pH-Dependent Formation of Oriented Zinc Oxide Nanostructures in the Presence of Tannic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnO-TA Nanostructures
2.3. Characterisation of ZnO-TA Nanostructures
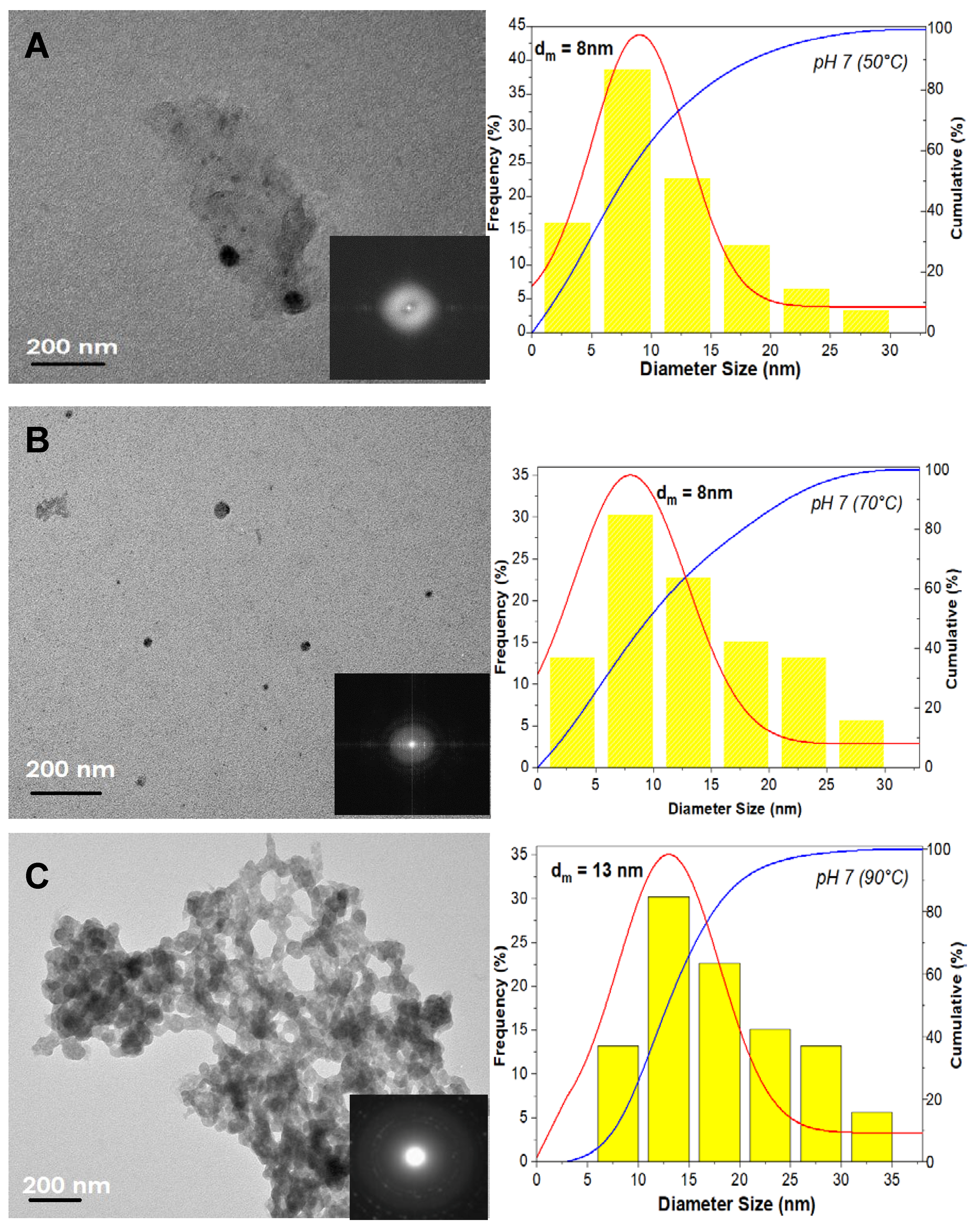
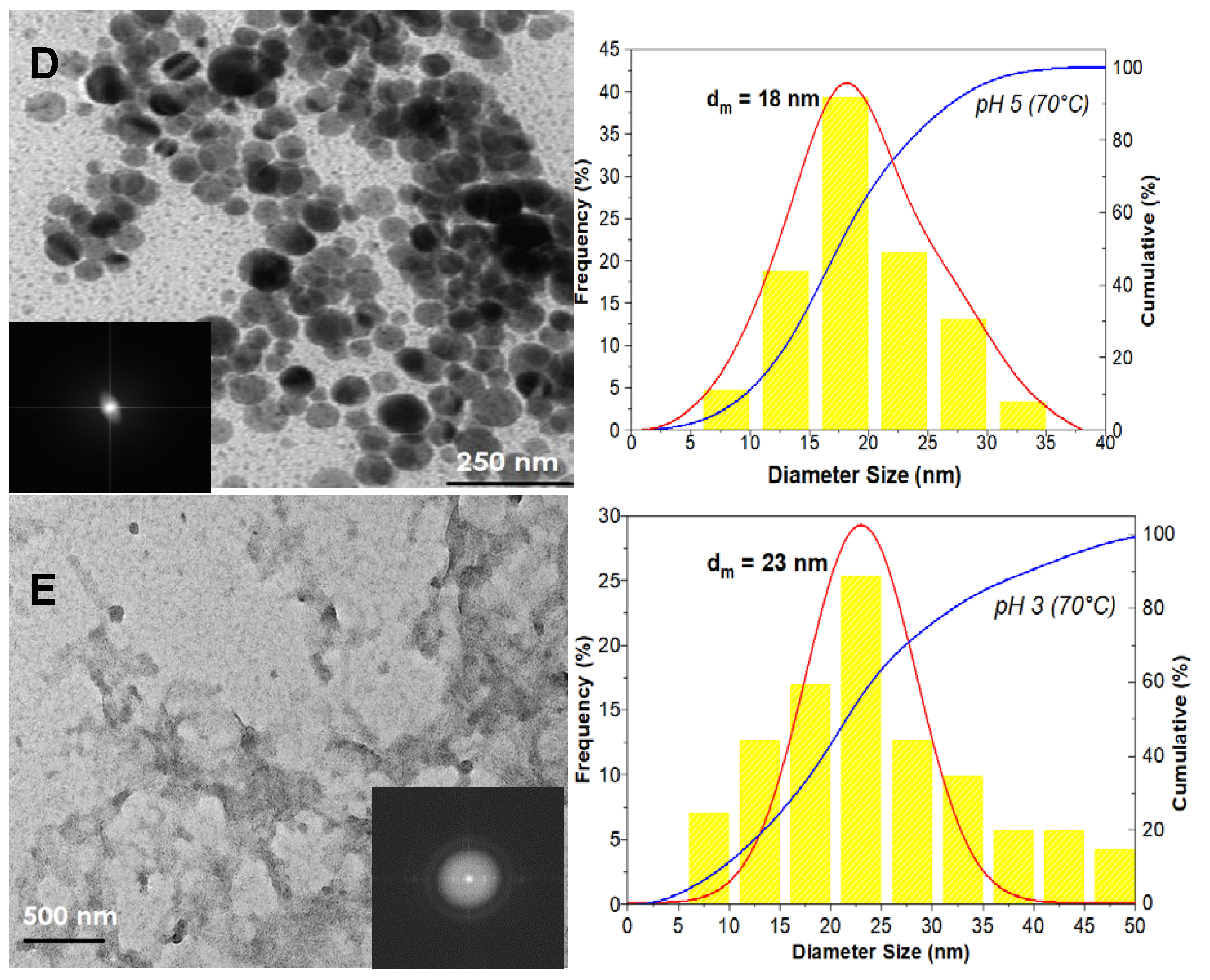
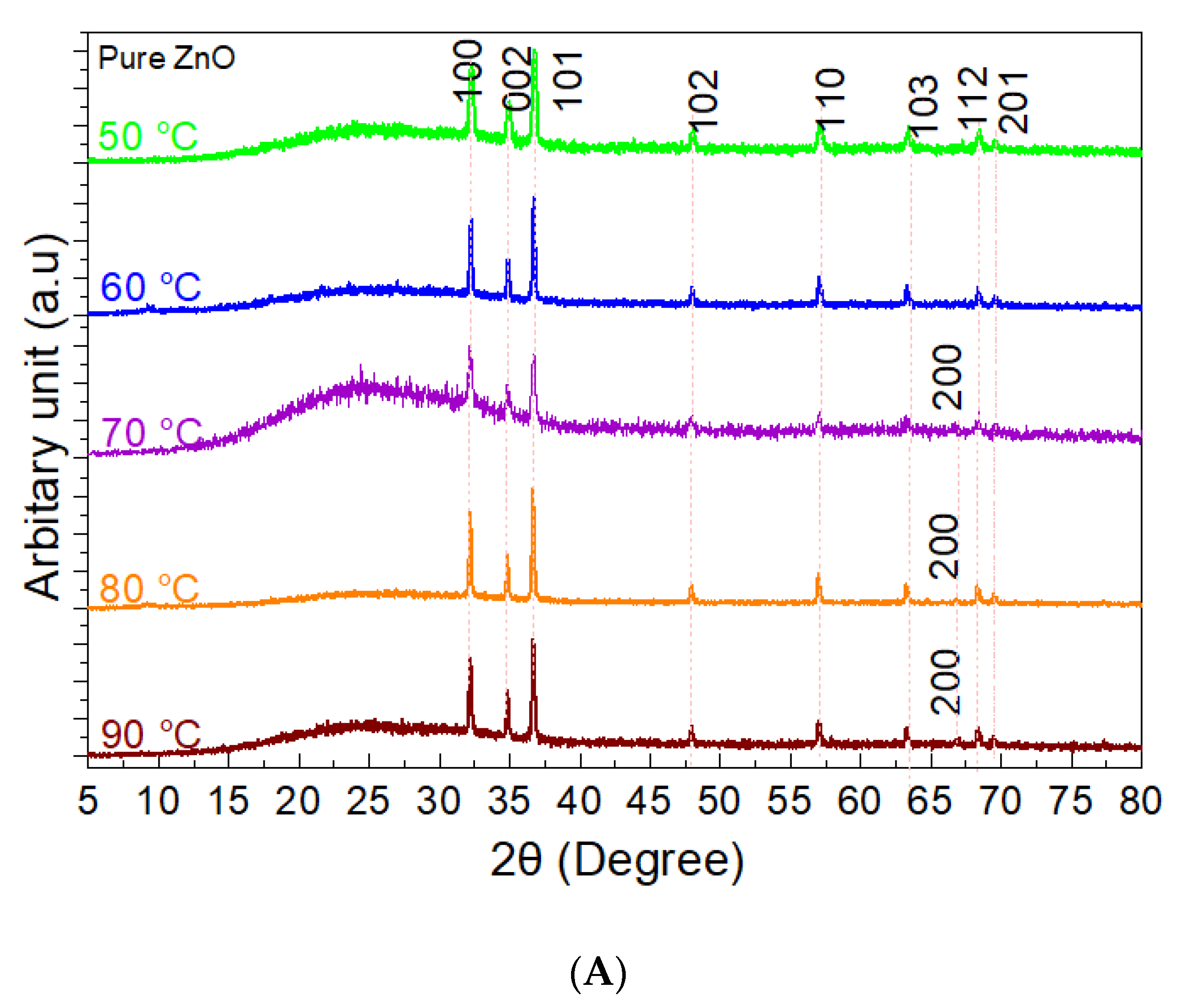
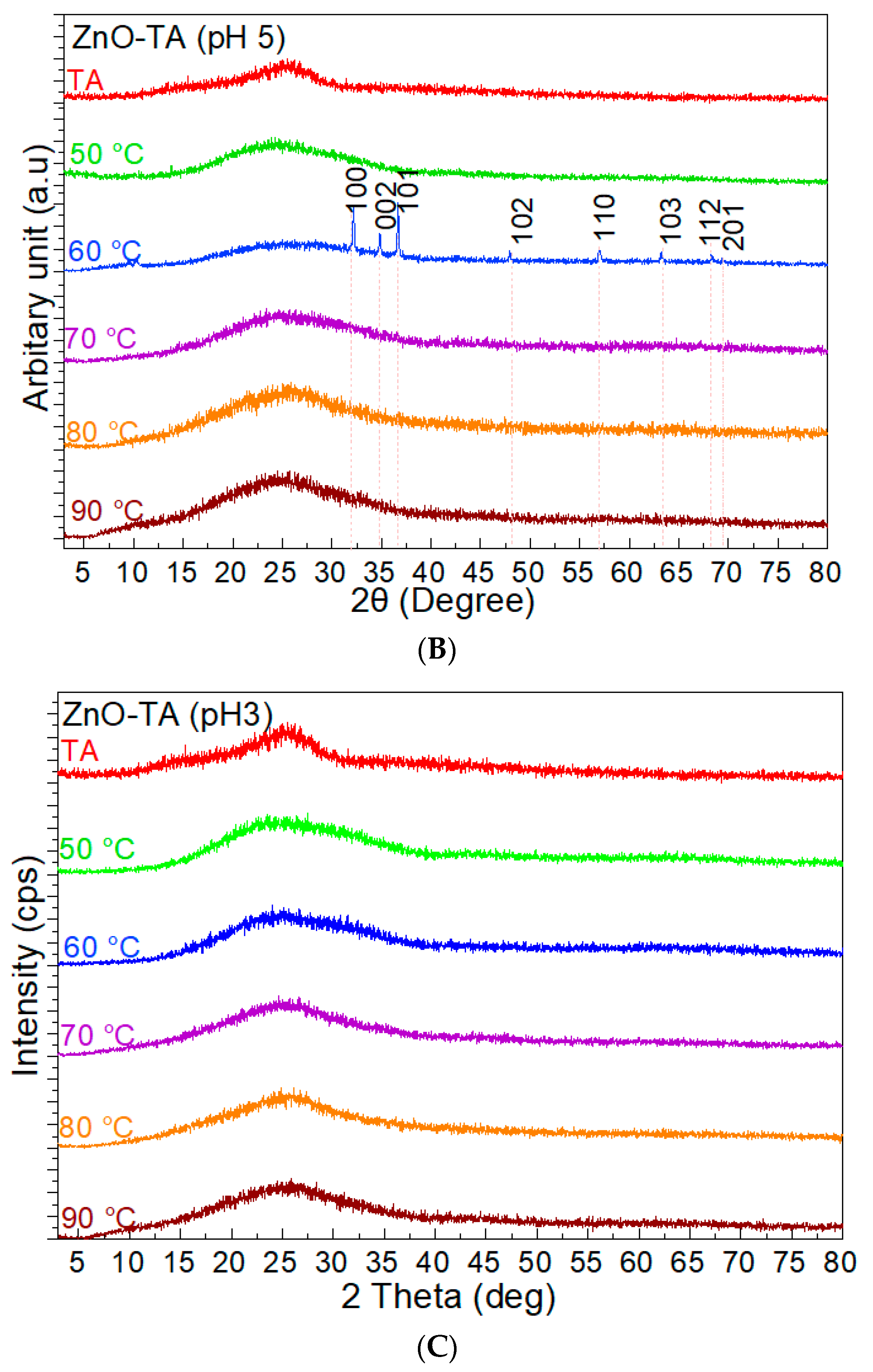
3. Results
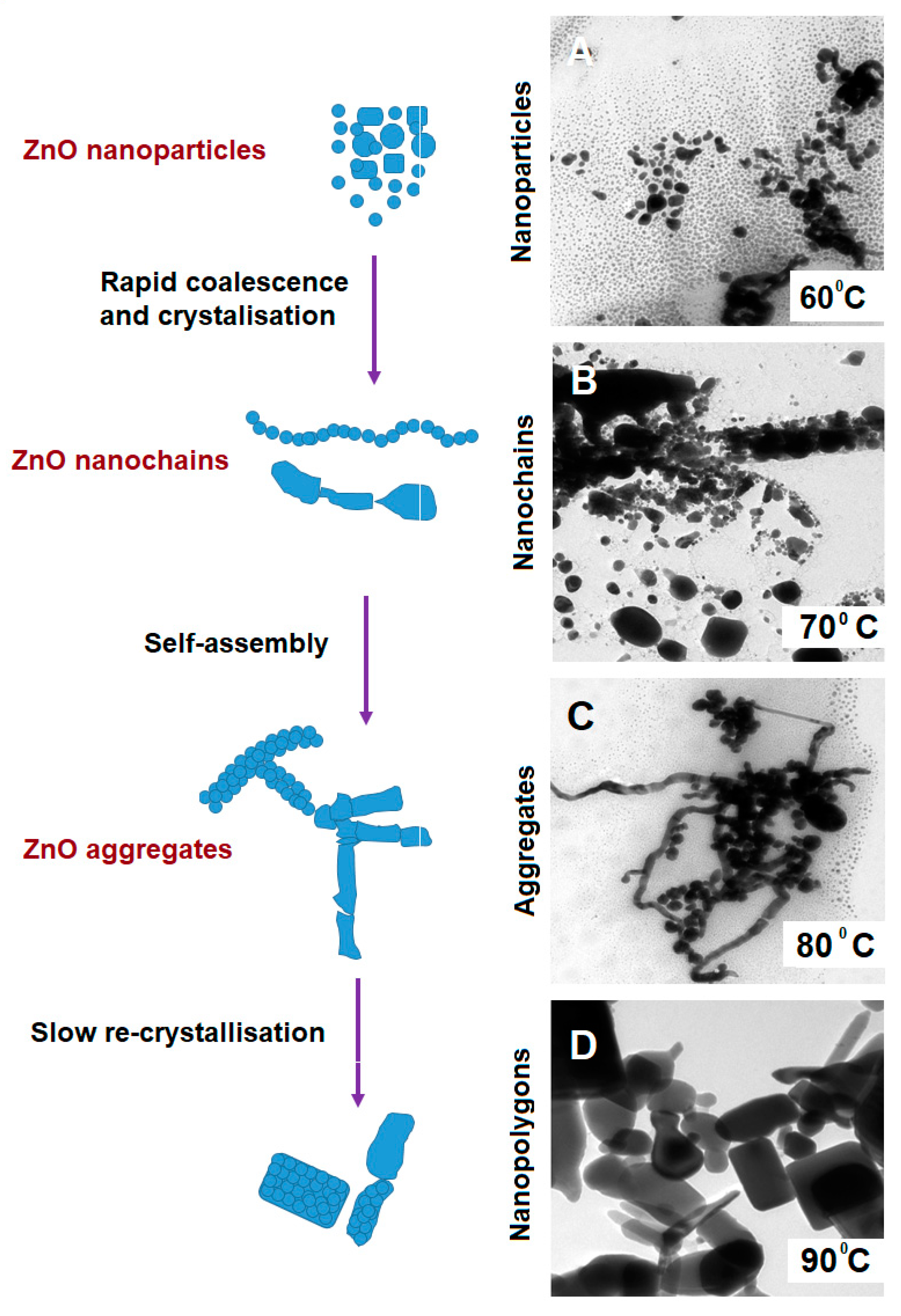
3.1. The Precipitation of ZnO Nanostructures
3.2. Assembly of Pure ZnO and ZnO-TA Nanostructures
3.2.1. Influence of the Reaction Temperature
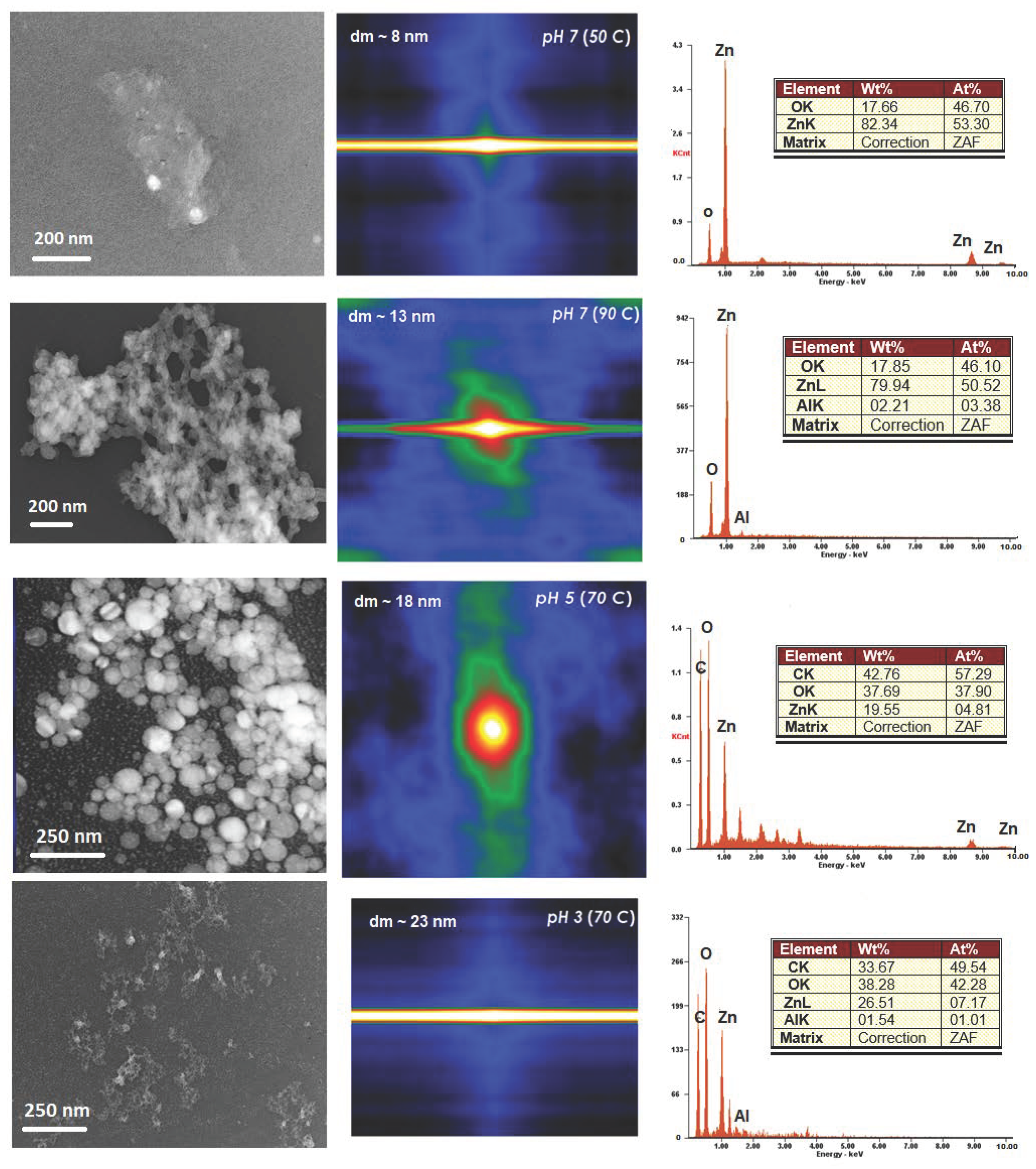
3.2.2. pH Influence
3.3. Autocorrelation
4. Summary
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penn, R.L.; Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H.K. Imperfect Oriented Attachment: Dislocation Generation in Defect-Free Nanocrystals. Science 1998, 281, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.L.; Banfield, J.F. Oriented attachment and growth, twinning, polytypism, and formation of metastable phases; insights from nanocrystalline TiO2. Am. Mineral. 1998, 83, 1077–1082. [Google Scholar] [CrossRef]
- Penn, R.L.; Soltis, J.A. Characterising crystal growth by oriented aggregation. CrystEngComm 2014, 16, 1409–1418. [Google Scholar] [CrossRef]
- Padhi, S.K.; Krishna, M.G. Non-Classical Crystal Growth Recipe using nanocrystalline ceria a detailed review. arXiv 2019, arXiv:1911.07454. [Google Scholar]
- Cao, D.; Gong, S.; Shu, X.; Zhu, D.; Liang, S. Preparation of ZnO Nanoparticles with High Dispersibility Based on Oriented Attachment (OA) Process. Nanoscale Res. Lett. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Hazarika, S.; Mohanta, D. Oriented attachment (OA) mediated characteristic growth of Gd2O3 nanorods from nanoparticle seeds. J. Rare Earths 2016, 34, 158–165. [Google Scholar] [CrossRef]
- Song, M.; Zhou, G.; Lu, N.; Lee, J.; Nakouzi, E.; Wang, H.; Li, D. Oriented attachment induces fivefold twins by forming and decomposing high-energy grain boundaries. Science 2019, 367, 40–45. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Kang, J.; Betzler, S.; Czarnik, C.; Zhang, X.; Ophus, C.; Yu, C.; Bustillo, K.C.; Pan, M.; et al. Formation of two-dimensional transition metal oxide nanosheets with nanoparticles as intermediates. Nat. Mater. 2019, 18, 970–976. [Google Scholar] [CrossRef]
- Sushko, M.L. Understanding the driving forces for crystal growth by oriented attachment through theory and simulations. J. Mater. Res. 2019, 34, 2914–2927. [Google Scholar] [CrossRef]
- Lah, N.A.C.; Kamaruzaman, A. The optical quantification measurement on aggregated aqueous ZnO nanostructures upon exposure to tannic acid. Mater. Res. Express 2019, 6, 125046. [Google Scholar] [CrossRef]
- Lah, N.A.C.; Mohamad, N.; Saari, M.M.; Johan, M.R. Nanoscopic tannic acid-ZnO colloid: Low temperature synthesis and the influence of pH on the aggregates. Mater. Res. Express 2019, 6, 065007. [Google Scholar] [CrossRef]
- Rajan, A.K.; Cindrella, L. w-ZnO nanostructures with distinct morphologies: Properties and integration into dye sensitized solar cells. Ceram. Int. 2020, 46, 8174–8184. [Google Scholar] [CrossRef]
- Basnet, P.; Chatterjee, S. Structure-directing property and growth mechanism induced by capping agents in nanostructured ZnO during hydrothermal synthesis—A systematic review. Nano Struct. Nano Objects 2020, 22, 100426. [Google Scholar] [CrossRef]
- Kuo, C.T.; Lee, J.C.; Chen, Y.; Wu, Y.F. Structural Investigations on Hydrothermally Grown ZnO Nanostructures. In Key Engineering Materials; Trans Tech Publ.: Zürich, Switzerland, 2020. [Google Scholar]
- Thbayh, D.K.; Talib, R.A.; Ziadan, K.M. The effect of polyaniline addition on ZnO for the fabrication of high performance photodetection. MS&E 2020, 757, 012074. [Google Scholar] [CrossRef]
- Nanev, C.N. Advancements (and challenges) in the study of protein crystal nucleation and growth; thermodynamic and kinetic explanations and comparison with small-molecule crystallisation. Prog. Cryst. Growth Charact. Mater. 2020, 66, 100484. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Nobile, C. Colloidal oxide-based heterostructured nanocrystals. In Colloidal Metal Oxide Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 401–470. [Google Scholar]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal synthesis of ZnO nanostructures with controllable morphology change. CrystEngComm 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rokhum, S.L.; Gupta, R.; Chatterjee, S. Zinc oxide supported silver nanoparticles as a heterogeneous catalyst for production of biodiesel from palm oil. Environ. Prog. Sustain. Energy 2020, 39, 13369. [Google Scholar] [CrossRef]
- Vill, R.; Gülcher, J.; Khalatur, P.; Wintergerst, P.; Stoll, A.; Mourran, A.; Ziener, U. Supramolecular polymerisation: Challenges and advantages of various methods in assessing the aggregation mechanism. Nanoscale 2019, 11, 663–674. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.-B.; Ge, Z. Understanding of perovskite crystal growth and film formation in scalable deposition processes. Chem. Soc. Rev. 2020, 49, 1653–1687. [Google Scholar] [CrossRef]
- Jose, N.; Lapkin, A. Influence of Hydrodynamics on Wet Syntheses of Nanomaterials. In Advanced Nanomaterials for Catalysis and Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–59. [Google Scholar]
- Rauf, A.; Abbas, Z.; Shehzad, S.A. Interactions of Active and Passive Control of Nanoparticles on Radiative Magnetohydrodynamics Flow of Nanofluid Over Oscillatory Rotating Disk in Porous Medium. J. Nanofluids 2019, 8, 1385–1396. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Al-Khateeb, A.N.; Kyritsis, D.C.; Abu-Nada, E. Four-way coupling of particle-wall and colloidal particle-particle interactions in direct absorption solar collectors. Energy Convers. Manag. 2019, 195, 7–20. [Google Scholar] [CrossRef]
- Loza, K.; Epple, M.; Maskos, M. Stability of Nanoparticle Dispersions and Particle Agglomeration. In Biological Responses to Nanoscale Particles; Springer: Berlin/Heidelberg, Germany, 2019; pp. 85–100. [Google Scholar]
- Zareei, M.; Yoozbashizadeh, H.; Hosseini, H.R.M. Investigating the effects of pH, surfactant and ionic strength on the stability of alumina/water nanofluids using DLVO theory. J. Therm. Anal. Calorim. 2018, 135, 1185–1196. [Google Scholar] [CrossRef]
- Lu, K.; Gervasio, M. Simulation Study of Nanoparticle–Polymer Organic Suspension Stability. Adv. Theory Simul. 2019, 2, 1900010. [Google Scholar] [CrossRef]
- Chaturbedi, A.K. A Combined Computational and Experimental Study of the Heteroaggregation of Dissimilar Adsorbent Particles; Rutgers University-School of Graduate Studies, Rutgers University: New Brunswick, NJ, USA, 2019. [Google Scholar]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.B. Physicochemical Principles Governing Agglomeration and Growth Kinetics. In Handbook of Pharmaceutical Wet Granulation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–35. [Google Scholar]
- Savoie, W.C. Effect of Shape and Particle Coordination on Collective Dynamics of Granular Matter; Georgia Institute of Technology: Atlanta, GA, USA, 2019. [Google Scholar]
- Nau, M. Functional Surface Coatings and Novel Materials Derived from Hydroxypropyl Cellulose; Technische Universität Berlin: Berlin, Germany, 2020. [Google Scholar]
- Rakhsha, A.H.; Abdizadeh, H.; Pourshaban, E.; Golobostanfard, M.R.; Mastelaro, V.R.; Montazerian, M. Ag and Cu doped ZnO nanowires: A pH-Controlled synthesis via chemical bath deposition. Materialia 2019, 5, 100212. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Lu, W.; Zhang, S.; Jiang, X.; Wu, H.; Yu, D.; Ouyang, X.-K. pH-sensitive ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite beads for colon-specific release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 128, 468–479. [Google Scholar] [CrossRef]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef]
- Jang, J.-M.; Kim, S.-D.; Choi, H.-M.; Kim, J.-Y.; Jung, W.-G. Morphology change of self-assembled ZnO 3D nanostructures with different pH in the simple hydrothermal process. Mater. Chem. Phys. 2009, 113, 389–394. [Google Scholar] [CrossRef]
- Esmaeili-Zare, M.; Behpour, M. CIS/CdS/ZnO/ZnO:Al modified photocathode for enhanced photoelectrochemical behavior under visible irradiation: Effects of pH and concentration of electrolyte solution. Int. J. Hydrogen Energy 2020, 45, 8273–8281. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Lah, N.A.; Kamaruzaman, A.; Trigueros, S. pH-Dependent Formation of Oriented Zinc Oxide Nanostructures in the Presence of Tannic Acid. Nanomaterials 2021, 11, 34. https://doi.org/10.3390/nano11010034
Che Lah NA, Kamaruzaman A, Trigueros S. pH-Dependent Formation of Oriented Zinc Oxide Nanostructures in the Presence of Tannic Acid. Nanomaterials. 2021; 11(1):34. https://doi.org/10.3390/nano11010034
Chicago/Turabian StyleChe Lah, Nurul Akmal, Aqilah Kamaruzaman, and Sonia Trigueros. 2021. "pH-Dependent Formation of Oriented Zinc Oxide Nanostructures in the Presence of Tannic Acid" Nanomaterials 11, no. 1: 34. https://doi.org/10.3390/nano11010034
APA StyleChe Lah, N. A., Kamaruzaman, A., & Trigueros, S. (2021). pH-Dependent Formation of Oriented Zinc Oxide Nanostructures in the Presence of Tannic Acid. Nanomaterials, 11(1), 34. https://doi.org/10.3390/nano11010034





