Nitrated Graphene Oxide Derived from Graphite Oxide: A Promising Energetic Two-Dimensional Material
Abstract
1. Introduction
2. Experimental Section
2.1. Calculation Methods
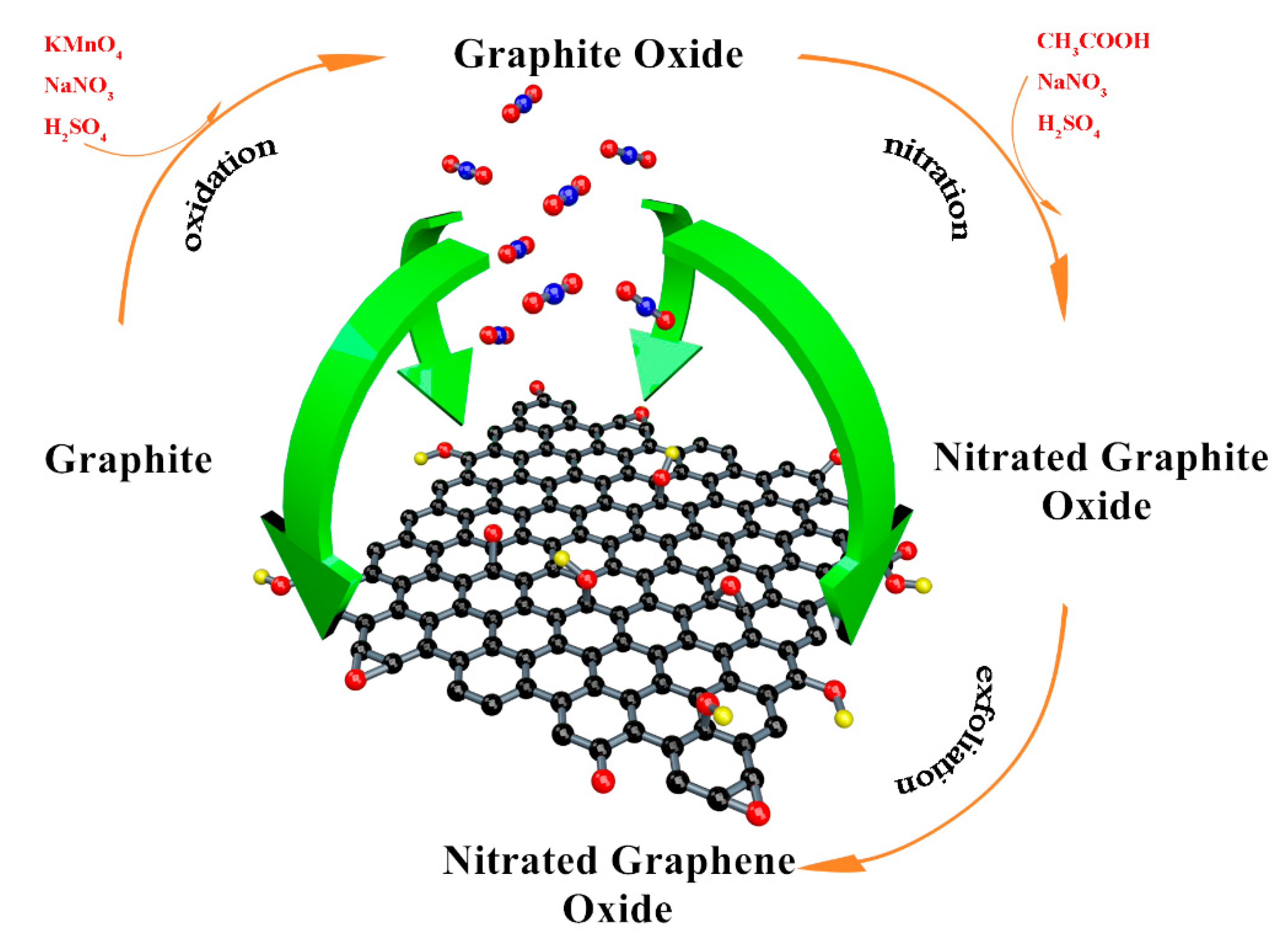
2.2. Sample Preparation
2.3. Materials Characterization
2.4. Thermal Analysis
2.5. Combustion Properties
3. Results and Discussion
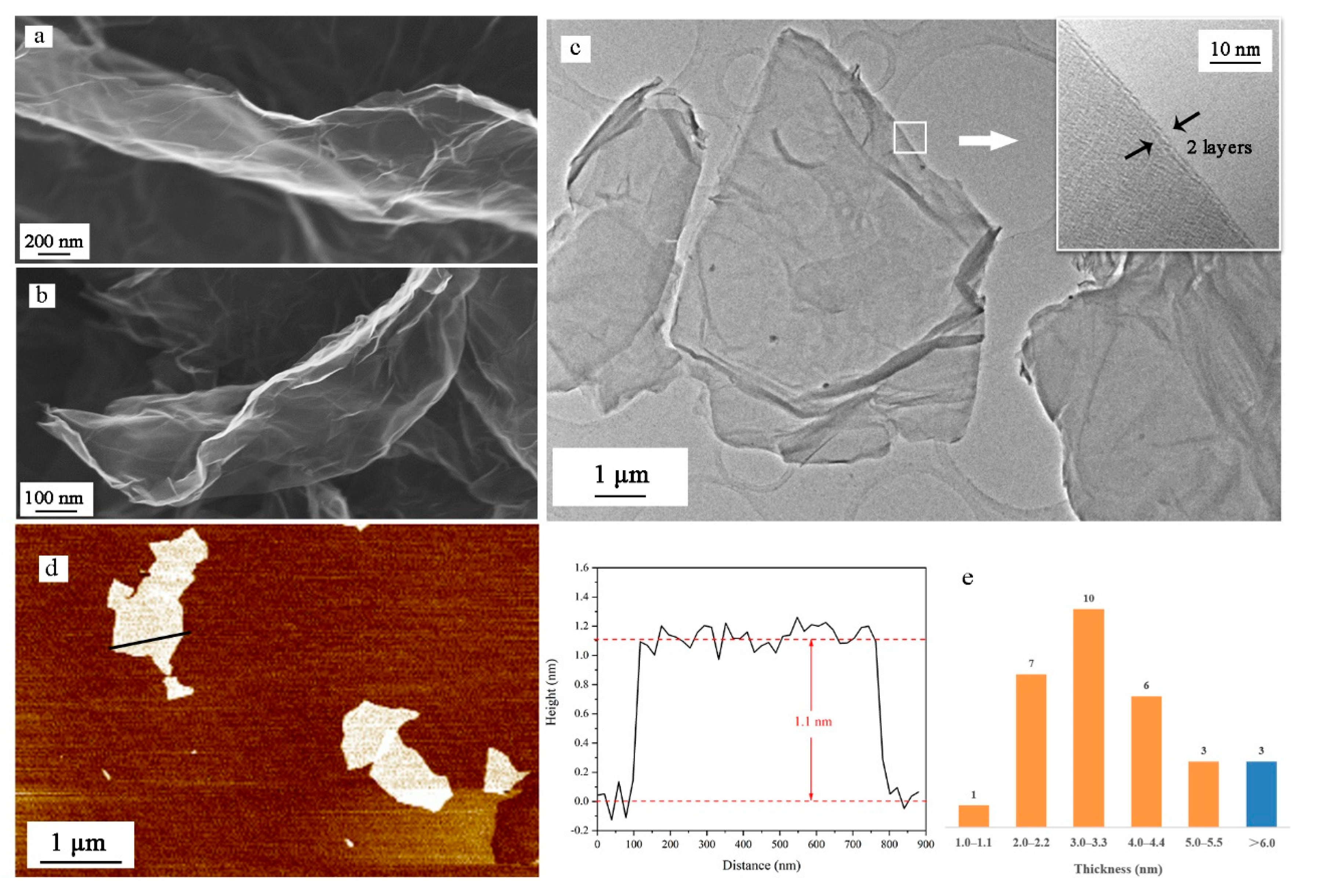
3.1. Morphological Analysis
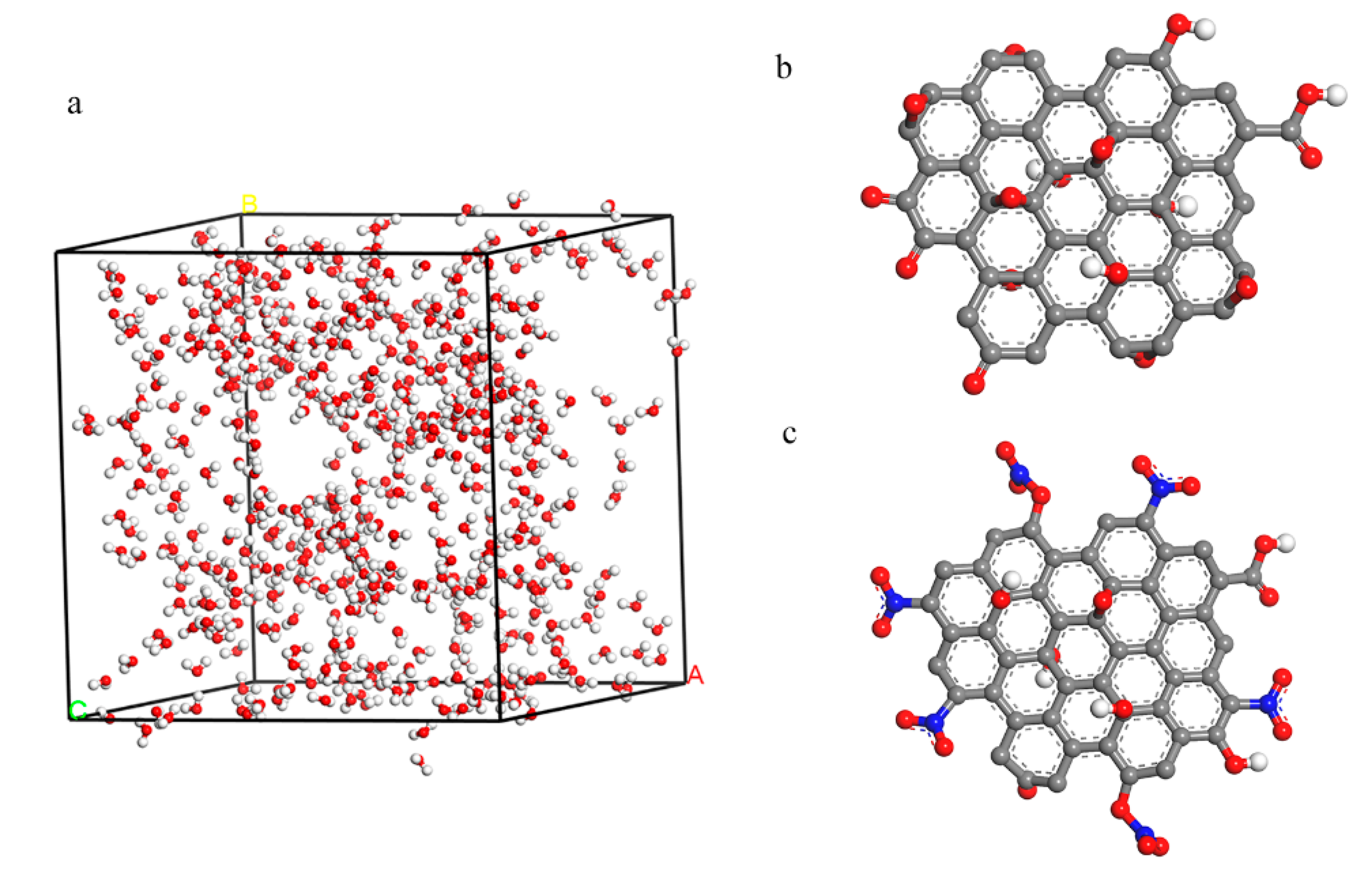
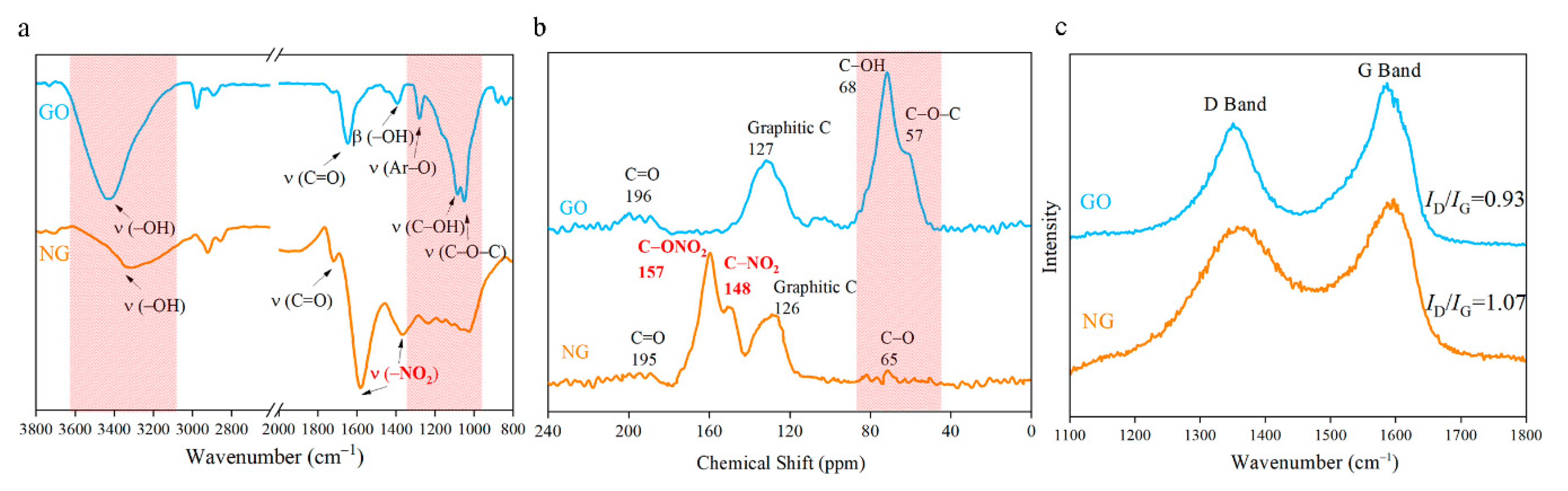
3.2. Structure Analysis
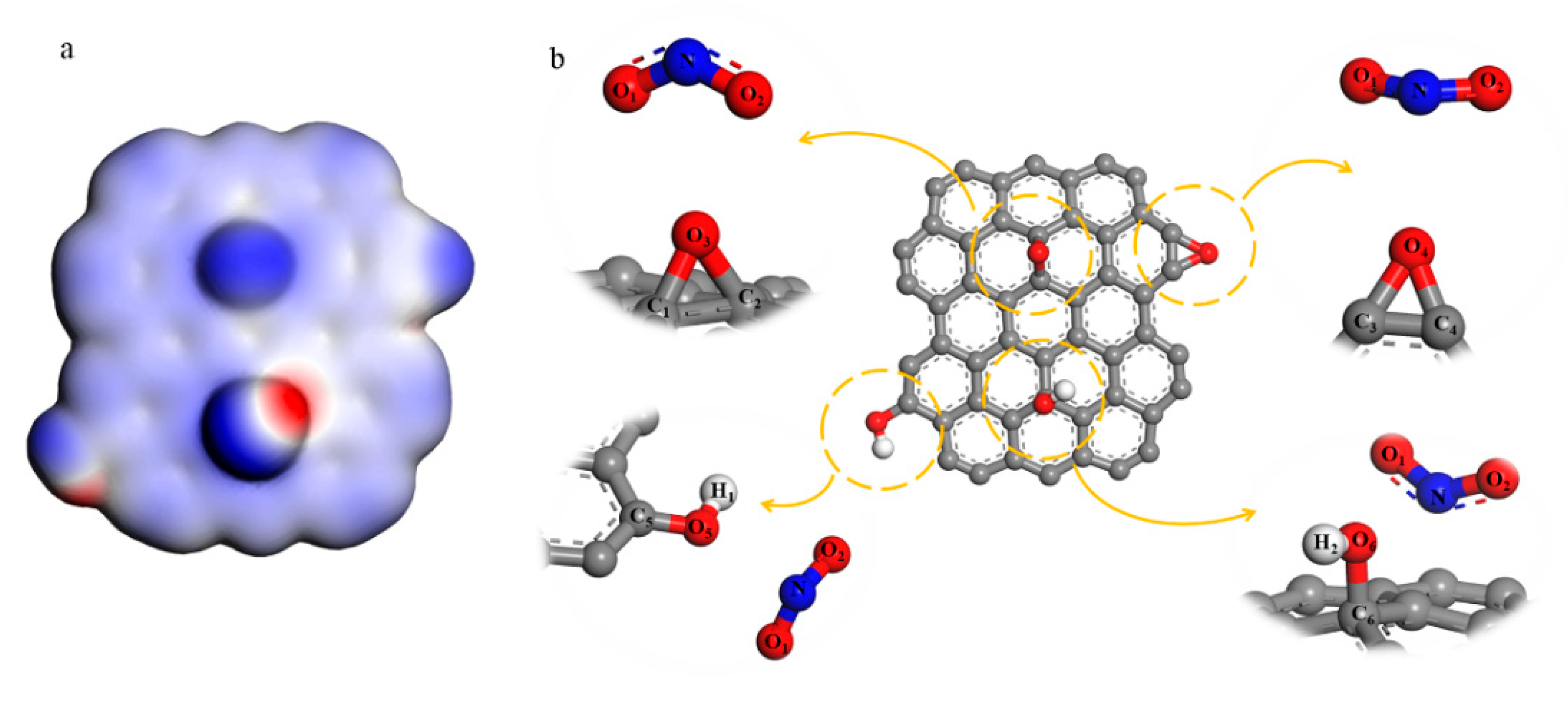
3.3. DFT Calculation Analysis
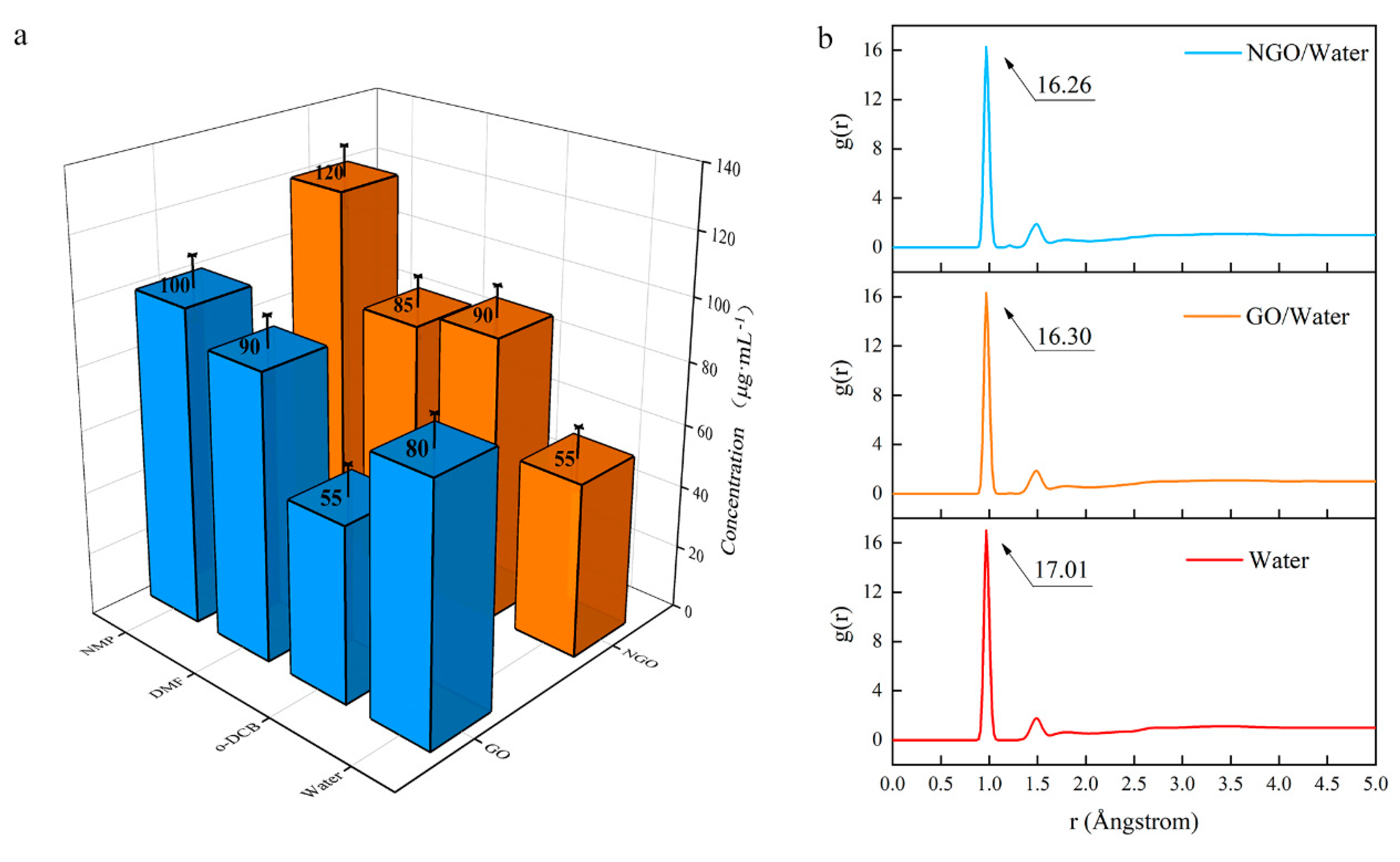
3.4. Dispersion and Hydrophobicity
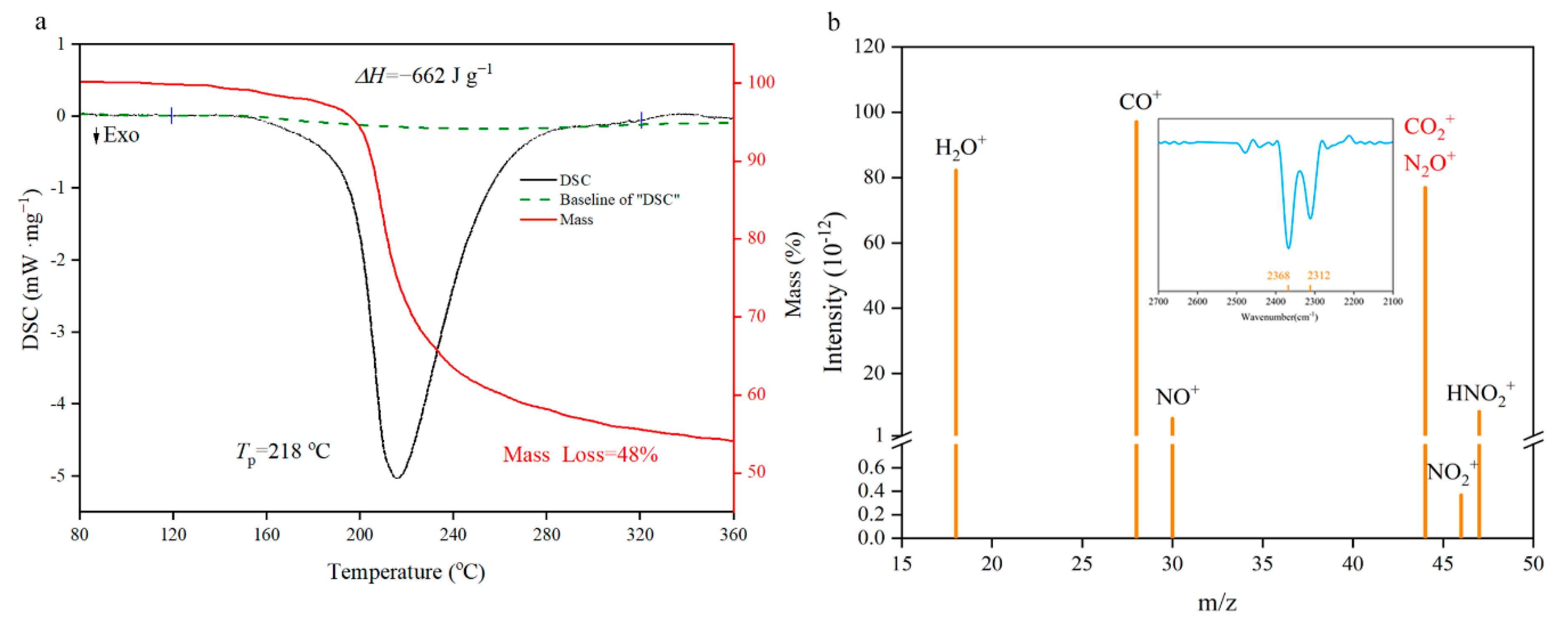
3.5. Thermal Analysis
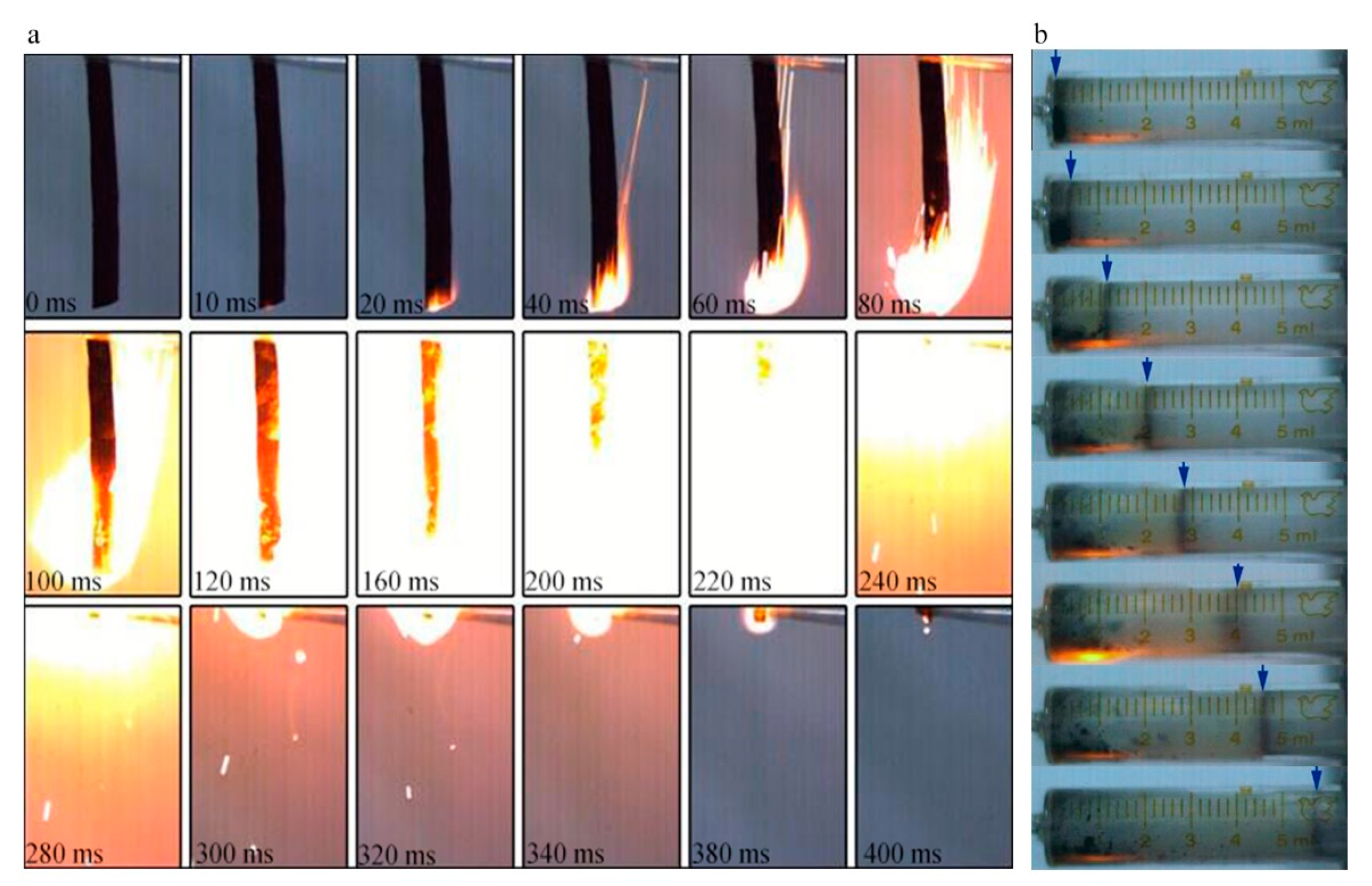
3.6. Energy Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Jung, I.; Pelton, M.; Piner, R.; Dikin, D.A.; Stankovich, S.; Watcharotone, S.; Hausner, M.; Ruoff, R.S. Simple Approach for High-Contrast Optical Imaging and Characterization of Graphene-Based Sheets. Nano Lett. 2007, 7, 3569–3575. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, J.; Tung, V.C.; Luo, J.; Kim, F.; Huang, J. Graphene oxide: Surfactant sheets. Pure Appl. Chem. 2010, 83, 95–110. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Chemistry with Graphene and Graphene Oxide-Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- He, H.; Riedl, T.; Lerf, A.; Klinowski, J. Solid-State NMR Studies of the Structure of Graphite Oxide. J. Phys. Chem. B 1996, 100, 19954–19958. [Google Scholar] [CrossRef]
- Dimiev, A.; Kosynkin, D.V.; Alemany, L.B.; Chaguine, P.; Tour, J.M. Pristine Graphite Oxide. J. Am. Chem. Soc. 2012, 134, 2815–2822. [Google Scholar] [CrossRef]
- Jonathan, P.R.; Priyanka, A.P.; Joseph, J.M.; Matthew, B.; Ian, A.K.; Robert, J.Y.; Neil, R.W. The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-like Sheets. Angew. Chem. Int. Ed. 2011, 50, 3173–3177. [Google Scholar]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.; Durstock, M.; Baek, J.-B.; Dai, L. Soluble P3HT-Grafted Graphene for Efficient Bilayer-Heterojunction Photovoltaic Devices. ACS Nano 2010, 4, 5633–5640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, Y.; Xu, L.; Zeng, L.; He, Y.; Kang, E.-T.; Zhang, J. Growing poly(N-vinylcarbazole) from the surface of graphene oxide via RAFT polymerization. J. Polym. Sci. Polym. Chem. 2011, 49, 2043–2050. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabó, T.; Szeri, A.; Dékány, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Krishnan, D.; Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L.J.; Jang, H.D.; Huang, J. Energetic graphene oxide: Challenges and opportunities. Nano Today 2012, 7, 137–152. [Google Scholar] [CrossRef]
- Olah, G.A.; Malhotra, R.; Narang, S.C. Nitration: Methods And Mechanisms; VCH Press: New York, NY, USA, 1989. [Google Scholar]
- Schofield, K. Aromatic Nitrations; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Zhang, W.; Luo, Q.; Duan, X.; Zhou, Y.; Pei, C. Nitrated graphene oxide and its catalytic activity in thermal decomposition of ammonium perchlorate. Mater. Res. Bull. 2014, 50, 73–78. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.; Luo, Q.; Duan, X.; Pei, C. Preparation and thermal decomposition properties of nitrated graphene oxide (NGO)/RDX nano-energetic composites. J. Therm. Anal. Calorim. 2019, 139, 1671–1679. [Google Scholar] [CrossRef]
- Robert, G.P.; Yang, W.T. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L.J.; Sohn, K.; Huang, J. Self-Propagating Domino-like Reactions in Oxidized Graphite. Adv. Funct. Mater. 2010, 20, 2867–2873. [Google Scholar] [CrossRef]
- Luo, J.; Kim, J.; Huang, J. Material processing of chemically modified graphene: Some challenges and solutions. Acc. Chem. Res. 2013, 46, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Arockiasamy, A.; Toghiani, H.; Oglesby, D.; Horstemeyer, M.F.; Bouvard, J.L.; King, R.L. TG-DSC-FTIR-MS study of gaseous compounds evolved during thermal decomposition of styrene-butadiene rubber. J. Therm. Anal. Calorim. 2013, 111, 535–542. [Google Scholar] [CrossRef]
- Ou, Y.; Sun, Y.; Guo, X.; Jiao, Q. Investigation on the thermal decomposition of hydroxyl terminated polyether based polyurethanes with inert and energetic plasticizers by DSC-TG-MS-FTIR. J. Anal. Appl. Pyrol. 2018, 132, 94–101. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Shan, M.X.; Xiao, Z.X.; Wang, J.S.; Jiao, Q.J. Kinetics of thermal decomposition of ε-hexanitrohexaazaisowurtzitane by TG-DSC-MS-FTIR. Korean J. Chem. Eng. 2015, 32, 1164–1169. [Google Scholar] [CrossRef]
- Cai, W.; Piner, R.D.; Stadermann, F.J.; Park, S.; Shaibat, M.A.; Ishii, Y.; Yang, D.; Velamakanni, A.; An, J.S.; Stoller, M.; et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 2008, 321, 1815–1817. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, G.; Chen, H.; Wang, S.; Zhang, G.; Zhang, F.; Fan, X. Sulfonated graphene as water-tolerant solid acid catalyst. Chem. Sci. 2011, 2, 484–487. [Google Scholar] [CrossRef]
- Hansen, J.P.; McDonald, I.R. Theory of Simple Liquids, 2nd ed.; Academic Press: London, UK, 1990. [Google Scholar]
- Wang, G.; Gong, X.; Liu, Y.; Xiao, H. A theoretical investigation on the structures, densities, detonation properties and pyrolysis mechanism of the nitro derivatives of toluenes. J. Hazard. Mater. 2010, 177, 703–710. [Google Scholar] [CrossRef]
- Collins, L.W.; Haws, L.D. The thermochemistry of explosives: A review. Thermochim. Acta 1977, 21, 1–38. [Google Scholar] [CrossRef]
- Waring, C.E.; Krastins, G. Kinetics and mechanism of the thermal decomposition of nitroglycerin. J. Phys. Chem. 1970, 74, 999–1006. [Google Scholar] [CrossRef]
- Roduit, B.; Borgeat, C.; Berger, B.; Folly, P.; Alonso, B.; Aebischer, J.N.; Stoessel, F. Advanced kinetic tools for the evaluation of decomposition reactions—Determination of thermal stability of energetic materials. J. Therm. Anal. Calorim. 2005, 80, 229–236. [Google Scholar] [CrossRef]
- Rossi, C.; Zhang, K.; Esteve, D.; Alphonse, P.; Tailhades, P.; Vahlas, C. Nanoenergetic materials for MEMS: A review. J. Micro. Microelectromech. Syst. 2007, 16, 919–931. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Liu, Z.R.; Qiu, G.; Yin, C.M. Influence of Different Pressure on the Thermal Decomposition of Energetic Materials at Liquid State. Chin. J. Energ. Mater. 2001, 9, 111–116. (In Chinese) [Google Scholar]
- Oyumi, Y.; Brill, T.B. Thermal decomposition of energetic materials 14. Selective product distributions evidenced in rapid, real-time thermolysis of nitrate esters at various pressures. Combust. Flame 1986, 66, 9–16. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 2002, 29, 1702–1706. [Google Scholar] [CrossRef]
- Chen, J.K.; Brill, T.B. Thermal decomposition of energetic materials 50. Kinetics and mechanism of nitrate ester polymers at high heating rates by SMATCH/FTIR spectroscopy. Combust. Flame 1991, 85, 479–488. [Google Scholar] [CrossRef]
- Chen, J.K.; Brill, T.B. Thermal decomposition of energetic materials 51. Kinetics of weight loss from nitrate ester polymers at low hating rate. Thermochim. Acta 1991, 181, 71–77. [Google Scholar] [CrossRef]
- Brill, T.B.; Gongwer, P.E. Thermal decomposition of energetic materials 69. Analysisi of the kinetics of nitrocellulose at 50 °C–500 °C. Propellants Explos. Pyrotech. 1997, 22, 38–44. [Google Scholar] [CrossRef]
- Liu, J.; Yan, T.; Li, Y.; Ren, H.; Wang, Q.; Guan, F.; Jiao, Q. Dual-mode response behavior of a graphene oxide implanted energetic system under different thermal stimuli. RSC Adv. 2020, 10, 10789–10798. [Google Scholar] [CrossRef]
- Ye, B.; An, C.; Zhang, Y.; Song, C.; Geng, X.; Wang, J. One-Step Ball Milling Preparation of Nanoscale CL-20/Graphene Oxide for Significantly Reduced Particle Size and Sensitivity. Nanoscale Res. Lett. 2018, 13, 42. [Google Scholar] [CrossRef]
- Wang, J.; Ye, B.; An, C.; Wu, B.; Li, H.; Wei, Y. Preparation and Properties of Surface-Coated HMX with Viton and Graphene Oxide. J. Energ. Mater. 2016, 34, 235–245. [Google Scholar] [CrossRef]
- Li, X.; Huang, B.; Li, R.; Zhang, H.; Qin, W.; Qiao, Z.; Liu, Y.; Yang, G. Laser-ignited relay-domino-like reactions in graphene oxide/CL-20 films for high-temperature pulse preparation of bi-layered photothermal membranes. Small 2019, 15, 1900338. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Guo, J.H.; Cao, C.K.; Liu, X.K.; Lv, J.Y.; Chen, S.W.; Liu, P.J.; Yan, Q.L. Catalytic Reactivity of Graphene Oxide Stabilized Transition Metal Complexes of Triaminoguanidine on Thermolysis of RDX. J. Phys. Chem. C 2018, 122, 14714–14724. [Google Scholar] [CrossRef]
- Deng, P.; Liu, Y.; Luo, P.; Wang, J.; Liu, Y.; Wang, D.; He, Y. Two-steps synthesis of sandwich-like graphene oxide/LLM-105 nanoenergetic composites using functionalized graphene. Mater. Lett. 2017, 194, 156–159. [Google Scholar] [CrossRef]
| O3 | O4 | O5 | O6 | |
|---|---|---|---|---|
| Mulliken atomic charges | −0.351 | −0.389 | −0.402 | −0.401 |
| f − (r) | 0.017 | 0.031 | 0.024 | 0.018 |
| β (°C·s−1) | Tp (°C) | ΔHd (J·g−1) |
|---|---|---|
| 5 | 201.4 | 425 |
| 10 | 209.7 | 473 |
| 15 | 213.7 | 577 |
| 20 | 218.0 | 662 |
| Ea = 166.6 kJ/mol | lgA = 6.10 | Rk = 0.9921 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, F.; Ren, H.; Yu, L.; Cui, Q.; Zhao, W.; Liu, J. Nitrated Graphene Oxide Derived from Graphite Oxide: A Promising Energetic Two-Dimensional Material. Nanomaterials 2021, 11, 58. https://doi.org/10.3390/nano11010058
Guan F, Ren H, Yu L, Cui Q, Zhao W, Liu J. Nitrated Graphene Oxide Derived from Graphite Oxide: A Promising Energetic Two-Dimensional Material. Nanomaterials. 2021; 11(1):58. https://doi.org/10.3390/nano11010058
Chicago/Turabian StyleGuan, Fayang, Hui Ren, Lan Yu, Qingzhong Cui, Wanjun Zhao, and Jie Liu. 2021. "Nitrated Graphene Oxide Derived from Graphite Oxide: A Promising Energetic Two-Dimensional Material" Nanomaterials 11, no. 1: 58. https://doi.org/10.3390/nano11010058
APA StyleGuan, F., Ren, H., Yu, L., Cui, Q., Zhao, W., & Liu, J. (2021). Nitrated Graphene Oxide Derived from Graphite Oxide: A Promising Energetic Two-Dimensional Material. Nanomaterials, 11(1), 58. https://doi.org/10.3390/nano11010058






