Acid-Responsive Adamantane-Cored Amphiphilic Block Polymers as Platforms for Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods
2.2.1. Synthesis of Star-Shaped Macroinitiator Ad-[P(LA-co-GA)-Br]4
2.2.2. Synthesis of Ad-[P(LA-co-GA)-b-PDEAEMA]4
2.2.3. Synthesis of Star-Shaped Polymer S-PLGA-D-P
2.2.4. Synthesis of Linear Polymer L-PLGA-D-P
2.3. Preparation of the Blank Polymeric Micelles and DOX-Loaded Micelles
2.4. Characterization and Measurement
2.5. CMC Measurement
2.6. Drug Loading and In Vitro Release
2.7. In Vitro Cytotoxicity and Anticancer Efficacy Assay
2.8. Dissipative Particle Dynamics (DPD) Simulation
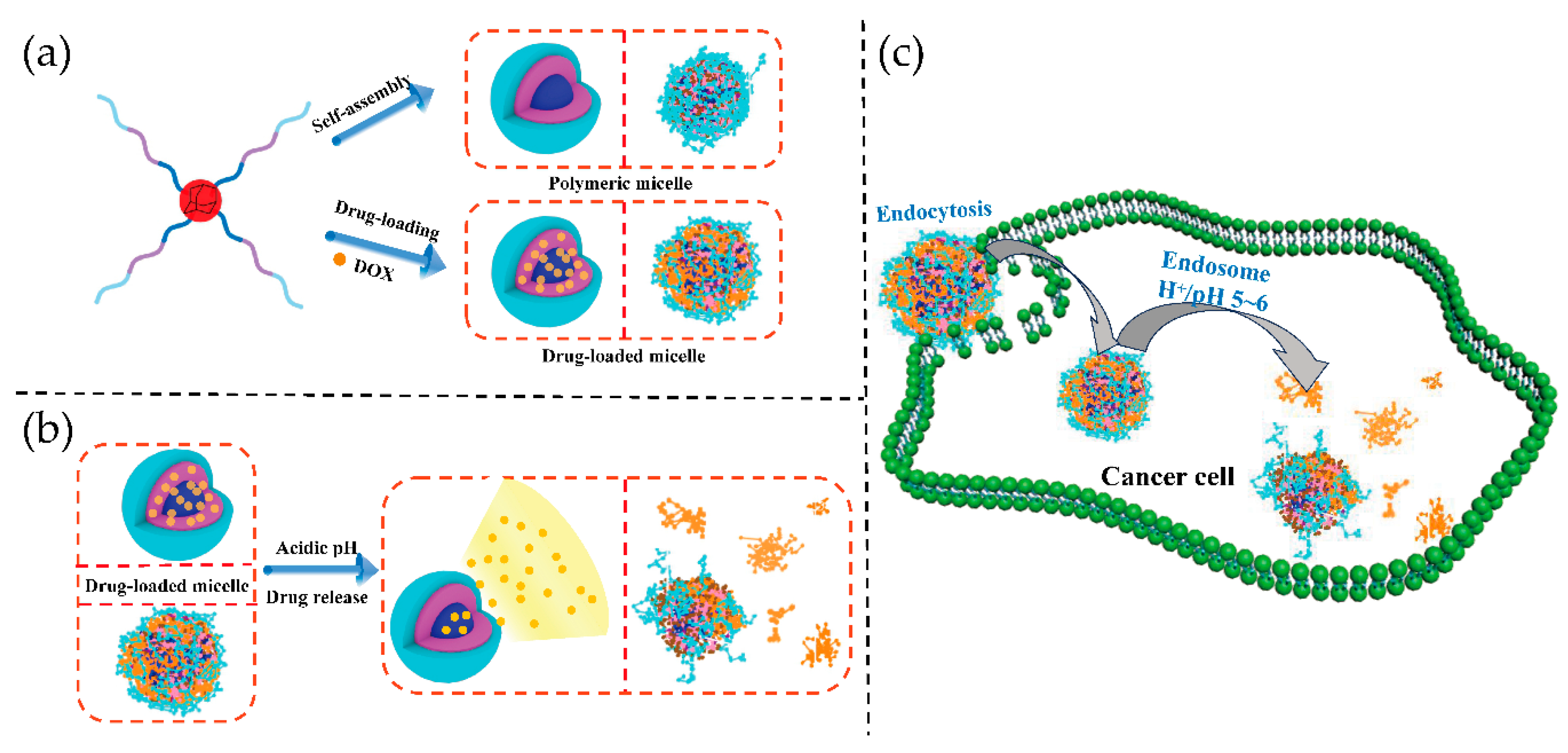
3. Results and Discussion
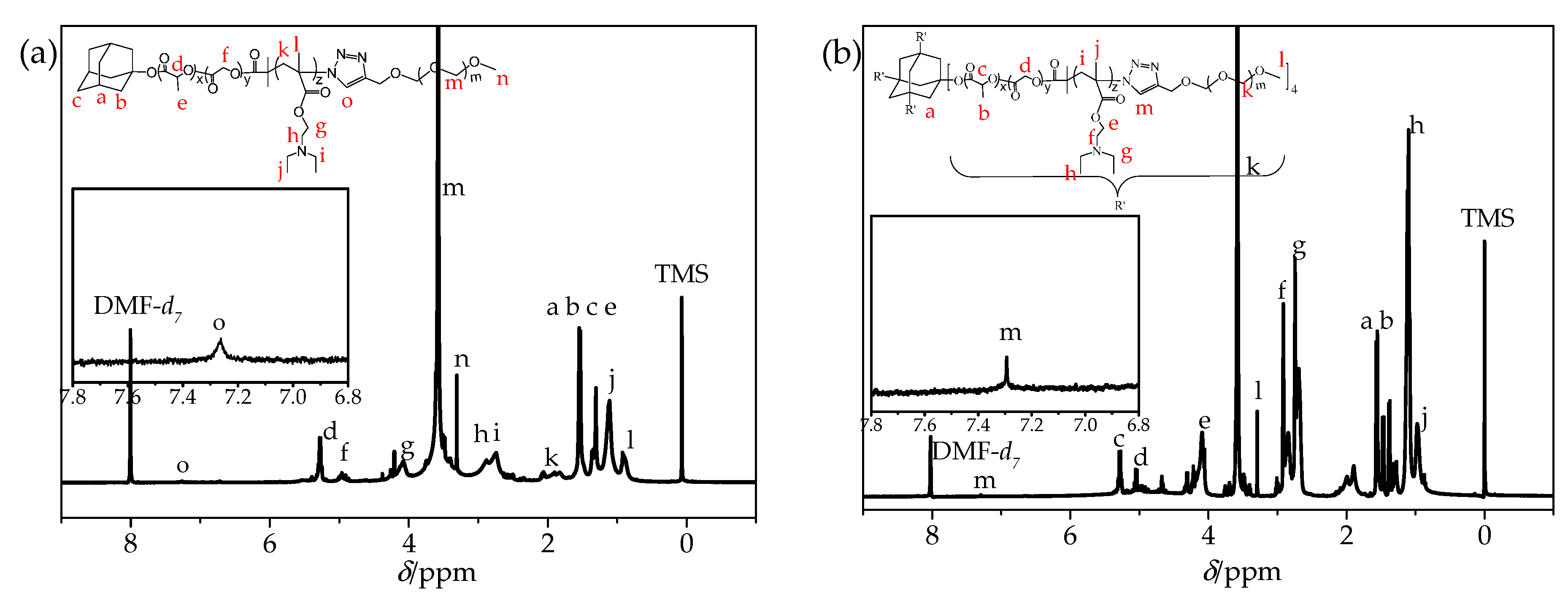
3.1. Synthesis and Characterization of Polymers
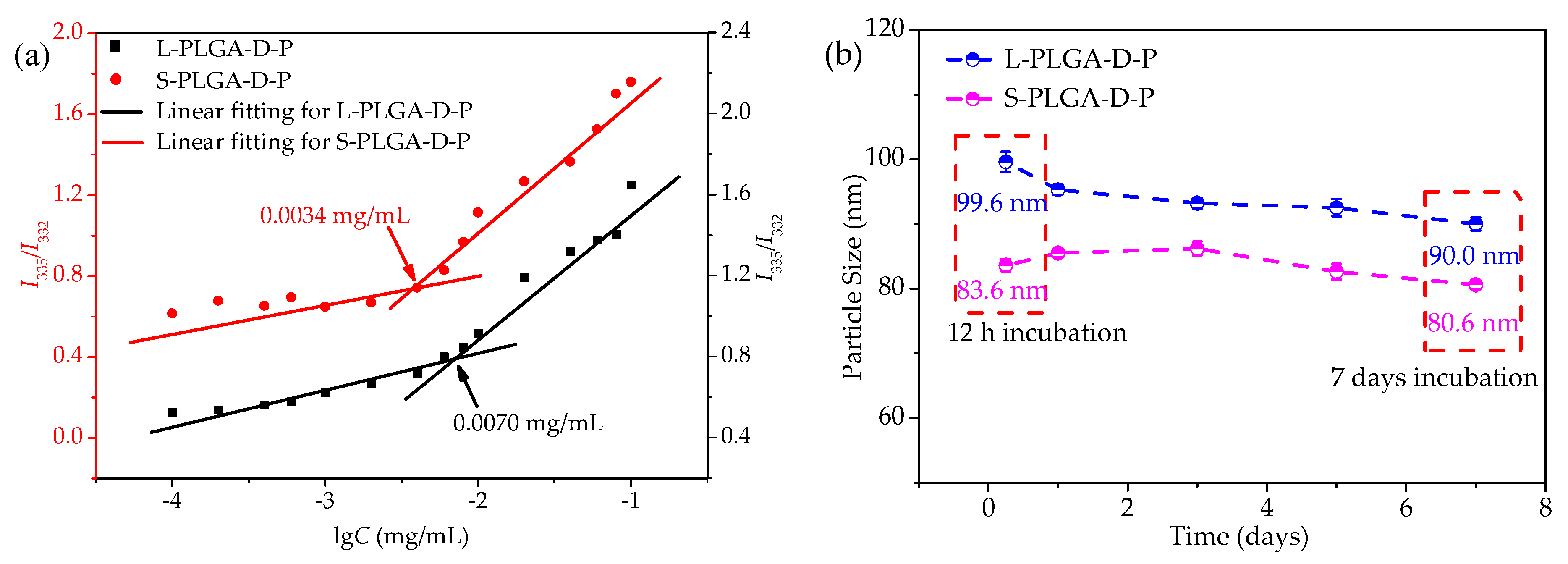
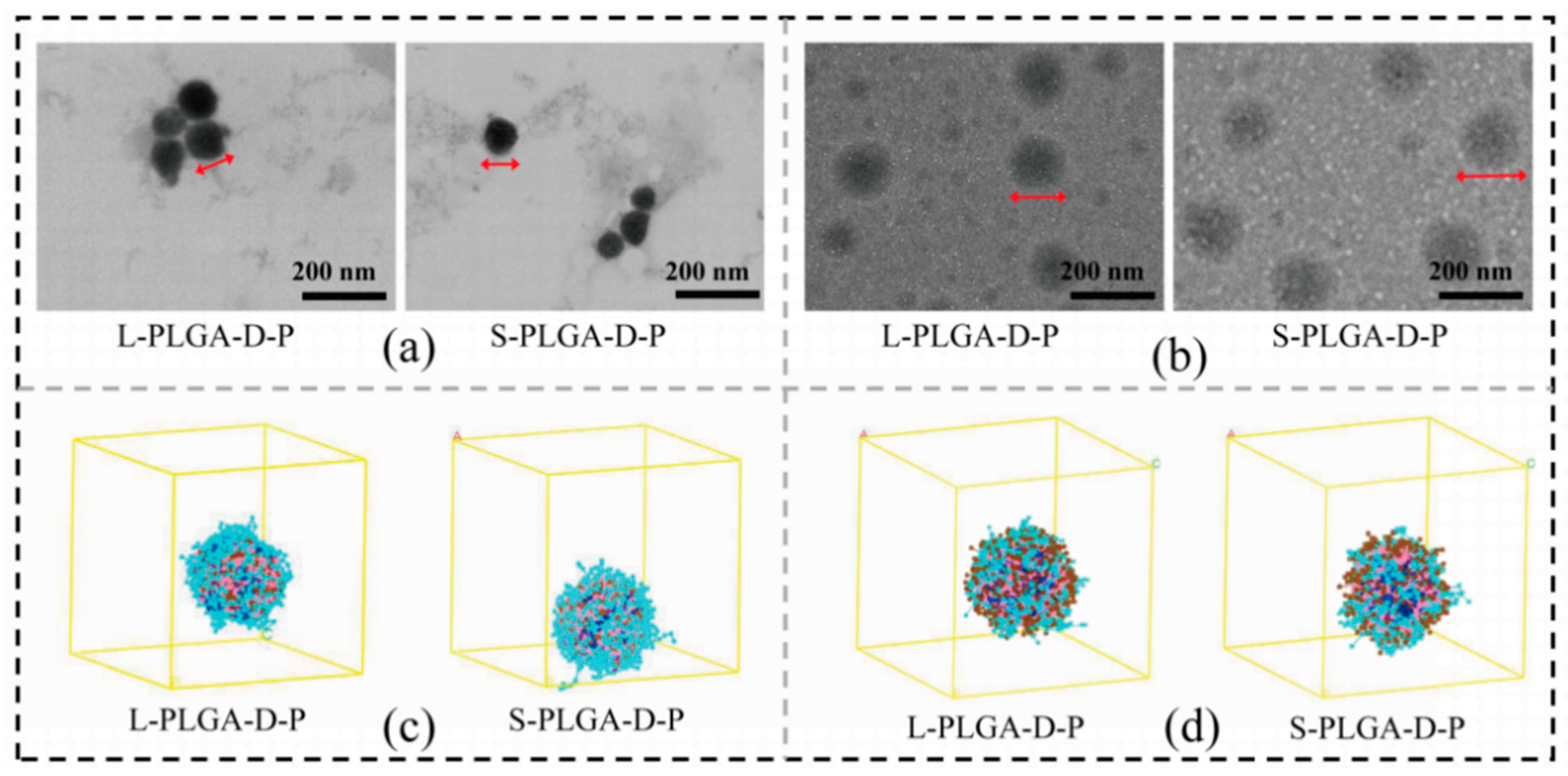
3.2. Self-Assembly and Stability of Amphiphilic Polymeric Micelles
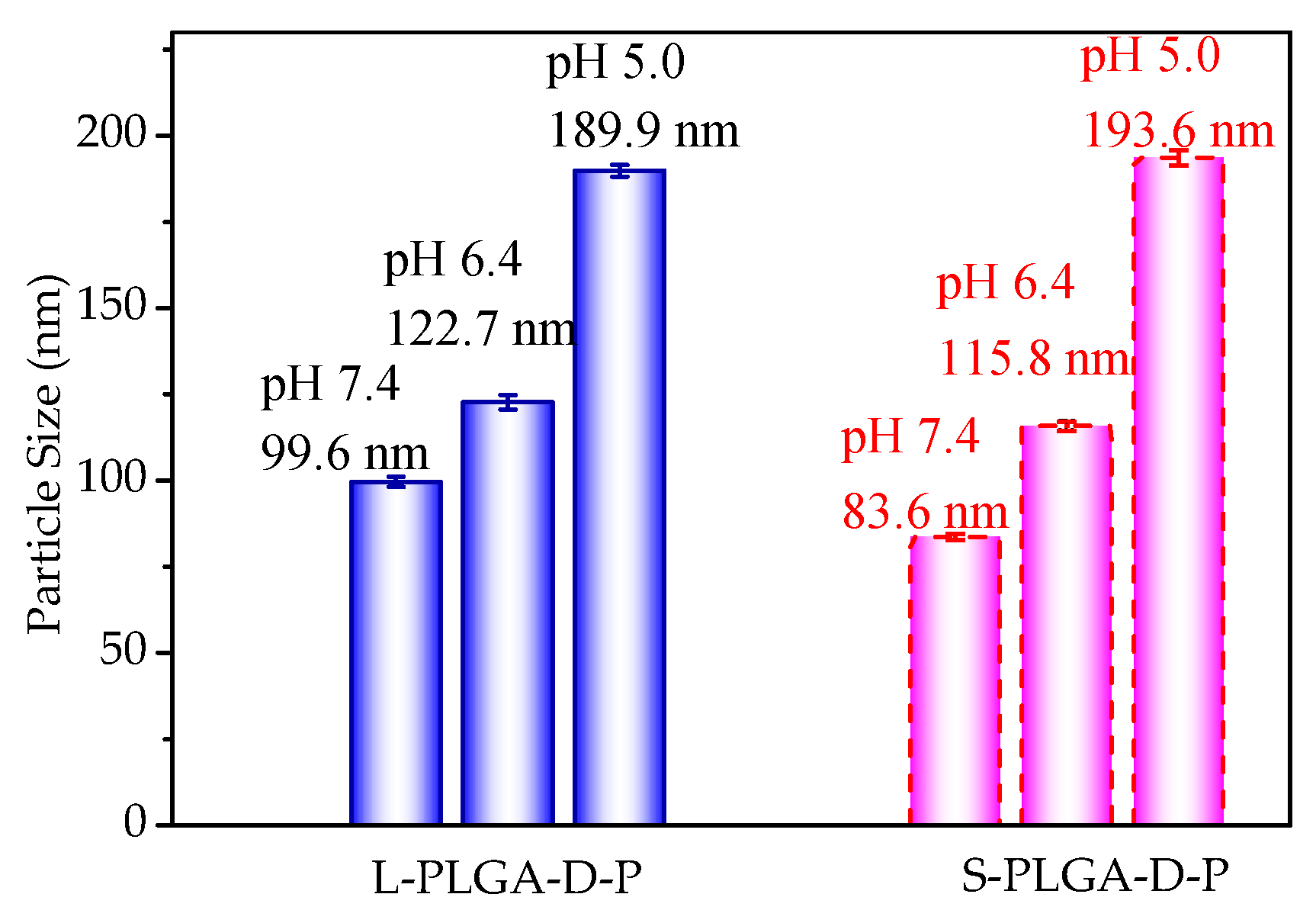
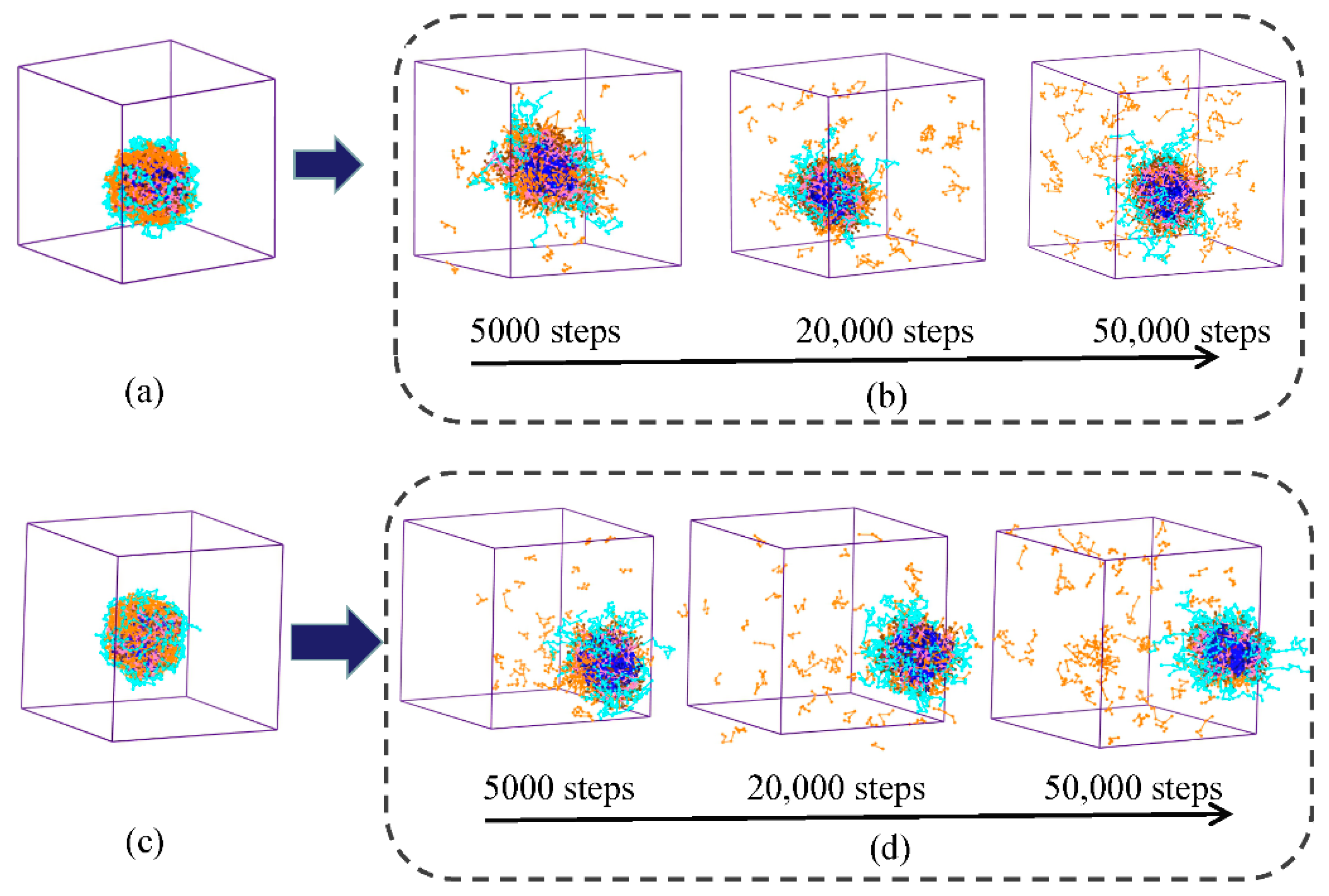
3.3. pH Response of Polymeric Micelles
3.4. Properties of DOX-Loaded Micelles
3.5. In Vitro Drug Release Behavior
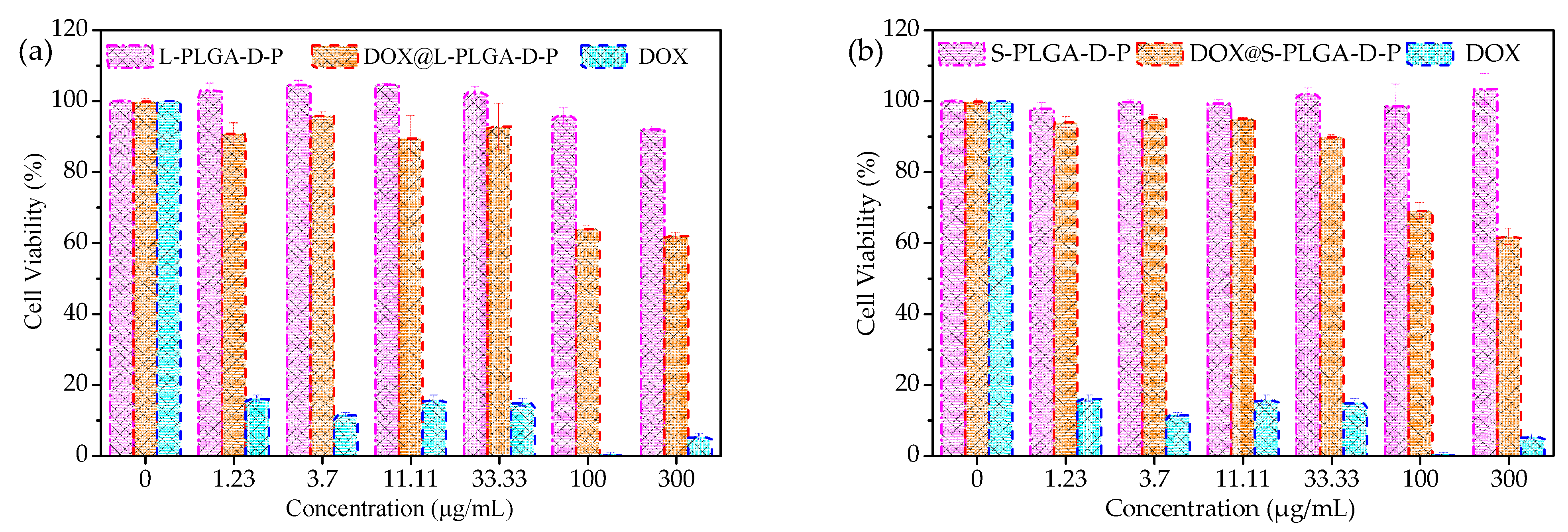
3.6. In Vitro Cytotoxicity
3.7. In Vitro Anticancer Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer Treatment and Survivorship Statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y.S. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Lu, H.Z.; Liang, S.M.; Xu, S.F. Dual Stable Nanomedicines Prepared by Cisplatin-crosslinked Camptothecin Prodrug Micelles for Effective Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 20649–20659. [Google Scholar] [CrossRef]
- Guo, X.; Shi, C.L.; Yang, G.Q.; Wang, J.; Cai, Z.H.; Zhou, S.B. Dual-responsive Polymer Micelles for Target-cell-specific Anticancer Drug Delivery. Chem. Mater. 2014, 26, 4405–4418. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Yu, L.; Ke, H.L.; Du, F.S.; Li, Z.C. Redox-responsive Fluorescent Polycarbonates Based on Selenide for Chemotherapy of Triple-negative Breast Cancer. Biomacromolecules 2019, 20, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Madrakian, T.; Ghoorchian, A.; Kamalabadi, M.; Afkhami, A. Stimuli-sensitive Drug Delivery Systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–59. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhang, Z.L.; Chen, M.H.; Xie, S.Z.; Wang, T.; Li, X.H. Synergistic Antitumor Efficacy of Hybrid Micelles with Mitochondrial Targeting and Stimuli-responsive Drug Release Behavior. J. Mater. Chem. B 2019, 7, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Nie, S.Y.; Zhong, Q.; Yang, Y.Q.; Cai, C.Z.; Wang, J.F.; Zhang, L.J. Amphiphilic Miktoarm Star Copolymer (PCL)3-(PDEAEMA-b-PPEGMA)3 as pH-Sensitive Micelles in the Delivery of Anticancer Drug. J. Mater. Chem. B 2014, 2, 4008. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Jia, F.; Chen, X.H.; Jin, Q.; Ji, J. ATP Suppression by pH-Activated Mitochondria-targeted Delivery of Nitric Oxide Nanoplatform for Drug Resistance Reversal and Metastasis Inhibition. Small 2020, 16, 2001747. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for pH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.R.; Du, H.L.; Zhang, W.J.; Zhai, G.X. Internal Stimuli-responsive Nanocarriers for Drug Delivery: Design Strategies and Applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.J.; Wen, W.Q.; Jia, Y.G.; Liu, S.; Guo, J.W. pH-Responsive Micelles Assembled by Three-armed Degradable Block Copolymers with A Cholic Acid Core for Drug Controlled-release. Polymers 2019, 11, 511. [Google Scholar] [CrossRef]
- Illy, N.; Corcé, V.; Zimbron, J.; Molinié, V.; Labourel, M.; Tresset, G.; Degrouard, J.; Salmain, M.; Guégan, P. pH-Sensitive Poly(ethylene glycol)/poly(ethoxyethyl glycidyl ether) Block Copolymers: Synthesis, Characterization, Encapsulation, and Delivery of A Hydrophobic Drug. Macromol. Chem. Phys. 2019, 220, 1900210. [Google Scholar] [CrossRef]
- Hu, D.D.; Xu, Z.P.; Hu, Z.Y.; Hu, B.H.; Yang, M.Y.; Zhu, L.J. pH-Triggered Charge-reversal Silk Sericin-based Nanoparticles for Enhanced Cellular Uptake and Doxorubicin Delivery. ACS Sustain. Chem. Eng. 2017, 5, 1638–1647. [Google Scholar] [CrossRef]
- Mao, J.; Li, Y.; Wu, T.; Yuan, C.H.; Zeng, B.R.; Xu, Y.T.; Dai, L.Z. A Simple Dual-pH Responsive Prodrug-based Polymeric Micelles for Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 17109–17117. [Google Scholar] [CrossRef]
- Qu, J.; Peng, S.; Wang, R.; Yang, S.T.; Zhou, Q.H.; Lin, J. Stepwise pH-Sensitive and Biodegradable Polypeptide Hybrid Micelles for Enhanced Cellular Internalization and Efficient Nuclear Drug Delivery. Colloids Surf. B Biointerfaces 2019, 181, 315–324. [Google Scholar] [CrossRef]
- Xu, Z.; Xue, P.; Gao, Y.E.; Liu, S.; Shi, X.; Hou, M.; Kang, Y. pH-Responsive Polymeric Micelles Based on Poly(ethyleneglycol)-b-poly(2-(diisopropylamino) ethyl methacrylate) Block Copolymer for Enhanced Intracellular Release of Anticancer Drugs. J. Colloid. Interface Sci. 2017, 490, 511–519. [Google Scholar] [CrossRef]
- Yang, C.F.; Xue, Z.L.; Liu, Y.L.; Xiao, J.Y.; Chen, J.R.; Zhang, L.J.; Guo, J.W.; Lin, W.J. Delivery of Anticancer Drug using pH-Sensitive Micelles from Triblock Copolymer MPEG-b-PBAE-b-PLA. Mater. Sci. Eng. C 2018, 84, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.B.; Chapman, R.; Chen, F.; Lu, H.; Stenzel, M.H. Swollen Micelles for The Preparation of Gated, Squeezable, pH-Responsive Drug Carriers. ACS Appl. Mater. Interfaces 2017, 9, 13865–13874. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Liu, W.P.; Zhao, L.; Xie, S.Z.; Chen, M.H.; Wang, T.; Li, X.H. Acid-labile Degradation of Injectable Fiber Fragments to Release Bioreducible Micelles for Targeted Cancer Therapy. Biomacromolecules 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.X.; Hou, M.L.; Ma, X.Q.; Bai, S.; Zhang, T.; Xue, P.; Zhang, X.L.; Liu, G.; Kang, Y.J.; Xu, Z.G. Starburst Diblock Polyprodrugs: Reduction-responsive Unimolecular Micelles with High Drug Loading and Robust Micellar Stability for Programmed Delivery of Anticancer Drugs. Biomacromolecules 2019, 20, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Yao, N.; Gu, H.W.; Wang, J.F.; Zhang, L.J. Stimuli-responsive Shell Cross-linked Micelles from Amphiphilic Four-arm Star Copolymers as Potential Nanocarriers for “pH/Redox-Triggered” Anticancer Drug Release. Polymer 2017, 114, 161–172. [Google Scholar] [CrossRef]
- Li, M.; Guo, J.W.; Wen, W.Q.; Chen, J.K. Biodegradable Redox-sensitive Star Polymer Nanomicelles for Enhancing Doxorubicin Delivery. Nanomaterials 2019, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, B.B.; Fan, Q.K.; Wu, J.N.; Wei, L.L.; Hou, J.; Guo, X.H.; Liu, Z.Y. Synthesis and Self-assembly Behavior of pH-Responsive Star-shaped POSS-(PCL-P(DMAEMA-co-PEGMA))16 Inorganic/Organic Hybrid Block Copolymer for The Controlled Intracellular Delivery of Doxorubicin. RSC Adv. 2016, 6, 61630–61640. [Google Scholar] [CrossRef]
- Shang, Y.Q.; Guo, L.X.; Wang, Z.G. Tetraphenylsilane-cored Star-shaped Amphiphilic Block Copolymers for pH-Responsive Anticancer Drug Delivery. Macromol. Chem. Phys. 2019, 220, 1900248. [Google Scholar] [CrossRef]
- Shang, Y.Q.; Zheng, N.; Wang, Z.G. Tetraphenylsilane-cored Star-shaped Polymer Micelles with pH/Redox Dual Response and Active Targeting Function for Drug-controlled Release. Biomacromolecules 2019, 20, 4602–4610. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st Century: Synthetic Polymers in Drug Delivery Applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Wang, G.Y.; Zhang, L.M. Synthesis, Self-assembly and pH Sensitivity of PDEAEMA–PEG–PDEAEMA Triblock Copolymer Micelles for Drug Delivery. React. Funct. Polym. 2016, 107, 1–10. [Google Scholar] [CrossRef]
- Yang, H.Y.; Guo, J.W.; Tong, R.; Yang, C.F.; Chen, J.K. pH-Sensitive Micelles Based on Star Copolymer Ad-(PCL-b-PDEAEMA-b-PPEGMA)4 for Controlled Drug Delivery. Polymers 2018, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Xiao, J.Y.; Xiao, W.F.; Lin, W.J.; Chen, J.R.; Chen, Q.; Zhang, L.J.; Zhang, C.Y.; Guo, J.W. Fabrication of PDEAEMA-Based pH-Responsive Mixed Micelles for Application in Controlled Doxorubicin Release. RSC Adv. 2017, 7, 27564–27573. [Google Scholar] [CrossRef]
- Yu, L.; Xie, M.M.; Li, Z.; Lin, C.Y.; Zheng, Z.; Zhou, L.Z.; Su, Y.; Wang, X.L. Facile Construction of Near-monodisperse and Dual Responsive Polycarbonate Mixed Micelles with the Ability of pH-Induced Charge Reversal for Intracellular Delivery of Antitumor Drugs. J. Mater. Chem. B 2016, 4, 6081–6093. [Google Scholar] [CrossRef]
- Wu, W.S.; Yi, P.; Zhang, J.; Cheng, Y.C.; Li, Z.W.; Hao, X.Y.; Chen, Q. 4/6-Herto-arm and 4/6-Mikto-arm Star-shaped Block Polymeric Drug-loaded Micelles and Their pH-Responsive Controlled Release Properties: A Dissipative Particle Dynamics Simulation. Phys. Chem. Chem. Phys. 2019, 21, 15222–15232. [Google Scholar] [CrossRef]
- Yang, C.F.; Yuan, C.; Liu, W.Y.; Guo, J.W.; Feng, D.C.; Yin, X.Q.; Lin, W.J.; Shuttleworth, P.S.; Yue, H.B. DPD Studies on Mixed Micelles Self-assembled from MPEG-PDEAEMA and MPEG-PCL for Controlled Doxorubicin Release. Colloids Surf. B 2019, 178, 56–65. [Google Scholar] [CrossRef]
- Lin, W.J.; Xue, Z.L.; Wen, L.Y.; Li, Y.Z.; Liang, Z.P.; Xu, J.C.; Yang, C.F.; Gu, Y.X.; Zhang, J.; Zu, X.H.; et al. Mesoscopic Simulations of Drug-loaded Diselenide Crosslinked Micelles: Stability, Drug Loading and Release Properties. Colloids Surf. B 2019, 182, 110313. [Google Scholar] [CrossRef]
- Xiong, X.B.; Binkhathlan, Z.; Molavi, O.; Lavasanifar, A. Amphiphilic Block Co-polymers: Preparation and Application in Nanodrug and Gene Delivery. Acta Biomater. 2012, 8, 2017–2033. [Google Scholar] [CrossRef]
- Yang, C.F.; Liu, W.Y.; Xiao, J.Y.; Yuan, C.; Chen, Y.X.; Guo, J.W.; Yue, H.B.; Zhu, D.Y.; Lin, W.J.; Tang, S.Q.; et al. pH-Sensitive Mixed Micelles Assembled from PDEAEMA-PPEGMA and PCL-PPEGMA for Doxorubicin Delivery: Experimental and DPD Simulations Study. Pharmaceutics 2020, 12, 170. [Google Scholar] [CrossRef]
- Ricotti, L.; Cafarelli, A.; Lacocacci, V.; Vannozzi, L.; Menciassi, A. Advanced Micro-nano-bio Systems for Future Targeted Therapies. Curr. Nanosci. 2015, 11, 144–160. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xie, X.X.; Lu, A.J.; He, B.; Chen, Y.W.; Gu, Z.W.; Luo, X.L. Cellular Internalization of Doxorubicin Loaded Star-Shaped Micelles with Hydrophilic Zwitterionic Sulfobetaine Segments. Biomaterials 2014, 35, 4517–4524. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J. Design and Preparation of Star Polymeric Micelles Drug/Gene Delivery Systems. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2016. [Google Scholar]
- Gao, Y.E.; Bai, S.; Ma, X.Q.; Zhang, X.L.; Hou, M.L.; Shi, X.X.; Huang, X.H.; Chen, J.C.; Wen, F.Q.; Xue, P.; et al. Codelivery of Doxorubicin and Camptothecin by Dual-responsive Unimolecular Micelle-based Beta-Cyclodextrin for Enhanced Chemotherapy. Colloids Surf. B 2019, 183, 110428. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Yao, N.; Qian, L.; Zhang, X.F.; Chen, Q.; Wang, J.F.; Zhang, L.J. pH-Responsive Unimolecular Micelle-gold Nanoparticles-drug Nanohybrid System for Cancer Theranostics. Acta Biomater. 2017, 58, 455–465. [Google Scholar] [CrossRef] [PubMed]



| Samples | aMn,th | Mn,GPC | Mw/Mn |
|---|---|---|---|
| Ad-P(LA-co-GA)-b-PDEAEMA | 5834 | 5110 | 1.36 |
| L-PLGA-D-P | 7834 | 7077 | 1.28 |
| Ad-[P(LA-co-GA)-b-PDEAEMA]4 | 22,772 | 19,437 | 1.37 |
| S-PLGA-D-P | 30,772 | 27,002 | 1.41 |
| Samples | DOX/Polymer (mg/mg) | LC (%) | EE (%) | aDh (nm) | b CMC (mg/mL) |
|---|---|---|---|---|---|
| DOX@L-PLGA-D-P | 0/10 | — | — | 99.6 (±2.1) | 0.0034 |
| 20/100 | 12.1 (±0.3) | 57.6 (±0.5) | 126.3 (±1.6) | ||
| 50/100 | 21.6 (±0.6) | 49.2 (±0.6) | 153.4 (±3.2) | ||
| DOX@S-PLGA-D-P | 0/10 | — | — | 83.6 (±3.0) | 0.0070 |
| 20/100 | 12.4 (±0.2) | 58.2 (±0.8) | 120.6 (±2.0) | ||
| 50/100 | 22.9 (±0.5) | 53.3 (±1.1) | 156.6 (±2.6) | ||
| PT-(PLGA-SS-mPEG)4 [23] | 20/100 | 6.66 | 39.7 | 132 | 0.0087 |
| (PCL)-(PDEAEMA-b-PPEGMA)3 [8] | 50/100 | 12.8–19.6 | 29.4–48.8 | 167–232 | 0.0035 |
| 4sPCLDEAS [42] | 20/100 | 11.4 | 51.5 | 113.7 | — |
| 6AS-PCL-PAA-PPEGMA [43] | 14/100 | 12.0 | — | — | — |
| β-CD-(PLA-PDMAEMA-PEtOxMA)21 [44] | 25/100 | 13.7 | 61.0 | — | — |
| 50/100 | 21.7 | 59.1 | — | ||
| 4AS-PLC-b-PDMAEMA [45] | 50/100 | 8.64 | 37.8 | 102.7 | 0.0041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, W.; Guo, C.; Guo, J. Acid-Responsive Adamantane-Cored Amphiphilic Block Polymers as Platforms for Drug Delivery. Nanomaterials 2021, 11, 188. https://doi.org/10.3390/nano11010188
Wen W, Guo C, Guo J. Acid-Responsive Adamantane-Cored Amphiphilic Block Polymers as Platforms for Drug Delivery. Nanomaterials. 2021; 11(1):188. https://doi.org/10.3390/nano11010188
Chicago/Turabian StyleWen, Weiqiu, Chong Guo, and Jianwei Guo. 2021. "Acid-Responsive Adamantane-Cored Amphiphilic Block Polymers as Platforms for Drug Delivery" Nanomaterials 11, no. 1: 188. https://doi.org/10.3390/nano11010188
APA StyleWen, W., Guo, C., & Guo, J. (2021). Acid-Responsive Adamantane-Cored Amphiphilic Block Polymers as Platforms for Drug Delivery. Nanomaterials, 11(1), 188. https://doi.org/10.3390/nano11010188






