Highly Flowable Nano TiO2/Porous Organic Polymer (POP) Supports for Efficient Metallocene Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
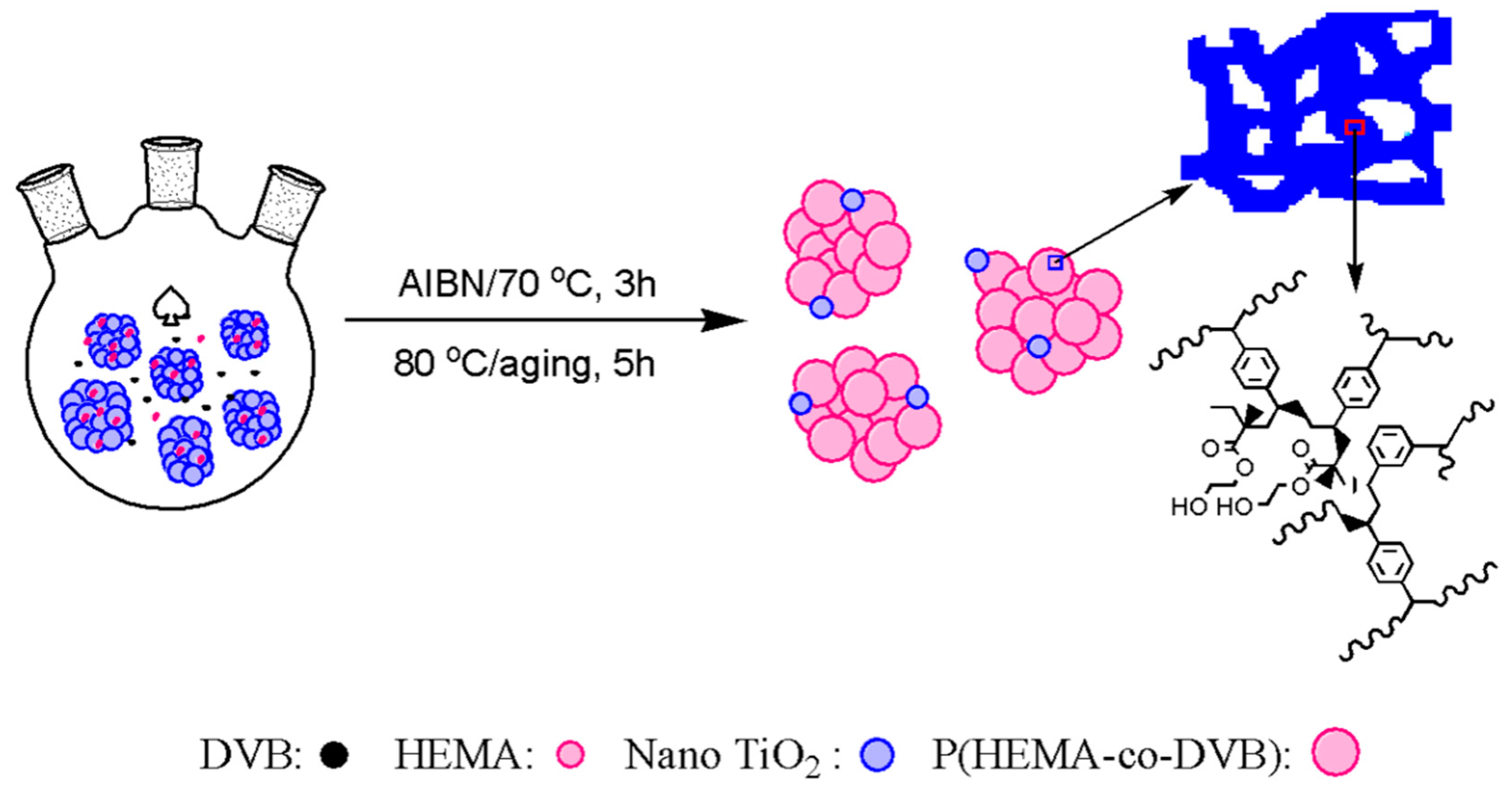
2.2. Preparation of TiO2/POP Supports
2.3. Immobilization of Metallocene Catalysts
2.4. Ethylene Polymerization
2.5. Characterization
3. Results and Discussion
3.1. Preparation of Nano TiO2/POP Particles
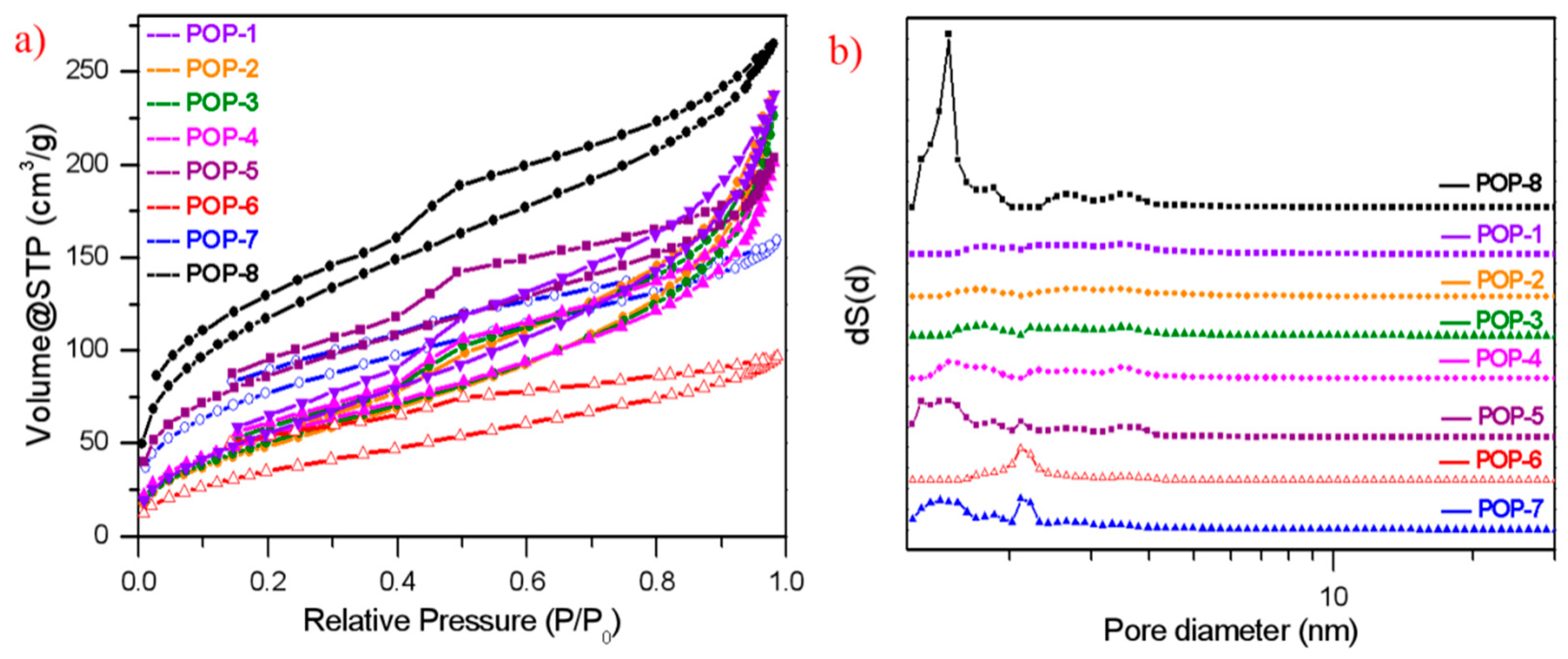
3.2. Pore Structure of the Prepared TiO2/POP Particles
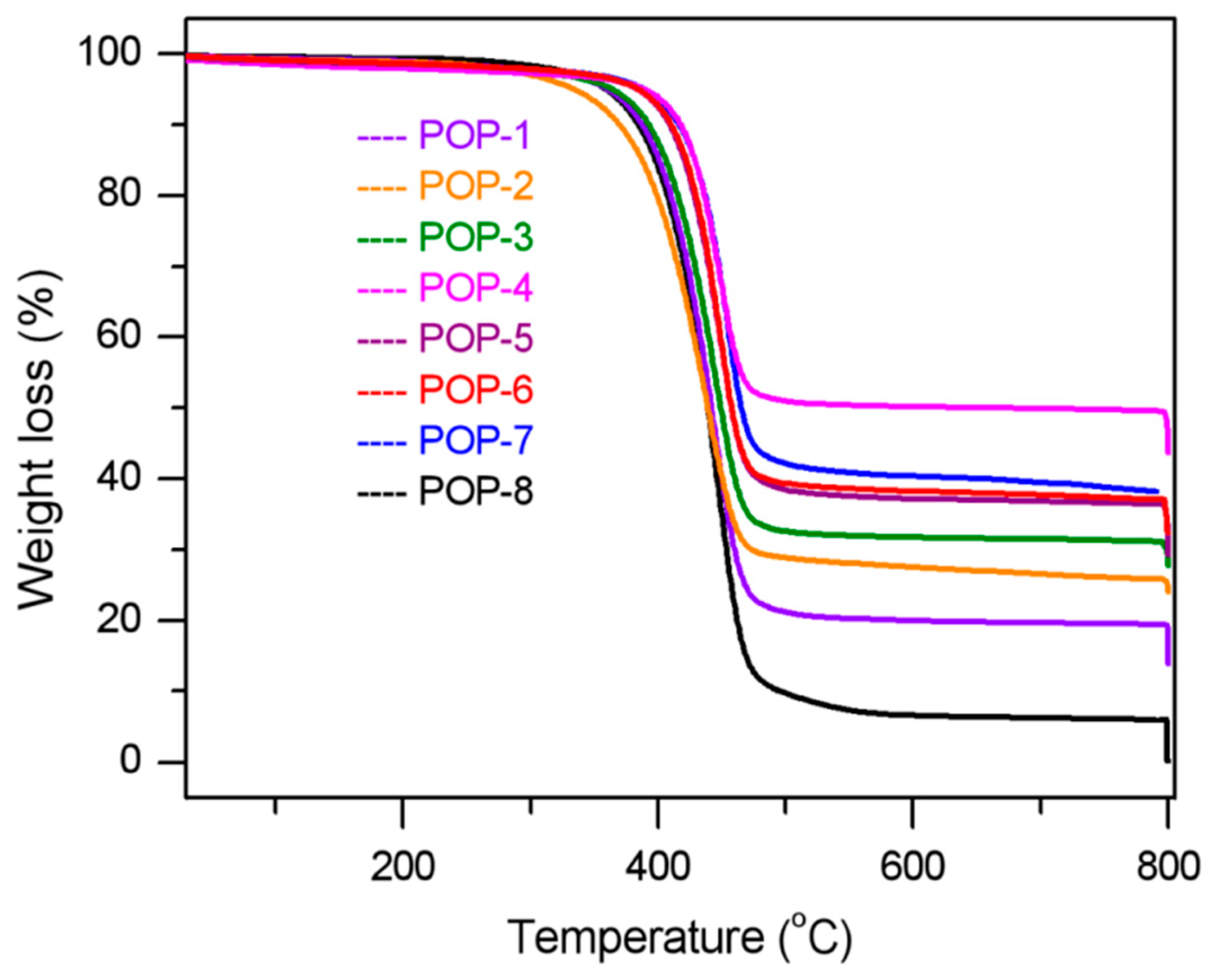
3.3. IR and TGA Analysis
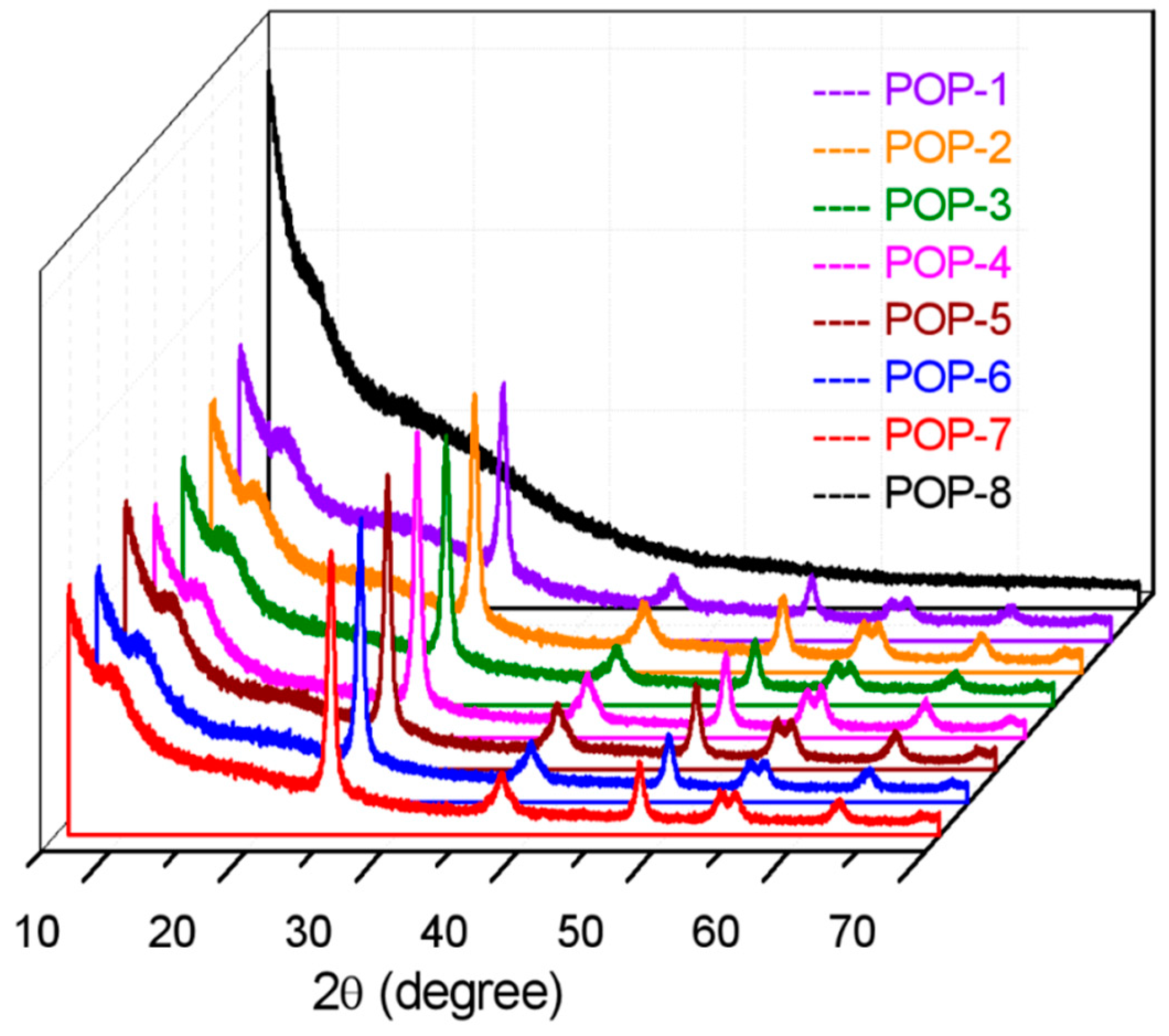
3.4. XRD Analysis
3.5. Particle Size and Particle Size Distribution
3.6. Surface Morphology
3.7. Ethylene Polymerization and Kinetic Curve of the Supported Metallocene Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauter, D.W.; Popoff, N.; Bashir, M.A.; Szeto, K.C.; Gauvin, R.M.; Delevoye, L.; Taoufik, M.; Boisson, C. The design of a bipodalbis(pentafluorophenoxy)aluminate supported on silica as an activator for ethylene polymerization using surface organometallic chemistry. Chem. Commun. 2016, 52, 4776–4779. [Google Scholar] [CrossRef] [PubMed]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Alt, H.G.; Köppl, A. Effect of the nature of metallocene complexes of group IV metals on their performance in catalytic ethylene and propylene polymerization. Chem. Rev. 2000, 100, 1205–1222. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.Y.; Xu, R.W. Polypropylene—Polymerization and Characterization of Mechanical and Thermal Properties. In Versatile Propylene-Based Polyolefins with Tunable Molecular Structure through Tailor-Made Catalysts and Polymerization Process; Wang, W.Y., Zeng, Y.M., Eds.; IntechOpen: London, UK, 2020; Chapter 2; ISBN 978-1-83880-414-5. [Google Scholar]
- Galli, P.; Vecellio, G. Technology: Driving force behind innovation and growth of polyolefins. Prog. Polym. Sci. 2001, 26, 1287–1336. [Google Scholar] [CrossRef]
- Yagaguchi, M.; Miyata, H.; Nitta, K.-H. Structure and properties for binary blends of isotactic-polypropylene with ethylene-α-olefin copolymer. 1. Crystallization and morphology. J. Polym. Sci. Part B 1997, 35, 953. [Google Scholar] [CrossRef]
- Phulkerd, P.; Funahashi, Y.; Ito, A.; Iwasaki, S.; Yamaguchi, M. Perpendicular orientation between dispersed rubber and polypropylene molecules in an oriented sheet. Polym. J. 2018, 50, 309. [Google Scholar] [CrossRef]
- Chum, P.S.; Swogger, K.W. Olefin polymerization technologies--History and recent progress at Dow Chemical Company. Prog. Polymer Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- CHang, M.; Liu, X.; Nelson, P.J.; MUnzing, G.R.; Gegan, T.A.; Kission, Y.V. Ziegler–Natta catalysts for propylene polymerization: Morphology and crystal structure of a fourth-generation catalyst. J. Catal. 2006, 239, 347. [Google Scholar] [CrossRef]
- Peil, K.P.; Neithamer, D.R.; Patrick, D.W.; WIlson, B.E.; Tucker, C.J. Applications of high throughput research at the Dow Chemical Company. Macromol. Rapid Commun. 2004, 25, 119. [Google Scholar] [CrossRef]
- Jüngling, S.; Koltzenberg, S.; Mülhaupt, R. Propene homo- and copolymerization using homogeneous and supported metallocene catalysts based on Me2Si (2-Me-Benz[e]lnd)2ZrCl2. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1–8. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1345. [Google Scholar] [CrossRef] [PubMed]
- Corradini, P.; Guera, G.; Cavallo, L. New Century Catalysts Unravel the Mechanism of Stereocontrol of Old Ziegler−Natta Catalysts? Acc. Chem. Res. 2004, 37, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W.; Waymouth, R.M. Oscillating stereocontrol:A strategy for the synthesis of thermoplastic elastomeric polyproopylene. Science 1995, 267, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Waymouth, R.M. 2-Arylindene metallocenes: Conformationally dynamic catalysts to control the structure and properties of Polypropylenes. Acc. Chem. Res. 2002, 35, 765–773. [Google Scholar] [CrossRef]
- Tisse, V.F.; Prades, F.; Briquel, R.; Boisson, C.; Mckenna, T.F.L. Role of silica properties in the polymerisation of ethylene using supported metallocene catalysts. Macromol. Chem. Phys. 2010, 211, 91–102. [Google Scholar] [CrossRef]
- Kumkaew, P.; Wanke, S.E.; Praserthdam, P.; Danumah, C.; Kaliaguine, S. Gas-phase ethylene polymerization using zirconocene supported on mesoporous molecular sieves. J. Appl. Polym. Sci. 2003, 87, 1161–1177. [Google Scholar] [CrossRef]
- Meshkova, I.N.; Kudinova, O.I.; Kovaleva, N.Y.; Grinev, V.G.; Ladygina, T.A.; Kiseleva, E.V.; Novokshonova, L.A. Effect of the zeolite support on the polymerization of propylene with immobilized ansa-zirconocene catalysts. Polym. Sci. Ser. B 2009, 51, 401–408. [Google Scholar] [CrossRef]
- Huang, R.; Duchateau, R.; Koning, C.E.; Chadwick, J.C. Zirconocene Immobilization and Activation on MgCl2-Based Supports: Factors Affecting Ethylene Polymerization Activity. Macromolecules 2008, 41, 579–590. [Google Scholar] [CrossRef]
- Michelotti, M.; Altomare, A.; Ciardelli, F.; Roland, E. Zeolite supported polymerization catalysts: Copolymerization of ethylene and α-olefins with metallocenes supported on HY zeolite. J. Mol. Catal. Part A Chem. 1998, 129, 241–248. [Google Scholar] [CrossRef]
- Severn, J.R.; Chadwick, J.C.; Duchateau, R.; Friederichs, N. “Bound but not gagged” immobilizing single-site α-olefin polymerization catalysts. Chem. Rev. 2005, 105, 4073–4147. [Google Scholar] [CrossRef]
- Vaya, V.I.; Belelli, P.G.; Santos, J.H.Z.; Ferreia, M.L.; Damiani, D.E. Influence of acidic support in metallocene catalysts for ethylene polymerization. J. Catal. 2001, 2004, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous organic polymers in catalysis: Opportunities and challenges. ACS Catal. 2011, 1, 819–835. [Google Scholar] [CrossRef]
- Lei, J.; Li, D.; Wang, H.; Zhou, G. Porous polyethylene spheres with nanofiber structure from Ziegler-Natta catalyst supported on porous polymer particles. Polymer 2011, 52, 602–605. [Google Scholar]
- Hong, S.C.; Teranishi, T.; Soga, K. Investigation on the polymer particle growth in ethylene polymerization with PS beads supported rac-Ph2Si(Ind)2ZrCl2 catalyst. Polymer 1998, 39, 7153–7157. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S.-L. Porous Organic Materials: Strategic Design and Structure–Function Correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Han, X.; Han, Z.; Bai, Y. Highly tunable porous organic polymer (POP) supports for metallocene-based ethylene polymerization. Appl. Surface Sci. 2017, 420, 496–503. [Google Scholar] [CrossRef]
- Rosco, S.B.; Frechet, J.M.; Walzer, J.F.; Dias, A.J. Polyolefin spheres from metallocenes supported on noninteracting polystyrene. Science 1998, 280, 270–273. [Google Scholar] [CrossRef]
- Nishida, H.; Uozumi, T.; Arai, T.; Soga, K. Polystyrene-supported metallocene catalysts for olefin polymerizations. Macromol. Rapid. Commun. 1995, 16, 821–830. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Ren, F.; Xu, R.; Bai, Y. Porous organic polymers-supported metallocene catalysts for ethylene/1-hexene copolymerization. Catalysts 2018, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, R.; Zhu, B.; Li, Y.; Han, X. Metal oxide as a template in the preparation of porous poly (2-hydroxyethylmethylacrylate-co-divinylbenzene) particles as a metallocene catalyst support. RSC Adv. 2016, 6, 52464–52474. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Zhu, B.; Li, Y.; Ma, Y. Synthesis and characterization of functional porous organic polymers as efficient metallocene catalyst supports. New J. Chem. 2016, 40, 8324–8333. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.-L.; Liu, W.-X.; Zhang, P.-S. Feasibility study on the design and synthesis of functional porous organic polymers with tunable pore structure as metallocene catalyst supports. Polymers 2018, 10, 944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure−Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef]


| Entry | TiO2 Adding Amount/Total Monomer wt% | DVB | DVB:HEMA (Molar Ratio) | Specific Surface Area m2/g | Total Pore Volume cm3/g | Average Pore Diameter nm | Bulk Density g/cm3 |
|---|---|---|---|---|---|---|---|
| POP-1 | 10% | 55% | 3:2 | 224 | 0.368 | 6.58 | 0.26 |
| POP-2 | 20% | 55% | 3:2 | 191 | 0.367 | 7.71 | 0.26 |
| POP-3 | 30% | 55% | 3:2 | 198 | 0.350 | 7.06 | 0.33 |
| POP-4 | 50% | 55% | 3:2 | 204 | 0.312 | 6.12 | 0.35 |
| POP-5 | 30% | 80% | 3:2 | 309 | 0.315 | 4.08 | 0.31 |
| POP-6 | 37.5% | 55% | 3:1 | 136 | 0.150 | 4.41 | 0.25 |
| POP-7 | 37.5% | 80% | 3:1 | 279 | 0.246 | 3.53 | 0.30 |
| POP-8 | 0 | 80% | 3:2 | 424 | 0.410 | 3.86 | 0.26 |
| Sample | Temperature Zone | Weight Loss, % | TiO2 Content (Calculated), % |
|---|---|---|---|
| POP-1 | 20 °C–300 °C | 1.94 | 13.8 |
| 20 °C–800 °C | 85.98 | ||
| POP-2 | 20 °C–300 °C | 2.51 | 24.0 |
| 20 °C–800 °C | 75.83 | ||
| POP-3 | 20 °C–300 °C | 1.72 | 27.9 |
| 20 °C–800 °C | 71.99 | ||
| POP-4 | 20 °C ~300 °C | 1.89 | 44.0 |
| 20 °C–800 °C | 55.88 | ||
| POP-5 | 20 °C–300 °C | 1.69 | 29.8 |
| 20 °C–800 °C | 70.08 | ||
| POP-6 | 20 °C–300 °C | 1.86 | 32.4 |
| 20 °C–800 °C | 67.44 | ||
| POP-7 | 20 °C–300 °C | 1.46 | 38.4 |
| 20 °C–800 °C | 61.48 | ||
| POP-8 | 20 °C–300 °C | 1.41 | 0 |
| 20 °C–800 °C | 99.79 |
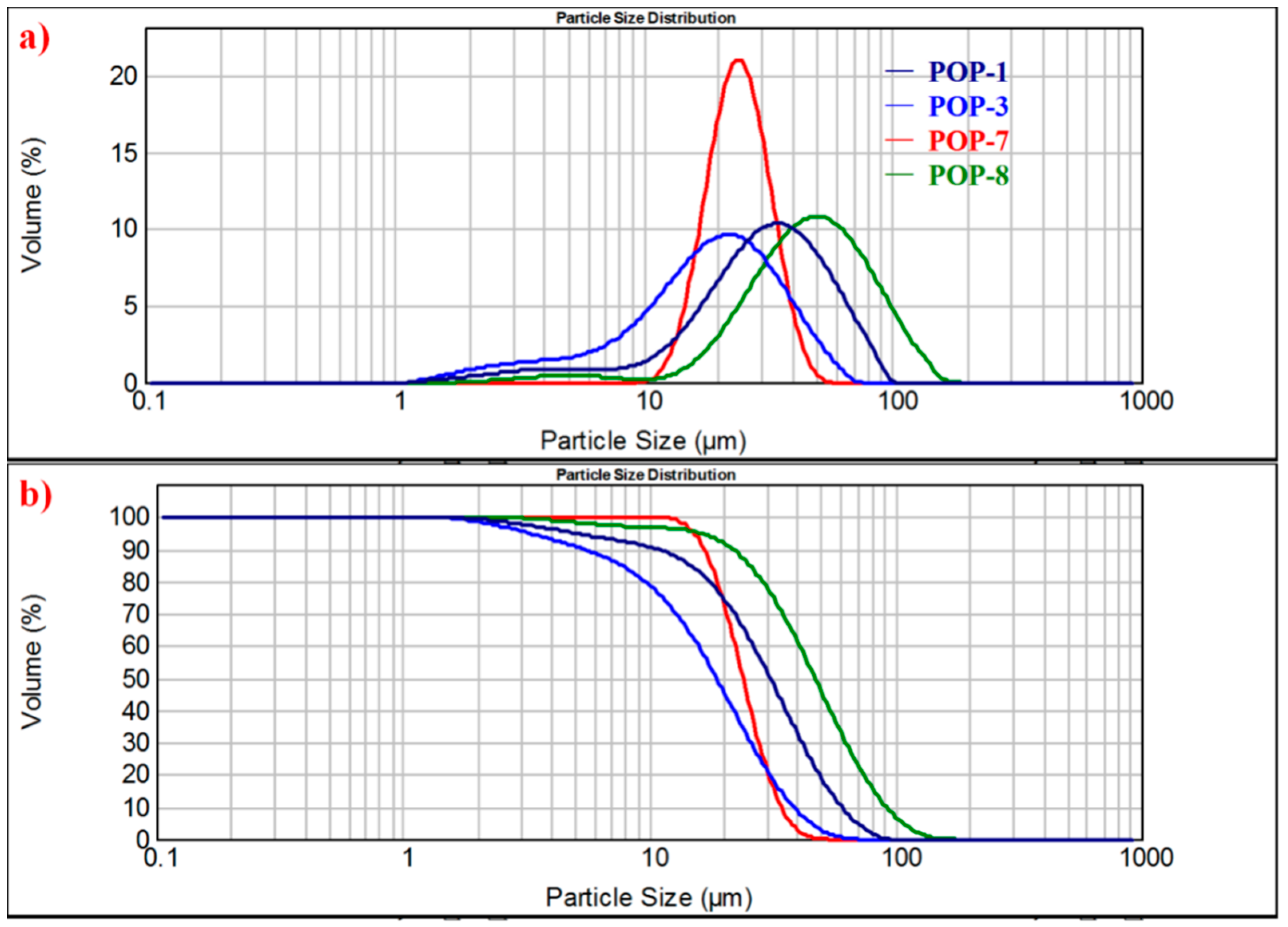
| Sample Δa | Dv(0.1) μm | Dv(0.5) μm | Dv(0.9) μm | Mode Δb μm | Span Δc |
|---|---|---|---|---|---|
| POP-1 | 10.8 | 31.0 | 60.7 | 34.9 | 1.61 |
| POP-2 | 8.89 | 28.3 | 56.2 | 33.1 | 1.67 |
| POP-3 | 5.41 | 18.8 | 38.6 | 22.0 | 1.77 |
| POP-4 | 9.21 | 17.6 | 35.3 | 17.2 | 1.48 |
| POP-5 | 8.10 | 24.3 | 52.2 | 26.7 | 1.82 |
| POP-6 | 15.8 | 24.7 | 38.3 | 24.8 | 0.909 |
| POP-7 | 16.9 | 24.1 | 34.2 | 24.1 | 0.717 |
| POP-8 | 21.8 | 47.3 | 91.9 | 49.8 | 1.48 |
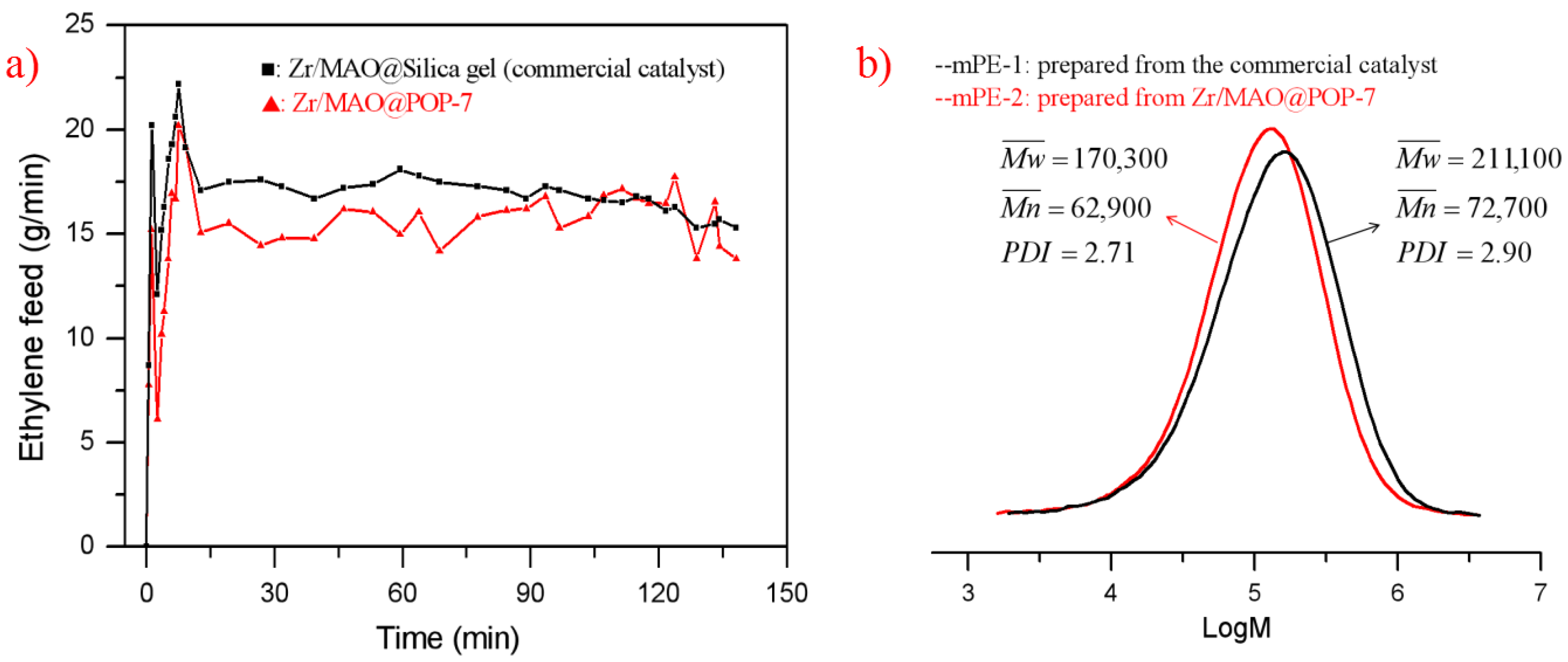
| Cat. | Zr (μmol/g) | Al/Zr Molar Ratio | Cat (mg) | Yield (g) | Activity (kg PE/mol.Zr.bar. h) | Productivity (g PE/g cat) | Bulk Density (g/mL) |
|---|---|---|---|---|---|---|---|
| Zr/MAO@POP-1 | 42.0 | 114 | 130 | 44.9 | 4112 | 345 | 0.26 |
| Zr/MAO@POP-4 | 43.5 | 112 | 135 | 37.4 | 3184 | 277 | 0.22 |
| Zr/MAO@POP-6 | 43.2 | 117 | 165 | 55.3 | 3879 | 335 | 0.30 |
| Zr/MAO@POP-7 | 40.6 | 123 | 140 | 54.5 | 4794 | 389 | 0.24 |
| Zr/MAO@silica *a | 47.3 | 103 | 132 | 24.2 | 1940 | 183 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Kang, W.; Gao, L.; Li, G.; Chen, X.; Guo, Y. Highly Flowable Nano TiO2/Porous Organic Polymer (POP) Supports for Efficient Metallocene Catalysts. Nanomaterials 2021, 11, 60. https://doi.org/10.3390/nano11010060
Wang X, Kang W, Gao L, Li G, Chen X, Guo Y. Highly Flowable Nano TiO2/Porous Organic Polymer (POP) Supports for Efficient Metallocene Catalysts. Nanomaterials. 2021; 11(1):60. https://doi.org/10.3390/nano11010060
Chicago/Turabian StyleWang, Xiong, Wenqian Kang, Lin Gao, Guangquan Li, Xuerong Chen, and Yi Guo. 2021. "Highly Flowable Nano TiO2/Porous Organic Polymer (POP) Supports for Efficient Metallocene Catalysts" Nanomaterials 11, no. 1: 60. https://doi.org/10.3390/nano11010060
APA StyleWang, X., Kang, W., Gao, L., Li, G., Chen, X., & Guo, Y. (2021). Highly Flowable Nano TiO2/Porous Organic Polymer (POP) Supports for Efficient Metallocene Catalysts. Nanomaterials, 11(1), 60. https://doi.org/10.3390/nano11010060





