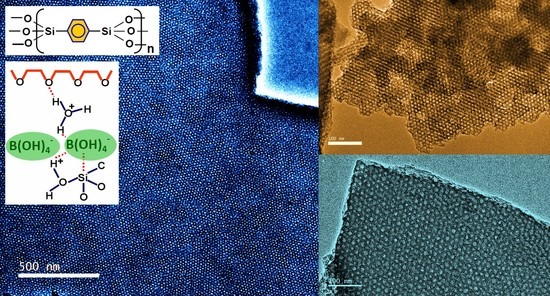
Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media
Abstract
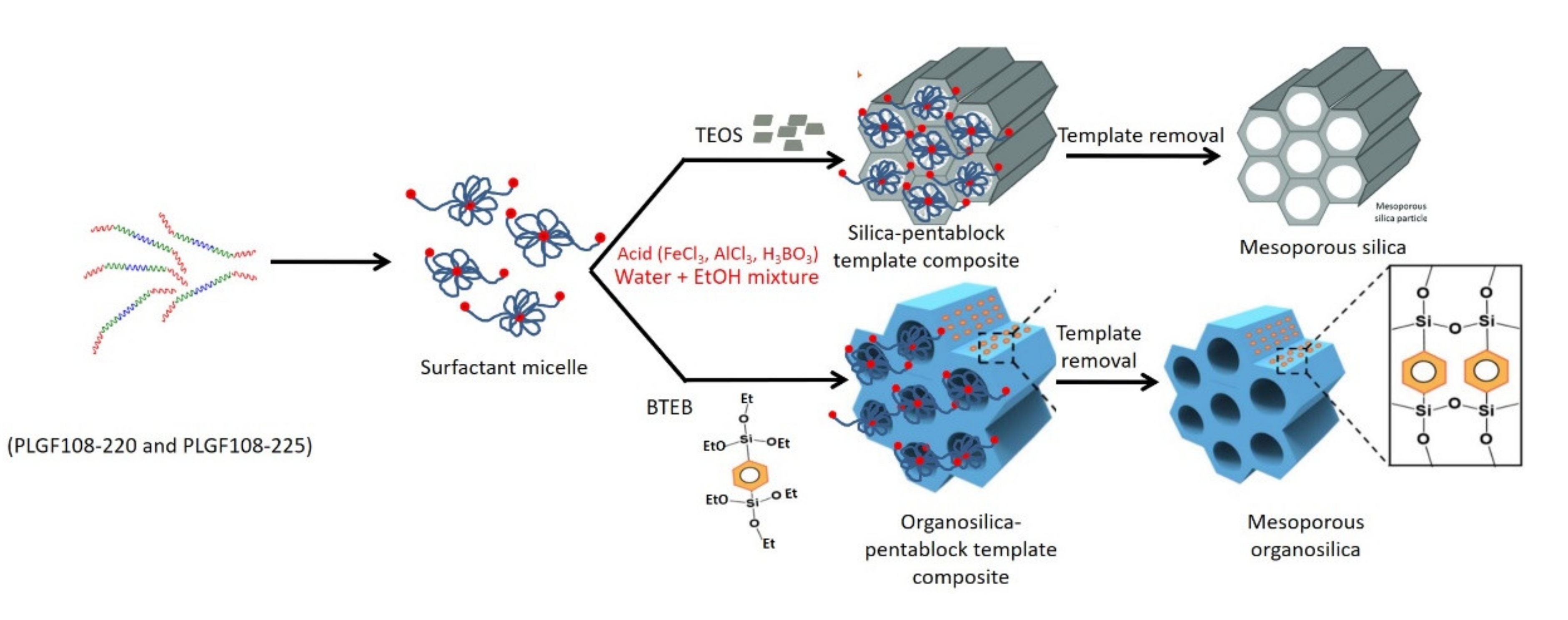
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis Procedure
2.3. Characterization
3. Results
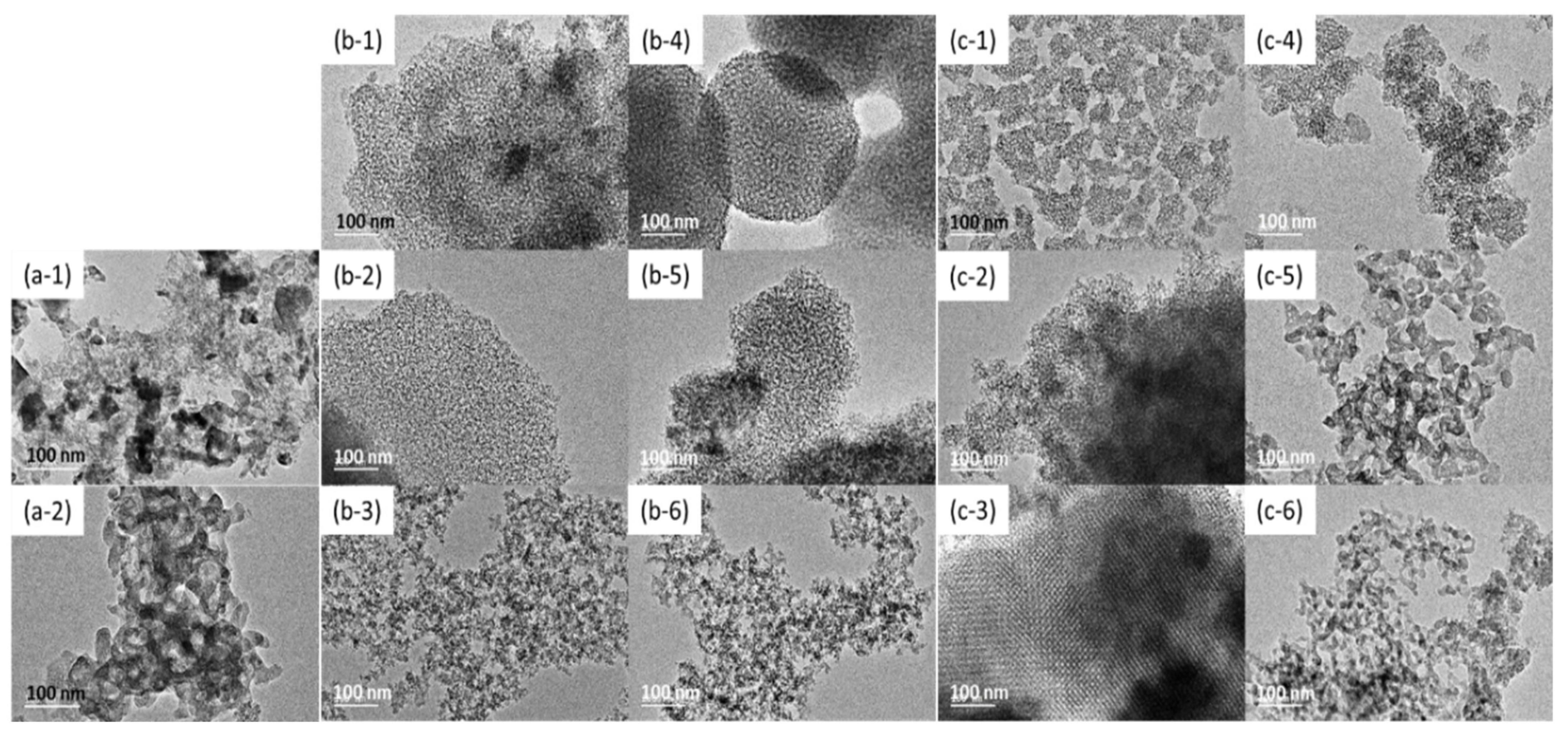
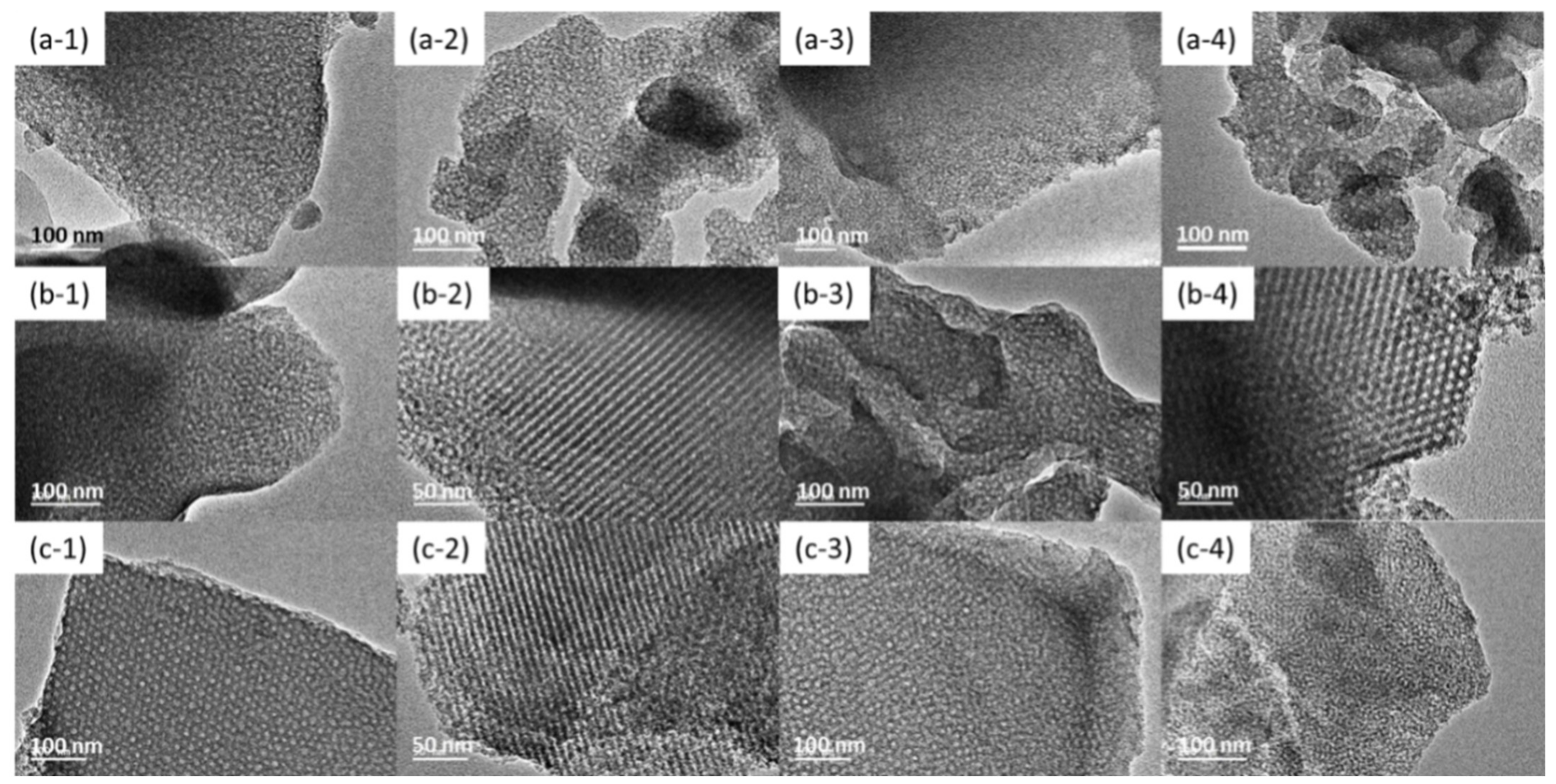
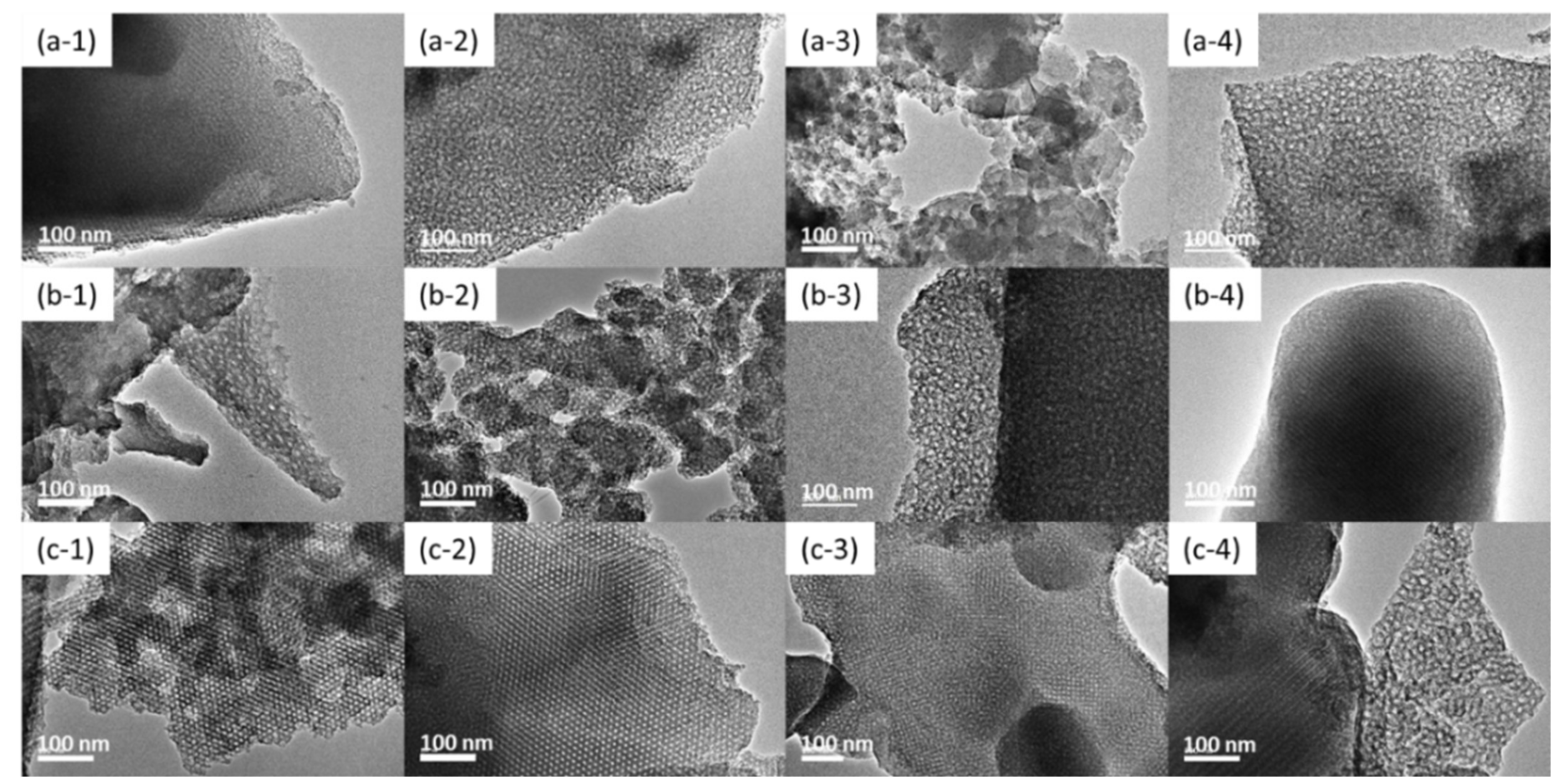
3.1. TEM Images
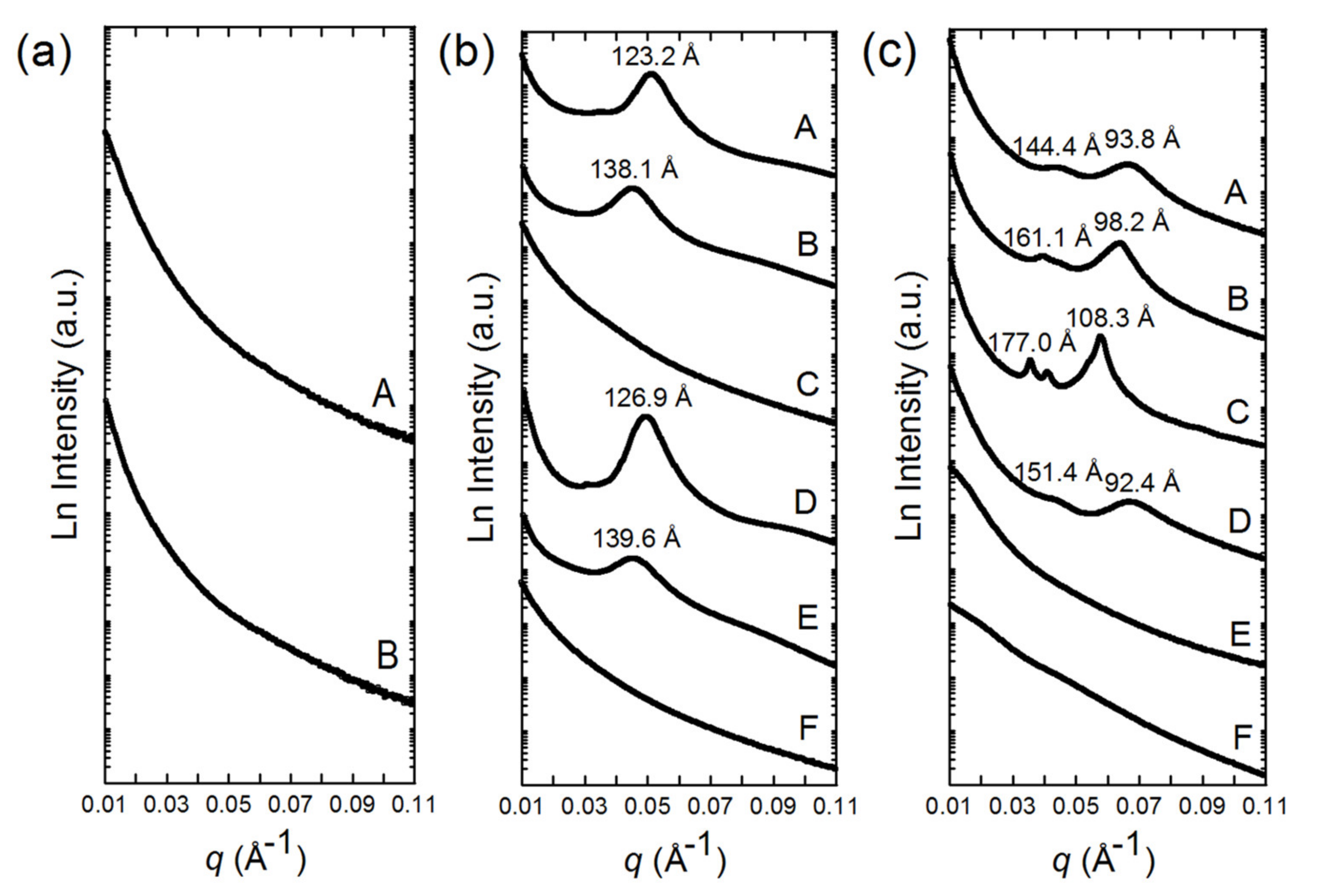
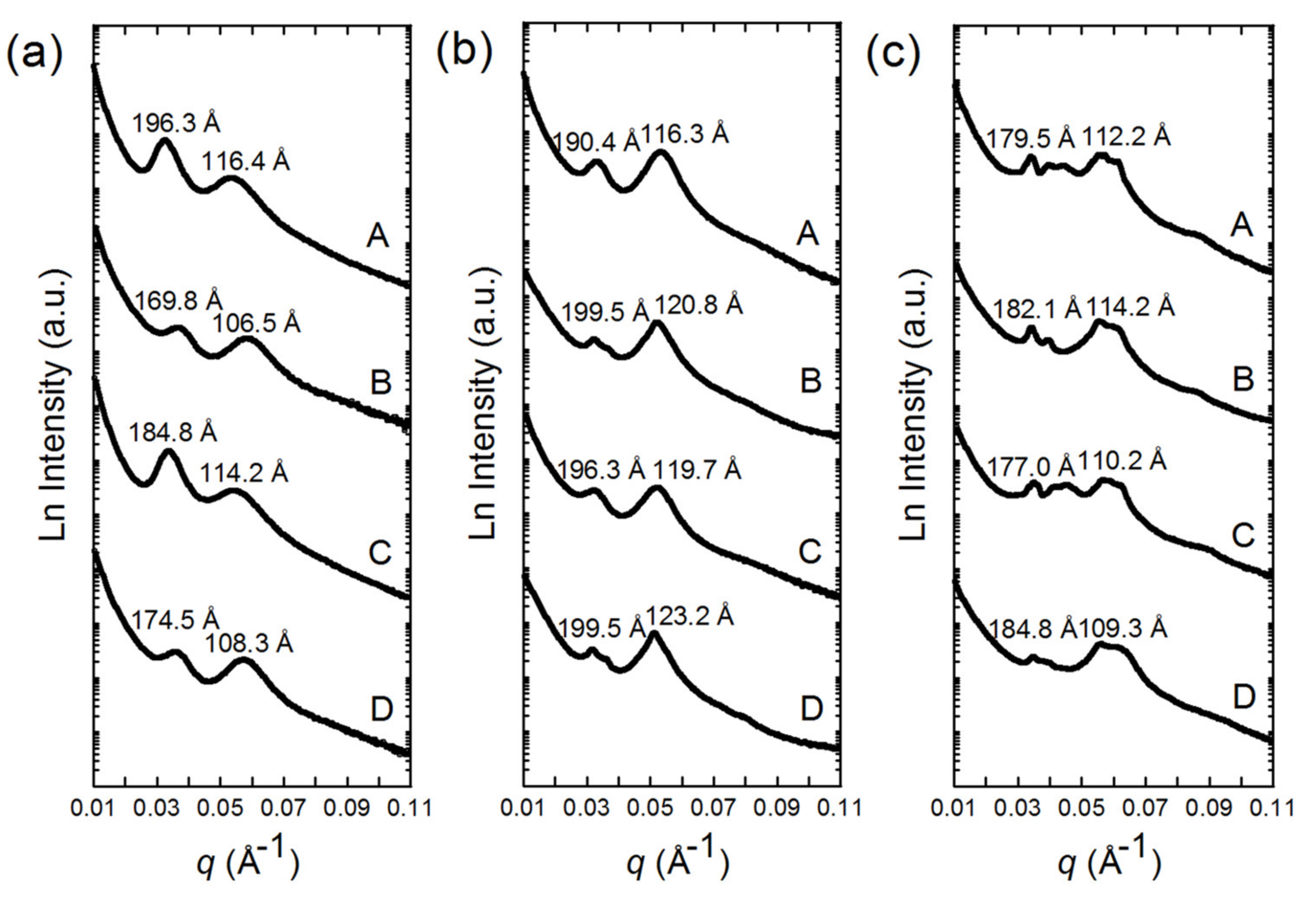
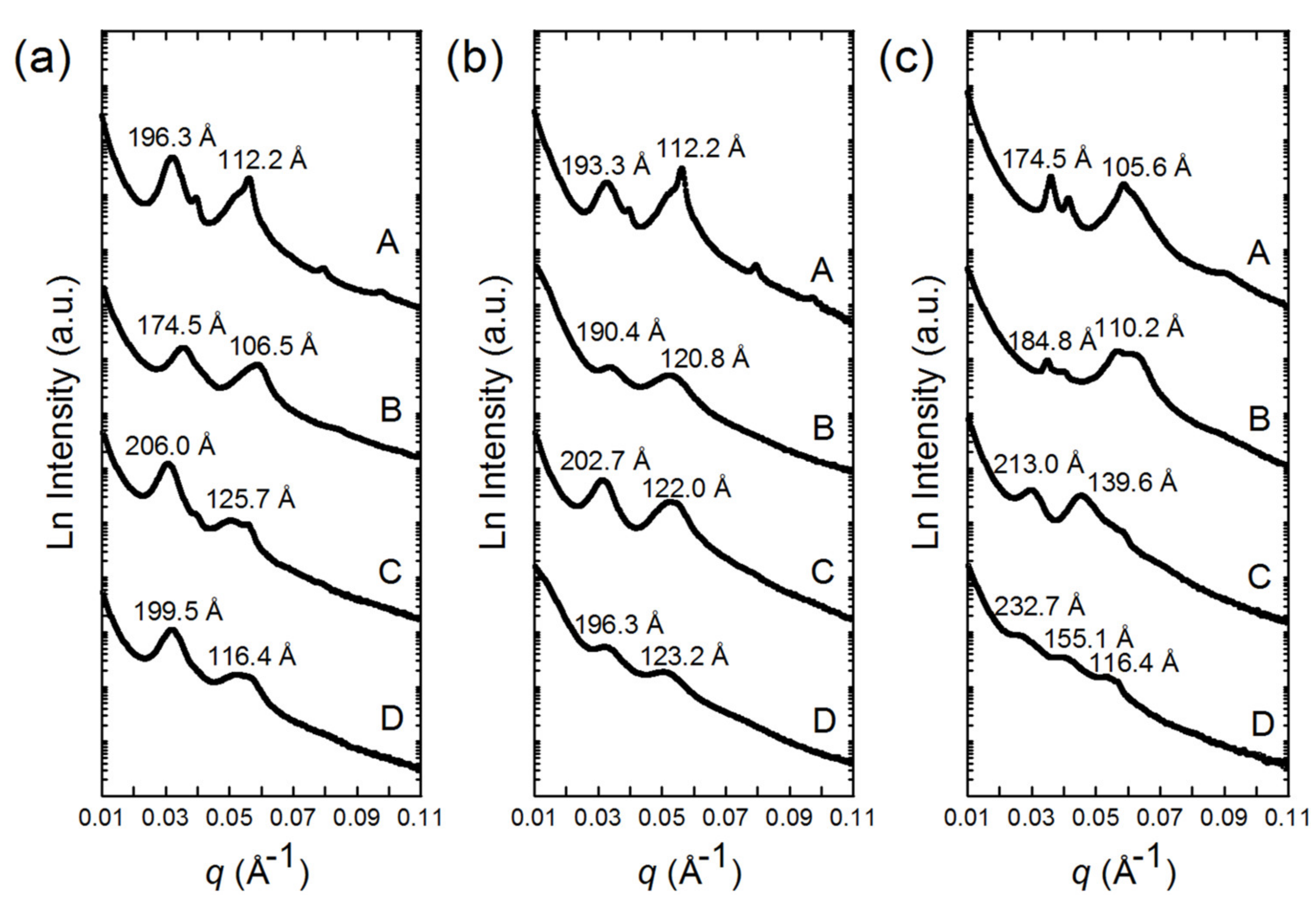
3.2. SAXS Patterns
3.3. FE-SEM Images
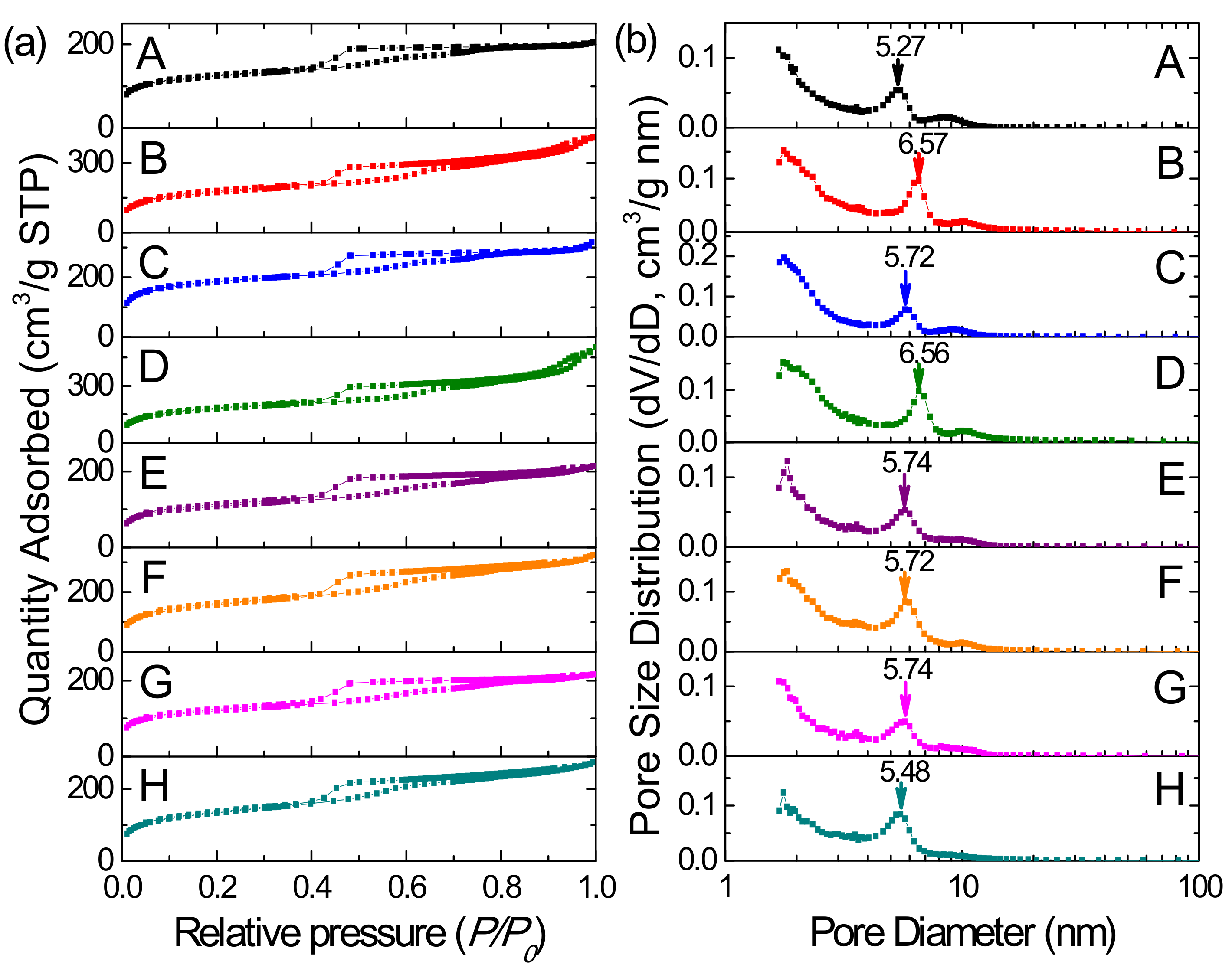
3.4. N2 Adsorption-Desorption Analysis
3.5. Elemental Analysis
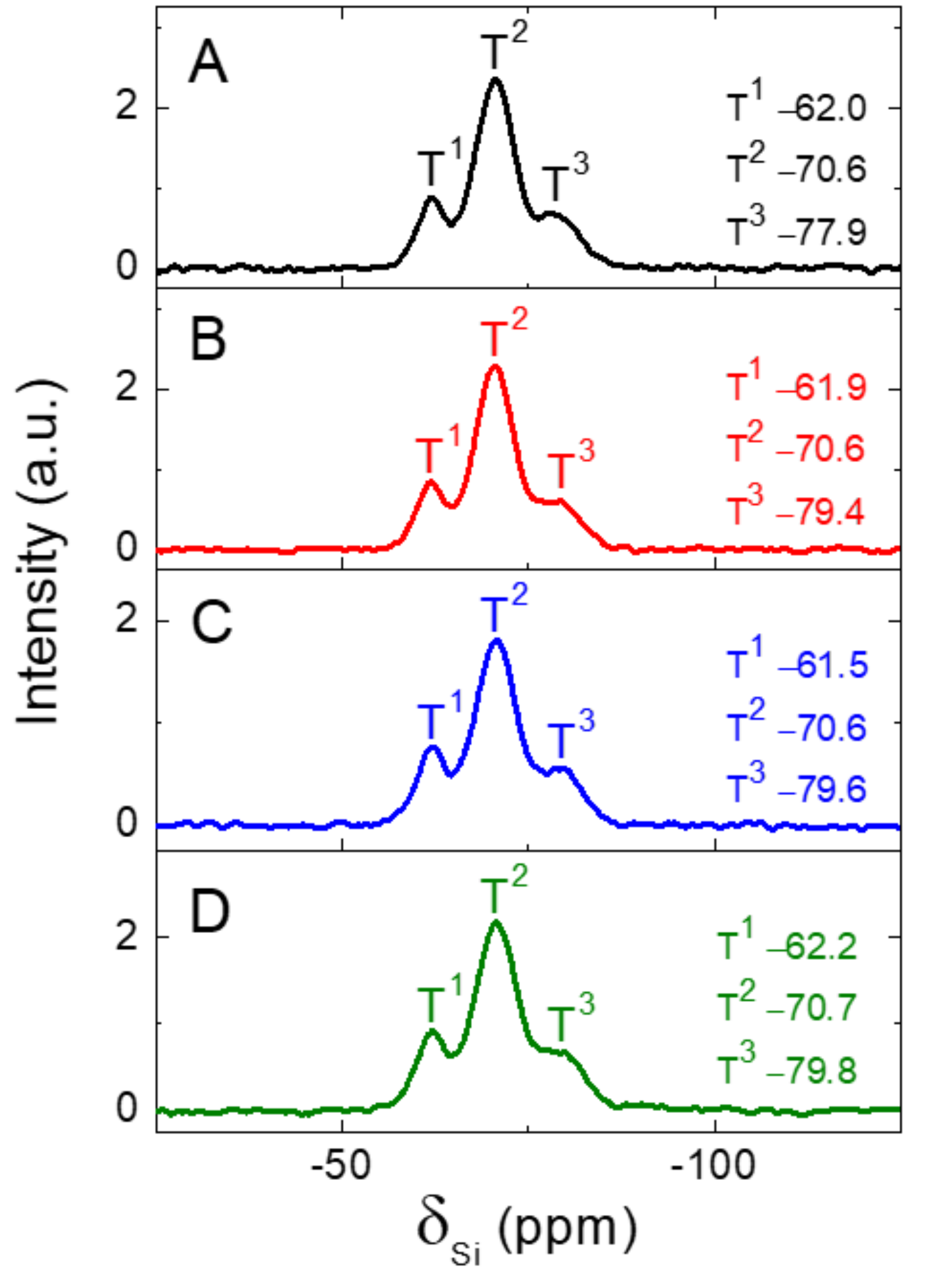
3.6. 29Si CP-MAS NMR
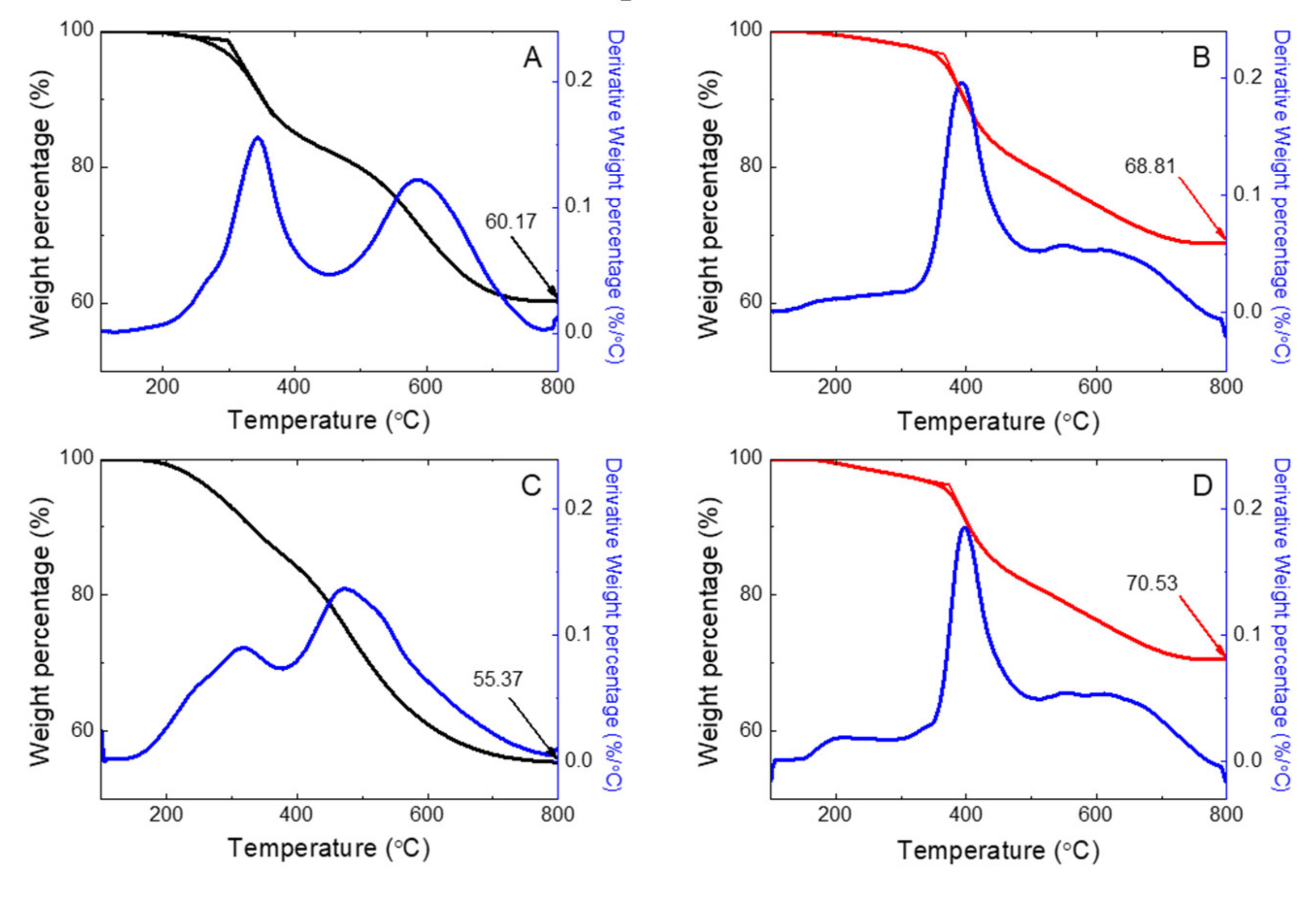
3.7. TG-DTA Thermogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, Y.; Shi, Y.; Zhao, D. Designed synthesis of mesoporous solids via nonionic-surfactant-templating approach. Chem. Commun. 2007, 9, 897–926. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Teng, Z. Facile method to efficiently fabricate large-size mesoporous organosilica nanosheets with uniform tunable pore size for robust separation membranes. Chem. Mater. 2019, 31, 3823–3830. [Google Scholar] [CrossRef]
- Choi, E.; Cho, E.-B.; Jaroniec, M. Preparation of highly ordered mesoporous ethane–silicas under weakly acidic conditions and their hydrothermal stability. J. Mater. Chem. A 2017, 5, 21378–21388. [Google Scholar] [CrossRef]
- Nagamune, N.; Ryohei, M. The effect of added inorganic salts on the micelle formation of nonionic surfactants in aqueous solutions. Bull. Chem. Soc. Jpn. 1977, 50, 1690–1694. [Google Scholar]
- Muramoto, N.; Sugiyama, T.; Matsuno, T.; Wada, H.; Kuroda, K.; Shimojima, A. Preparation of periodic mesoporous organosilica with large mesopores using silica colloidal crystals as templates. Nanoscale 2020, 12, 21155–21164. [Google Scholar] [CrossRef] [PubMed]
- Kipkemboi, P.; Fogden, A.; Alfredsson, V.; Flodström, K. Triblock copolymers as templates in mesoporous silica formation: Structural dependence on polymer chain length and synthesis temperature. Langmuir 2001, 17, 5398–5402. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Pal, N.; Sim, S.; Cho, E.-B. Multifunctional periodic mesoporous benzene-silicas for evaluation of CO2 adsorption at standard temperature and pressure. Microporous Mesoporous Mater. 2020, 293, 109816. [Google Scholar] [CrossRef]
- Lee, G.; Choi, E.; Yang, S.; Cho, E.-B. Tailoring pore size, structure, and morphology of hierarchical mesoporous silica using diblock and pentablock copolymer templates. J. Phys. Chem. C 2018, 122, 4507–4516. [Google Scholar] [CrossRef]
- Li, Y.S.; Shi, J.L.; Hua, Z.L.; Chen, H.R.; Ruan, M.L.; Yan, D.S. Hollow spheres of mesoporous aluminosilicate with a three-dimensional pore network and extraordinarily high hydrothermal stability. Nano Lett. 2003, 3, 609–612. [Google Scholar] [CrossRef]
- Kuang, D.B.; Brezesinski, T.; Smarsly, B. Hierarchical porous silica materials with a trimodal pore system using surfactant templates. J. Am. Chem. Soc. 2004, 126, 10534–10535. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Brunella, V.; Berlier, G.; Ugazio, E.; Scalarone, D. Effect of multimodal pore channels on cargo release from mesoporous silica nanoparticles. J. Nanomater. 2016, 2016, 1325174. [Google Scholar]
- Cho, E.-B.; Kim, D.; Mandal, M.; Gunathilake, C.A.; Jaroniec, M. Benzene-silica with hexagonal and cubic ordered mesostructures synthesized in the presence of block copolymers and weak acid catalysts. J. Phys. Chem. C 2012, 116, 16023–16029. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, X.; Cui, X. A two-step route to synthesis of small-pored and thick-walled SBA-16-type mesoporous silica under mildly acidic conditions. J. Colloid Interface Sci. 2007, 307, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-B.; Kim, D.; Górka, J.; Jaroniec, M. Periodic mesoporous benzene- and thiophene-silicas prepared using aluminum chloride as an acid catalyst: Effect of aluminum salt/organosilane ratio and stirring time. J. Phys. Chem. C 2009, 113, 5111–5119. [Google Scholar] [CrossRef]
- Cho, E.-B.; Mandal, M.; Jaroniec, M. Periodic mesoporous benzene-silicas prepared using boric acid as catalyst. Chem. Mater. 2011, 23, 1971–1976. [Google Scholar] [CrossRef]
- Seong, H.; Cho, E.-B.; Oh, J.; Chang, T. Synthesis and micellar characterization of CBABC type PLGA-PEO-PPO-PEO-PLGA pentablock copolymers. Bull. Korean Chem. Soc. 2014, 35, 2342–2348. [Google Scholar] [CrossRef][Green Version]
- Jaroniec, M.; Solovyov, L.A. Improvement of the Kruk-Jaroniec-Sayari method for pore size analysis of ordered silicas with cylindrical mesopores. Langmuir 2006, 22, 6757–6760. [Google Scholar] [CrossRef]
- Xie, W.; Hu, L.; Yang, X. Basic ionic liquid supported on mesoporous SBA-15 silica as an efficient heterogeneous catalyst for biodiesel production. Ind. Eng. Chem. Res. 2015, 54, 1505–1512. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Kuroda, K. Synthesis of highly ordered mesoporous materials from a layered polysilicate. J. Chem. Soc. Chem. Commun. 1993, 8, 680–682. [Google Scholar] [CrossRef]
- Pal, N.; Cho, E.-B.; Kim, D. Synthesis of ordered mesoporous silica/ceria–silica composites and their high catalytic performance for solvent-free oxidation of benzyl alcohol at room temperature. RSC Adv. 2014, 4, 9213–9222. [Google Scholar] [CrossRef]
- Ballem, M.A.; Córdoba, J.M.; Odén, M. Influence of synthesis temperature on morphology of SBA-16 mesoporous materials with a three-dimensional pore system. Microporous Mesoporous Mater. 2010, 129, 106–111. [Google Scholar] [CrossRef][Green Version]
- Mesa, M.; Sierra, L.; Patarin, J.; Guth, J.-L. Morphology and porosity characteristics control of SBA-16 mesoporous silica. Effect of the triblock surfactant Pluronic F127 degradation during the synthesis. Solid State Sci. 2005, 7, 990–997. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Tanev, P.T.; Chibwe, M.; Pinnavaia, T.J. Titanium-containing mesoporous molecular sieves for catalytic oxidation of aromatic compounds. Nature 1994, 368, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.; Carcel, C.; Oliviero, E.; Toquer, G.; Trens, P.; Bartlett, J.R.; Wong, M.; Man, C. Single-template periodic mesoporous organosilica with organized bimodal mesoporosity. Microporous Mesoporous Mater. 2020, 297, 110042. [Google Scholar] [CrossRef]
- Baù, L.; Bártová, B.; Arduini, M.; Mancin, F. Surfactant-free synthesis of mesoporous and hollow silica nanoparticles with an inorganic template. Chem. Commun. 2009, 48, 7584–7586. [Google Scholar] [CrossRef] [PubMed]


| Sample | 1H NMR | GPC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Repeating Unit | Molecular Formula | Mn (g/mol) | wLG | Mn (g/mol) | Mw (g/mol) | PDI | ||||
| PO | EO | LA | GA | |||||||
| PLGF108-220 | 54 | 282 | 44 | 14 | (LA22GA7)EO141PO54EO141(LA22GA7) | 19,462 | 0.201 | 11,100 | 15,000 | 1.35 |
| PLGF108-225 | 54 | 282 | 57 | 18 | (LA28GA9)EO141PO54EO141(LA28GA9) | 20,630 | 0.247 | 10,800 | 15,000 | 1.40 |
| A | |||||
| Sample | Template | TEOS (g) | Ethanol (g) | H2O (g) | Acid Concentration |
| PMSF-1 | PLGF108-220 | 0.8 | 10 | 60 | FeCl3·6H2O/TEOS = 2 |
| PMSF-2 | PLGF108-225 | 0.8 | 10 | 60 | FeCl3·6H2O/TEOS = 2 |
| PMSA-1 | PLGF108-220 | 1.2 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-2 | PLGF108-220 | 0.8 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-3 | PLGF108-220 | 0.4 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-4 | PLGF108-225 | 1.2 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-5 | PLGF108-225 | 0.8 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-6 | PLGF108-225 | 0.4 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSB-1 | PLGF108-220 | 1.2 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-2 | PLGF108-220 | 0.8 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-3 | PLGF108-220 | 0.4 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-4 | PLGF108-225 | 1.2 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-5 | PLGF108-225 | 0.8 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-6 | PLGF108-225 | 0.4 | 10 | 60 | H3BO3/TEOS = 2 |
| B | |||||
| Sample | Template | BTEB (g) | Ethanol (g) | H2O (g) | Acid concentration |
| PMOF-1 | PLGF108-220 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOF-2 | PLGF108-220 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOF-3 | PLGF108-225 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOF-4 | PLGF108-225 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOA-1 | PLGF108-220 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOA-2 | PLGF108-220 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOA-3 | PLGF108-225 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOA-4 | PLGF108-225 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOB-1 | PLGF108-220 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOB-2 | PLGF108-220 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| PMOB-3 | PLGF108-225 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOB-4 | PLGF108-225 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| C | |||||
| Sample | Template | BTEB (g) | Ethanol (g) | H2O (g) | Acid concentration |
| PMOFH-1 | PLGF108-220 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOFH-2 | PLGF108-220 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOFH-3 | PLGF108-225 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOFH-4 | PLGF108-225 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOAH-1 | PLGF108-220 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOAH-2 | PLGF108-220 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOAH-3 | PLGF108-225 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOAH-4 | PLGF108-225 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOBH-1 | PLGF108-220 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOBH-2 | PLGF108-220 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| PMOBH-3 | PLGF108-225 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOBH-4 | PLGF108-225 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | DKJS (nm) | d (nm) |
| PMSA-2 | 755 | 1.03 | 1.01 | 0.87 | 9.50 | 13.81 |
| PMSA-4 | 731 | 0.76 | 0.75 | 0.69 | 3.64, 8.08 | 12.69 |
| PMSB-2 | 737 | 0.69 | 0.61 | 0.51 | 5.69 | 16.11 |
| PMSB-3 | 836 | 0.81 | 0.74 | 0.59 | 2.89, 6.58 | 17.70 |
| PMSB-4 | 388 | 0.74 | 0.52 | 0.21 | 5.45 | 15.14 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | DKJS (nm) | d (nm) |
| PMOA-1 | 457 | 0.32 | 0.22 | 0.09 | 5.27 | 19.04 |
| PMOA-2 | 630 | 0.63 | 0.51 | 0.33 | 6.57 | 19.95 |
| PMOA-3 | 691 | 0.49 | 0.31 | 0.19 | 5.72 | 19.63 |
| PMOA-4 | 659 | 0.78 | 0.61 | 0.32 | 6.56 | 19.95 |
| PMOB-1 | 320 | 0.27 | 0.26 | 0.19 | 5.74 | 17.95 |
| PMOB-2 | 578 | 0.50 | 0.41 | 0.35 | 5.72 | 18.21 |
| PMOB-3 | 437 | 0.33 | 0.25 | 0.27 | 5.74 | 17.70 |
| PMOB-4 | 484 | 0.42 | 0.36 | 0.29 | 5.48 | 18.48 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | DKJS (nm) | d (nm) |
| PMOBH-1 | 339 | 0.27 | 0.20 | 0.20 | 4.87, 8.93 | 17.45 |
| PMOBH-2 | 361 | 0.36 | 0.31 | 0.23 | 5.49 | 18.48 |
| PMOBH-3 | 337 | 0.28 | 0.21 | 0.20 | 5.50, 11.24 | 21.30 |
| PMOBH-4 | 210 | 0.39 | 0.26 | 0.03 | 6.00, 13.66 | 23.27 |
| Sample | Al (ppm) | B (ppm) |
|---|---|---|
| PMOA-2 | 665.4 | - |
| PMOA-4 | 650.3 | - |
| PMOB-1 | - | 46.68 |
| PMOB-3 | - | 20.45 |
| PMOBH-1 | - | 142.25 |
| PMOBH-3 | - | 168.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, N.; Sunwoo, Y.; Park, J.-S.; Kim, T.; Cho, E.-B. Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media. Nanomaterials 2021, 11, 2522. https://doi.org/10.3390/nano11102522
Pal N, Sunwoo Y, Park J-S, Kim T, Cho E-B. Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media. Nanomaterials. 2021; 11(10):2522. https://doi.org/10.3390/nano11102522
Chicago/Turabian StylePal, Nabanita, Young Sunwoo, Jae-Seo Park, Taeyeon Kim, and Eun-Bum Cho. 2021. "Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media" Nanomaterials 11, no. 10: 2522. https://doi.org/10.3390/nano11102522
APA StylePal, N., Sunwoo, Y., Park, J.-S., Kim, T., & Cho, E.-B. (2021). Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media. Nanomaterials, 11(10), 2522. https://doi.org/10.3390/nano11102522