Novel Nanofluidic Cells Based on Nanowires and Nanotubes for Advanced Chemical and Bio-Sensing Applications
Abstract
1. Introduction
2. Synthesis Approaches
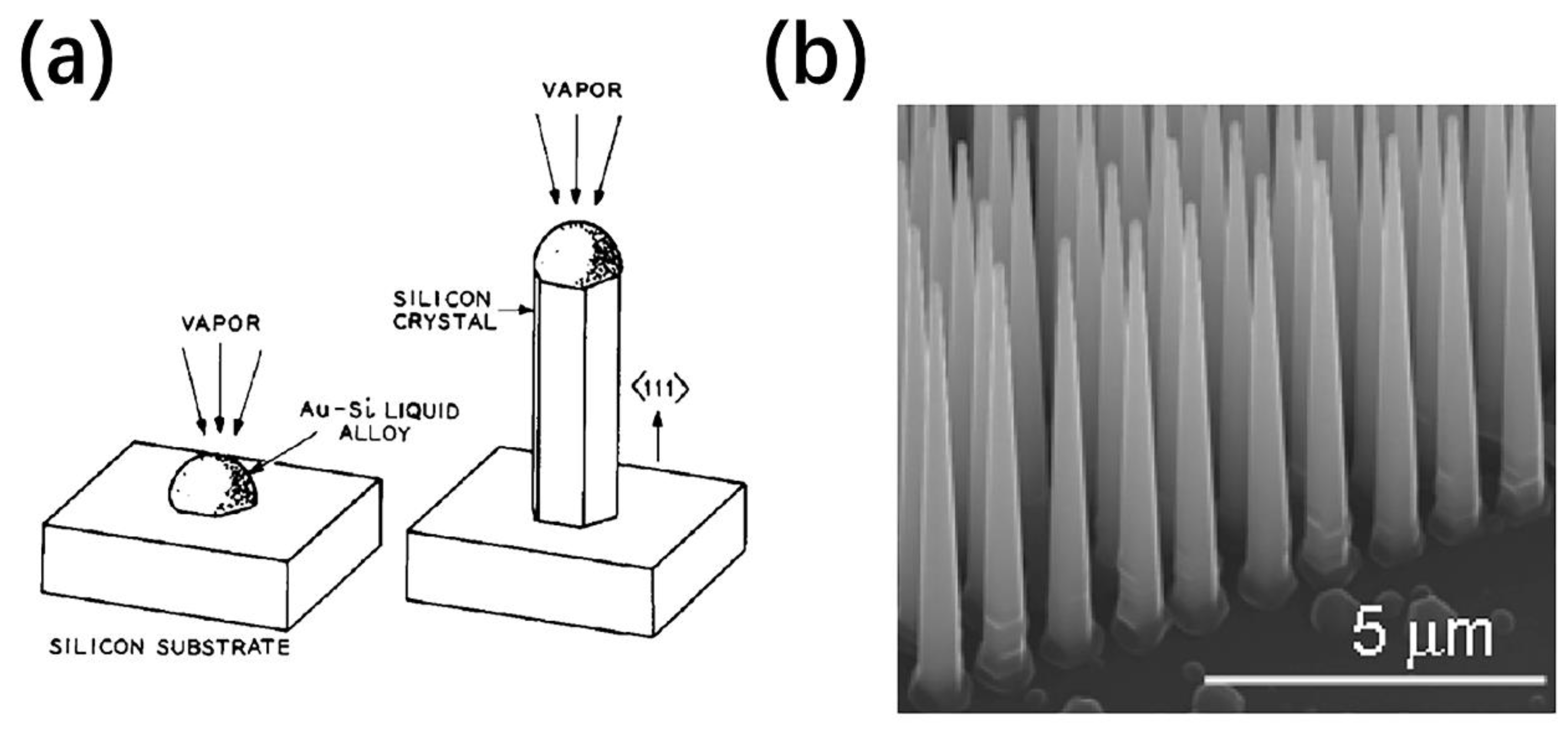
2.1. Vapor-Liquid-Solid (VLS) Growth
2.2. Membrane-Template Synthesis
2.3. Nanowire Template Approach

3. Device Mechanisms
3.1. Working Principle of Nanofluidic Cells Based on Nanowires/Nanotubes (NWs/NTs)
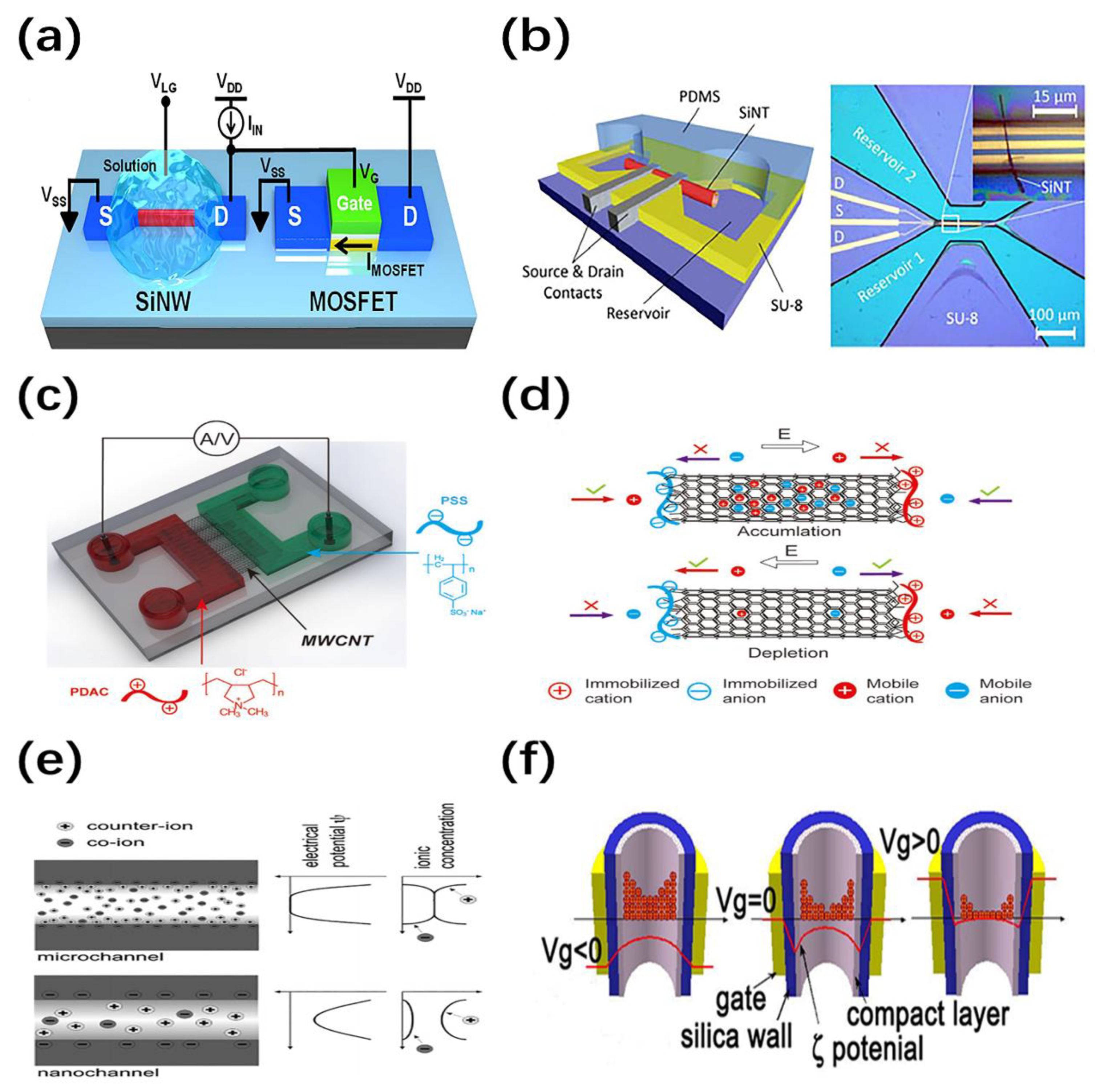
3.2. Sensing Mechanism of Biochemical Sensors Based on NW/NT Nanofluidic Cells

4. Device Applications for Chemical and Bio-Sensing
4.1. Chemical Sensing
4.1.1. Ion Sensing
4.1.2. Gas Sensing
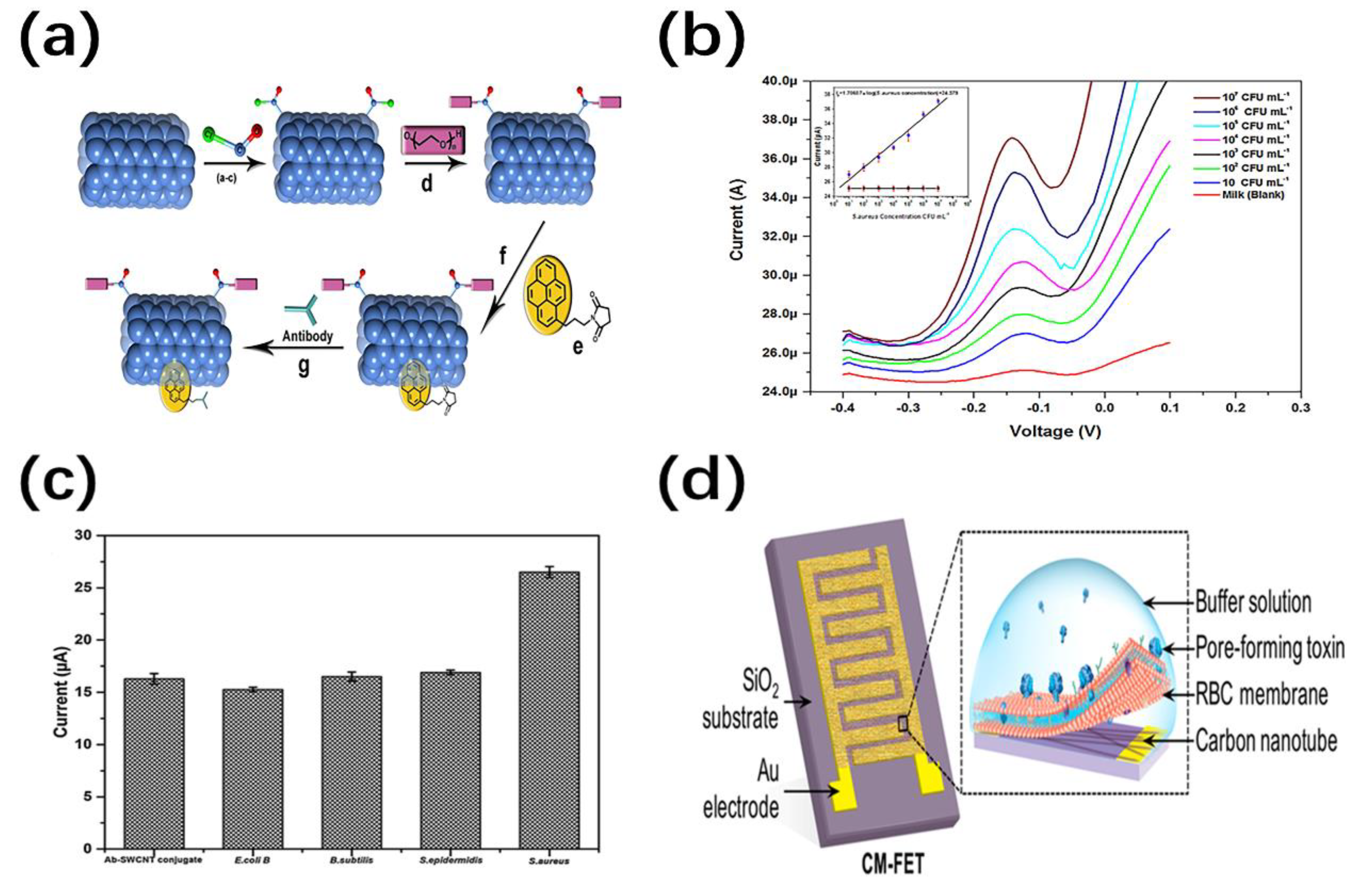
4.2. Bio-Sensing
4.2.1. Molecular Detection
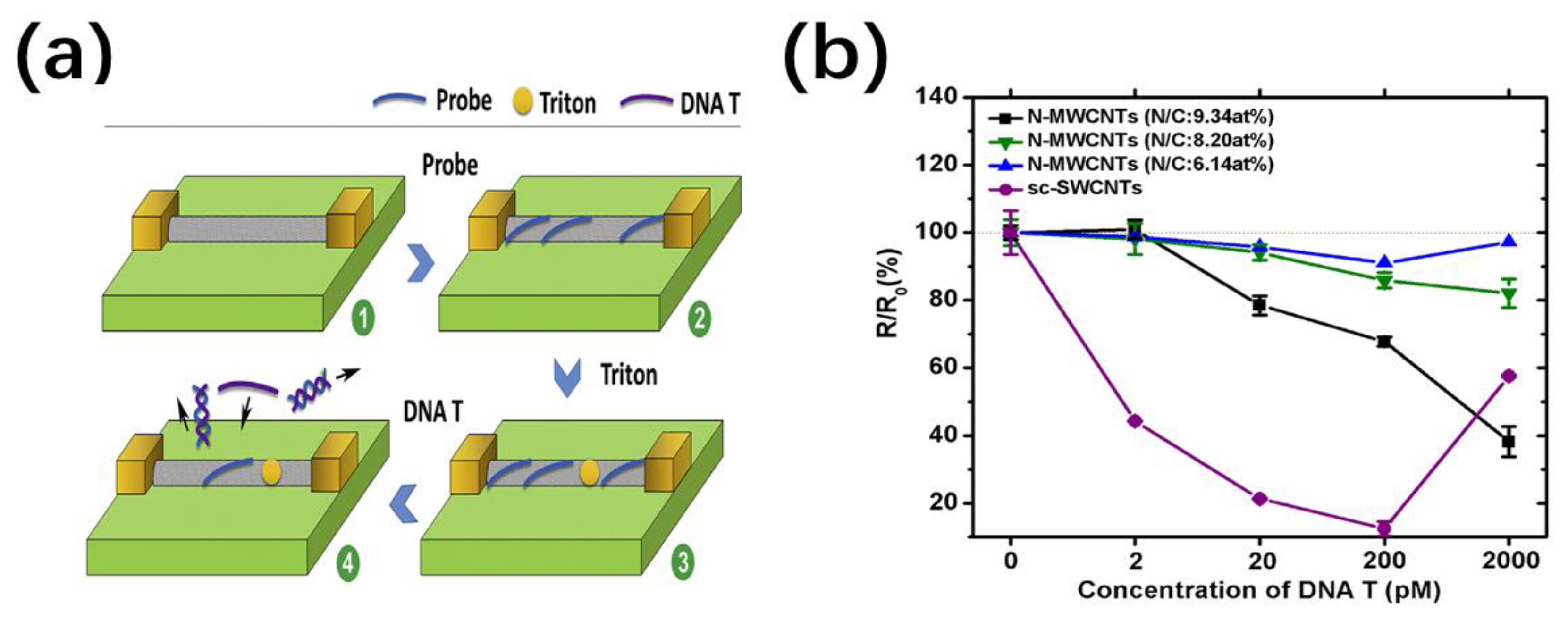
4.2.2. DNA Sequencing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Lu, D. Intensification of chemical separation engineering by nanostructured channels and nanofluidics: From theories to applications. Chin. J. Chem. Eng. 2019, 27, 1439–1448. [Google Scholar] [CrossRef]
- Radha, B.; Esfandiar, A.; Wang, F.C.; Rooney, A.P.; Gopinadhan, K.; Keerthi, A.; Mishchenko, A.; Janardanan, A.; Blake, P.; Fumagalli, L.; et al. Molecular transport through capillaries made with atomic-scale precision. Nature 2016, 538, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Kasianowicz, J.J.; Brandin, E.; Branton, D.; Deamer, D.W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA 1996, 93, 13770–13773. [Google Scholar] [CrossRef] [PubMed]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Zhang, P.; Zhang, J.; Chen, G.; Feng, X. Mechanically strong MXene/Kevlar nanofiber composite membranes as high-performance nanofluidic osmotic power generators. Nat. Commun. 2019, 10, 2920. [Google Scholar] [CrossRef]
- Mogg, L.; Zhang, S.; Hao, G.P.; Gopinadhan, K.; Barry, D.; Liu, B.L.; Cheng, H.M.; Geim, A.K.; Lozada-Hidalgo, M. Perfect proton selectivity in ion transport through two-dimensional crystals. Nat. Commun. 2019, 10, 4243. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Guo, X.; Zhang, M.; Chen, B.; Wei, G.; Li, X.; Li, X.; Li, S.; Ma, L. Laminated self-standing covalent organic framework membrane with uniformly distributed subnanopores for ionic and molecular sieving. Nat. Commun. 2020, 11, 599. [Google Scholar] [CrossRef]
- Ji, D.; Wen, Q.; Cao, L.; Kang, Q.; Lin, S.; Zhang, X.; Jiang, L.; Guo, W. Electrokinetically Controlled Asymmetric Ion Transport through 1D/2D Nanofluidic Heterojunctions. Adv. Mater. Technol. 2019, 4, 1800742. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Peng, R.; Pan, Y.; Li, Z.; Zhang, S.; Wheeler, A.R.; Tang, X.; Liu, X. Ionotronics Based on Horizontally Aligned Carbon Nanotubes. Adv. Funct. Mater. 2020, 30, 2003177. [Google Scholar] [CrossRef]
- Ambhorkar, P.; Wang, Z.; Ko, H.; Lee, S.; Koo, K.I.; Kim, K.; Cho, D.D. Nanowire-Based Biosensors: From Growth to Applications. Micromachines 2018, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, V.; Ingebrandt, S. Biologically sensitive field-effect transistors: From ISFETs to NanoFETs. Essays Biochem. 2016, 60, 81–90. [Google Scholar] [PubMed]
- Smith, R.; Geary, S.M.; Salem, A.K. Silicon Nanowires and Their Impact on Cancer Detection and Monitoring. ACS Appl. Nano Mater. 2020, 3, 8522–8536. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-Liquid-Solid Mechanism Of Single Crystal Growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Morales, A.M.; Lieber, C.M. A laser ablation method for the synthesis of crystalline semiconductor nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef]
- Hu, P.; Dong, S.; Zhang, D.; Fang, C.; Zhang, X. Catalyst-assisted synthesis of core–shell SiC/SiO2 nanowires via a simple method. Ceram. Int. 2016, 42, 1581–1587. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Nickel oxide nanowires: Vapor liquid solid synthesis and integration into a gas sensing device. Nanotechnology 2016, 27, 205701. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Li, J.; Tian, S.; Fei, T.; Li, H. Effects of deposition temperature and time on HfC nanowires synthesized by CVD on SiC-coated C/C composites. Ceram. Int. 2016, 42, 5623–5628. [Google Scholar] [CrossRef]
- Haffner, T.; Zeghouane, M.; Bassani, F.; Gentile, P.; Gassenq, A.; Chouchane, F.; Pauc, N.; Martinez, E.; Robin, E.; David, S.; et al. Growth of Ge1−xSnx Nanowires by Chemical Vapor Deposition via Vapor-Liquid-Solid Mechanism Using GeH4 and SnCl4. Phys. Status Solidi A 2018, 215, 1700743. [Google Scholar] [CrossRef]
- Wu, R.; Yang, Z.; Fu, M.; Zhou, K. In-situ growth of SiC nanowire arrays on carbon fibers and their microwave absorption properties. J. Alloy. Compd. 2016, 687, 833–838. [Google Scholar] [CrossRef]
- Dong, S.; Hu, P.; Zhang, X.; Cheng, Y.; Fang, C.; Xu, J.; Chen, G. Facile synthesis of silicon nitride nanowires with flexible mechanical properties and with diameters controlled by flow rate. Sci. Rep. 2017, 7, 45538. [Google Scholar] [CrossRef] [PubMed]
- Terasako, T.; Kawasaki, Y.; Yagi, M. Growth and morphology control of β-Ga2O3 nanostructures by atmospheric-pressure CVD. Thin Solid Films 2016, 620, 23–29. [Google Scholar] [CrossRef]
- Terasako, T.; Kohno, K.; Yagi, M. Vapor-liquid-solid growth of SnO2 nanowires utilizing alternate source supply and their photoluminescence properties. Thin Solid Films 2017, 644, 3–9. [Google Scholar] [CrossRef]
- Krylyuk, S.; Davydov, A.V.; Levin, I. Tapering Control of Si Nanowires Grownfrom SiCl4 at Reduced Pressure. ACS Nano 2010, 5, 656–664. [Google Scholar] [CrossRef]
- Küpers, H.; Lewis, R.B.; Tahraoui, A.; Matalla, M.; Krüger, O.; Bastiman, F.; Riechert, H.; Geelhaar, L. Diameter evolution of selective area grown Ga-assisted GaAs nanowires. Nano Res. 2018, 11, 2885–2893. [Google Scholar] [CrossRef]
- DeJarld, M.; Teran, A.; Luengo-Kovac, M.; Yan, L.; Moon, E.S.; Beck, S.; Guillen, C.; Sih, V.; Phillips, J.; Milunchick, J.M. The effect of doping on low temperature growth of high quality GaAs nanowires on polycrystalline films. Nanotechnology 2016, 27, 495605. [Google Scholar] [CrossRef]
- Akbari, M.; Mohajerzadeh, S. Highly patterned growth of SnO2 nanowires using a sub-atmospheric vapor–liquid–solid deposition. J. Phys. D-Appl. Phys. 2017, 50, 305104. [Google Scholar] [CrossRef]
- Fan, H.J.; Werner, P.; Zacharias, M. Semiconductor nanowires: From self-organization to patterned growth. Small 2006, 2, 700–717. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Takahashi, T.; Okamoto, N.; Sato, M. Position-Controlled VLS Growth of Nanowires on Mask-Patterned GaAs Substrates for Axial GaAsSb/InAs Heterostructures. Phys. Status Solidi A 2018, 215, 1870015. [Google Scholar] [CrossRef]
- Khayyat, M.M.; Wacaser, B.A.; Reuter, M.C.; Ross, F.M.; Sadana, D.K.; Chen, T.C. Nanoscale chemical templating of Si nanowires seeded with Al. Nanotechnology 2013, 24, 235301. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Senz, S.; Gosele, U. Diameter-dependent growth direction of epitaxial silicon nanowires. Nano Lett. 2005, 5, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Westerbeek, E.Y.; van den Berg, A.; Segerink, L.I.; Shui, L.; Eijkel, J.C.T. Mass Transport Determined Silica Nanowires Growth on Spherical Photonic Crystals with Nanostructure-Enabled Functionalities. Small 2020, 16, 2001026. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Ling, L.; Luo, J.; Zhang, K.; Xu, Y.; Lu, H.; Yao, Y. Remote catalyzation for growth of boron nitride nanotubes by low pressure chemical vapor deposition. Chem. Phys. Lett. 2016, 652, 27–31. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.; Wang, N.; Zhou, Y.; Li, T. Magnetic and microwave absorption properties of Fe/TiO2 nanocomposites prepared by template electrodeposition. J. Alloy. Compd. 2018, 763, 421–429. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Xu, L. Dual-templating approach to ordered mesoporous Pt nanowires with various morphologies. Mater. Lett. 2018, 223, 97–101. [Google Scholar] [CrossRef]
- Narula, C.; Chauhan, R.P. Size dependent properties of one dimensional CdSe micro/nanostructures. Physica B 2017, 521, 381–388. [Google Scholar] [CrossRef]
- Movsesyan, L.; Schubert, I.; Yeranyan, L.; Trautmann, C.; Eugenia Toimil-Molares, M. Influence of electrodeposition parameters on the structure and morphology of ZnO nanowire arrays and networks synthesized in etched ion-track membranes. Semicond. Sci. Technol. 2016, 31, 014006. [Google Scholar] [CrossRef]
- Yang, B.; Niu, G.; Liu, X.-D.; Yang, Y.; He, W.; Zhu, Y.; Yu, B.; Zhou, X.-W.; Wu, W.-D. Preparation of size controllable porous polymethylmethacrylate template and Cu micro/nanowire arrays. RSC Adv. 2016, 6, 88656–88663. [Google Scholar] [CrossRef]
- He, G.; Chen, H.-J.; Liu, D.; Feng, Y.; Yang, C.; Hang, T.; Wu, J.; Cao, Y.; Xie, X. Fabrication of Various Structures of Nanostraw Arrays and Their Applications in Gene Delivery. Adv. Mater. Interfaces 2018, 5, 1701535. [Google Scholar] [CrossRef]
- De la Torre Medina, J.; da Camara Santa Clara Gomes, T.; Velazquez Galvan, Y.G.; Piraux, L. Large-scale 3-D interconnected Ni nanotube networks with controlled structural and magnetic properties. Sci Rep 2018, 8, 14555. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martin-Gonzalez, M. Three-Dimensional Bi2Te3 Networks of Interconnected Nanowires: Synthesis and Optimization. Nanomaterials 2018, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Wang, L.C.; Liu, W.S.; Wang, C.C.; Perng, T.P. Photocatalysis and Hydrogen Evolution of Al- and Zn-Doped TiO2 Nanotubes Fabricated by Atomic Layer Deposition. Semicond. Sci. Technol. 2018, 10, 33287–33295. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.Y.; Han, H.; Kim, J.W.; Lee, S.-M.; Ha, J.S.; Shim, J.H.; Han, C.-S. Sub-5 nm nanostructures fabricated by atomic layer deposition using a carbon nanotube template. Nanotechnology 2016, 27, 265301. [Google Scholar] [CrossRef] [PubMed]
- Rabin, O.; Herz, P.R.; Lin, Y.M.; Akinwande, A.I.; Cronin, S.B.; Dresselhaus, M.S. Formation of Thick Porous Anodic Alumina Films and Nanowire Arrays on Silicon Wafers and Glass. Adv. Funct. Mater. 2003, 13, 631–638. [Google Scholar] [CrossRef]
- Sander, M.S.; Gao, H. Aligned arrays of nanotubes and segmented nanotubes on substrates fabricated by electrodeposition onto nanorods. J. Am. Chem. Soc. 2005, 127, 12158–12159. [Google Scholar] [CrossRef]
- Guiliani, J.; Cadena, J.; Monton, C. Template-assisted electrodeposition of Ni and Ni/Au nanowires on planar and curved substrates. Nanotechnology 2018, 29, 075301. [Google Scholar] [CrossRef]
- Goldberger, J.; Fan, R.; Yang, P.D. Inorganic nanotubes: A novel platform for nanofluidics. Acc. Chem. Res. 2006, 39, 239–248. [Google Scholar] [CrossRef]
- Jia, X.; Zhu, X.; Tian, W.; Ding, Y.; Tian, X.; Cheng, B.; Cheng, L.; Bai, S.; Qin, Y. Nanowire templated CVD synthesis and morphological control of MoS2 nanotubes. J. Mater. Chem. C 2020, 8, 4133–4138. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Guo, Y.; Wang, Y.; Liu, R.; Chen, B.; Zhong, H.; Zou, B. Growth of CdS nanotubes and their strong optical microcavity effects. Nanoscale 2019, 11, 5325–5329. [Google Scholar] [CrossRef]
- Ishiyama, T.; Nakagawa, S.; Wakamatsu, T.; Fujiwara, N. Synthesis of β-FeSi2 nanowires by using silicon nanowire templates. AIP Adv. 2018, 8, 085324. [Google Scholar] [CrossRef]
- Zhu, H.; Li, H.; Robertson, J.W.F.; Balijepalli, A.; Krylyuk, S.; Davydov, A.V.; Kasianowicz, J.J.; Suehle, J.S.; Li, Q. Novel nanofluidic chemical cells based on self-assembled solid-state SiO2 nanotubes. Nanotechnology 2017, 28, 435601. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.; French, J.S.; Balgarkashi, A.; Tappy, N.; Fontcuberta, I.M.A.; Idrobo, J.C.; Sutter, P. Single-Crystalline gamma-Ga2S3 Nanotubes via Epitaxial Conversion of GaAs Nanowires. Nano Lett. 2019, 19, 8903–8910. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yu, D.; Zhou, J.; Lu, X.; Yang, Y.; Gao, F. Ultrathin Wall (1 nm) and Superlong Pt Nanotubes with Enhanced Oxygen Reduction Reaction Performance. Small 2018, 14, 1704503. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, H.; Chen, S.; Wang, C.; Ma, T. Nanowire-Templated Synthesis of FeNx -Decorated Carbon Nanotubes as Highly Efficient, Universal-pH, Oxygen Reduction Reaction Catalysts. Chemistry 2019, 25, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, G.; Asadollahi, A.; Hellström, P.E.; Garidis, K.; Östling, M. Silicon nanowires integrated with CMOS circuits for biosensing application. Solid-State Electron. 2014, 98, 26–31. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Choi, B.; Yoon, J.; Kim, J.Y.; Choi, Y.K.; Kim, D.M.; Kim, D.H.; Choi, S.J. A Highly Responsive Silicon Nanowire/Amplifier MOSFET Hybrid Biosensor. Sci. Rep. 2015, 5, 12286. [Google Scholar] [CrossRef]
- Lee, R.; Kwon, D.W.; Kim, S.; Kim, S.; Mo, H.-S.; Kim, D.H.; Park, B.-G. Nanowire size dependence on sensitivity of silicon nanowire field-effect transistor-based pH sensor. Jpn. J. Appl. Phys. 2017, 56, 124001. [Google Scholar] [CrossRef]
- Schwartz, M.; Thanh Chien, N.; Xuan Thang, V.; Wagner, P.; Thoelen, R.; Ingebrandt, S. Impedimetric Sensing of DNA with Silicon Nanowire Transistors as Alternative Transducer Principle. Phys. Status Solidi A 2018, 215, 1700740. [Google Scholar] [CrossRef]
- Guo, J.; He, J.; Zeng, B. Carbon Nanotube Based Nanopore and Nanofluidic Devices Towards Sensing Applications. Curr. Nanosci. 2016, 12, 421–428. [Google Scholar] [CrossRef][Green Version]
- Hibst, N.; Steinbach, A.M.; Strehle, S. Fluidic and Electronic Transport in Silicon Nanotube Biosensors. MRS Adv. 2016, 1, 3761–3766. [Google Scholar] [CrossRef]
- Haywood, D.G.; Saha-Shah, A.; Baker, L.A.; Jacobson, S.C. Fundamental studies of nanofluidics: Nanopores, nanochannels, and nanopipets. Anal. Chem. 2015, 87, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, H.; Wang, D.; Yin, Y.; Stroeve, P.; Zhang, Y.; Sheng, Z.; Chen, B.; Zhan, K.; Hou, X. Dynamic Curvature Nanochannel-Based Membrane with Anomalous Ionic Transport Behaviors and Reversible Rectification Switch. Adv. Mater. 2019, 31, 1805130. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Fan, R.; Reed, M.A. Field-effect reconfigurable nanofluidic ionic diodes. Nat. Commun. 2011, 2, 506. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Chen, L.; Zhang, Z.; Xie, G.; Li, P.; Kong, X.Y.; Wen, L.; Jiang, L. A Tunable Ionic Diode Based on a Biomimetic Structure-Tailorable Nanochannel. Angew.Chem.Int. Ed. 2017, 56, 8168–8172. [Google Scholar] [CrossRef] [PubMed]
- Abgrall, P.; Nguyen, N.T. Nanofluidic devices and their applications. Anal. Chem. 2008, 80, 2326–2341. [Google Scholar] [CrossRef]
- Fan, R.; Yue, M.; Karnik, R.; Majumdar, A.; Yang, P. Polarity switching and transient responses in single nanotube nanofluidic transistors. Phys. Rev. Lett. 2005, 95, 086607. [Google Scholar] [CrossRef]
- Wilson, J.; Di Ventra, M. Single-base DNA discrimination via transverse ionic transport. Nanotechnology 2013, 24, 415101. [Google Scholar] [CrossRef]
- Schneider, G.F.; Kowalczyk, S.W.; Calado, V.E.; Pandraud, G.; Zandbergen, H.W.; Vandersypen, L.M.K.; Dekker, C. DNA Translocation through Graphene Nanopores. Nano Lett. 2010, 10, 3163–3167. [Google Scholar] [CrossRef]
- Fan, R.; Karnik, R.; Yue, M.; Li, D.Y.; Majumdar, A.; Yang, P.D. DNA translocation in inorganic nanotubes. Nano Lett. 2005, 5, 1633–1637. [Google Scholar] [CrossRef]
- Roy, S.; Gao, Z. Nanostructure-based electrical biosensors. Nano Today 2009, 4, 318–334. [Google Scholar] [CrossRef]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Rackauskas, S.; Barbero, N.; Barolo, C.; Viscardi, G. ZnO Nanowire Application in Chemoresistive Sensing: A Review. Nanomaterials 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Kondo, T.; Sato, Y.; Kinoshita, M.; Shankar, P.; Mintcheva, N.N.; Honda, M.; Iwamori, S.; Kulinich, S.A. Room temperature ethanol sensor based on ZnO prepared via laser ablation in water. Jpn. J. Appl. Phys. 2017, 56, 080304. [Google Scholar] [CrossRef]
- Sun, P.; Cai, Y.; Du, S.; Xu, X.; You, L.; Ma, J.; Liu, F.; Liang, X.; Sun, Y.; Lu, G. Hierarchical α-Fe2O3/SnO2 semiconductor composites: Hydrothermal synthesis and gas sensing properties. Sens. Actuator B-Chem. 2013, 182, 336–343. [Google Scholar] [CrossRef]
- Zeb, S.; Peng, X.; Yuan, G.; Zhao, X.; Qin, C.; Sun, G.; Nie, Y.; Cui, Y.; Jiang, X. Controllable synthesis of ultrathin WO3 nanotubes and nanowires with excellent gas sensing performance. Sens. Actuator B-Chem. 2020, 305, 127435. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.Q.; Park, H.K.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Amorim, C.A.; Blanco, K.C.; Costa, I.M.; Vicente, E.F.; da S Petruci, J.F.; Contiero, J.; Leite, E.R.; Chiquito, A.J. Active-electrode biosensor of SnO2 nanowire for cyclodextrin detection from microbial enzyme. Nanotechnology 2020, 31, 165501. [Google Scholar] [CrossRef]
- Choi, S.; Mo, H.-S.; Kim, J.; Kim, S.; Lee, S.M.; Choi, S.-J.; Kim, D.M.; Park, D.-W.; Kim, D.H. Experimental extraction of stern-layer capacitance in biosensor detection using silicon nanowire field-effect transistors. Curr. Appl. Phys. 2020, 20, 828–833. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.B.; Shiddiky, M.J.A. Nanozyme-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sun, C.; Guo, Y.; Nie, G.; Xu, L. Peroxidase-like activity of apoferritin paired gold clusters for glucose detection. Biosens. Bioelectron. 2015, 64, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, S.; Wang, Y.; Li, T. Gold nanoparticle modified silicon nanowire array based sensor for low-cost, high sensitivity and selectivity detection of mercury ions. Mater. Res. Express 2020, 7, 035017. [Google Scholar] [CrossRef]
- Cao, D.; Pang, P.; Liu, H.; He, J.; Lindsay, S.M. Electronic sensitivity of a single-walled carbon nanotube to internal electrolyte composition. Nanotechnology 2012, 23, 085203. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, B.H.; Kim, S.Y.; Park, J.Y.; Bae, H.; Choi, Y.K.; Ahn, J.H. Nanoscale FET-Based Transduction toward Sensitive Extended-Gate Biosensors. ACS Sens. 2019, 4, 1724–1729. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A. A novel electrochemical sensor based on silver/halloysite nanotube/molybdenum disulfide nanocomposite for efficient nitrite sensing. Biosens. Bioelectron. 2018, 109, 279–285. [Google Scholar] [CrossRef]
- Song, C.; Li, C.; Yin, Y.; Xiao, J.; Zhang, X.; Song, M.; Dong, W. Preparation and gas sensing properties of partially broken WO3 nanotubes. Vacuum 2015, 114, 13–16. [Google Scholar] [CrossRef]
- Liu, H.; Wei, D.; Yan, Y.; Li, A.; Chuai, X.; Lu, G.; Wang, Y. Silver Nanowire Templating Synthesis of Mesoporous SnO2 Nanotubes: An Effective Gas Sensor for Methanol with a Rapid Response and Recovery. ChemistrySelect 2018, 3, 7741–7748. [Google Scholar] [CrossRef]
- Choi, K.-S.; Chang, S.-P. Effect of structure morphologies on hydrogen gas sensing by ZnO nanotubes. Mater. Lett. 2018, 230, 48–52. [Google Scholar] [CrossRef]
- Huang, M.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Liang, Y. Pd-loaded In2O3 nanowire-like network synthesized using carbon nanotube templates for enhancing NO2 sensing performance. RSC Adv. 2015, 5, 30038–30045. [Google Scholar] [CrossRef]
- Choi, K.S.; Park, S.; Chang, S.-P. Enhanced ethanol sensing properties based on SnO2 nanowires coated with Fe2O3 nanoparticles. Sens. Actuator B-Chem. 2017, 238, 871–879. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, C.; Zhang, M.; Zhang, Z.; Xie, E.; Zhou, J.; Han, W. Doping effect of In2O3 on structural and ethanol-sensing characteristics of ZnO nanotubes fabricated by electrospinning. Appl. Surf. Sci. 2015, 349, 615–621. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Poli, N.; Zappa, D.; Sberveglieri, G. NiO/ZnO Nanowire-heterostructures by Vapor Phase Growth for Gas Sensing. Procedia Eng. 2016, 168, 1140–1143. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Sun, J.S.; Li, Y.; Xue, S.X.; Chen, X.Q.; Li, X.S.; Du, G.X. Facile synthesis of multifunctional multi-walled carbon nanotube for pathogen Vibrio alginolyticus detection in fishery and environmental samples. Talanta 2014, 128, 311–318. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sens. Actuator B-Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Gong, H.; Chen, F.; Huang, Z.; Gu, Y.; Zhang, Q.; Chen, Y.; Zhang, Y.; Zhuang, J.; Cho, Y.K.; Fang, R.H.; et al. Biomembrane-Modified Field Effect Transistors for Sensitive and Quantitative Detection of Biological Toxins and Pathogens. ACS Nano 2019, 13, 3714–3722. [Google Scholar] [CrossRef]
- Kitikul, J.; Satienperakul, S.; Preechaworapun, A.; Pookmanee, P.; Tangkuaram, T. A Simple Flow Amperometric Electrochemical Biosensor Based on Chitosan Scaffolds and Gold Nanowires Modified on a Glassy Carbon Electrode for Detection of Glutamate in Food Products. Electroanalysis 2017, 29, 264–271. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Qin, Y.; Zhang, L.; Chen, Y. Construct of Carbon Nanotube-Supported Fe2O3 Hybrid Nanozyme by Atomic Layer Deposition for Highly Efficient Dopamine Sensing. Front. Chem. 2020, 8, 564968. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Tao, Z.; Gao, X.; Wang, S.; Liu, Y. A Asp/Ce nanotube-based colorimetric nanosensor for H2O2-free and enzyme-free detection of cysteine. Talanta 2019, 196, 556–562. [Google Scholar] [CrossRef]
- Spain, E.; McCooey, A.; Joyce, K.; Keyes, T.E.; Forster, R.J. Gold nanowires and nanotubes for high sensitivity detection of pathogen DNA. Sens. Actuator B-Chem. 2015, 215, 159–165. [Google Scholar] [CrossRef]
- Tahir, M.A.; Hameed, S.; Munawar, A.; Amin, I.; Mansoor, S.; Khan, W.S.; Bajwa, S.Z. Investigating the potential of multiwalled carbon nanotubes based zinc nanocomposite as a recognition interface towards plant pathogen detection. J. Virol. Methods 2017, 249, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Danielson, E.; Dhamodharan, V.; Porkovich, A.; Kumar, P.; Jian, N.; Ziadi, Z.; Grammatikopoulos, P.; Sontakke, V.A.; Yokobayashi, Y.; Sowwan, M. Gas-Phase Synthesis for Label-Free Biosensors: Zinc-Oxide Nanowires Functionalized with Gold Nanoparticles. Sci. Rep. 2019, 9, 17370. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Romay, V.; Liu, Y.; Ibarlucea, B.; Baraban, L.; Khavrus, V.; Oswald, S.; Bachmatiuk, A.; Ibrahim, I.; Rümmeli, M.; et al. Chemiresistive biosensors based on carbon nanotubes for label-free detection of DNA sequences derived from avian influenza virus H5N1. Sens. Actuator B-Chem. 2017, 249, 691–699. [Google Scholar] [CrossRef]
- Tang, X.; Bansaruntip, S.; Nakayama, N.; Yenilmez, E.; Chang, Y.-L.; Wang, Q. Carbon nanotube DNA sensor and sensing mechanism. Nano Lett. 2006, 6, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Bunimovich, Y.L.; Shin, Y.S.; Yeo, W.-S.; Amori, M.; Kwong, G.; Heath, J.R. Quantitative real-time measurements of DNA hybridization with alkylated nonoxidized silicon nanowires in electrolyte solution. J. Am. Chem. Soc. 2006, 128, 16323–16331. [Google Scholar] [CrossRef]
- Fortunati, S.; Rozzi, A.; Curti, F.; Giannetto, M.; Corradini, R.; Careri, M. Novel amperometric genosensor based on peptide nucleic acid (PNA) probes immobilized on carbon nanotubes-screen printed electrodes for the determination of trace levels of non-amplified DNA in genetically modified (GM) soy. Biosens. Bioelectron. 2019, 129, 7–14. [Google Scholar] [CrossRef]
- Xu, G.; Abbott, J.; Ham, D. Optimization of CMOS-ISFET-Based Biomolecular Sensing: Analysis and Demonstration in DNA Detection. IEEE Trans. Electron Devices 2016, 63, 3249–3256. [Google Scholar] [CrossRef]




| Materials | Concentration(ppm) | Response |
|---|---|---|
| WO3 | 300 | 16.9 |
| WO3 nanoplates | 400 | ~12 |
| WO3 hollow spheres | 500 | 6.14 |
| WO3 nanotube bundles | 400 | ~38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.-Y.; Wang, B.-R.; Gu, Y.; Zhu, H.; Chen, L.; Sun, Q.-Q. Novel Nanofluidic Cells Based on Nanowires and Nanotubes for Advanced Chemical and Bio-Sensing Applications. Nanomaterials 2021, 11, 90. https://doi.org/10.3390/nano11010090
Zhu X-Y, Wang B-R, Gu Y, Zhu H, Chen L, Sun Q-Q. Novel Nanofluidic Cells Based on Nanowires and Nanotubes for Advanced Chemical and Bio-Sensing Applications. Nanomaterials. 2021; 11(1):90. https://doi.org/10.3390/nano11010090
Chicago/Turabian StyleZhu, Xin-Yi, Bo-Ran Wang, Yi Gu, Hao Zhu, Lin Chen, and Qing-Qing Sun. 2021. "Novel Nanofluidic Cells Based on Nanowires and Nanotubes for Advanced Chemical and Bio-Sensing Applications" Nanomaterials 11, no. 1: 90. https://doi.org/10.3390/nano11010090
APA StyleZhu, X.-Y., Wang, B.-R., Gu, Y., Zhu, H., Chen, L., & Sun, Q.-Q. (2021). Novel Nanofluidic Cells Based on Nanowires and Nanotubes for Advanced Chemical and Bio-Sensing Applications. Nanomaterials, 11(1), 90. https://doi.org/10.3390/nano11010090





