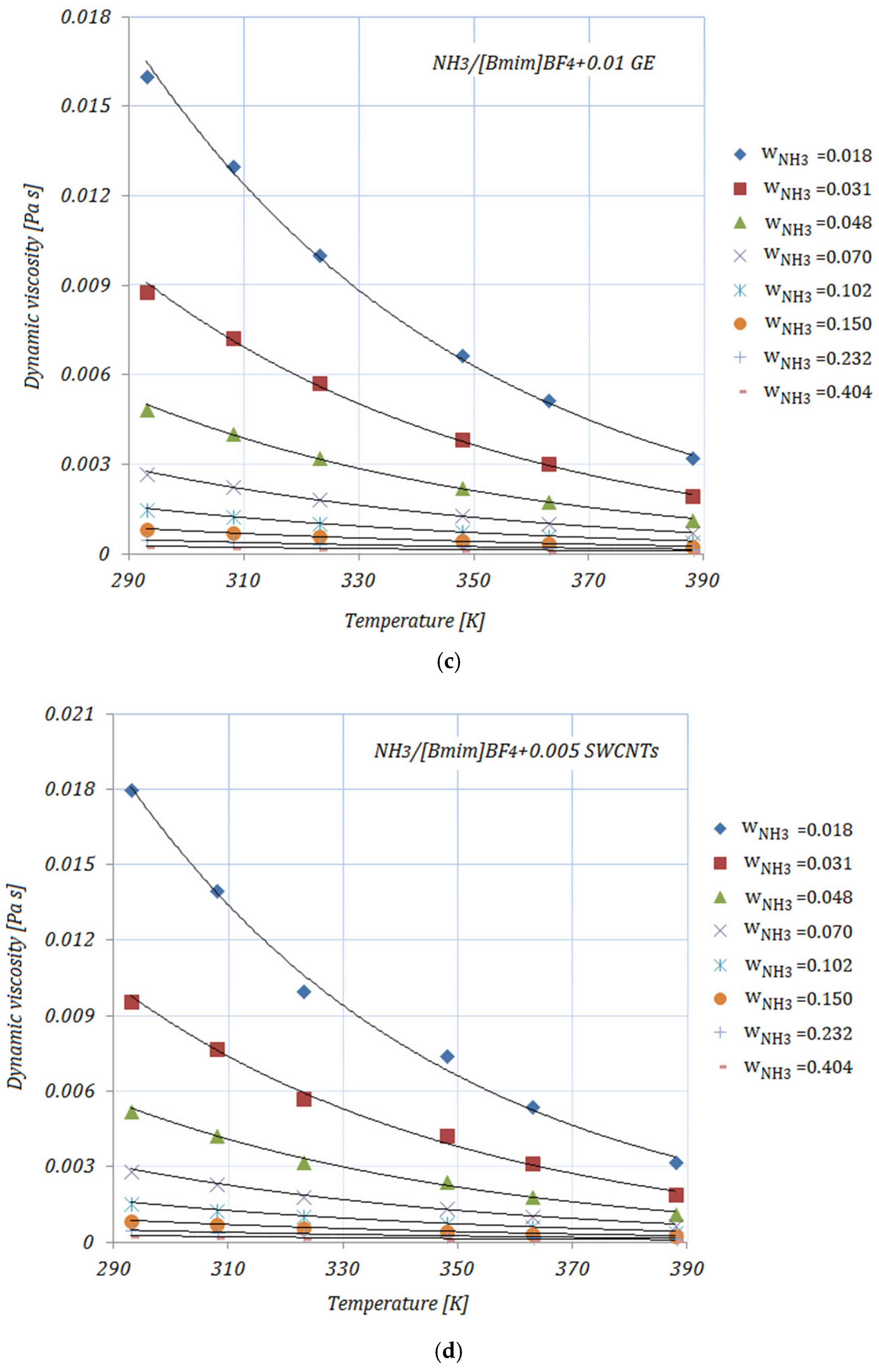
Figure 2a–e depict the variation in the viscosity of the solutions, with various NH
3 fractions, at rising temperatures. As shown in
Figure 2, the viscosities of the solutions decrease exponentially with higher temperatures. By adding the carbon nanomaterials into the ionic liquid, a reduction in viscosity may be seen compared to the base solution. Higher fractions of carbon nanomaterials lead to an increase in the viscosity of the studied solutions, but these viscosity values do not exceed those of the base solution. With higher NH
3 fractions, a decrease in viscosity may also be seen. The diminution in viscosity is more obvious at the lower mass fractions of NH
3 in the solutions. The viscosities of the NH
3/IL+CNMs solutions are lower than that of the base solution, indicating that these solutions are suitable for NH
3 absorption. The reduction in the viscosity of the studied solutions—calculated as
—at minimum and maximum NH
3 fractions—
and
, respectively—is shown in
Table 4:
From
Table 4, it may be seen that at a temperature of 293 K the maximum reduction in viscosity is achieved by
, while the minimum is seen in the case of
for both NH
3 fractions. With increasing temperature,
and
show similar values of viscosity reduction. In addition, a descending trend in viscosity may be seen at higher NH
3 fractions in the solutions, with the viscosity values of the NH
3/IL+CNMs being similar to the values of the base solution.
The data related to the dynamic viscosity of ionic liquids are still contradictory. Most experimental studies indicate an increase in viscosity with the addition of nanoparticles to the ionic liquid, while on the other hand there are studies that have found a decrease in viscosity. The reduction in viscosity can be explained by the interaction between the molecules of the ionic liquid and the nanoparticles, as well as by the lubricating properties of the nanoparticles.
The dynamic viscosity values are correlated by means of an exponential equation as a function of temperature:
The coefficient values
,
, and
are given in
Table 5.
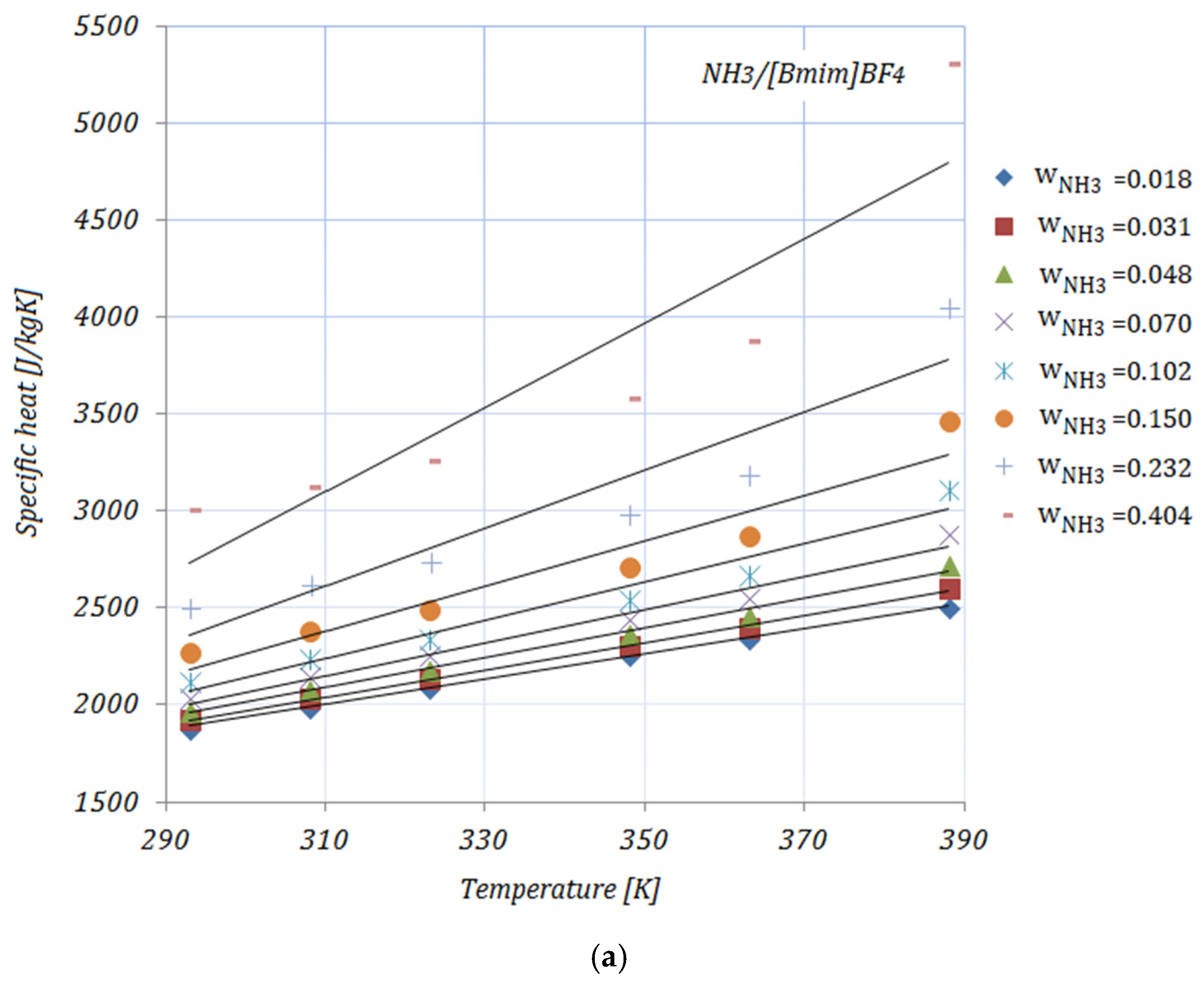
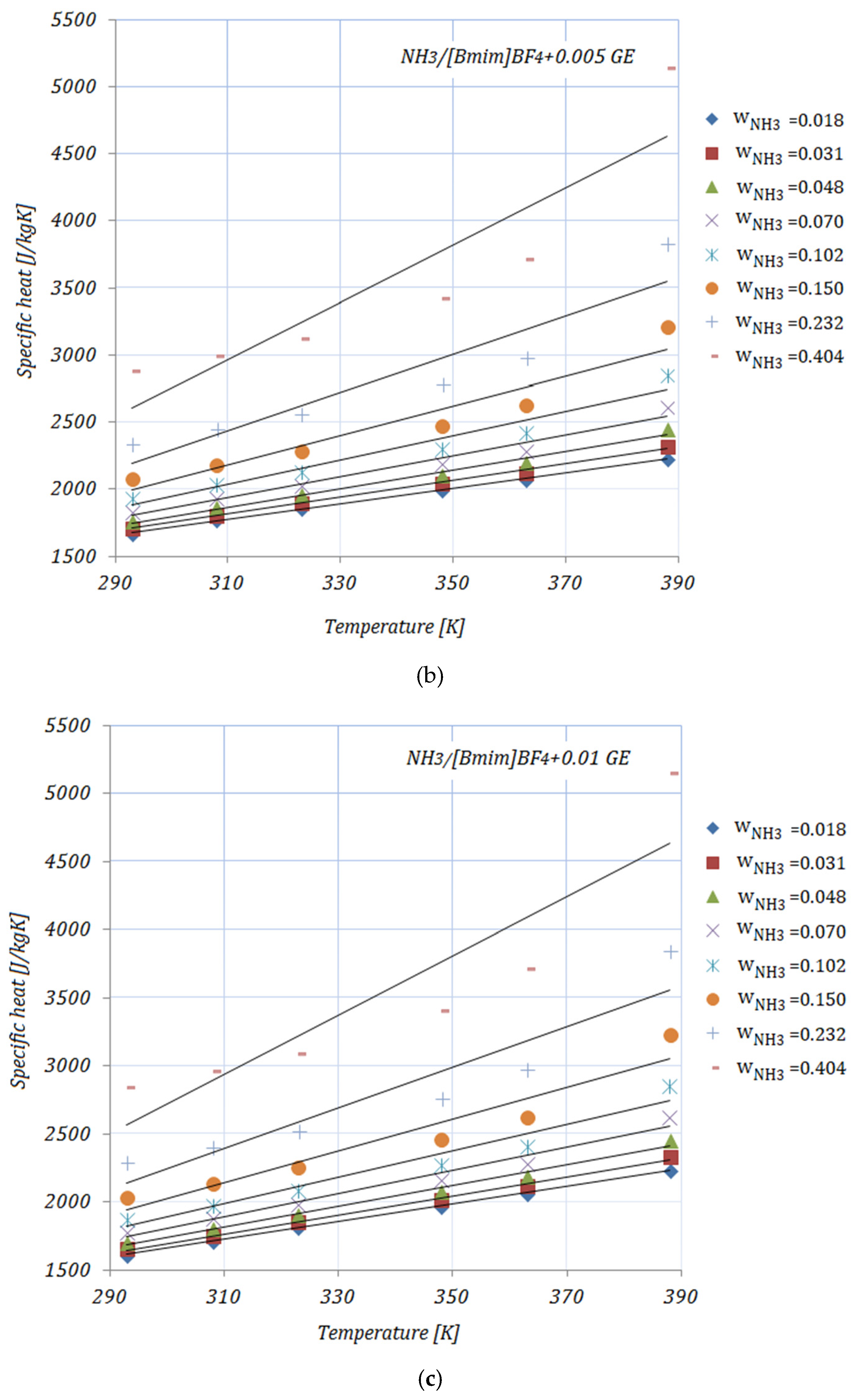
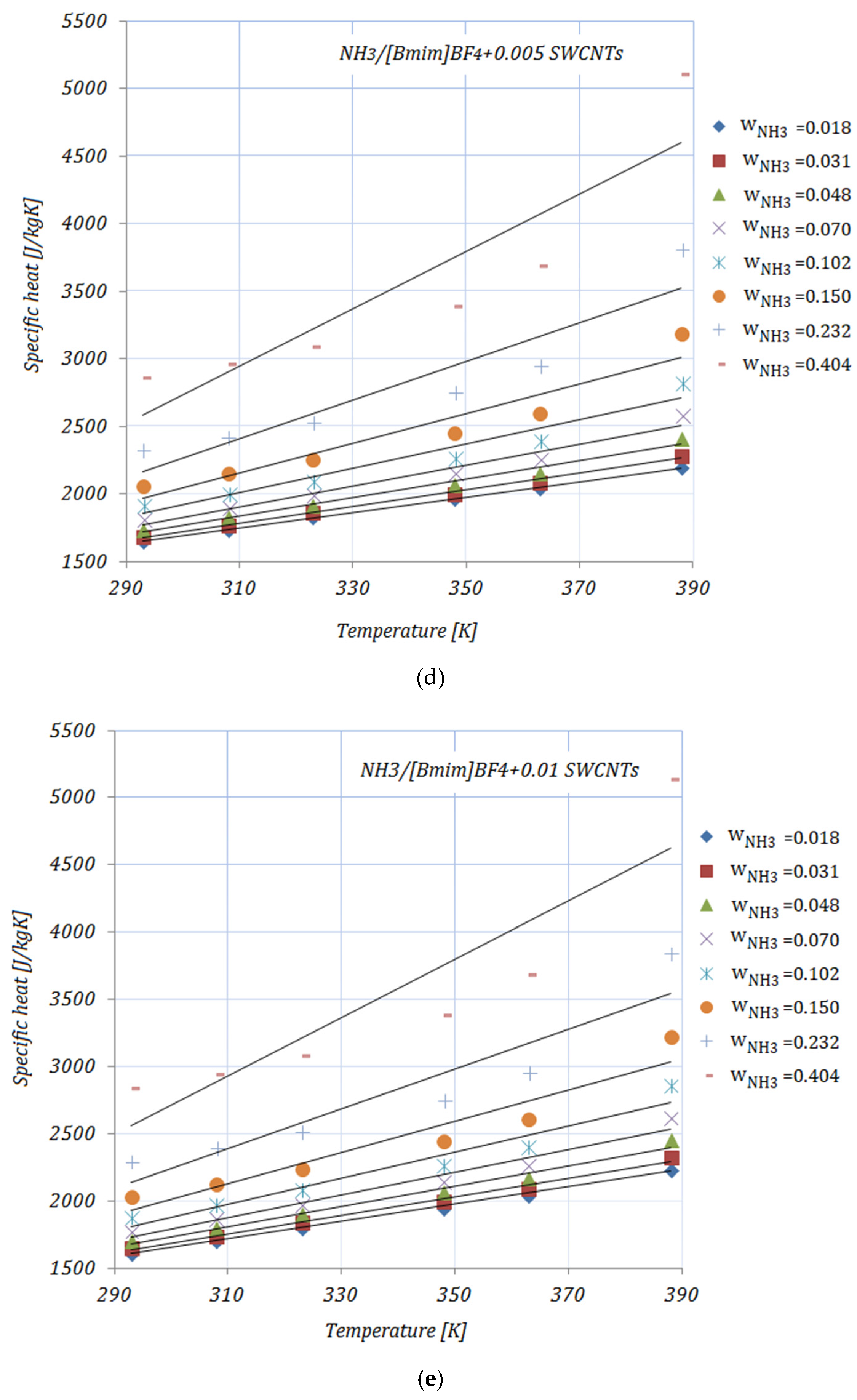
3.3. Specific Heat
Figure 3a–e illustrate the variation in the specific heat of the solutions, with various NH
3 fractions, at rising temperatures. As shown in
Figure 3, the specific heat of the solutions increases with both higher temperatures and higher fractions of NH
3. In addition, by adding nanoparticles to the ionic liquid, a reduction in specific heat may be seen compared to the base solution. Higher CNMs fractions led to a decrease in the specific heat of all the solutions. The presented results are according to an equation proposed by Raud et al. [
34], which indicates the increase in a solution’s specific heat with rising temperatures, and also the reduction in specific heat by the addition of nanomaterials into the base solution.
The reduction in the specific heat of the studied solutions—calculated as
—at minimum and maximum NH
3 fractions—
and
, respectively—is shown in
Table 6:
The maximum reduction in specific heat was achieved by both solutions with a 0.01 fraction of nanomaterials, and , while the minimum can be seen in the case of , for both NH3 fractions, at a temperature of . With higher temperatures, the maximum reduction was recorded for . With higher fractions of NH3, the reduction in specific heat was significant.
The data available in the open literature related to the specific heat of ionic liquids are, as in the case of viscosity, contradictory. The main reasons for this are the interaction between the molecules of the nanomaterials and the ionic liquid and the chemical structure of the ionic liquid.
The specific heat values are correlated by means of a linear equation as a function of temperature:
In
Table 7, the coefficient values
,
, and
are given:
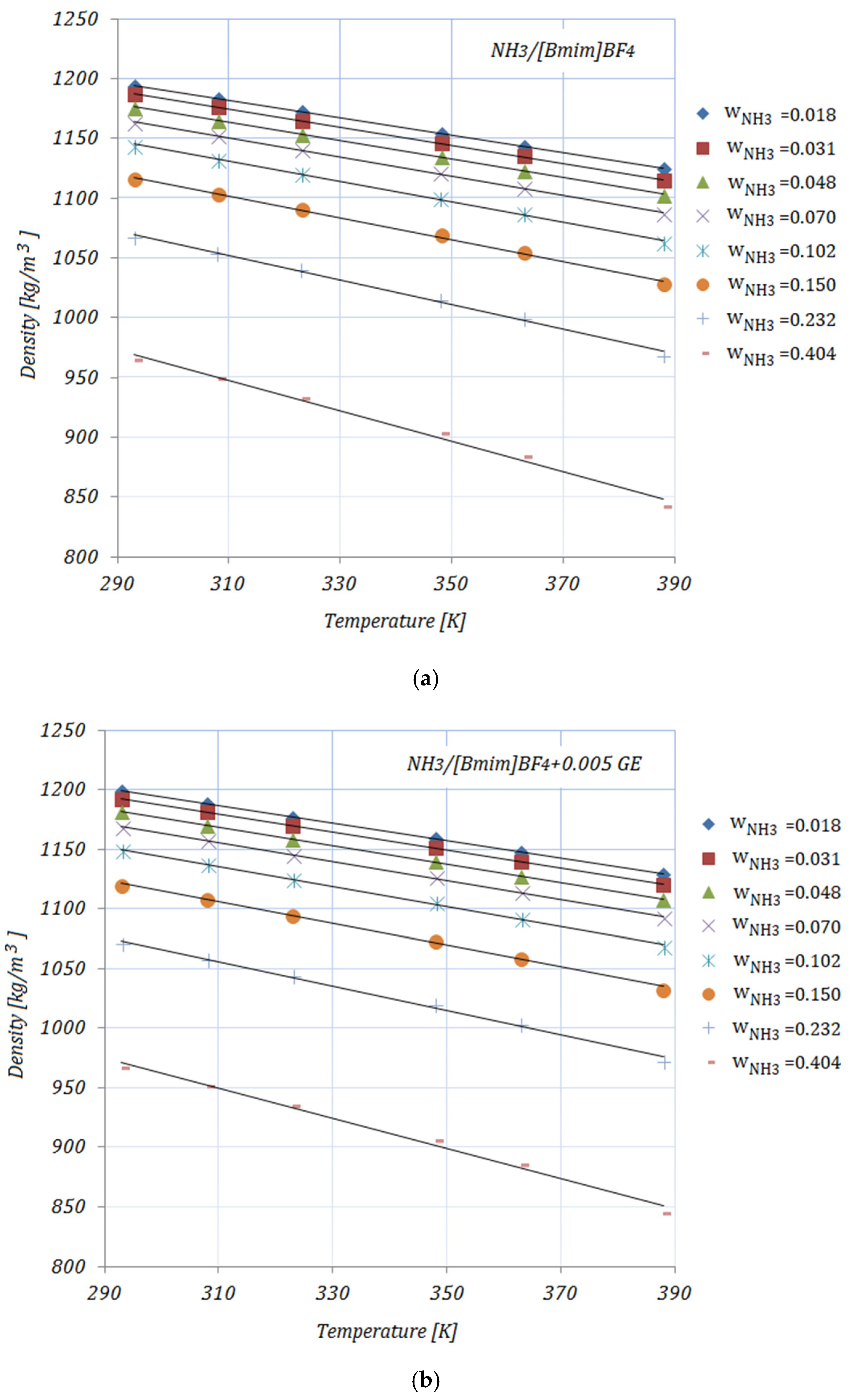
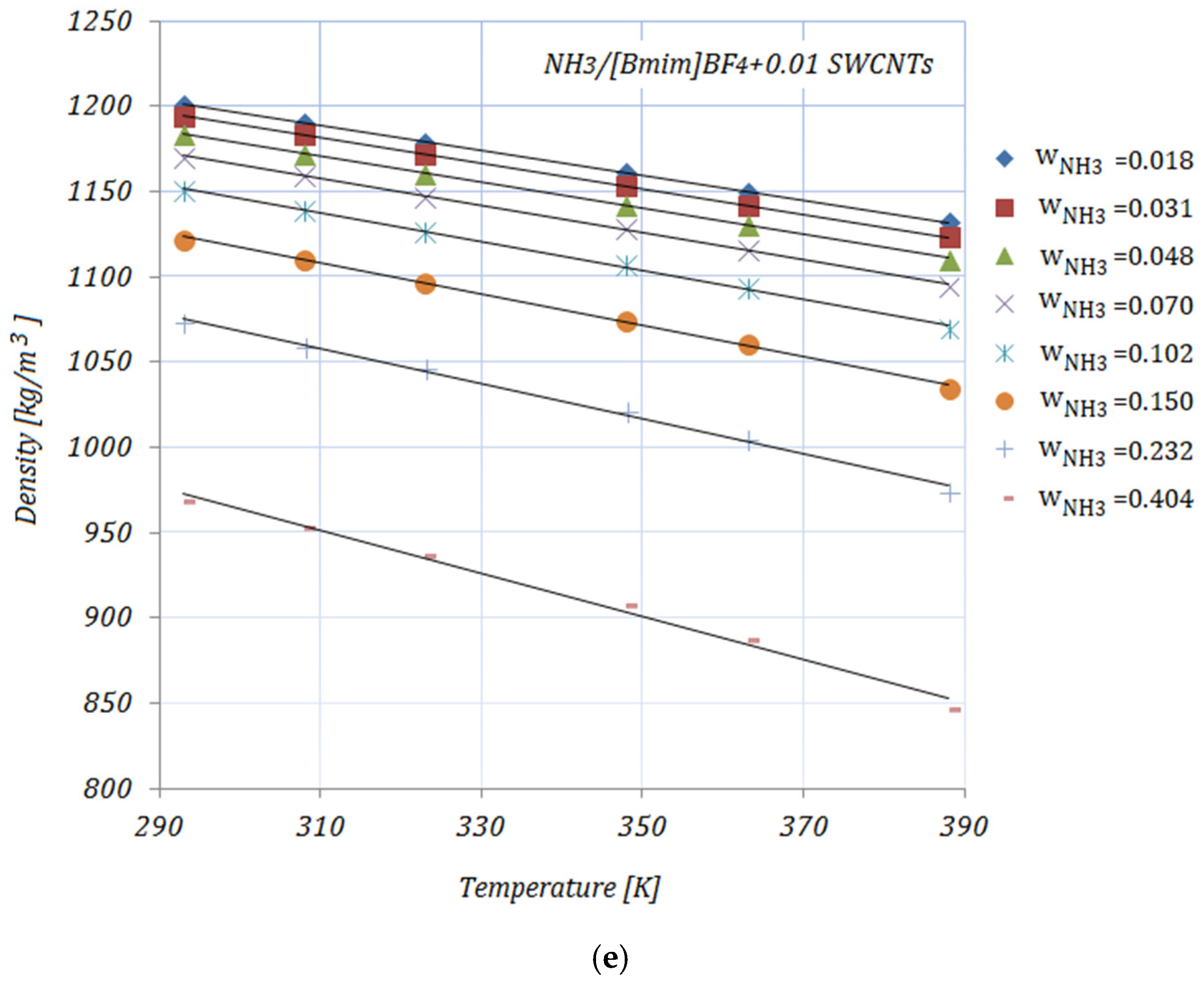
3.4. Density
The densities of the solutions, with various NH
3 fractions and at rising temperatures, are illustrated in
Figure 4a–e. As can be seen, the density decreases with both higher temperature and higher NH
3 fractions. The addition of carbon nanomaterials into the ionic liquid increases the solution’s density compared to the base solution. In addition, higher CNMs fractions lead to increased density for all solutions. Most experimental studies regarding the density of ionic liquids report an increase in density with the addition of nanoparticles and a decrease with higher temperatures. The presented results show the same trend as the experimental results obtained by other studies [
35].
The enhancement in the density of the studied solutions—calculated as
—at minimum and maximum NH
3 fractions—
and
, respectively—is shown in
Table 8:
At a temperature of , the maximum enhancement in density was achieved by , while the minimum can be seen in the case of for both NH3 fractions. At higher temperatures, the maximum enhancement was recorded for .
The density values are correlated by means of a linear equation as a function of temperature:
The coefficient values
,
, and
are given in
Table 9.
4. Conclusions
In this study, the thermophysical properties of ammonia and carbon nanomaterials (CNMs), dispersed into [Bmim]BF4 ionic liquid, were analyzed and discussed. The results showed that the thermal conductivity of the solutions decreases with higher NH3 fractions. By adding carbon nanomaterials into the ionic liquid, an enhancement in the solution’s thermal conductivity may be seen compared to the base solution, with the maximum enhancement in thermal conductivity having been achieved by. The viscosities of the NH3/IL+CMNs solutions were lower than that of the base solution, indicating that these solutions are suitable for NH3 absorption. In this case, the maximum reduction in viscosity was recorded for. In addition, by adding CMNs to the ionic liquid, a reduction in the specific heat of the solutions may be seen compared to the base solution. At a temperature of , the maximum reduction in specific heat was achieved by the solutions with a 0.01 fraction of nanomaterials ( and . Moreover, the addition of CMNs to the ionic liquid led to an increase in the solution’s density. At a temperature of , the maximum enhancement in density was achieved by. Finally, correlations for all studied properties were proposed.
The results of this study may contribute to the consolidation of the property database of NH3/IL+NMs for applications in absorption refrigeration. Further investigations concerning the thermophysical characteristics of ammonia with other types of ionic liquids are needed. In addition, for the practical implementation of NH3/ILs+CNMs in absorption refrigeration systems, experimental studies to support the reported theoretical results are needed.