Electrophoretic Deposition of Aged and Charge Controlled Colloidal Copper Sulfide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
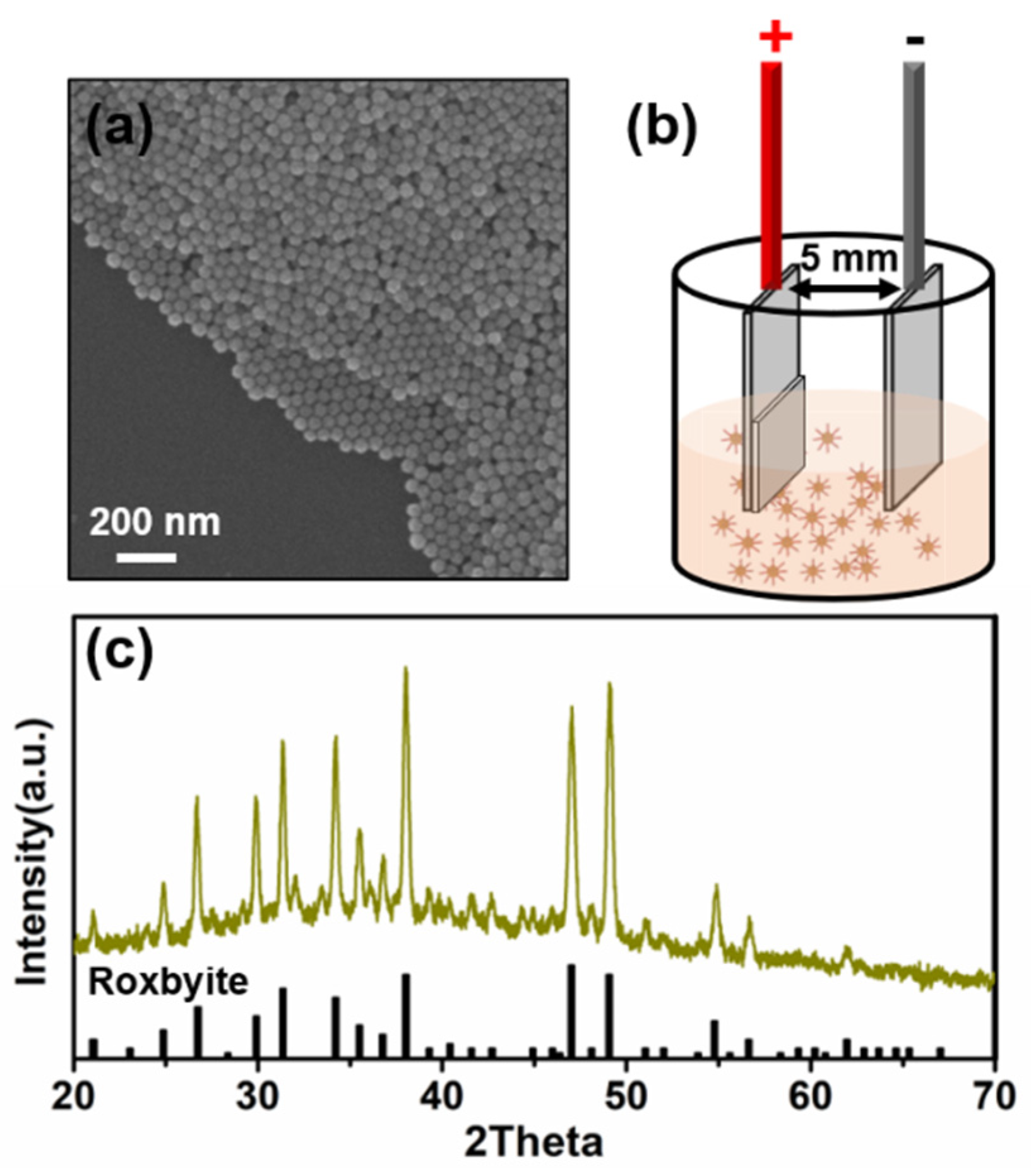
2.2. Synthesis of Colloidal Cu2-xS Nanoparticles
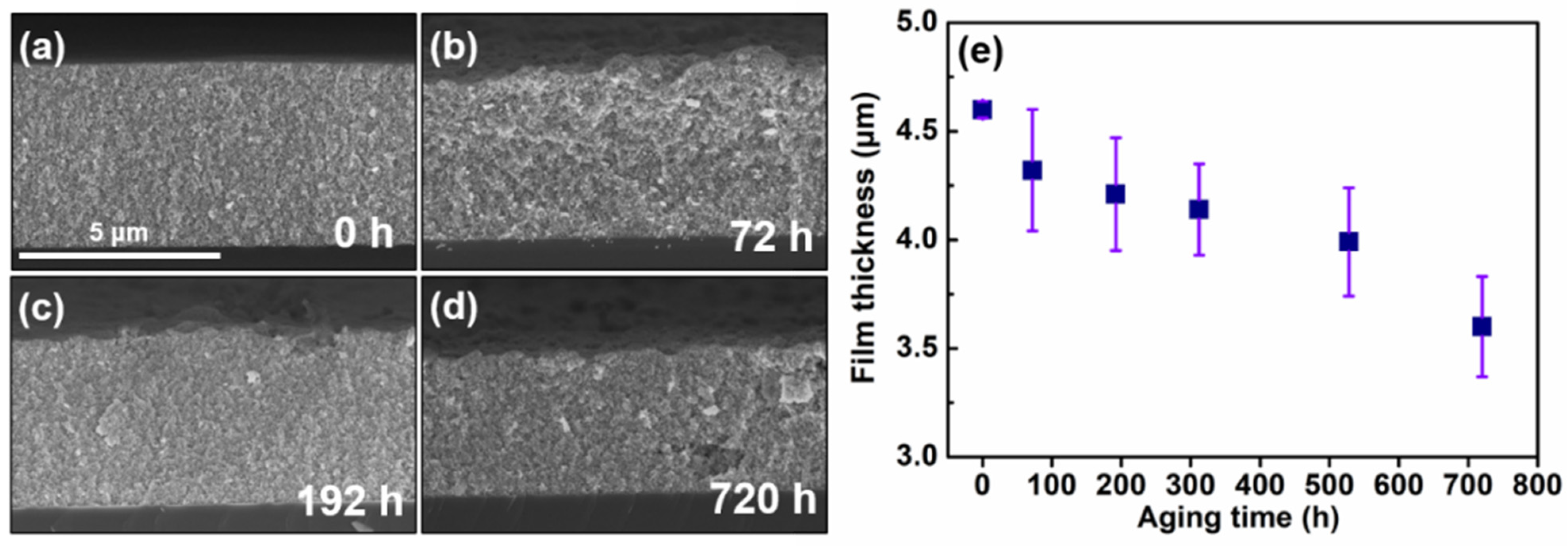
2.3. Preparation of Nanoparticle Film via the EPD Process
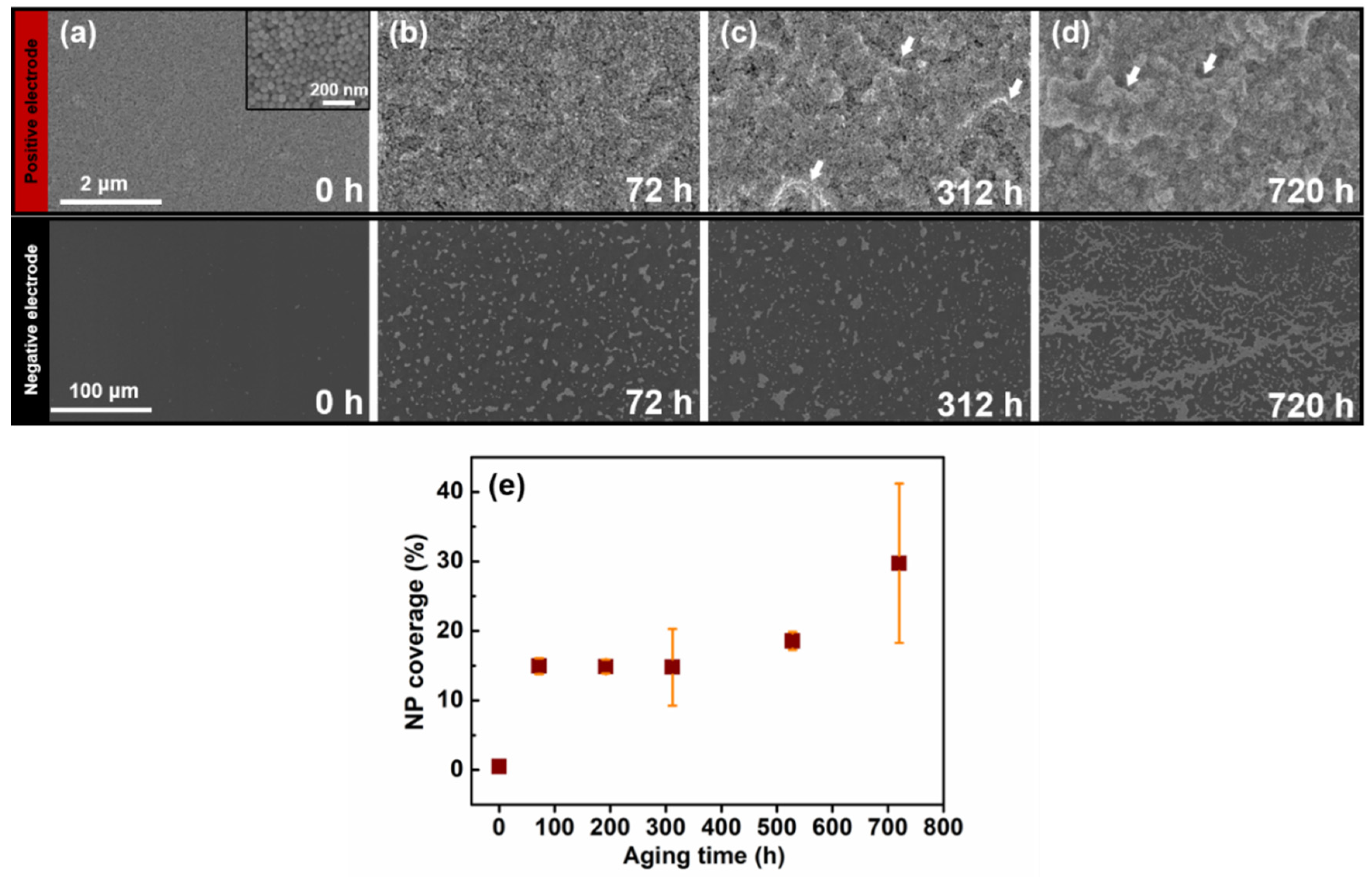
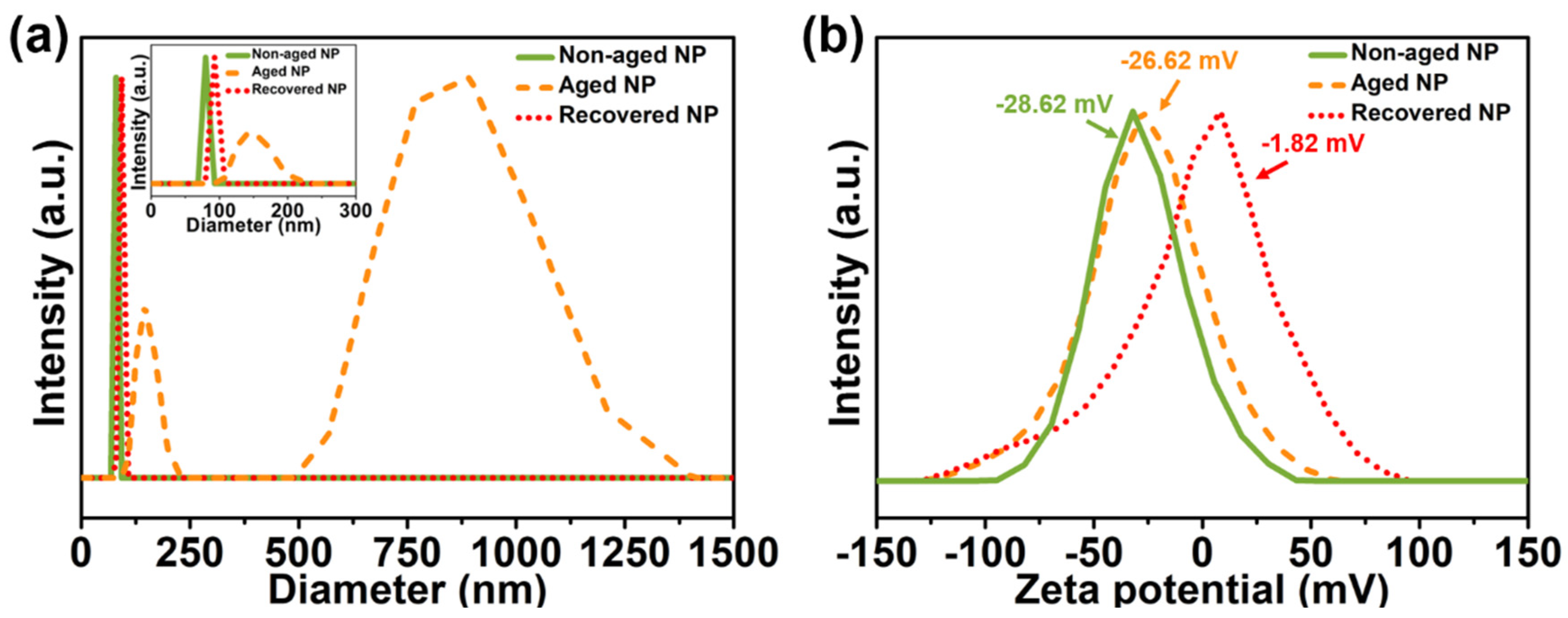
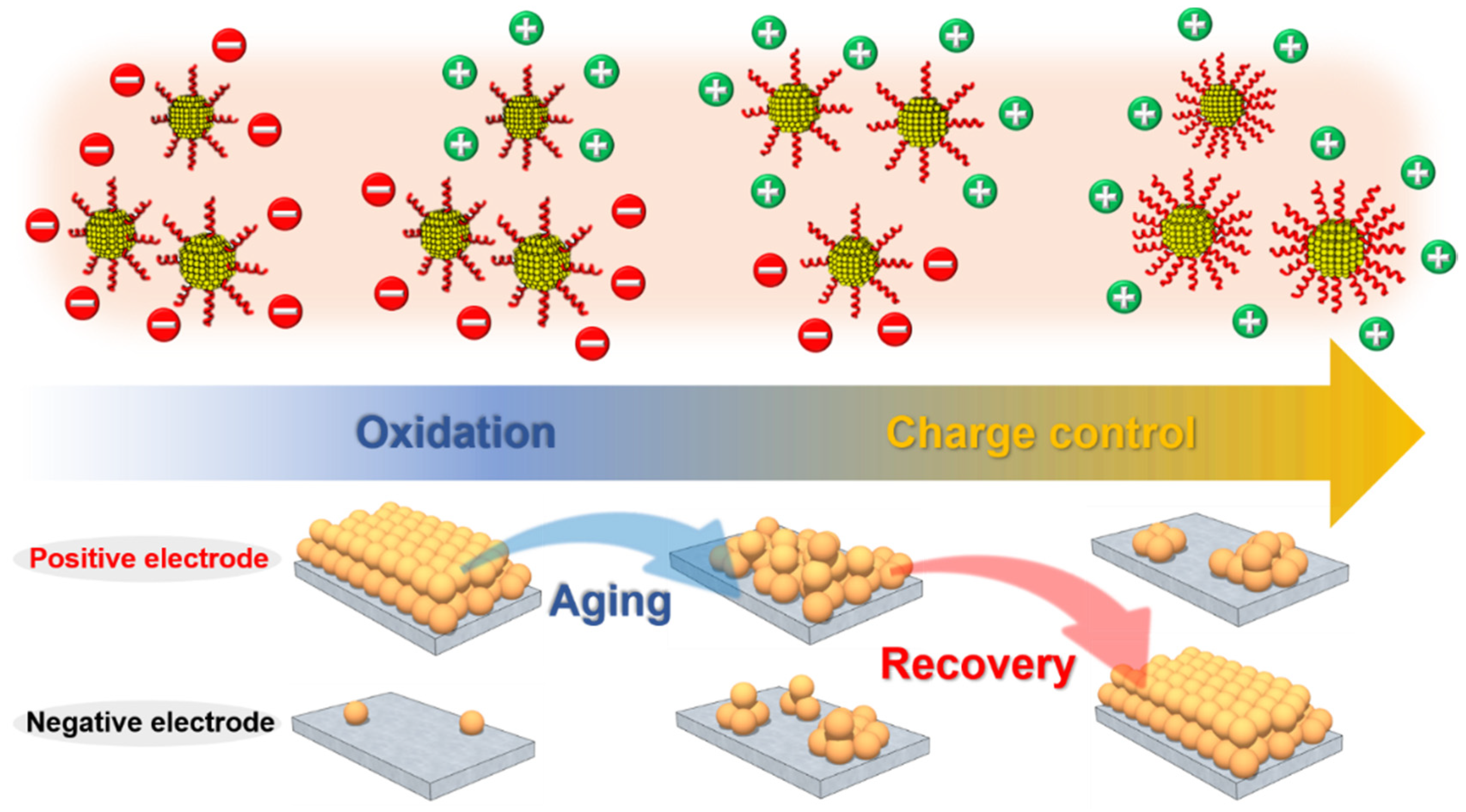
2.4. Preparation and Recovery of Aged Nanoparticles
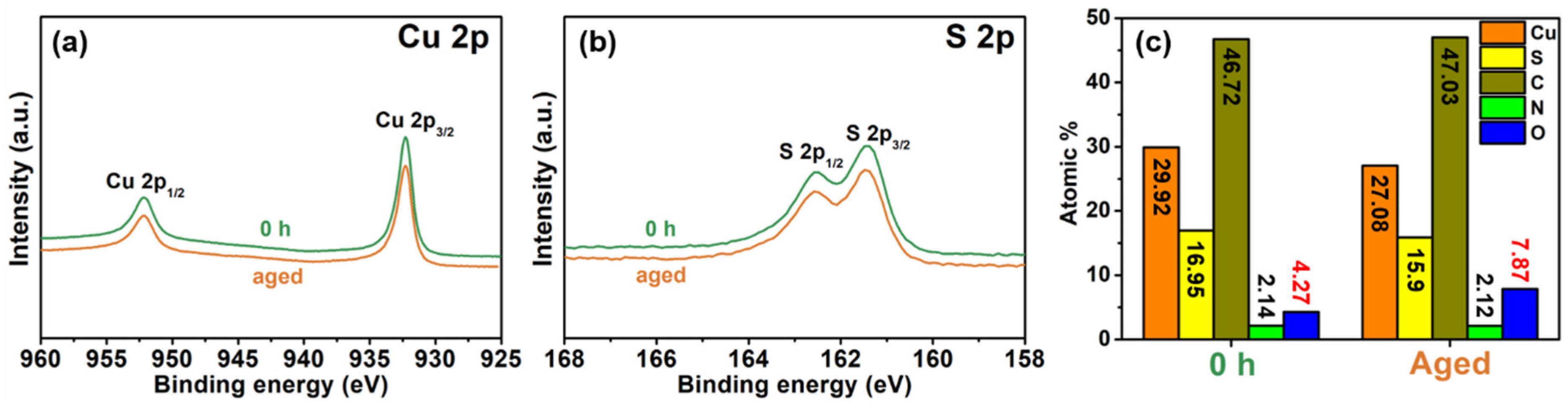
2.5. Materials Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lohse, S.E.; Murphy, C.J. Applications of Colloidal Inorganic Nanoparticles: From Medicine to Energy. J. Am. Chem. Soc. 2012, 134, 15607–15620. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Pérez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: Controlling the morphology, composition and crystallinity. CrystEngComm 2015, 17, 3727–3762. [Google Scholar] [CrossRef]
- He, M.; Protesescu, L.; Caputo, R.; Krumeich, F.; Kovalenko, M.V. A General Synthesis Strategy for Monodisperse Metallic and Metalloid Nanoparticles (In, Ga, Bi, Sb, Zn, Cu, Sn, and Their Alloys) via in Situ Formed Metal Long-Chain Amides. Chem. Mater. 2015, 27, 635–647. [Google Scholar] [CrossRef]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, L.; Han, W.; Xin, X.; Lin, Z. A general and robust strategy for the synthesis of nearly monodisperse colloidal nanocrystals. Nat. Nanotechnol. 2013, 8, 426–431. [Google Scholar] [CrossRef]
- Hafiz, S.B.; Al Mahfuz, M.M.; Scimeca, M.R.; Lee, S.; Oh, S.J.; Sahu, A.; Ko, D.-K. Ligand engineering of mid-infrared Ag2Se colloidal quantum dots. Phys. E 2020, 124, 114223. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef]
- Ha, D.-H.; Islam, M.A.; Robinson, R.D. Binder-Free and Carbon-Free Nanoparticle Batteries: A Method for Nanoparticle Electrodes without Polymeric Binders or Carbon Black. Nano Lett. 2012, 12, 5122–5130. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, C.-H.; Wang, C.-M.; Li, Y.-J.; Chu, H.-Y.; Chan, H.-H.; Huang, Y.-W.; Liao, W.-S. Large Area Nanoparticle Alignment by Chemical Lift-Off Lithography. Nanomaterials 2018, 8, 71. [Google Scholar] [CrossRef]
- Edley, M.E.; Opasanont, B.; Conley, J.T.; Tran, H.; Smolin, S.Y.; Li, S.; Dillon, A.D.; Fafarman, A.T.; Baxter, J.B. Solution processed CuSbS2 films for solar cell applications. Thin Solid Films 2018, 646, 180–189. [Google Scholar] [CrossRef]
- Song, K.W.; Costi, R.; Bulović, V. Electrophoretic Deposition of CdSe/ZnS Quantum Dots for Light-Emitting Devices. Adv. Mater. 2013, 25, 1420–1423. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Tsybeskov, L.; Alam, M.; Hafiz, S.B.; Ko, D.K.; Bratkovsky, A.M.; Wu, X.; Lockwood, D.J. Photoluminescence in PbS nanocrystal thin films: Nanocrystal density, film morphology and energy transfer. J. Appl. Phys. 2020, 128, 134301. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Černohorský, O.; Grym, J.; Yatskiv, R.; Pham, V.H.; Dickerson, J.H. Insight into Nanoparticle Charging Mechanism in Nonpolar Solvents to Control the Formation of Pt Nanoparticle Monolayers by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2016, 8, 19680–19690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2-xS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper(I) Sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Riedinger, A.; Prato, M.; Casu, A.; Genovese, A.; Guardia, P.; Sottini, S.; Sangregorio, C.; Miszta, K.; Ghosh, S.; et al. Copper Sulfide Nanocrystals with Tunable Composition by Reduction of Covellite Nanocrystals with Cu+ Ions. J. Am. Chem. Soc. 2013, 135, 17630–17637. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sakamoto, M.; Haruta, M.; Nemoto, T.; Sato, R.; Kurata, H.; Teranishi, T. Tin Ion Directed Morphology Evolution of Copper Sulfide Nanoparticles and Tuning of Their Plasmonic Properties via Phase Conversion. Langmuir 2016, 32, 7582–7587. [Google Scholar] [CrossRef]
- Ren, K.; Yin, P.; Zhou, Y.; Cao, X.; Dong, C.; Cui, L.; Liu, H.; Du, X. Localized Defects on Copper Sulfide Surface for Enhanced Plasmon Resonance and Water Splitting. Small 2017, 13, 1700867. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Widmer, R.; Erni, R.; Dubey, R.J.C.; Krumeich, F.; Kovalenko, M.V.; Bodnarchuk, M.I. Copper sulfide nanoparticles as high-performance cathode materials for Mg-ion batteries. Sci. Rep. 2019, 9, 7988. [Google Scholar] [CrossRef] [PubMed]
- Toe, C.Y.; Zheng, Z.; Wu, H.; Scott, J.; Amal, R.; Ng, Y.H. Transformation of Cuprous Oxide into Hollow Copper Sulfide Cubes for Photocatalytic Hydrogen Generation. J. Phys. Chem. C 2018, 122, 14072–14081. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, S.W.; Kim, E.J.; Park, J. In-Situ Growth of Copper Sulfide Nanocrystals on Multiwalled Carbon Nanotubes and Their Application as Novel Solar Cell and Amperometric Glucose Sensor Materials. Nano Lett. 2007, 7, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.; Guo, L.; Li, Y.; Yan, B.; Lu, W. Hollow Copper Sulfide Nanoparticle-Mediated Transdermal Drug Delivery. Small 2012, 8, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Dhasade, S.S.; Patil, J.S.; Han, S.H.; Rath, M.C.; Fulari, V.J. Copper sulfide nanorods grown at room temperature for photovoltaic application. Mater. Lett. 2013, 90, 138–141. [Google Scholar] [CrossRef]
- Otelaja, O.O.; Ha, D.-H.; Ly, T.; Zhang, H.; Robinson, R.D. Highly Conductive Cu2–xS Nanoparticle Films through Room-Temperature Processing and an Order of Magnitude Enhancement of Conductivity via Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2014, 6, 18911–18920. [Google Scholar] [CrossRef]
- Ha, D.-H.; Ly, T.; Caron, J.M.; Zhang, H.; Fritz, K.E.; Robinson, R.D. A General Method for High-Performance Li-Ion Battery Electrodes from Colloidal Nanoparticles without the Introduction of Binders or Conductive-Carbon Additives: The Cases of MnS, Cu2-xS, and Ge. ACS Appl. Mater. Interfaces 2015, 7, 25053–25060. [Google Scholar] [CrossRef]
- Jia, S.; Banerjee, S.; Herman, I.P. Mechanism of the Electrophoretic Deposition of CdSe Nanocrystal Films: Influence of the Nanocrystal Surface and Charge. J. Phys. Chem. C 2008, 112, 162–171. [Google Scholar] [CrossRef]
- Bass, J.D.; Ai, X.; Bagabas, A.; Rice, P.M.; Topuria, T.; Scott, J.C.; Alharbi, F.H.; Kim, H.-C.; Song, Q.; Miller, R.D. An Efficient and Low-Cost Method for the Purification of Colloidal Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 6538–6542. [Google Scholar] [CrossRef]
- Dickerson, J.H. Electrophoretic Deposition of Nanocrystals in Non-polar Solvents. In Electrophoretic Deposition of Nanomaterials; Dickerson, J.H., Boccaccini, A.R., Eds.; Springer: New York, NY, USA, 2012; pp. 131–155. [Google Scholar]
- Islam, M.A.; Xia, Y.; Telesca, D.A.; Steigerwald, M.L.; Herman, I.P. Controlled Electrophoretic Deposition of Smooth and Robust Films of CdSe Nanocrystals. Chem. Mater. 2004, 16, 49–54. [Google Scholar] [CrossRef]
- Li, W.; Shavel, A.; Guzman, R.; Rubio-Garcia, J.; Flox, C.; Fan, J.; Cadavid, D.; Ibáñez, M.; Arbiol, J.; Morante, J.R.; et al. Morphology evolution of Cu2-xS nanoparticles: From spheres to dodecahedrons. Chem. Commun. 2011, 47, 10332–10334. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Bai, P.; Lucas, J.M.; Alivisatos, A.P.; Xu, T. Size-Dependent Assemblies of Nanoparticle Mixtures in Thin Films. J. Am. Chem. Soc. 2013, 135, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Zabet-Khosousi, A.; Dhirani, A.-A. Charge Transport in Nanoparticle Assemblies. Chem. Rev. 2008, 108, 4072–4124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Wang, J.S.; Smith, H.E.; Brown, G.J. Stability and aging studies of lead sulfide quantum dot films: Photoabsorption, morphology, and chemical state characteristics. Mater. Chem. Phys. 2015, 154, 44–52. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; Ten Brinck, S.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef]
- Mallevre, F.; Alba, C.; Milne, C.; Gillespie, S.; Fernandes, T.F.; Aspray, T.J. Toxicity Testing of Pristine and Aged Silver Nanoparticles in Real Wastewaters Using Bioluminescent Pseudomonas putida. Nanomaterials 2016, 6, 49. [Google Scholar] [CrossRef]
- Pang, C.; Zhang, P.; Mu, Y.; Ren, J.; Zhao, B. Transformation and Cytotoxicity of Surface-Modified Silver Nanoparticles Undergoing Long-Term Aging. Nanomaterials 2020, 10, 2255. [Google Scholar] [CrossRef]
- Krejci, A.J.; Gonzalo-Juan, I.; Dickerson, J.H. Evolution of Ordering in Iron Oxide Nanoparticle Monolayers Using Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2011, 3, 3611–3615. [Google Scholar] [CrossRef]
- Singh, A.; English, N.J.; Ryan, K.M. Highly Ordered Nanorod Assemblies Extending over Device Scale Areas and in Controlled Multilayers by Electrophoretic Deposition. J. Phys. Chem. B 2013, 117, 1608–1615. [Google Scholar] [CrossRef]
- Zhu, D.; Tang, A.; Peng, L.; Liu, Z.; Yang, C.; Teng, F. Tuning the plasmonic resonance of Cu2−xS nanocrystals: Effects of the crystal phase, morphology and surface ligands. J. Mater. Chem. C 2016, 4, 4880–4888. [Google Scholar] [CrossRef]
- Tang, A.; Qu, S.; Li, K.; Hou, Y.; Teng, F.; Cao, J.; Wang, Y.; Wang, Z. One-pot synthesis and self-assembly of colloidal copper(I) sulfide nanocrystals. Nanotechnology 2010, 21, 285602. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Zhu, Y.; Qin, Y.; Shi, X.; Chen, L. Electrical and thermal transports of binary copper sulfides CuxS with x from 1.8 to 1.96. APL Mater. 2016, 4, 104805. [Google Scholar] [CrossRef]
- Ha, D.-H.; Caldwell, A.H.; Ward, M.J.; Honrao, S.; Mathew, K.; Hovden, R.; Koker, M.K.A.; Muller, D.A.; Hennig, R.G.; Robinson, R.D. Solid–Solid Phase Transformations Induced through Cation Exchange and Strain in 2D Heterostructured Copper Sulfide Nanocrystals. Nano Lett. 2014, 14, 7090–7099. [Google Scholar] [CrossRef]
- Jiang, P.; Prendergast, D.; Borondics, F.; Porsgaard, S.; Giovanetti, L.; Pach, E.; Newberg, J.; Bluhm, H.; Besenbacher, F.; Salmeron, M. Experimental and theoretical investigation of the electronic structure of Cu2O and CuO thin films on Cu(110) using X-ray photoelectron and absorption spectroscopy. J. Chem. Phys. 2013, 138, 024704. [Google Scholar] [CrossRef]
- Shan, J.; Pulkkinen, P.; Vainio, U.; Maijala, J.; Merta, J.; Jiang, H.; Serimaa, R.; Kauppinen, E.; Tenhu, H. Synthesis and characterization of copper sulfide nanocrystallites with low sintering temperatures. J. Mater. Chem. 2008, 18, 3200–3208. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Lin, J.; Hou, K.; Yang, Z.; Pi, P.; Xu, S.; Wen, X.; Cheng, J. Matchstick-Like Cu2S@CuxO Nanowire Film: Transition of Superhydrophilicity to Superhydrophobicity. J. Phys. Chem. C 2017, 121, 19716–19726. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Yu, C.-H.; Chen, Y.-C.; Lin, Y.-G. Synthesis of novel Cu2O micro/nanostructural photocathode for solar water splitting. Electrochim. Acta 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Tarachand; Hussain, S.; Lalla, N.P.; Kuo, Y.-K.; Lakhani, A.; Sathe, V.G.; Deshpande, U.; Okram, G.S. Thermoelectric Properties of Ag-Doped CuS Nanocomposites Synthesized by a Facile Polyol Method. Phys. Chem. Chem. Phys. 2018, 20, 5926–5935. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Stegemeier, J.; Levard, C.; Dale, J.G.; Noack, C.W.; Yang, T.; Brown, G.E.; Lowry, G.V. Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide. Environ. Sci. Nano 2014, 1, 347–357. [Google Scholar] [CrossRef]
- Mou, J.; Li, P.; Liu, C.; Xu, H.; Song, L.; Wang, J.; Zhang, K.; Chen, Y.; Shi, J.; Chen, H. Ultrasmall Cu2-xS nanodots for highly efficient photoacoustic imaging-guided photothermal therapy. Small 2015, 11, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, W. Synthesis and Reaction Temperature-tailored Self-assembly of Copper Sulfide Nanoplates. Nanoscale 2011, 3, 5096–5102. [Google Scholar] [CrossRef]
- Yang, P.; Tretiak, S.; Ivanov, S. Influence of Surfactants and Charges on CdSe Quantum Dots. J. Clust. Sci. 2011, 22, 405. [Google Scholar] [CrossRef]
- Rossi, L.M.; Fiorio, J.L.; Garcia, M.A.S.; Ferraz, C.P. The role and fate of capping ligands in colloidally prepared metal nanoparticle catalysts. Dalton Trans. 2018, 47, 5889–5915. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Kang, H.; Jeong, W.; Son, H.; Ha, D.-H. Electrophoretic Deposition of Aged and Charge Controlled Colloidal Copper Sulfide Nanoparticles. Nanomaterials 2021, 11, 133. https://doi.org/10.3390/nano11010133
Park Y, Kang H, Jeong W, Son H, Ha D-H. Electrophoretic Deposition of Aged and Charge Controlled Colloidal Copper Sulfide Nanoparticles. Nanomaterials. 2021; 11(1):133. https://doi.org/10.3390/nano11010133
Chicago/Turabian StylePark, Yoonsu, Hyeri Kang, Wooseok Jeong, Hyungbin Son, and Don-Hyung Ha. 2021. "Electrophoretic Deposition of Aged and Charge Controlled Colloidal Copper Sulfide Nanoparticles" Nanomaterials 11, no. 1: 133. https://doi.org/10.3390/nano11010133
APA StylePark, Y., Kang, H., Jeong, W., Son, H., & Ha, D.-H. (2021). Electrophoretic Deposition of Aged and Charge Controlled Colloidal Copper Sulfide Nanoparticles. Nanomaterials, 11(1), 133. https://doi.org/10.3390/nano11010133






