3.1. MAI Dissociation in Thermal Deposition
In
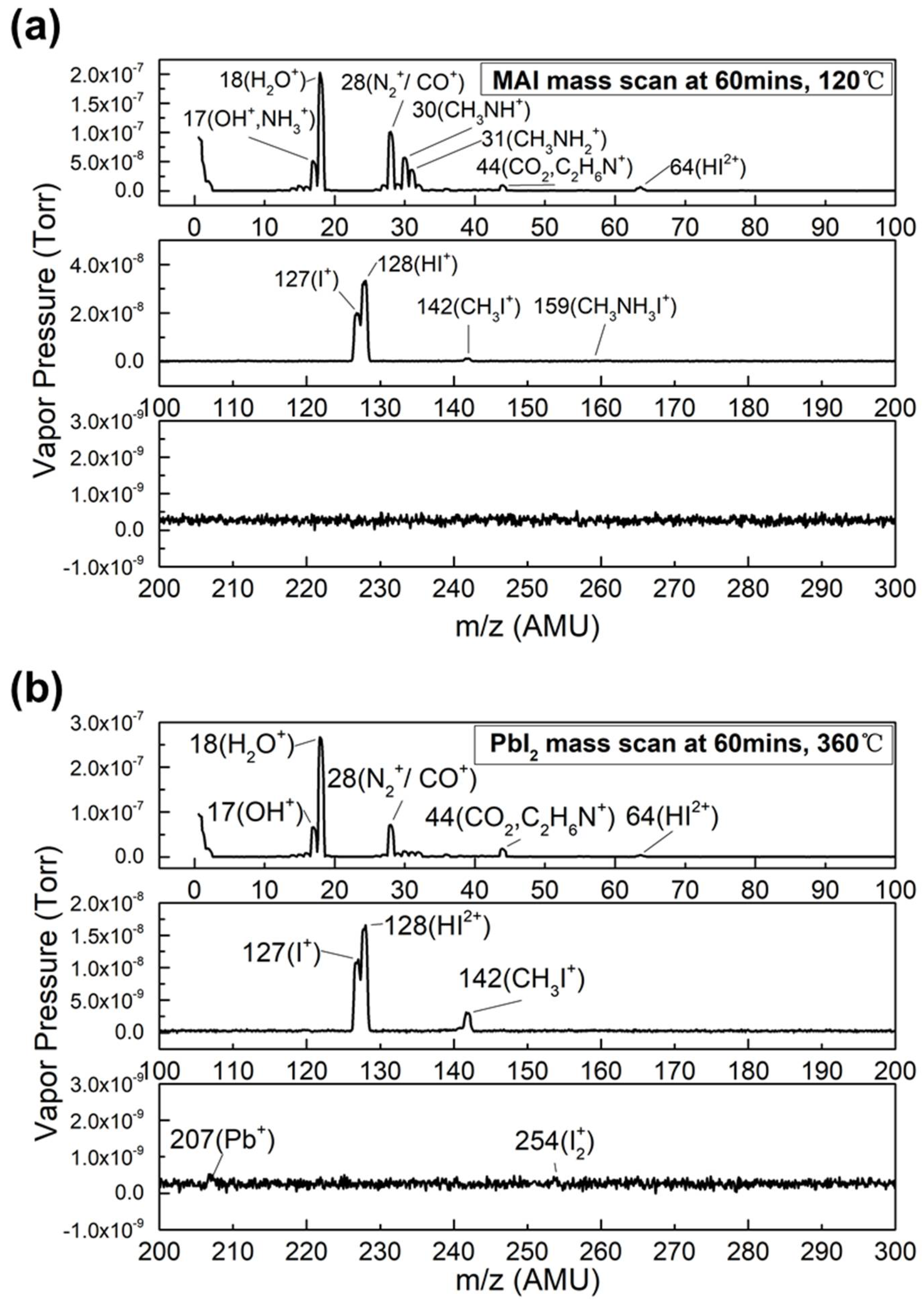
Figure 1, the mass scans are presented from 0 to 300 AMU for (a) MAI boat temperature of 120 °C and (b) PbI
2 boat temperature of 360 °C.
Figure 1a shows that water and water fragment (AMU 17) (with pressures of 2.02 × 10
−7 and 5.10 × 10
−8 Torr, respectively) were the dominant peaks in the spectrum. The other noticeable peaks are the MAI-dissociated products CO, CH
3NH
2, CH
3NH, HI, and I. No MAI peak (AMU 159) was detected by RGA. In
Figure 1b, the pressures of water and OH
+ are both higher than that of MAI evaporation because of the higher evaporation temperature of PbI
2. Additionally, the pressures of all MAI-dissociated products, except for CH
3I, were greatly reduced. This suggests that even without MAI evaporation, MAI-dissociated products existed in the chamber. For both evaporations, Pb
+ and I
2+ were not detected by RGA.
Table 1 shows the most noticeable peaks along with the most likely compounds that they are associated with. During the MAI evaporation, the main MAI peak was not detected by RGA, but the peaks of the MAI-dissociated products CO, CH
3NH
2, CH
3NH, HI, and I were noticeable. This indicates that MAI did not evaporate as a whole molecule; instead, it thermally decomposed into its components in the vacuum during the evaporation. These results agree well with the observations of Bækbo, et al. [
17]. For PbI
2 evaporation, Pb (AMU 207) and I
2 (AMU 254) peaks were not detected (as seen in
Figure 1b), which suggests that PbI
2 evaporates without dissociation. I, HI, and CH
3I were the residual compounds from MAI evaporation.
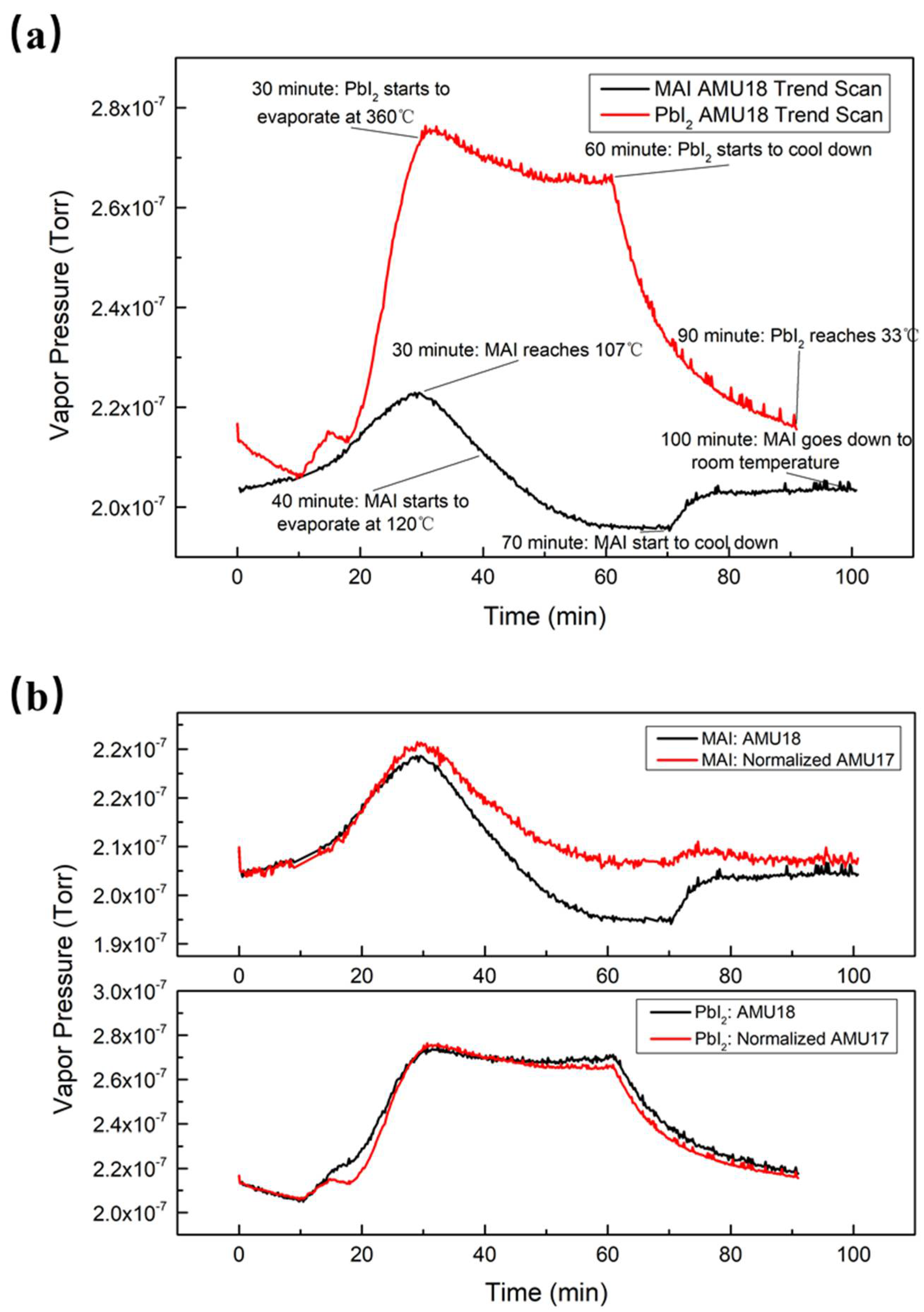
The dynamics of the evaporation were monitored by observing the time evolution of the RGA scans. In
Figure 2a, a typical evolution pattern of PbI
2 evaporation is shown. The partial pressure of water initially increased as the temperature rose. Once the temperature was stabilized, the water pressure slightly decreased as the new equilibrium state was established. When the evaporation was finished, the water pressure returned to its initial pressure. The partial pressures of water vapor behaved differently in MAI and in PbI
2 evaporation, as shown in
Figure 2a. For MAI evaporation, the water vapor pressure increased from the beginning of heating to about 30 min due to the heating of both the MAI boat and its surrounding area that desorbed the water molecules from the boat and the inner chamber wall. As the evaporation progressed, the water pressure decreased from its peak value of 2.20 × 10
−7 Torr at 30 min to below the base value of 2.02 × 10
−7 Torr after 47 min, indicating the occurrence of a chemical process that consumed water during the evaporation.
The water pressure behavior during the PbI2 evaporation was qualitatively different from that during MAI evaporation. The water pressure increased in the initial stage of evaporation, reaching a maximum value of 2.75 × 10−7 Torr at 30 min, similar to that of MAI. The maximum water vapor pressure was higher than that in MAI evaporation because the evaporation temperature of PbI2 is much higher than that of MAI. The pressure stabilizes after 50 min at 2.67 × 10−7 Torr, far above the baseline of water and in sharp contrast to the case of MAI. The reduction of water during evaporation may have been caused by the absorption of water by MAI on the inner chamber wall and reabsorption by the inner chamber wall. After turning off the evaporation power supply, both temperatures quickly decreased and the water vapor pressures returned to their initial values.
As indicated in
Figure 1 and
Table 1, AMU 17 could be both NH
3+ and OH
+ as water fragment. We therefore compared the H
2O
+ peak at AMU 18 to that at AMU 17 to see if there was any NH
3+ contribution.
Figure 2b shows a comparison of water and normalized AMU 17 trend scans in both MAI and PbI
2 evaporation. The two trend scans can be seen to practically overlap for PbI
2 evaporation, while the water trend was further decreased than that of AMU 17 for MAI. The residual gas molecules were electron impact-ionized, and then the quadrupole separated all the ions so that we could measure their mass-to-charge ratios and current. These ions could have been molecular fragments or from a mixture of molecules: this is the cracking pattern of each molecule. The ionization energy of our RGA was 70 eV; under this configuration, we observed approximately 25% water molecules cracking into OH
− [
21]. However, NH
3 has the same molecule weight of 17, which is the same as OH
−. To further investigate AMU 17’s composition, we normalized AMU 17’s trend scan to reach the same initial value as that of the water in
Figure 2b. Water and AMU 17’s curves were almost coincident in PbI
2 evaporation, which suggests that most of AMU 17 was OH
−. However, AMU 17 did not decrease as much as water in MAI evaporation, which indicates that AMU 17 could be a mixture of OH
− and NH
3.
3.2. MAI Dissociation Mechanism
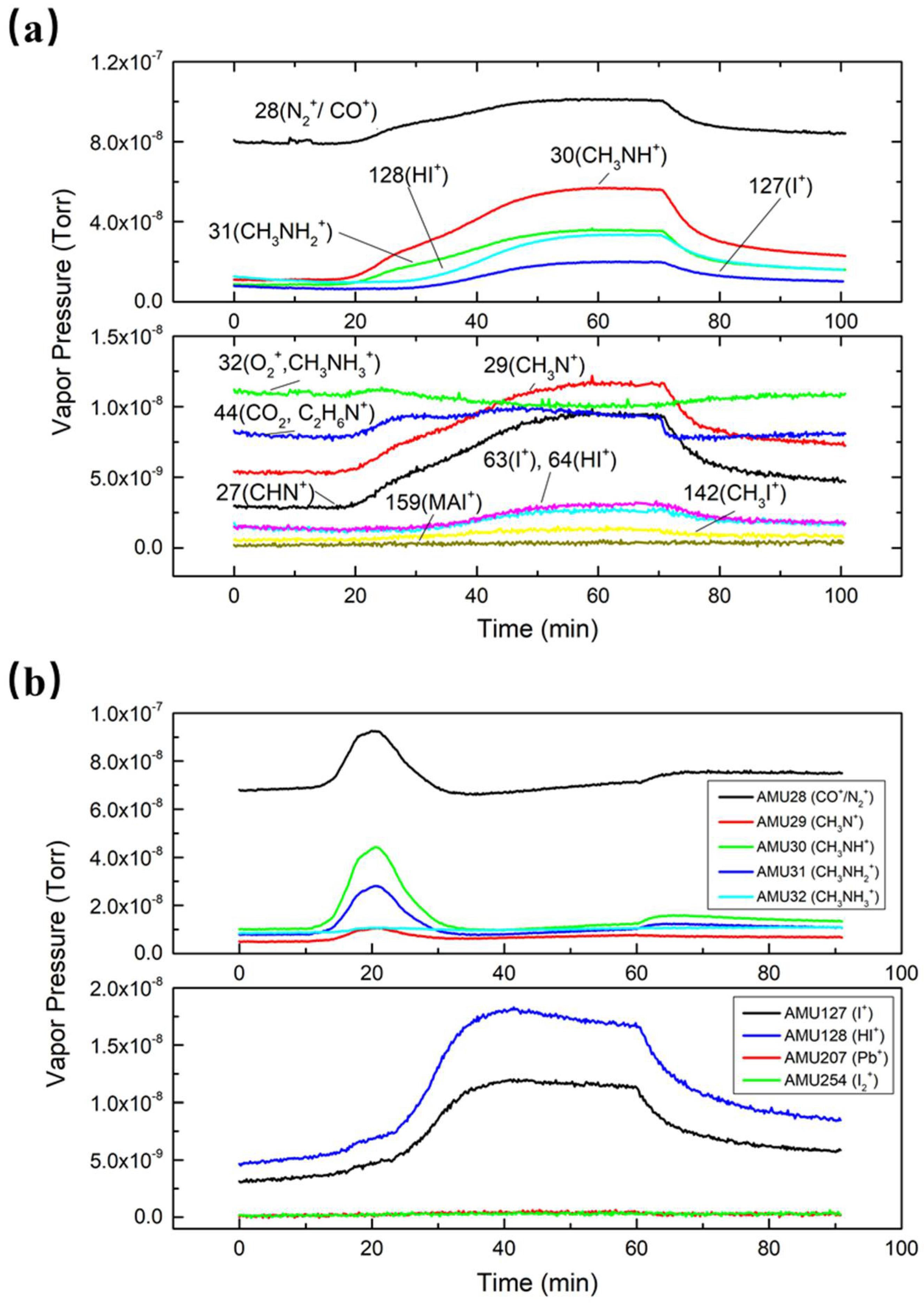
All residual gases were monitored and recorded with RGA. The trend scans of MAI-dissociated products are shown in
Figure 3a, which illustrates the partial pressure changes for the whole heating–evaporation–cooling process. We first degassed the RGA filament and then started evaporation at 10 min when the detected pressures were stabilized. It took about 30 min to reach the evaporation temperature. As can be seen in the figure, the vapor pressures of MAI-dissociated products rose with the increase in the temperature while the MAI curve stayed flat, which means there was no MAI detected by RGA. These results support the notion that MAI does not evaporate as a whole compound but instead dissociates into fragments, as observed by Bækbo et al. [
17].
In order to compare partial pressure changes for PbI
2 and MAI, we also collected data for each individual compound in PbI
2 evaporation.
Figure 3b shows that there were no increases for both Pb (AMU 207) and I
2 (AMU 254) during the PbI
2 evaporation process, which further proves that PbI
2 evaporates as a whole compound. However, due to the limitations of the RGA, PbI
2 molecules were out of the detecting range. I and HI were suspended and accumulated in the evaporation chamber after each MAI evaporation. Once the temperature increased, I and HI left the inner chamber wall, thus explaining the strong presence of the two compounds. Meanwhile, at the early stage of PbI
2 evaporation, several MAI-dissociated products were also detected, even though MAI was not evaporated. This further confirms that these dissociated products were floating in the chamber and sticking to the PbI
2 boat and nearby areas.
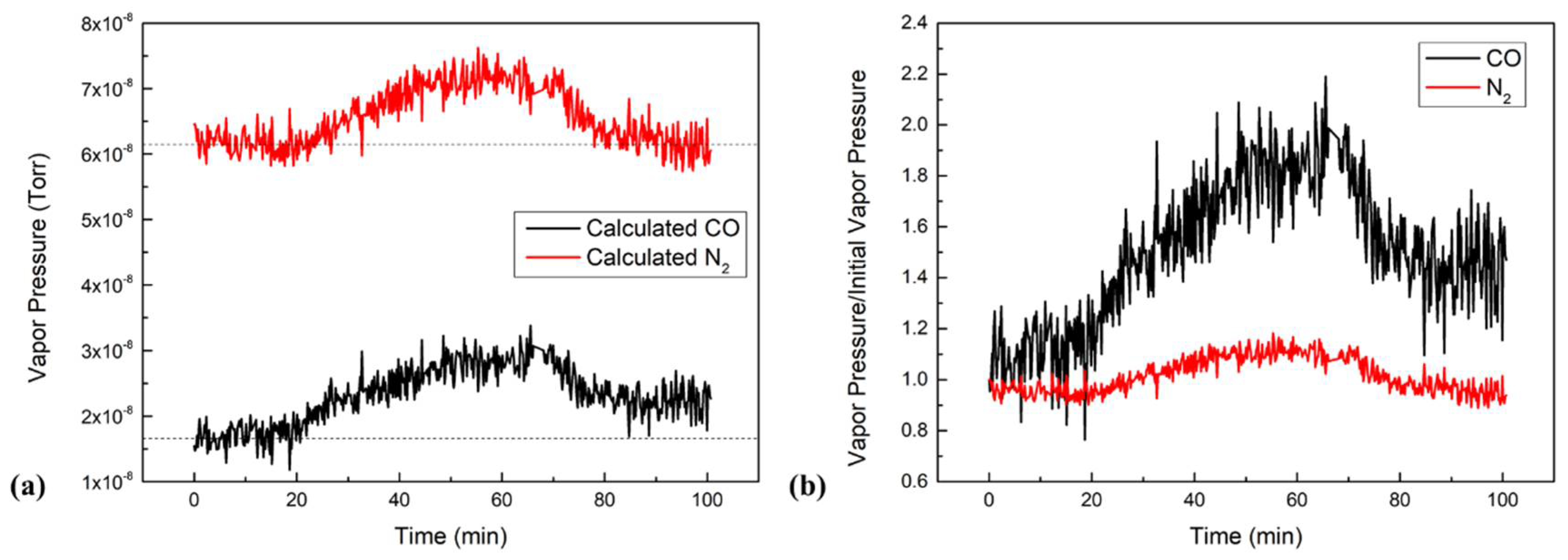
Among all the MAI-dissociated products, AMU 28 increased the most. However, it was hard to identify whether CO or N
2 was produced because their trend scan to reach the same initial value as that peaks coincided with each other. To further investigate the composition of AMU 28, we used the cracking patterns from the RGA manual provided by the manufacturer to calculate the partial pressures for each component. AMU 28 was found to consist of three parts: N2, CO, and CO
2 fragments. We found ~11.4% of AMU 44 that was actually in AMU 28. AMU 12 was found to represent ~4.5% of CO. Therefore, the pressure of N
2 could be obtained by subtracting CO and CO
2 fragments from total pressure of AMU 28. Following this calculation, the trend scans for both CO and N
2 shown in
Figure 4a were obtained. Both curves increased during the heating stage then stabilized when the boats reached evaporation temperatures, and they both decreased after turning off the power supplies for the MAI boat. However, N
2′s final pressure was 6.16 × 10
−8 Torr (which was similar to its initial pressure), while CO’s final pressure was 2.30 × 10
−8 Torr (which was higher than its initial pressure of 1.69 × 10
−8 Torr). The increase of N
2 pressure was due to the heating effect of the evaporation boat and its surroundings, and it returned to its equilibrium position when the temperature again decreased. To have a more comparable figure, we plotted
Figure 4b by dividing each vapor pressure by its initial pressure. Then, we obtained the ratio of the on-going vapor pressure and the initial pressure. The figure shows that CO pressure increased by about 90% during the evaporation, while N
2 only increased by 10%. After evaporation, CO still increased by about 50% and N
2 fell back to its initial pressure. Therefore, the total pressure of N
2 did not increase, indicating that N
2 was not produced during evaporation. On the contrary, the increase in total CO pressure suggests that CO was produced during the evaporation.
The data of
Figure 4 show that the partial pressures of CO and N
2 increased by 1.23 × 10
−8 and 1.14 × 10
−8 Torr, respectively. Since N
2 did not participate in the reaction, the increase was due to the heating effect that occurred as it was degassed from MAI during the heating process, which means that it increased 19% from its initial pressure. We assumed that the heating effect also applied to CO and NH
3, so we were able to deduce that the increases of 3.20 × 10
−9 and 4.10 × 10
−10 Torr of CO and NH
3, respectively, were due to heating, which were the 19% of their initial pressure. Therefore, the net increases of the CO and NH
3 due to the water reaction were 9.10 × 10
−9 and 1.94 × 10
−9 Torr, respectively.
Based on this discussion, we propose the following reaction for water during MAI evaporation:
In order to understand whether this reaction is thermodynamically spontaneous, its Gibbs free energy of formation Δ
fG needed to be calculated. The standard Gibbs free energy of formation Δ
fGo was found to be 51.06 kJ/mol, which means the reaction is not thermodynamically spontaneous in the standard state. However, when using the states from the evaporation period (including pressure and temperature for each reactant and product), Δ
fG was found to be negative. In this case, we used the pressures of CO, NH
3, H
2, CH
3NH
2, and H
2O at 120 °C for 60 min. Among the five chemical substances, the pressures of CH
3NH
2 and H
2 were 3.57 × 10
−9 and 1.68 × 10
−9 Torr, respectively, as directly obtained by RGA. Those for H
2O, CO, and NH
3 were 2.61 × 10
−8, 2.92 × 10
−8, and 4.50 × 10
−9 Torr, respectively. These values were calculated based on the cracking patterns and the results discussed earlier [
21]. R is ideal gas constant and T is 393.15 K, plugging these values into the Equation (1) revealed that Δ
fG for this reaction is −124.65 kJ/mol under the evaporation conditions, thus implying that the reaction is thermodynamically spontaneous. The reaction may occur on the sample surface during evaporation.
Our observations and discussion suggest that when we evaporated MAI, it decomposed into CH3NH2, HI, and other dissociated products. Thus, water vapor could have collided and reacted with CH3NH2 when it was dissociated from MAI. The consumption of CH3NH2 also reduced the amount of CH3NH2 that reached the sample surface, which led to an imbalance of the CH3NH2, HI, and PbI2 ratio and resulted in an excess of PbI2. As such, water vapor may have reduced the conversion rate of perovskite precursors and the elemental ratio of the sample surface. In this case, to grow perovskite film on the sample surface, both CH3NH2 and HI needed to be present at the same time as PbI2 to form perovskite.
3.3. Effects from Different Evaporation Conditions
We evaporated a sample under a water vapor pressure of 1.0 × 10−6 Torr and an MAI temperature of 139 °C (HW/HT), and then we used XPS to see whether the chemical composition was different from the sample grown at 2.08 × 10−9 Torr of water vapor pressure and 139 °C of MAI temperature (LW/HT). First, we evaporated two 200 Å thick perovskite film on a highly oriented pyrolytic graphite (HOPG) substrate under water vapor pressures of 1.0 × 10−6 and 2.08 × 10−9 Torr. The high water vapor pressure was controlled by a leak valve that was connected to a distilled water tube. By leaking in distilled water, we manually set the water vapor pressure to 1.0 × 10−6 Torr, stabilized it for 20 min, and then started evaporation. The lower water vapor pressure was achieved with an ion pump. The evaporation conditions for the two samples was the same as those of precursor evaporation. After the evaporation, the samples were transferred to the analytical chamber in a vacuum-sealed transfer arm for XPS measurements.
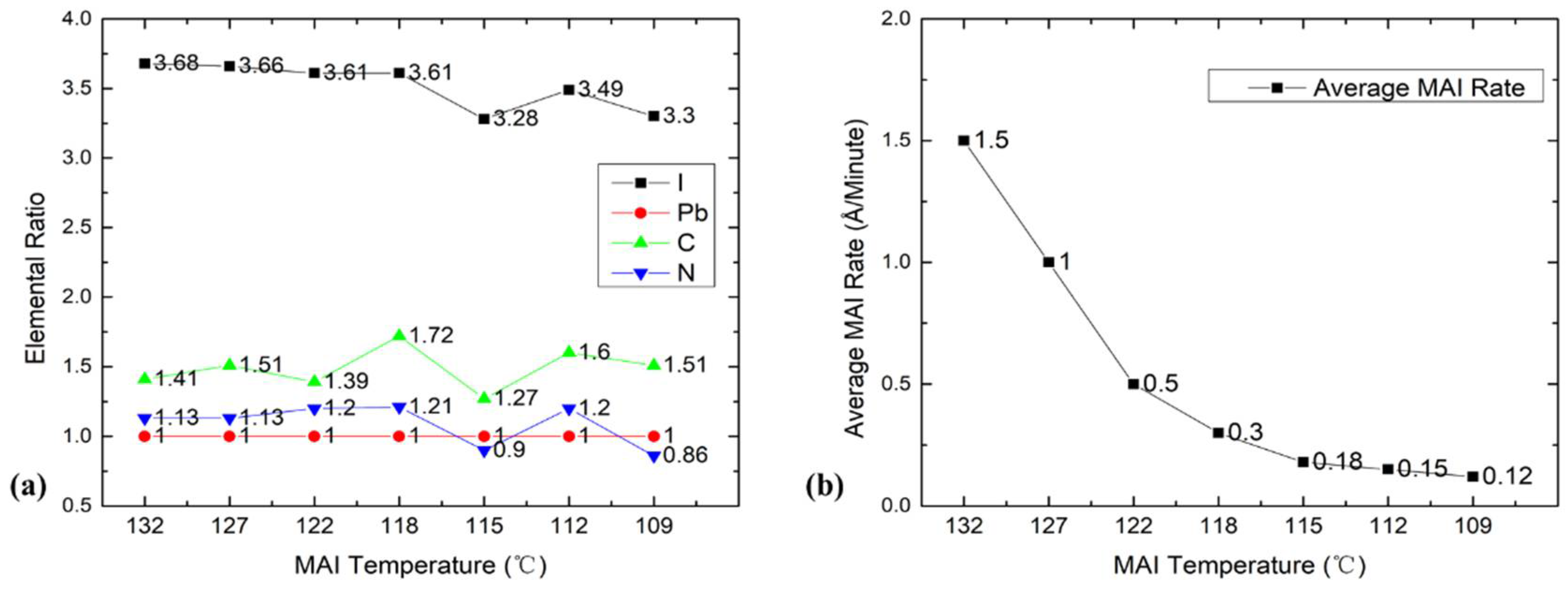
To examine the effects of different MAI temperatures, we evaporated seven MAPbI
3 films at different MAI evaporation temperatures from 132 to 109 °C at 1.75 × 10
−8 Torr of water vapor pressure, and then we analyzed film composition with XPS. The results are shown in
Figure 5. The MAI evaporation rates according to the QCM for these temperatures were 1.5, 1.0, 0.5, 0.3, 0.18, 0.15, and 0.12 Å/min. From 132 to 118 °C, the chemical composition of the film was quite uniform for all four elements, except a spike of C at 118 °C that was likely spurious because we did not see it in our many repeating measurements. Once we lowered the temperature to 109 °C (LW/LT), the iodine and nitrogen ratios were obviously lower than their initial ratios at 132 °C. It is worth noting that the ion pump consumed water and produced H
2. The pressures of H
2 before and after turning off the ion pump are displayed in
Figure S2, which shows that H
2 pressure was 2.50 × 10
−8 Torr when the ion pump was on and quickly dropped to 1.30 × 10
−8 Torr when the ion pump was turned off, which suggests that the ion pump produced H
2 at 1.20 × 10
−8 Torr. The pressures of related chemical substances can be seen in
Figure S3. To check the effect of this portion of H
2 on the thermodynamics of reaction, we calculated the Gibbs free energy of formation at 30 min with raw H
2 pressures and the net residual H
2 pressure; the results were −94.11 and −95.52 kJ/mol, respectively. These two values were similar and still thermodynamically favored the reaction. We found that the water vapor pressure first increased to 3.55 × 10
−8 Torr at 132 °C and then slowly dropped to 2.38 × 10
−8 Torr when the MAI temperature reached 109 °C. Though the MAI rate dropped from 1.5 to 0.12 Å/min, the iodine ratio only dropped to 3.3. Even QCM reading of MAI at 109 °C is 1/8 of PbI
2 showed that the film stoichiometry remained OK. The drop in the elemental ratios was not as large as that of evaporation rate, which indicates that the MAI QCM reading underestimated the amount of MAI. Thus, we had an excess of MAI in the chamber, and the real MAI rate was much higher than that shown by the QCM reading; thus, 2 × 10
−8 Torr of water pressure may not be enough to make a dramatic change with a much stronger MAI flow in terms of chemical composition. The drop of C, N, and I at 115 °C may be explained by the oversupply of MAI, as well as fluctuations in the choice of XPS measurement spot. (More LW/LT ratios can be found in
Table S2.) In this case, high water pressure could have made a considerable difference with a lower MAI evaporation temperature. Kim et al. also reported similar QCM reading underestimations of MAI flux. They explained that due to the poor adhesion of MAI on the surface of the sensor crystals, the deposition rate of MAI could be increased in the presence of PbI
2 [
22]. In order to investigate how water pressure affected the evaporation at a low MAI temperature, we increased water pressure to 1.0 × 10
−6 Torr and then measured sample growth at an MAI temperature of 109 °C (HW/LT). The RGA spectra are shown in
Figure S4. The calculated pressures at CO, NH
3, H
2, CH
3NH
2, and H
2O at 109 °C for 150 min were 9.60 × 10
−8, 3.40 × 10
−8, 2.03 × 10
−8, 1.89 × 10
−8, and 1.16 × 10
−7 Torr, respectively. The Gibbs free energy for this condition was −108.38 kJ/mol, which still supports our previous assumption.
3.4. Characterization of MAPbI3 Films
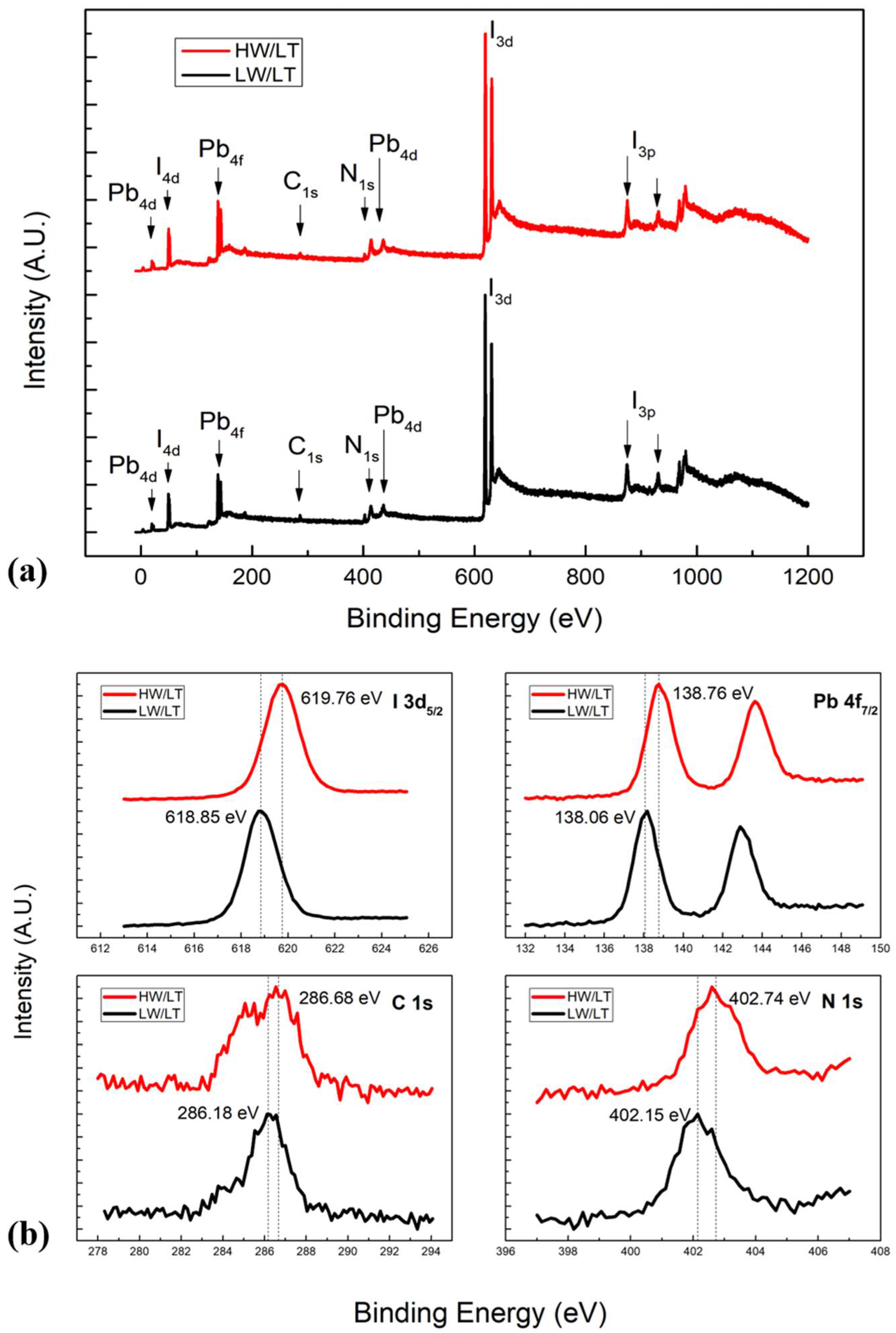
An elemental ratio comparison of LW/LT and HW/LT samples is shown in
Table 2. It is clear that we had an overall ratio reduction for every element, which is different from the results shown in
Figure 5. In the presence of high water pressure and an MAI temperate of 109 °C, perovskite carbon (286 eV) decreased by 46.4%, nitrogen decreased by 27.9%, and iodine decreased by 13%. This result shows that water vapor strongly affects perovskite film formation with a low MAI flux. This can be explained by the fact that partial CH
3NH
2 and other MAI-dissociated products were consumed by water vapor during the evaporation, as well as by the fact that the reaction left excess HI in the chamber that caused iodine to reduce less. A comparison of XPS survey scans is shown in
Figure 6, where XPS features can be seen to be similar to each other, indicating their similar chemical compositions. Since both samples were grown under the same conditions except for water vapor pressure, comparing the core level spectra allowed us to find that there was no significant change in the spectral shape but a rigid shift of about 0.59 eV to the higher binding energy (BE) in the HW/LT condition. The observed rigid shift was unlikely due to charging because MAPbI
3 film is relatively thin (200 Å), the material is of relatively high conductivity [
23], and both the HW/LT and LW/LT samples are of the same thickness. The results suggest that the presence of high water vapor pressure moved the Fermi level of the perovskite to the higher binding energy region by 0.59 eV, indicating that the Fermi level is much closer to the bottom of the conduction band. This was because water vapor reduced the amount of MAI in the HW/LT sample and made it rich in PbI
2, resulting in the n-doping of the sample (as previously observed in PbI
2-rich films) [
4,
9,
12]. In that sense, water vapor acts as an n-dopant of MAPbI
3.
Figure 3b shows that MAI-dissociated products were floating and accumulated in the chamber after each evaporation. They also enhanced the iodine-rich environment in the chamber, which may have led to an iodine surplus on the sample surface. Therefore, the existence of water vapor in the evaporation chamber could have caused the elemental ratio deficiency of the film. The excess iodine may have served as the interface recombination center, thus limiting the performance of the perovskite device [
24]. The evaporated film may have become less stable in the ambient environment when a considerable amount of water vapor was present during evaporation, which may have caused the rapid degradation of the film. Accordingly, water vapor pressure should be kept as low as possible during evaporation. In order to obtain a well-controlled MAPbI
3 thin film with good chemical stoichiometry, a water-free chamber environment is needed. An attached ion pump could reduce water vapor pressure to as low as 10
−9 Torr region, as well as reducing the pressure of other residual chemical substances from previous MAI evaporation to keep the chamber environment relatively clean.
Figures S5 and S6 show the chamber environment before HW and LW evaporations and the chamber setup. Those of AMU 18 and AMU 28 are the most noticeable peaks in the spectra. During HW evaporation, water was the dominate peak because it was manually injected into the chamber to increase the water vapor pressure. However, in LW evaporation, water was effectively reduced with an ion pump and AMU 28 became the dominate peak. The spectra show that there was no major MAI-dissociated products before the evaporations, indicating that our chamber provided a clean environment. In addition,
Figure S7 shows that the majority of the AMU 32 in MAI evaporation was CH
3NH
3+. And it was consumed during the evaporation. The impact of the evaporation boat was also examined. We heated an empty boat to 120 °C and tracked the pressure of several compounds. The results are shown in
Figure S8, indicating the pressure change was neglectable before and after heating.
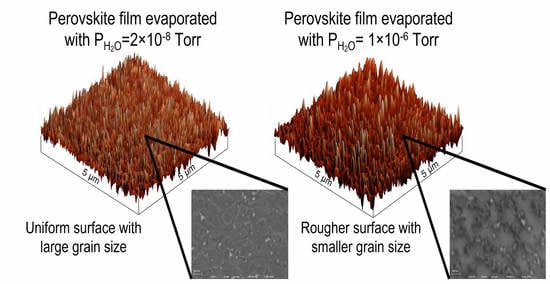
We also performed AFM and SEM to investigate effects on sample morphology. AFM and SEM image comparisons of LW and HW samples are shown in
Figure 7a,b,d,e. AFM measurements were performed at room temperature (RT) and 25.1% relative humidity (RH). The root mean square (RMS) of the LW-evaporated film was 2.08 nm, which suggests that film surface was extremely uniform. However, the RMS of the HW-evaporated film significantly increased to 13.78 nm. The other statistic parameters of AFM can be found in
Table S3. SEM images further confirmed the surface uniformity difference between the LW and HW samples. The average grain sizes of the LW and HW samples were about 100 and 50 nm, respectively. The voids on the HW sample were much bigger than those on the LW ones. This indicates that water vapor played a destructive role in terms of surface uniformity during the evaporation. The grain size of perovskite plays an important role in device performance. Previous reports have shown that with the increasing grain size of perovskites, all photovoltaic parameters such as open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), and power conversion efficiency (PCE) are improved. In addition, a large grain size could reduce bulk defects and pin holes at grain boundaries, thus enhancing the long term stability of a device [
25,
26,
27,
28]. Surface morphology also has a great impact on the optical properties. Droseros et al. found that photoluminescence (PL) is enhanced with grain size reductions of MAPbBr
3 thin film [
29]. Yan et al. reported that the grain size of MAPbBr
3 thin film can be modified by changing the molar ratio of the organic to inorganic precursor, and PL emissions can reach their maximum when the molar ratio is two [
30]. Falsini et al. showed that there are two PL emissions in CsPbCl
3 thin film, and these emissions are attributed to the different morphologies of the crystallites [
31].
Furthermore, the XRD spectra shown in
Figure 7c,f demonstrate the crystallinity of the LW- and HW-evaporated films, respectively. The thin films were grown on an Au-coated Si wafer with a thickness of 800Å because the graphite substrate became uneven after multiple exfoliations, which made it unsuitable for XRD measurement because XRD requires larger film thicknesses. For both films, the perovskite, PbI
2, and Au from the substrate can be identified in the spectra. Two perovskite peaks were observed in the LW sample at 14.07° and 28.31°, and these were assigned to the (100) and (200) lattice planes, respectively. The other three peaks at 38.29°, 44.32°, and 64.68° correspond to the (111), (200), and (220) diffractions of the Au substrate, respectively. The PbI
2 peak located at 12.65° corresponded to the (001) lattice plane. For LW sample, it is obvious that perovskite peaks were the dominant features of the spectrum but PbI
2 had a weak intensity, suggesting that the surface only had a small amount of PbI
2. On the contrary, for the HW sample, the intensities of PbI
2 and Au peaks greatly increased and even overtook the domination of perovskite peak. Based on our discussion, these can be explained by the idea that some MAI were consumed by water vapor during the evaporation, which led to excess PbI
2 on the surface. Due to the crystallization of PbI
2, voids may have been generated in the film and further exposed the Au substrate as a PbI
2 crystal aggregate, thus leading to the increase of Au peak intensity.