Air–Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanomaterials
2.2. Characterization of MNMs in Suspension
2.3. Determination of the Effective Particle Density and the Relevant In Vitro Dose (RID)
2.4. Generation of NP Aerosols
2.5. Cell Culture and Submerged Exposure
2.6. ALI Cell Exposure
2.7. Determination of Deposited Dose after ALI Exposure
2.8. LDH Release
2.9. AlamarBlue® Reduction
2.10. MTS Reduction
2.11. IL-8 Release
2.12. RT-qPCR
2.13. Detection of DNA Strand Breaks and Alkali-Labile Sites
2.14. Statistics
3. Results
3.1. Particle Characterization
3.2. Aerosol Characterization
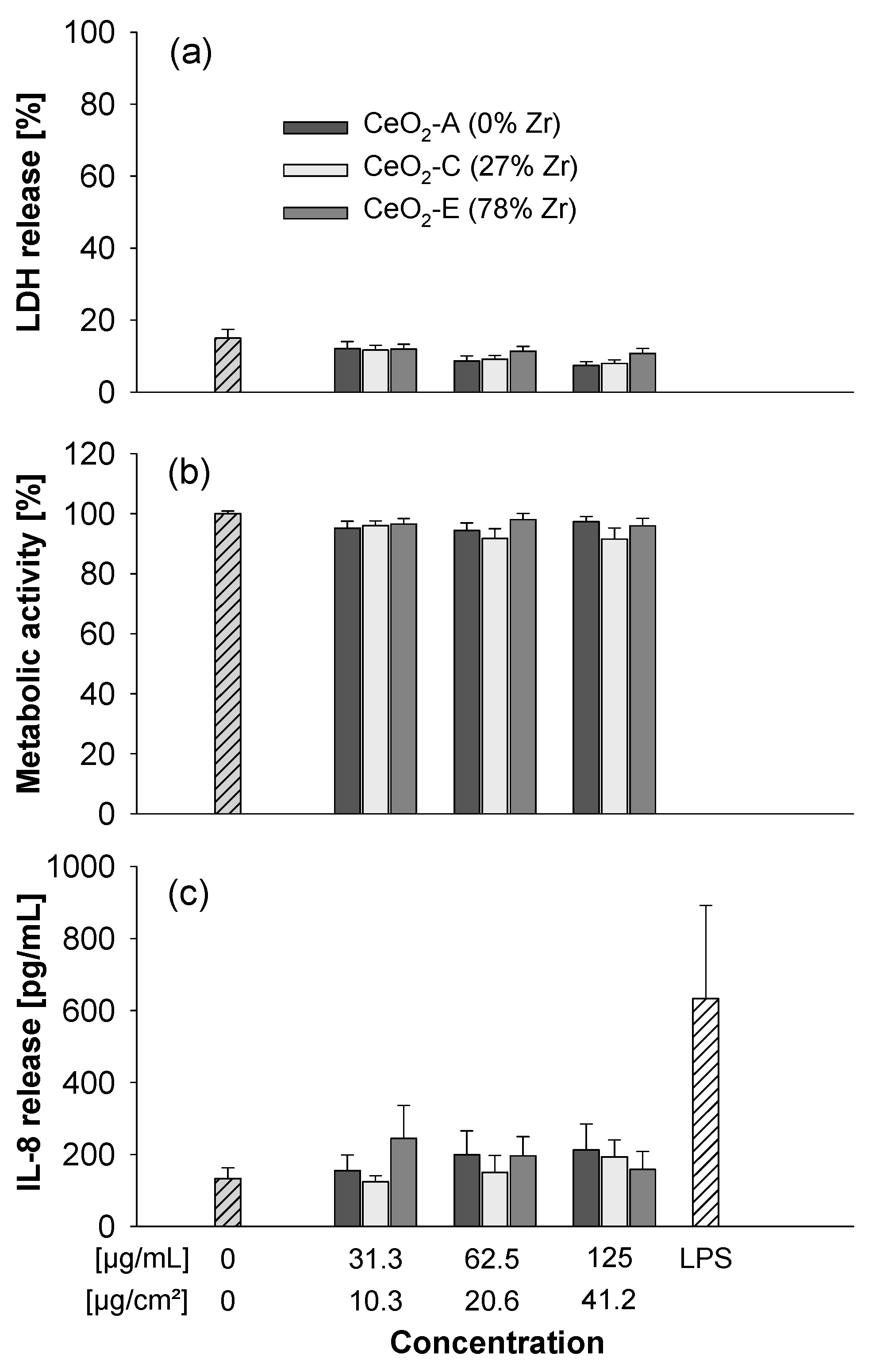
3.3. Submerged Exposure to MNMs
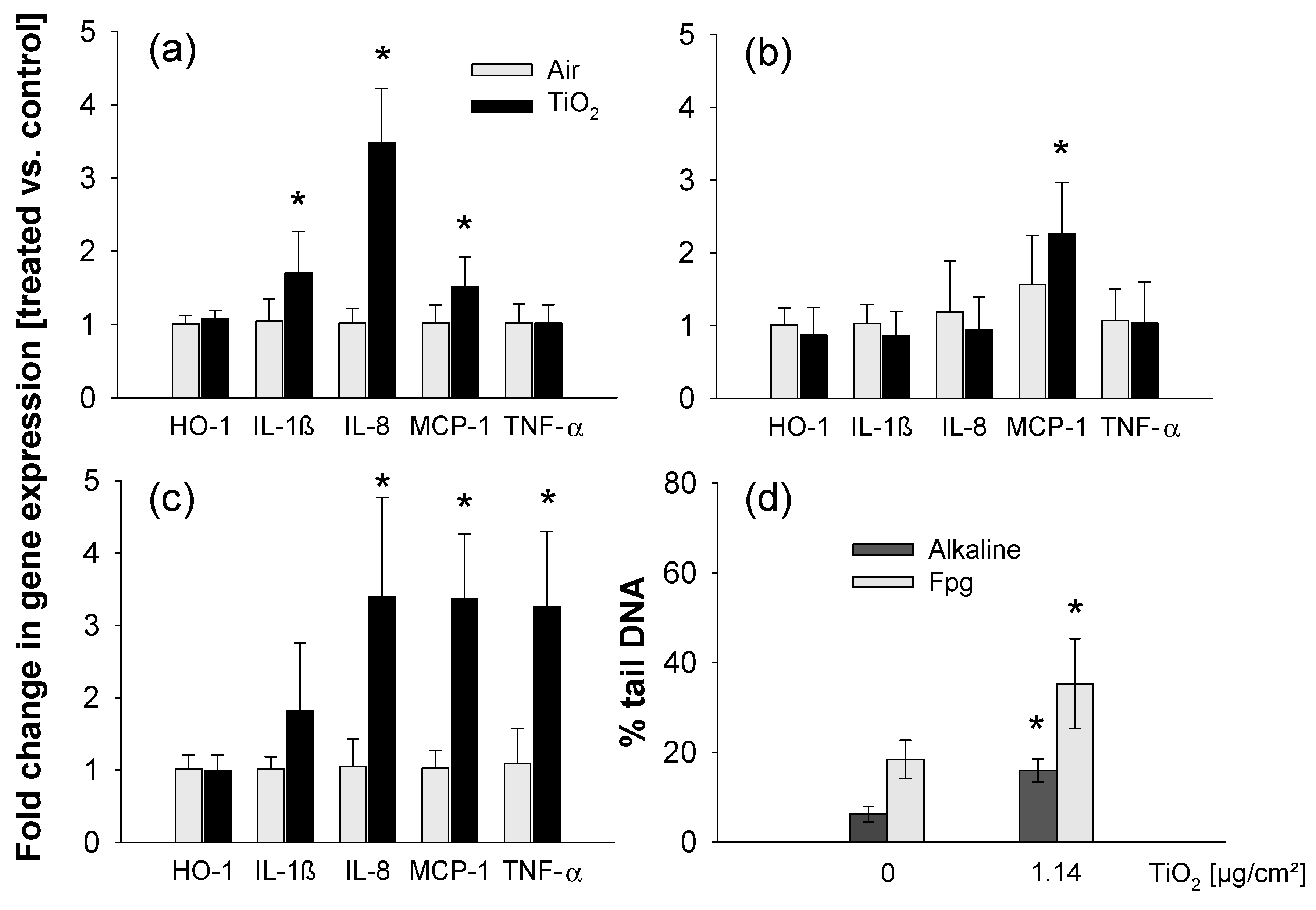
3.4. ALI Exposure to NP Aerosols
4. Discussion
4.1. Effects of Ceria NPs Studied in In Vitro Experiments under Submerged or ALI Conditions and Comparison to In Vivo Findings
4.2. Effects of Titania NPs Studied in In Vitro Experiments under Submerged or ALI Conditions and Comparison to In Vivo Findings
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebel, T.; Foth, H.; Damm, G.; Freyberger, A.; Kramer, P.J.; Lilienblum, W.; Röhl, C.; Schupp, T.; Weiss, C.; Wollin, K.M.; et al. Manufactured nanomaterials: Categorization and approaches to hazard assessment. Arch. Toxicol. 2014, 88, 2191–2211. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Weiss, C.; Valsami-Jones, E. A strategy for grouping of nanomaterials based on key physio-chemical descriptors as a basis for safer-by-design NMs. Nano Today 2014, 9, 266–270. [Google Scholar] [CrossRef]
- Secondo, L.E.; Liu, N.J.; Lewinski, N.A. Methodological considerations when conducting in vitro, air-liquid interface exposures to engineered nanoparticle aerosols. Crit. Rev. Toxicol. 2017, 47, 225–262. [Google Scholar] [CrossRef] [PubMed]
- Paur, H.R.; Fissan, H.; Rothen-Rutishauser, B.; Teeguarden, J.G.; Diabaté, S.; Aufderheide, M.; Kreyling, W.; Cassee, F.R.; Hänninen, O.; Kasper, G.; et al. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—A dialogue between aerosol science and biology. J. Aerosol Sci. 2011, 42, 668–692. [Google Scholar] [CrossRef]
- Paur, H.R.; Mülhopt, S.; Weiss, C.; Diabaté, S. In-vitro exposure systems and bioassays for the assessment of toxicity of nanoparticles to the human lung. J. Verbrauch. Lebensm. 2008, 3, 319–329. [Google Scholar] [CrossRef]
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B.; et al. Air-liquid interface in vitro models for respiratory toxicological research: Consensus workshop and recommendations. Appl. In Vitro Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Pitek, A.S.; Lynch, I.; Dawson, K.A. Formation and characterization of the nanoparticle-protein corona. Methods Mol. Biol. 2013, 1025, 137–155. [Google Scholar]
- Panas, A.; Marquardt, C.; Nalcaci, O.; Bockhorn, H.; Baumann, W.; Paur, H.R.; Mülhopt, S.; Diabaté, S.; Weiss, C. Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology 2013, 7, 259–273. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef]
- Diabaté, S.; Mülhopt, S.; Paur, H.R.; Krug, H.F. Pro-inflammatory effects in lung cells after exposure to fly ash aerosol via the atmosphere or the liquid phase. Ann. Occup. Hyg. 2002, 46, 382–385. [Google Scholar]
- Diabaté, S.; Mülhopt, S.; Paur, H.R.; Krug, H.F. The response of a co-culture lung model to fine and ultrafine particles of incinerator fly ash at the air-liquid interface. Altern. Lab. Anim. 2008, 36, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Mülhopt, S.; Diabaté, S.; Krebs, T.; Weiss, C.; Paur, H.R. Lung toxicity determination by in vitro exposure at the air-liquid interface with an integrated online dose measurement. J. Phys. 2009, 170, 012008. [Google Scholar]
- Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.R.; et al. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J. Nanotechnol. 2014, 5, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Mülhopt, S.; Dilger, M.; Diabaté, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Wäscher, T.; Weiss, C.; et al. Toxicity testing of combustion aerosols at the air-liquid interface with a self-contained and easy-to-use exposure system. J. Aerosol Sci. 2016, 96, 18. [Google Scholar] [CrossRef]
- Sapcariu, S.C.; Kanashova, T.; Dilger, M.; Diabaté, S.; Oeder, S.; Passig, J.; Radischat, C.; Buters, J.; Sippula, O.; Streibel, T.; et al. Metabolic profiling as well as stable isotope assisted metabolic and proteomic analysis of RAW 264.7 macrophages exposed to ship engine aerosol emissions: Different effects of heavy fuel oil and refined diesel fuel. PLoS ONE 2016, 11, e0157964. [Google Scholar] [CrossRef]
- Oeder, S.; Kanashova, T.; Sippula, O.; Sapcariu, S.C.; Streibel, T.; Arteaga-Salas, J.M.; Passig, J.; Dilger, M.; Paur, H.R.; Schlager, C.; et al. Particulate matter from both heavy fuel oil and diesel fuel shipping emissions show strong biological effects on human lung cells at realistic and comparable in vitro exposure conditions. PLoS ONE 2015, 10, e0126536. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities und uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- IARC. Carbon Black, Titanium Dioxide, and Talc; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93, pp. 1–466. [Google Scholar]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Unfried, K.; Albrecht, C.; Klotz, L.O.; von Mikecz, A.; Grether-Beck, S.; Schins, R.P.F. Cellular responses to nanoparticles: Target structures and mechanisms. Nanotoxicology 2007, 1, 1–20. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef]
- Dekkers, S.; Miller, M.R.; Schins, R.P.F.; Romer, I.; Russ, M.; Vandebriel, R.J.; Lynch, I.; Belinga-Desaunay, M.F.; Valsami-Jones, E.; Connell, S.P.; et al. The effect of zirconium doping of cerium dioxide nanoparticles on pulmonary and cardiovascular toxicity and biodistribution in mice after inhalation. Nanotoxicology 2017, 11, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, A.; Rao, P.J.; Selvam, G.; Murthy, P.B.; Reddy, P.N. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicol. Lett. 2011, 205, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Demokritou, P.; Gass, S.; Pyrgiotakis, G.; Cohen, J.M.; Goldsmith, W.; McKinney, W.; Frazer, D.; Ma, J.; Schwegler-Berry, D.; Brain, J.; et al. An in vivo and in vitro toxicological characterisation of realistic nanoscale CeO2 inhalation exposures. Nanotoxicology 2013, 7, 1338–1350. [Google Scholar] [CrossRef]
- Gosens, I.; Mathijssen, L.E.; Bokkers, B.G.; Muijser, H.; Cassee, F.R. Comparative hazard identification of nano- and micro-sized cerium oxide particles based on 28-day inhalation studies in rats. Nanotoxicology 2014, 8, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Aalapati, S.; Ganapathy, S.; Manapuram, S.; Anumolu, G.; Prakya, B.M. Toxicity and bio-accumulation of inhaled cerium oxide nanoparticles in CD1 mice. Nanotoxicology 2014, 8, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Wohlleben, W.; Ma-Hock, L.; Strauss, V.; Gröters, S.; Küttler, K.; Wiench, K.; Herden, C.; Oberdörster, G.; van Ravenzwaay, B.; et al. Time course of lung retention and toxicity of inhaled particles: Short-term exposure to nano-Ceria. Arch. Toxicol. 2014, 88, 2033–2059. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Pulmonary toxicity of well-dispersed cerium oxide nanoparticles following intratracheal instillation and inhalation. J. Nanopart. Res. 2015, 17, 442. [Google Scholar] [CrossRef]
- Schwotzer, D.; Ernst, H.; Schaudien, D.; Kock, H.; Pohlmann, G.; Dasenbrock, C.; Creutzenberg, O. Effects from a 90-day inhalation toxicity study with cerium oxide and barium sulfate nanoparticles in rats. Part. Fibre Toxicol. 2017, 14, 23. [Google Scholar] [CrossRef]
- Loret, T.; Peyret, E.; Dubreuil, M.; Aguerre-Chariol, O.; Bressot, C.; le, B.O.; Amodeo, T.; Trouiller, B.; Braun, A.; Egles, C.; et al. Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part. Fibre Toxicol. 2016, 13, 58. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Leseman, D.L.; Deciga-Alcaraz, A.; He, R.; Gremmer, E.R.; Fokkens, P.H.B.; Flores-Flores, J.O.; Cassee, F.R.; Chirino, Y.I. Differences in cytotoxicity of lung epithelial cells exposed to titanium dioxide nanofibers and nanoparticles: Comparison of air-liquid interface and submerged cell cultures. Toxicol. In Vitro 2020, 65, 104798. [Google Scholar] [CrossRef]
- Steinritz, D.; Möhle, N.; Pohl, C.; Papritz, M.; Stenger, B.; Schmidt, A.; Kirkpatrick, C.J.; Thiermann, H.; Vogel, R.; Hoffmann, S.; et al. Use of the Cultex(R) Radial Flow System as an in vitro exposure method to assess acute pulmonary toxicity of fine dusts and nanoparticles with special focus on the intra- and inter-laboratory reproducibility. Chem. Biol. Interact. 2013, 206, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Fuhst, R.; Rittinghausen, S.; Creutzenberg, O.; Bellmann, B.; Koch, W.; Levsen, K. Chronic inhalation exposure of Wistar rats and 2 different strains of mice to diesel-engine exhaust, carbon-black, and titanium-dioxide. Inhal. Toxicol. 1995, 7, 533–556. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Burkhardt, S.; Strauss, V.; Gamer, A.O.; Wiench, K.; Van, R.B.; Landsiedel, R. Development of a short-term inhalation test in the rat using nano-titanium dioxide as a model substance. Inhal. Toxicol. 2009, 21, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Ferin, J.; Lehnert, B.E. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994, 102 (Suppl. 5), 173–179. [Google Scholar]
- Relier, C.; Dubreuil, M.; Lozano, G.O.; Cordelli, E.; Mejia, J.; Eleuteri, P.; Robidel, F.; Loret, T.; Pacchierotti, F.; Lucas, S.; et al. Study of TiO2 P25 nanoparticles genotoxicity on lung, blood, and liver cells in lung overload and non-overload conditions after repeated respiratory exposure in rats. Toxicol. Sci. 2017, 156, 527–537. [Google Scholar]
- Lester, E.; Blood, P.; Denyer, J.; Giddings, D.; Azzopardi, B.; Poliakoff, M. Reaction engineering: The supercritical water hydrothermal synthesis of nano-particles. J. Supercrit. Fluid 2006, 37, 209–214. [Google Scholar] [CrossRef]
- Lester, E.; Tang, S.V.Y.; Khlobystov, A.; Loczenski Rose, V.; Buttery, L.; Roberts, C.J. Producing nanotubes of biocompatible hydroxyapatite by continuous hydrothermal synthesis. Cryst. Eng. Comm. 2013, 15, 3256. [Google Scholar] [CrossRef]
- Rasmussen, K.; Mast, J.; De Temmerman, P.J.; Verleysen, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Van Doren, E.; Jensen, K.A.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and Physico-Chemical Properties; European Union; Publications Office of the European Union: Luxembourg, 2014; pp. 1–218. [Google Scholar]
- DeLoid, G.; Cohen, J.M.; Darrah, T.; Derk, R.; Rojanasakul, L.; Pyrgiotakis, G.; Wohlleben, W.; Demokritou, P. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat. Commun. 2014, 5, 3514. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc 2017, 12, 355–371. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Pirela, S.V.; Pal, A.; Liu, J.; Srebric, J.; Demokritou, P. Advanced computational modeling for in vitro nanomaterial dosimetry. Part. Fibre Toxicol. 2015, 12, 32. [Google Scholar] [CrossRef]
- Kowoll, T.; Fritsch-Decker, S.; Diabaté, S.; Nienhaus, G.U.; Gerthsen, D.; Weiss, C. Assessment of in vitro particle dosimetry models at the single cell and particle level by scanning electron microscopy. J. Nanobiotechnol. 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Leibe, R.; Hsiao, I.L.; Fritsch-Decker, S.; Kielmeier, U.; Wagbo, A.M.; Voss, B.; Schmidt, A.; Hessman, S.D.; Duschl, A.; Oostingh, G.J.; et al. The protein corona suppresses the cytotoxic and pro-inflammatory response in lung epithelial cells and macrophages upon exposure to nanosilica. Arch. Toxicol. 2019, 93, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Sauer, U.G.; Ma-Hock, L.; Schnekenburger, J.; Wiemann, M. Pulmonary toxicity of nanomaterials: A critical comparison of published in vitro assays and in vivo inhalation or instillation studies. Nanomedicine 2014, 9, 2557–2585. [Google Scholar] [CrossRef] [PubMed]
- Mülhopt, S.; Schlager, C.; Berger, M.; Murugadoss, S.; Hoet, P.H.; Krebs, T.; Paur, H.R.; Stapf, D. A novel TEM grid sampler for airborne particles to measure the cell culture surface dose. Sci. Rep. 2020, 10, 8401. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, I.; Haase, A.; Anguissola, S.; Rocks, L.; Jacobs, A.; Willems, H.; Riebeling, C.; Luch, A.; Piret, J.P.; Toussaint, O.; et al. Improving quality in nanoparticle-induced cytotoxicity testing by a tiered inter-laboratory comparison study. Nanomaterials 2020, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; MacCormack, T.J.; Clark, R.J.; Ede, J.D.; Ortega, V.A.; Felix, L.C.; Dang, M.K.; Ma, G.; Fenniri, H.; Veinot, J.G.; et al. Widespread nanoparticle-assay interference: Implications for nanotoxicity testing. PLoS ONE 2014, 9, e90650. [Google Scholar] [CrossRef]
- Armand, L.; Tarantini, A.; Beal, D.; Biola-Clier, M.; Bobyk, L.; Sorieul, S.; Pernet-Gallay, K.; Marie-Desvergne, C.; Lynch, I.; Herlin-Boime, N.; et al. Long-term exposure of A549 cells to titanium dioxide nanoparticles induces DNA damage and sensitizes cells towards genotoxic agents. Nanotoxicology 2016, 10, 913–923. [Google Scholar] [CrossRef]
- Jugan, M.L.; Barillet, S.; Simon-Deckers, A.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology 2012, 6, 501–513. [Google Scholar] [CrossRef]
- Biola-Clier, M.; Beal, D.; Caillat, S.; Libert, S.; Armand, L.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Comparison of the DNA damage response in BEAS-2B and A549 cells exposed to titanium dioxide nanoparticles. Mutagenesis 2017, 32, 161–172. [Google Scholar] [CrossRef]
- Gohlsch, K.; Muckter, H.; Steinritz, D.; Aufderheide, M.; Hoffmann, S.; Gudermann, T.; Breit, A. Exposure of 19 substances to lung A549 cells at the air liquid interface or under submerged conditions reveals high correlation between cytotoxicity in vitro and CLP classifications for acute lung toxicity. Toxicol. Lett. 2019, 316, 119–126. [Google Scholar] [CrossRef]
- Kooter, I.; Ilves, M.; Grollers-Mulderij, M.; Duistermaat, E.; Tromp, P.C.; Kuper, F.; Kinaret, P.; Savolainen, K.; Greco, D.; Karisola, P.; et al. Molecular Signature of Asthma-Enhanced Sensitivity to CuO Nanoparticle Aerosols from 3D Cell Model. Acs Nano 2019, 13, 6932–6946. [Google Scholar] [CrossRef]
- Kanashova, T.; Sippula, O.; Oeder, S.; Streibel, T.; Passig, J.; Czech, H.; Kaoma, T.; Sapcariu, S.C.; Dilger, M.; Paur, H.R.; et al. Emissions from a modern log wood masonary heater and wood pellet boiler: Composition and biological impact on air-liquid interface exposed human lung cancer cells. J. Mol. Clin. Med. 2018, 1, 23–35. [Google Scholar]
- Ihantola, T.; Di Bucchianico, S.; Happo, M.; Ihalainen, M.; Uski, O.; Bauer, S.; Kuuspalo, K.; Sippula, O.; Tissari, J.; Oeder, S.; et al. Influence of wood species on toxicity of log-wood stove combustion aerosols: A parallel animal and air-liquid interface cell exposure study on spruce and pine smoke. Part. Fibre Toxicol. 2020, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Raemy, D.O.; Limbach, L.K.; Rothen-Rutishauser, B.; Grass, R.N.; Gehr, P.; Birbaum, K.; Brandenberger, C.; Gunther, D.; Stark, W.J. Cerium oxide nanoparticle uptake kinetics from the gas-phase into lung cells in vitro is transport limited. Eur. J. Pharm. Biopharm. 2011, 77, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Rothen-Rutishauser, B.; Grass, R.N.; Blank, F.; Limbach, L.K.; Mühlfeld, C.; Brandenberger, C.; Raemy, D.O.; Gehr, P.; Stark, W.J. Direct combination of nanoparticle fabrication and exposure to lung cell cultures in a closed setup as a method to simulate accidental nanoparticle exposure of humans. Environ. Sci. Technol. 2009, 43, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Schoch, S.; Wall, J.; Strauch, B.M.; Hartwig, A. Toxicity and Gene Expression Profiling of Copper- and Titanium-Based Nanoparticles Using Air-Liquid Interface Exposure. Chem. Res. Toxicol. 2020, 33, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Rach, J.; Budde, J.; Möhle, N.; Aufderheide, M. Direct exposure at the air-liquid interface: Evaluation of an in vitro approach for simulating inhalation of airborne substances. J. Appl. Toxicol 2014, 34, 506–515. [Google Scholar] [CrossRef]
- Sauer, U.G.; Vogel, S.; Aumann, A.; Hess, A.; Kolle, S.N.; Ma-Hock, L.; Wohlleben, W.; Dammann, M.; Strauss, V.; Treumann, S.; et al. Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol. Appl. Pharmacol. 2014, 276, 1–20. [Google Scholar] [CrossRef]
- Cappellini, F.; Di Bucchianico, S.; Karri, V.; Latvala, S.; Malmlof, M.; Kippler, M.; Elihn, K.; Hedberg, J.; Odnevall Wallinder, I.; Gerde, P.; et al. Dry Generation of CeO2 Nanoparticles and Deposition onto a Co-Culture of A549 and THP-1 Cells in Air-Liquid Interface-Dosimetry Considerations and Comparison to Submerged Exposure. Nanomaterials 2020, 10, 618. [Google Scholar] [CrossRef]
- Wiemann, M.; Vennemann, A.; Sauer, U.G.; Wiench, K.; Ma-Hock, L.; Landsiedel, R. An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. J. Nanobiotechnol. 2016, 14, 16. [Google Scholar] [CrossRef]
- Steiner, S.; Mueller, L.; Popovicheva, O.B.; Raemy, D.O.; Czerwinski, J.; Comte, P.; Mayer, A.; Gehr, P.; Rothen-Rutishauser, B.; Clift, M.J. Cerium dioxide nanoparticles can interfere with the associated cellular mechanistic response to diesel exhaust exposure. Toxicol. Lett. 2012, 214, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Kooter, I.M.; Gröllers-Mulderij, M.; Steenhof, M.; Duistermaat, E.; van Acker, F.A.A.; Staal, Y.C.M.; Tromp, P.C.; Schoen, E.; Kuper, C.F.; van Someren, E. Cellular effects in an in vitro human 3D cellular airway model and A549/BEAS-2B in vitro cell cultures following air exposure to cerium oxide particles at an air-liquid interface. Appl. In Vitro Toxicol 2016, 2, 56–66. [Google Scholar] [CrossRef]
- Donaldson, K.; Borm, P.J.; Oberdörster, G.; Pinkerton, K.E.; Stone, V.; Tran, C.L. Concordance between in vitro and in vivo dosimetry in the proinflammatory effects of low-toxicity, low-solubility particles: The key role of the proximal alveolar region. Inhal. Toxicol. 2008, 20, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Ma-Hock, L.; Hofmann, T.; Wiemann, M.; Strauss, V.; Treumann, S.; Wohlleben, W.; Groters, S.; Wiench, K.; Van, R.B. Application of short-term inhalation studies to assess the inhalation toxicity of nanomaterials. Part. Fibre Toxicol. 2014, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Hansjosten, I.; Rapp, J.; Reiner, L.; Vatter, R.; Fritsch-Decker, S.; Peravali, R.; Palosaari, T.; Joossens, E.; Gerloff, K.; Macko, P.; et al. Microscopy-based high-throughput assays enable multi-parametric analysis to assess adverse effects of nanomaterials in various cell lines. Arch. Toxicol. 2018, 92, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Monteiller, C.; Tran, L.; MacNee, W.; Faux, S.; Jones, A.; Miller, B.; Donaldson, K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: The role of surface area. Occup. Environ. Med. 2007, 64, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shi, T.; Duffin, R.; Albrecht, C.; van Berlo, D.; Höhr, D.; Fubini, B.; Martra, G.; Fenoglio, I.; Borm, P.J.; et al. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: Role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007, 222, 141–151. [Google Scholar] [CrossRef]
- Loret, T.; Rogerieux, F.; Trouiller, B.; Braun, A.; Egles, C.; Lacroix, G. Predicting the in vivo pulmonary toxicity induced by acute exposure to poorly soluble nanomaterials by using advanced in vitro methods. Part. Fibre Toxicol. 2018, 15, 25. [Google Scholar] [CrossRef]
- Gordon, S.; Daneshian, M.; Bouwstra, J.; Caloni, F.; Constant, S.; Davies, D.E.; Dandekar, G.; Guzman, C.A.; Fabian, E.; Haltner, E.; et al. Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. ALTEX 2015, 32, 327–378. [Google Scholar] [CrossRef]
- Chary, A.; Serchi, T.; Moschini, E.; Hennen, J.; Cambier, S.; Ezendam, J.; Blomeke, B.; Gutleb, A.C. An in vitro coculture system for the detection of sensitization following aerosol exposure. ALTEX 2019, 36, 403–418. [Google Scholar] [CrossRef]
- Kletting, S.; Barthold, S.; Repnik, U.; Griffiths, G.; Loretz, B.; Schneider-Daum, N.; de Souza Carvalho-Wodarz, C.; Lehr, C.M. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX 2018, 35, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, K.E.; Gehr, P.; Castañeda, A.; Crapo, J.D. Architecture and cellular composition of the air-blood tissue barrier. In Comparative Biology of the Normal Lung, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 105–117. [Google Scholar]
- Chortarea, S.; Clift, M.J.; Vanhecke, D.; Endes, C.; Wick, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Repeated exposure to carbon nanotube-based aerosols does not affect the functional properties of a 3D human epithelial airway model. Nanotoxicology 2015, 9, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Chortarea, S.; Barosova, H.; Clift, M.J.D.; Wick, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Human Asthmatic Bronchial Cells Are More Susceptible to Subchronic Repeated Exposures of Aerosolized Carbon Nanotubes At Occupationally Relevant Doses Than Healthy Cells. ACS Nano 2017, 11, 7615–7625. [Google Scholar] [CrossRef] [PubMed]

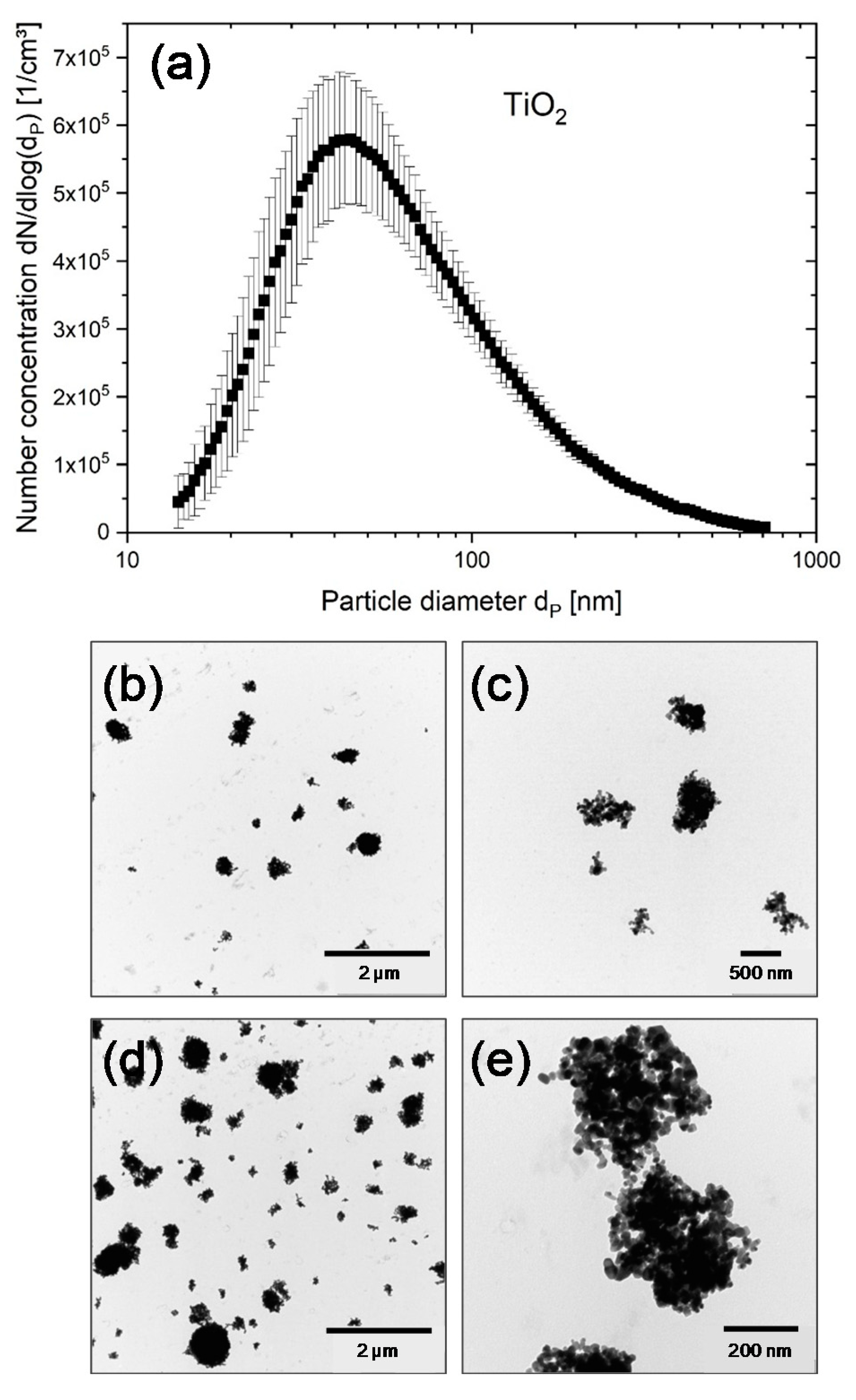


| CeO2-A (Undoped) | CeO2-C (27% Zr) | CeO2-E (78% Zr) | TiO2 a | |
|---|---|---|---|---|
| Nominal diameter b [nm] | 20 | 20 | 20 | 21 |
| z-average in water [nm] | 44.3 c | 58.1 c | 100 c | 165 ± 19 d |
| z-average in RPMI-FBS, 0 h [nm] | 3063 ± 437 c PDI: 0.257 | 6355 ± 590 c PDI: 0.347 | 7724 ± 322 c PDI: 0.389 | 1918 ± 309 e PDI: 0.263 |
| z-average in RPMI-FBS, 24 h [nm] | 4167 ± 178 c PDI: 0.206 | 6451 ± 935 c PDI: 0.477 | 8858 ± 1582 c PDI: 0.586 | 2903 ± 68 e PDI: 0.187 |
| Material density [g/cm3] | 7.22 f | 6.80 f | 6.02 f | 4.23 b |
| Effective density in RPMI-FBS [g/cm3] | 1.24 e | 1.12 e | 1.09 e | 1.32 e |
| CeO2-A (Undoped) | CeO2-C (27% Zr) | CeO2-E (78% Zr) | TiO2 | |
|---|---|---|---|---|
| Modal value xM [nm]c/6g | 49/1.31 | 52/1.34 | 48/1.32 | 47/1.24 |
| Total number concentration cN [1/cm3] | 1.75 × 105 | 1.37 × 105 | 2.07 × 105 | 2.8 × 105 |
| Mass concentration cMS [mg/m3] a | 1.77 | 1.66 | 2.24 | 2.2 |
| Dose 0.5 h − EF [µg/cm2] b | 0.02–0.03 | |||
| Dose 4 h − EF [µg/cm2] b | 0.19 ± 0.09 | 0.18 ± 0.02 | 0.24 ± 0.01 | 0.17 |
| Dose 0.5 h + EF [µg/cm2] c | 0.15–0.18 | |||
| Dose 4 h + EF [µg/cm2] c | 0.93 ± 0.44 | 0.88 ± 0.09 | 1.19 ± 0.07 | 1.14 |
| Endpoints | Marker | Lung Exposure | ALI Exposure | Submerged Exposure |
|---|---|---|---|---|
| Cytotoxicity | LDH | 0.15 a | 0.09 b | 41.2 b |
| Genotoxicity | Comet Assay | 0.03 c | 0.57 d | 5.7 d |
| DSBs | 0.57 e | 0.09 f | n.d. | |
| Inflammation | IL-8 | 0.57 g | 0.09 h | 10.3 g |
| TNF-α | 0.57 g | 0.57 h | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diabaté, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Béal, D.; Arnal, M.-E.; Biola-Clier, M.; Ambrose, S.; et al. Air–Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects. Nanomaterials 2021, 11, 65. https://doi.org/10.3390/nano11010065
Diabaté S, Armand L, Murugadoss S, Dilger M, Fritsch-Decker S, Schlager C, Béal D, Arnal M-E, Biola-Clier M, Ambrose S, et al. Air–Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects. Nanomaterials. 2021; 11(1):65. https://doi.org/10.3390/nano11010065
Chicago/Turabian StyleDiabaté, Silvia, Lucie Armand, Sivakumar Murugadoss, Marco Dilger, Susanne Fritsch-Decker, Christoph Schlager, David Béal, Marie-Edith Arnal, Mathilde Biola-Clier, Selina Ambrose, and et al. 2021. "Air–Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects" Nanomaterials 11, no. 1: 65. https://doi.org/10.3390/nano11010065
APA StyleDiabaté, S., Armand, L., Murugadoss, S., Dilger, M., Fritsch-Decker, S., Schlager, C., Béal, D., Arnal, M.-E., Biola-Clier, M., Ambrose, S., Mülhopt, S., Paur, H.-R., Lynch, I., Valsami-Jones, E., Carriere, M., & Weiss, C. (2021). Air–Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects. Nanomaterials, 11(1), 65. https://doi.org/10.3390/nano11010065








